Abstract
Urate is a cause of gout, kidney stones, and acute kidney injury from tumor lysis syndrome, but its relationship to kidney disease, cardiovascular disease, and diabetes remains controversial. A scientific workshop organized by the National Kidney Foundation was held in September 2016 to review current evidence. Cell culture studies and animal models suggest that elevated serum urate concentrations can contribute to kidney disease, hypertension, and metabolic syndrome. Epidemiologic evidence also supports elevated serum urate concentrations as a risk factor for the development of kidney disease, hypertension, and diabetes, but differences in methodologies and inpacts on serum urate concentrations by even subtle changes in kidney function render conclusions uncertain. Mendelian randomization studies generally do not support a causal role of serum urate in kidney disease, hypertension, or diabetes, although interpretation is complicated by nonhomogeneous populations, a failure to consider environmental interactions, and a lack of understanding of how the genetic polymorphisms affect biological mechanisms related to urate. Although several small clinical trials suggest benefits of urate-lowering therapies on kidney function, blood pressure, and insulin resistance, others have been negative, with many trials having design limitations and insufficient power. Thus, whether uric acid has a causal role in kidney and cardiovascular diseases requires further study.
Introduction
The association of serum urate with kidney, cardiovascular (CVD), and metabolic disease has been described since the 19th century, but whether urate metabolism has a role in these diseases is controversial. Experimental and clinical evidence were presented at a Scientific Workshop held by the National Kidney Foundation in September 2016. We present a meeting summary and discuss current controversies.
Serum Urate Regulation
Urate is the end product of nucleic acid metabolism and is also generated during the breakdown of high-energy nucleotides (eg, adenosine triphosphate [ATP]). It is generated intracellularly by xanthine oxidoreductase (XOR) and transported into and exists in the circulation as plasma sodium urate. Intracellular urate concentration likely varies, but may be orders of magnitude lower than it is in serum.1
In most mammals, serum urate concentrations are low (1–3 mg/dL [60–180 μM]) due to the enzyme uricase, which degrades uric acid to 5-hydroxyisourate and allantoin. Humans and apes lack uricase due to inactivating mutations that occurred during hominoid evolution,2,3 resulting in higher circulating urate concentrations. The lower range of urate concentrations, described in people consuming traditional non-Western diets, is 2 to 4 mg/dL (120–240 μM).4 Serum urate concentrations are higher in industrialized populations (3–8 mg/dL [180–480 μM]), reflecting diets richer in purines and fructose (both of which generate urate), greater alcohol intake, increased prevalence of factors that reduce kidney urate excretion (eg, insulin resistance, renal vasoconstriction associated with hypertension, and decreased kidney function),4 and interpopulation genetic differences.5
In humans, serum urate is excreted by the kidney (two-thirds) and gut (one-third). Some circulating urate is also removed by reaction with oxidants or nitric oxide. Urate production by XOR contributes to serum concentrations,6 but the molecular physiology of epithelial urate transport is most relevant to the genetics of hyper- and hypouricemia.7
Kidney Urate Excretion
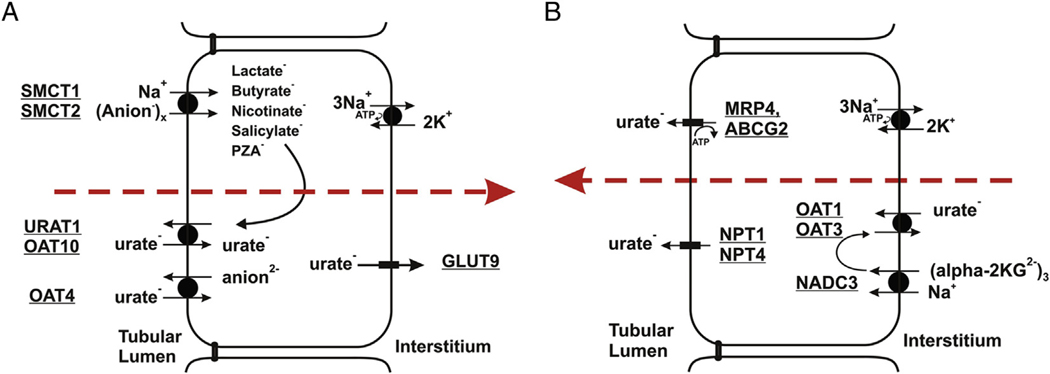
Urate is freely filtered at the glomerulus, followed by reabsorption and secretion in the proximal tubule. However, reabsorption is dominant, resulting in fractional urate excretion ≤ 10% (Fig 1).
Figure 1.
Transport pathways for urate in proximal tubule cells. (A) Urate reabsorption. Sodium-dependent anion transport by SMCT1 and SMCT2 increases intracellular concentrations of monovalent anions that exchange with luminal urate (URAT1/OAT10). OAT4 appears to exchange urate with divalent anions. GLUT9 is the exit pathway for urate at the basolateral membrane. (A) Urate secretion. Urate enters the cell at the basolateral membrane by exchange with α-ketoglutarate, mediated by OAT1 and OAT3. At the apical membrane, urate is secreted by MRP4, ABCG2, NPT1, and/or NPT4. Figure is copyright Annual Reviews and is reproduced from Mandal and Mount.7
Urate reabsorption in the proximal tubule occurs by urate/monocarboxylate exchange (Fig 1A). Organic monocarboxylate reabsorption by the apical sodium/monocarboxylate cotransporters SMCT1 and SMCT28,9 results in higher intracellular concentrations of anions that exchange with luminal urate by means of urate-anion exchangers (Fig 1A). Higher concentrations of SMCT substrates (including nicotinate, pyrazinoate, lactate, and ketones) in the circulation can lead to hyperuricemia10–13 arising from elevated apical uptake of these filtered anions, greater intracellular concentrations in proximal tubular cells, and increased apical urate/anion exchange.14 In humans, URAT1 is the main apical urate/anion exchanger.15 The “orphan” organic anion transporter known variously as ORCTL3 or OAT10 also mediates urate/nicotinate and urate/pyrazinoate exchange.16 Human OAT4 reportedly functions as an apical urate/anion exchanger17; however, unlike OAT10 and URAT1, OAT4 exchanges urate with divalent organic anions. At the basolateral membrane, GLUT9 (glucose transporter 9) is the sole pathway for urate exit during urate reabsorption. Initially identified as a fructose transporter,18 GLUT9 functions instead as a urate uniporter.19
A separate set of transporters function in urate secretion (Fig 1B). At the basolateral membrane, OAT1 and OAT3 transport urate into proximal tubular cells.20 This basolateral uptake is driven by intracellular concentration of divalent anions that exchange with urate by means of OAT1 and OAT3. Uptake of these anions is mediated by the sodium-dependent dicarboxylate transporter NaDC3 (Fig 1B). Efflux at the apical membrane is mediated by the ATP-driven pumps MRP4 (multidrug resistance protein 4)21 and ABCG2.22,23 There are also electrogenic apical urate transporters (NPT124,25 and NPT426) that function in secretion.
Gut Urate Excretion
Urate is also transported in the gut, where as much as one-third can be degraded by uricolytic bacteria. Mechanisms for urate transport in the gut are uncertain, but both GLUT9 and ABCG2 transport urate into the gut, and knockout of intestinal GLUT9 can cause hyperuricemia.27 Knockout of ABCG2 results in hyperuricemia and “overload” uricosuria.28
Definition of Hyperuricemia
Hyperuricemia is defined as serum urate concentrations > 7 mg/dL (>420 μM) in men and >6 mg/dL (>360 μM) in women. For children and adolescents, a concentration ≥ 5.5 mg/dL (≥330 μM) is considered abnormal.29 Serum urate concentrations are lower in premenopausal women due to the uricosuric effects of estrogen, and following menopause, urate increases to concentrations similar to those observed in men. The concentration of 7 mg/dL (420 μM) is viewed as abnormal because it nearly matches the solubility of urate in water; however, urate is more soluble in plasma and concentrations may be >10 mg/dL (>600 μM) without crystal deposition.
In gout, hyperuricemia results from both dietary purine excess30 and reduced urinary urate excretion. In the steady state, urinary excretion reflects the rate of production; notably, the fractional excretion of urate can increase rapidly in response to a purine load.30
Biological Actions of Urate
Antioxidant Effects
Urate can function as an antioxidant, especially in the extracellular environment.31 Urate reacts with superoxide to generate allantoin and with peroxynitrite to form triuret. These effects may be important in neurologic disease, in which acute administration of urate reduces neurologic injury in models of ischemic stroke32 or multiple sclerosis.33 In contrast, the reaction of urate with peroxynitrite generates aminocarbonyl and triuretcarbonyl radicals,34 and the reaction with myeloperoxidase generates the pro-oxidant urate hydroperoxide.35
Immune Effects
Urate may aid the immune response by release from dying cells, facilitating recognition of apoptotic cells by dendritic cells and activation of CD8 cells.36,37
Proinflammatory Effects
Although urate is an extracellular antioxidant, intracellular urate functions as a pro-oxidant, stimulating reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.38–41 The effects of exogenous urate on cells can be prevented if cellular uptake of urate is prevented by probenecid (an organic anion transport inhibitor). Likewise, biological effects of endogenously produced urate can be prevented by blocking its synthesis with XOR inhibitors (XORIs).39,42
Urate exerts autocrine, paracrine, and endocrine effects. High intracellular urate concentrations stimulate mitogen activated protein kinases, proinflammatory transcription factors (nuclear factor κB [NF-κB]), growth factors, vasoconstrictive substances (angiotensin II, thromboxane, and endothelin), chemokines, and mitochondrial dysfunction.39,43–45 Urate also reduces endothelial nitric oxide bioavailability by a variety of mechanisms and inhibits endothelial cell proliferation and migration.44,46–48 High urate concentrations induce proximal tubular dysfunction with release of inflammatory chemokines, vascular cell muscle proliferation, fat synthesis in hepatocytes, oxidative stress in islet cells, and decreased adiponectin synthesis in adipocytes.39,49–53
Models of Hypertension and Kidney Injury
The classic model of hyperuricemia in the rat involves administering a uricase inhibitor (oxonic acid) to double or triple serum urate concentration. Hyperuricemic rats develop modest hypertension mediated by activation of renal and systemic renin-angiotensin-aldosterone systems (RAAS), oxidative stress, and loss of endothelial nitric oxide.54–57 Over time, microvascular and inflammatory changes in the kidney drive hypertension independent of serum urate concentrations.58 Afferent arteriolar disease also results in impaired renal autoregulation with glomerular hypertension while simultaneously reducing renal blood flow.59 These effects can both cause chronic kidney disease (CKD)60 and accelerate existing CKD43 with histologic and renal hemodynamic features similar to those observed in persons with longstanding gout and/or hypertension.61 Experimental studies also confirmed a role of urate in animal models of diabetic kidney disease, calcineurin inhibitor nephrotoxicity, and acute kidney injury (AKI) (Table 1).43,54,55,59,62–64,66–76
Table 1.
Experimental Models in Which Lowering Serum Urate Prevents or Improves Kidney Injury
| Model | Hyperuricemia | UA-Lowering Drug | References |
|---|---|---|---|
| Oxonic acid (rat) | Yes | Allopurinol, febuxostat, benziodarone, thiadiazolopyrimidin-5-one analogue | 54, 55, 62–64 |
| Oxonic acid + 5/6 nephrectomy (rat) | Yes | Allopurinol, febuxostat | 43, 59, 65 |
| Oxonic acid + cyclosporine (rat) | Yes | 66 | |
| Tacrolimus-induced nephrotoxicity (rat) | Yes | Febuxostat | 67 |
| Cyclosporine-induced nephrotoxicity (rat) | Yes | Allopurinol, benzbromarone | 68 |
| Diabetic nephropathy associated with type 1 diabetes (rat) | No | Febuxostat | 69 |
| Diabetic nephropathy associated with type 2 diabetes (rat, mouse, in vitro culture) | Yes | Febuxostat, allopurinol | 70–72 |
| Spontaneously hypertensive rat | Not reported | Allopurinol | 73 |
| Rhabdomyolysis-induced AKI (rat) | Not reported | Allopurinol | 74 |
| Oxonic acid + cisplatin-induced AKI (rat) | Yes | Rasburicase | 49 |
| Unilateral ureteral obstructive nephropathy (rat) | Yes | Febuxostat | 75 |
| Renal ischemia-reperfusion injury (rat) | No | Febuxostat | 76 |
Abbreviations: AKI, acute kidney injury; UA, uric acid.
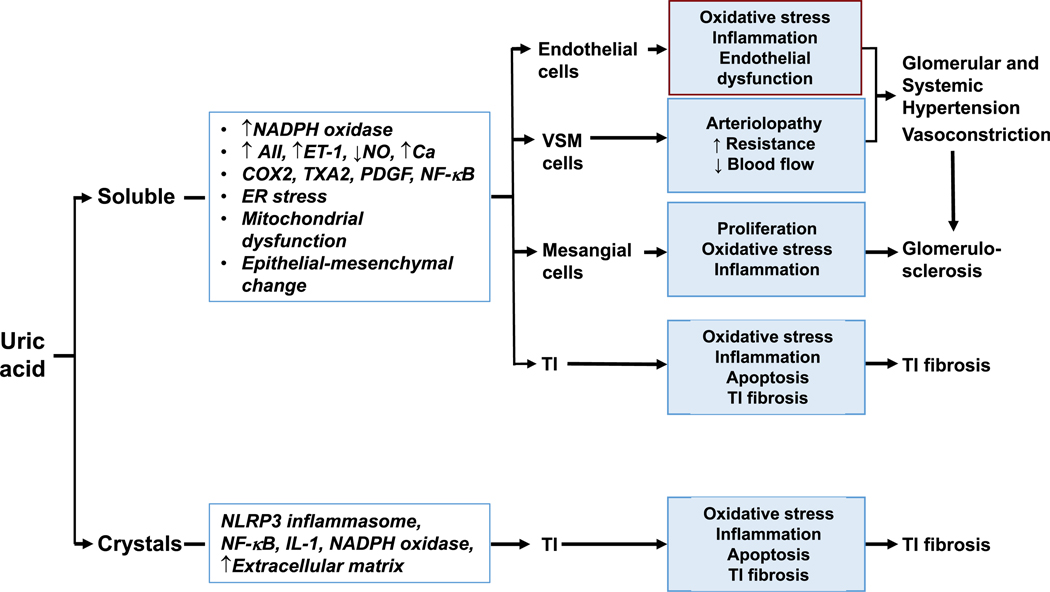
Although many effects of urate appear to be mediated by its intracellular action, kidney injury in humans can occur with hyperuricosuria, especially in the setting of urate crystalluria and acidic urinary pH. Soluble urate and urate crystals activate inflammasomes, causing local inflammation and tubular injury.77,78 Hyperuricosuria and/or hyperuricemia may also play a role in AKI (eg, rhabdomyolysis and radiocontrast administration).74,79,80 Figure 2 summarizes mechanisms by which urate induces kidney damage through crystal-independent and crystal-dependent mechanisms. Figure 3 shows a possible mechanism for urate-induced hypertension.
Figure 2.
Potential mechanisms by which urate may cause kidney disease. Urate may induce renal damage in its soluble (crystal-independent) or crystal form. After entering renal cells, soluble uric acid can activate various cascades and responses that lead to damaging inflammatory, proliferative, and maladaptive changes in glomeruli and the tubulointerstitium (TI). Crystalline uric acid seems to be confined to the TI, where it may elicit similar changes. Abbreviation: VSM, vascular smooth muscle.
Figure 3.
Mechanism of hyperuricemia-induced hypertension. Hyperuricemia-induced hypertension has been proposed to be a consequence of the effect of serum and/or intracellular urate to stimulate the renin-angiotensin-aldosterone system, lower endothelial nitric oxide, induce oxidative stress, and stimulate vascular smooth muscle cell proliferation, resulting in systemic and renal vasoconstriction and arteriolosclerosis leading to hypertension.
Fructose and Metabolic Syndrome
Fructose is distinct from most energy sources in its ability to induce features of metabolic syndrome.81 Experimental studies suggest that this occurs secondary to a decrease in ATP concentrations during fructose metabolism that leads to intracellular urate generation, mitochondrial oxidative stress, and inhibition of adenosine monophosphate (AMP)-activated protein kinase.39,52,82,83 Urate also stimulates aldose reductase (which can lead to more fructose generation) and fructokinase (which amplifies the pathway).53,84 Reducing serum and intracellular urate concentrations has been found to block features of metabolic syndrome in fructose-dependent and -independent models.85,86 The side effects of thiazides to induce features of metabolic syndrome could also be prevented by lowering serum urate concentrations with allopurinol.87
The observation that knocking down the urate transporter GLUT9 in the intestine causes hyperuricemia and metabolic syndrome that can be ameliorated by XORIs further supports a role for uric acid in the causality of metabolic syndrome.27 In contrast, with hepatic knockout of GLUT9, hyperuricemia develops without hypertension or metabolic syndrome.88
An Evolutionary Perspective
The observation that parallel inactivating mutations occurred in uricase during the Oligocene and Miocene epochs in the ancestors of humans and great apes and also in lesser apes suggests a survival advantage to higher serum urate concentrations.2,3 Various hypotheses have been proposed, including the possibility that urate may have carried a survival advantage as an antioxidant31 or as a means to help increase blood pressure (BP) during a period when salt intake was low58 or to help store fat during a time of global cooling when fruit was less available.89 Viewed in this context, the uricase mutation may have functioned as a “thrifty gene,” being protective during periods of starvation in the past, but harmful with ample access to food.
Epidemiology
Epidemiologic Studies
Many studies have evaluated whether serum urate is independently associated with CKD, hypertension, and metabolic syndrome/type 2 diabetes.90,91 Many studies are limited by disparate covariate adjustment strategies and exposure and outcome definitions, which introduce uncertainty when attempting to integrate evidence. Nevertheless, longitudinal studies have shown that elevated serum urate concentration is independently associated with hypertension in 22 of 23 published studies, including 2 meta-analyses.92–94 The relation of serum urate with the development of hypertension meets Bradford Hill criteria for a likely causal relation (Box 1).29,54,62,95–97
Box 1. Bradford Hill Criteria for Causality: Urate and Hypertension.
Strength (effect size): An elevated serum uric acid consistently predicts a 1.5- to 2-fold increased risk for hypertension within 5–10 y.92
Consistency (reproducibility): An elevated serum uric acid independently predicts the development of hypertension in 22 of 23 studies.92
Specificity: The risk for developing hypertension in those with elevated serum urate level persists after controlling for other cardiovascular risk factors. In adolescents, new-onset essential hypertension is associated with an elevated serum urate (≥5.5 mg/dL) in 90% of cases; by contrast, hyperuricemia occurs in just 30% of those with secondary hypertension and is rare in normotensive and white-coat hypertensive adolescent patients.29
Temporality: Although not all individuals with hypertension have hyperuricemia, an elevated serum urate frequently precedes the development of hypertension, and in adolescents, 90% with primary hypertension have been reported to have hyperuricemia.92 These data are most consistent with hyperuricemia being a major cause of hypertension in adolescents.
Biological gradient: A linear relationship is observed between serum uric acid and level of blood pressure in adolescents with primary hypertension (r=0.80).29
Plausibility: Experimental studies found that hyperuricemia in rats results in hypertension that is mediated by activation of the renin-angiotensin system, induction of oxidative stress, and inhibition of endothelial function.54–57
Coherence: Lowering serum urate in hyperuricemic hypertensive adolescents was observed to correct blood pressure in 86% of cases whose serum urate was lowered to <5 mg/dL.96 This was also found to be associated with a reduction in plasma renin activity consistent with experimental studies that the hypertension is dependent on the renin-angiotensin system.54,55
Experiment: Rats with experimental hyperuricemia develop hypertension that can be corrected by either a xanthine oxidase inhibitor or an uricosuric agent.54,62
Analogy: Experimentally one can also induce hypertension by stimulation of the renin-angiotensin system, blocking endothelial nitric oxide synthase, or inducing oxidative stress, all mechanisms mediated by uric acid.97
In 23 of 24 published studies, including meta-analyses,98–100 higher serum urate concentrations are independently associated with metabolic syndrome and type 2 diabetes in men, women, or both and may also be independently associated with obesity.101 Serum urate concentration was reported to independently predict incident CKD in 17 of 18 published studies and in meta-analyses in people with and without diabetes.102–107 However, serum urate is not consistently associated with progression of CKD in patients with pre-existing CKD (reviewed in108). Hyperuricemia also predicts AKI following surgery or radiocontrast exposure.79,109
In contrast, serum urate concentration is not consistently associated with CVD,110–112 likely reflecting complex causal linkages among potential risk factors used in multivariable analysis.113 Nevertheless, one study found hyperuricemia to be associated with hypertension, obesity, and CKD in Japanese adults who at baseline did not have elevated Quételet (body mass) index, had normal BP, and had normal glucose tolerance.114
A major problem with these epidemiologic studies is that serum urate concentrations are affected by kidney function, and the relationship may be subject to confounding by other factors.113,115,116 A confounder is associated with both the exposure and the outcome, but does not constitute the causal pathway between exposure and outcome. Epidemiologic studies attempt to account for confounding through multivariable adjustment and other strategies; however, if there is residual confounding, the association between serum urate and CKD may be significant even in the absence of causality.113 For example, if the true risk factor for CKD were oxidative stress (which is not directly measured) rather than hyperuricemia, the presence of hyperuricemia as a proxy for oxidative stress could lead to the incorrect conclusion that hyperuricemia causes CKD. Box 2 summarizes factors affecting the association of urate with outcomes in observational studies and clinical trials.
Box 2. Factors Affecting the Association of Serum Urate With Kidney and Cardiovascular Outcomes in Epidemiologic Studies.
Heterogeneity of patients
Heterogeneity of baseline GFR
Heterogeneity of risk factors
Limitations with GFR prediction
Competing risks (multiple hits) and competing outcomes
Varying outcome definitions and lack of a core outcomes set
Varying exposure definitions
Varying follow-up time
Adjusting for factors in the causal pathway
Unmeasured and unadjusted confounding
Abbreviation: GFR, glomerular filtration rate.
Mendelian Randomization Studies
Genome-wide association studies (GWAS) have identified approximately 30 loci controlling serum urate.117,118 The loci with the strongest effects encode uric acid transporters (eg, GLUT9, ABCG2, and URAT1)117,118 or regulatory transporter-associated proteins (eg, PDZK1).117,119 In general, loss-of-function mutations in reabsorptive urate transporters cause hypouricemia,15,117,120–123 whereas loss-of-function mutations in secretory transporters result in hyperuricemia.23,26,28 Other loci with weaker effects encode genes involved in glycolysis, consistent with a role for hepatic metabolism in urate homeostasis. However, for most loci, causal genes and causal variants have not been identified. Predictably, many of the urate-controlling loci are associated with gout.117,124,125
The identification of genetic polymorphisms that influence serum urate concentrations has allowed investigation of whether these polymorphisms also increase the risk for hypertension or kidney disease. Specifically, large populations in which GWAS have been performed can be used to develop a “genetic score” to identify individuals with genetic predisposition to hyperuricemia and gout (as evidence for validation), CKD, hypertension, and type 2 diabetes. Because persons with urate-raising and urate-lowering genetic variants have been exposed to these variants since conception and provided that genetic variants are not themselves associated with confounders or do not exhibit pleiotropic effects, these Mendelian randomization studies can mitigate confounding.126
Using this approach, many adequately powered studies have been unable to find associations between a genetic urate score with hypertension,117,127 type 2 diabetes,127–129 or the development of CKD,127,130,131 arguing against a causal role of urate in these disease states. However, the absence of associations in these studies does not conclusively dismiss causality. First, intracellular urate drives its metabolic and vascular effects (rather than extracellular concentrations or crystal deposition), and polymorphisms that affect serum urate may not have the same effect on intracellular and hepatic urate.27,88,132 Second, environment and/or diet can influence the effects that genetic polymorphisms exert on urate disposition.133,134 Third, most published studies focus on polymorphisms involved in renal urate handling without considering alternative pathways. For example, genetic polymorphisms of XOR have been linked with CVD.135,136 Finally, the inability to show an association does not mean that lowering serum urate concentrations may not have beneficial effects on hypertension or CKD; inhibitors of the renin-angiotensin system (RAAS), for example, are beneficial in the management of hypertension and CKD despite GWAS failing to identify genetic polymorphisms of the RAAS associated with hypertension and CKD.
Other Mendelian randomization studies have identified associations of genetic polymorphisms that influence serum urate concentrations with hypertension, obesity, metabolic syndrome, CVD, or CKD.137–143 These studies differ from others because they either focus on more homogeneous populations, such as Italian144,145 or Native American populations,139,146 or assess interactions of the genetic score with potential confounders, such as body mass index147 or asymmetric dimethylarginine concentrations.138
GWAS Loci Predicting Both CKD and Serum Urate Concentration
A GWAS has identified approximately 50 loci associated with estimated glomerular filtration rate (eGFR) and CKD.148 Of these, 9 are also associated with serum urate concentrations (Table 2), none of which encode urate transporters. Interestingly, patterns are the same for most shared loci, suggesting that (as yet unidentified) genetic variants associated with serum urate and kidney function are likely to be the same. However, there is inconsistency in the direction of effect, with the serum urate–increasing allele associated with better kidney function in some cases and worse kidney function in others. These data suggest that shared pathologic mechanism(s) between effectors of kidney function and determination of hyperuricemia further complicating interpretation.
Table 2.
Shared Loci for eGFR and Serum Urate Control
| Locus (function) | SNP Association | Urate | eGFR |
|---|---|---|---|
| A1CF (regulation of lipoprotein synthesis) | Same | ↑ | ↑ |
| BCAS3 | Same, additional signal in eGFR | ↑ | ↑ |
| GCKR (glycolysis) | Same | ↑ | ↑ |
| INHBC | Same | ↑ | ↓ |
| LRP2 | Different | — | — |
| PRKAG2 (energy) | Same | ↑ | ↓ |
| STC1 | Same | ↑ | ↓ |
| UBE2Q2 | Same | ↑ | ↓ |
| VEGFA | Same | ↑ | ↓ |
Note: Arrows indicate whether the effect allele of the most associated SNP at each locus increases or decreases urate concentrations or eGFR.
Abbreviations: A1CF, apolipoprotein B messenger RNA editing enzyme, catalytic polypeptide 1 complementation factor; BCAS3, breast carcinoma amplified sequence 3; eGFR, estimated glomerular filtration rate; GCKR, glucokinase regulatory protein; INHBC, inhibin beta C chain; LRP2, lipoprotein-related protein 2; PRKAG2, protein kinase adenosine-monophosphate-activated non-catalytic sub-unit gamma 2; SNP, single-nucleotide polymorphism; STC1, stanniocalcin 1; UBE2Q2, ubiquitin-conjugating enzyme E2 Q2; VEGFA, vascular endothelial growth factor A.
Clinical Trials
Hypertension
Studies of the role of urate in hypertension have largely focused on children and adolescents, reflecting experimental studies showing that hyperuricemia has its greatest effect on BP early in life, before kidney microvascular and inflammatory changes occur.55,58 Adolescents with primary hypertension have elevated serum urate concentrations (>5.5 mg/dL) in nearly 90% of cases, correlating directly with systolic BP (SBP).29 A small pilot study reported that lowering serum urate concentration to <5.0 mg/dL (<300 μM) normalized BP in 86% of patients compared to 3% during the placebo phase.96 A double-blind randomized study in which obese prehypertensive adolescents were given placebo, probenecid (a uricosuric), or allopurinol (an XORI) showed a significant decrease in BP in both the probenecid -and allopurinol-treated groups, and lowering serum urate concentration was associated with less weight gain.149 The therapeutic equivalence of probenecid and allopurinol suggests that urate-lowering mediated the effect. A third double-blind placebo-controlled study in older (>65 years) patients after ischemic stroke who had prehypertension found that treatment with allopurinol in patients with normal serum urate concentrations resulted in a decrease in clinically assessed SBP and central SBP and a reduction in carotid intimal thickness compared to placebo.150 Allopurinol lowered SBP and diastolic BP in obese middle-aged adults with prehypertension and modestly elevated serum urate concentrations (6.0–6.2 mg/dL [360–372 μM]).151 One study of asymptomatic hyperuricemia (serum urate ≥ 8 mg/dL [≥480 μM]) found a decrease in SBP with improvement in eGFR following allopurinol treatment compared to placebo.152 In contrast, 2 double-blind placebo-controlled trials found that lowering serum urate concentrations in patients with modestly elevated serum urate concentrations (6–7 mg/dL [360–480 μM] range) did not lower BP, although interpretation is limited by the fact that the mean blood pressure of participants was in the normotensive range prior to starting therapy.153–155
Chronic Kidney Disease
Small studies have reported that treatment with XORIs can slow CKD progression.156–161 A double-blind placebo-controlled trial randomly assigned 93 hyperuricemic patients with CKD stage 3 or higher to febuxostat (or placebo) for 6 months.158 Febuxostat attenuated the decline in kidney function, with 38% showing a >10% decline in eGFR versus 54% in the placebo group (P = 0.004). Febuxostat also lowered SBP (−13 vs −4 mm Hg).158
Two randomized trials reported that lowering serum urate concentrations with allopurinol could slow CKD progression in patients with CKD stage 3,156,157 with one trial showing a reduction in cardiovascular events.157,162 In another trial, 109 hyperuricemic patients with CKD stage 3 or higher and who had previously undergone cardiac surgery were randomly assigned to allopurinol or febuxostat for 6 months. Febuxostat reduced serum urate concentrations and led to favorable effects on SBP, eGFR, and albuminuria.163 Post hoc analyses of other studies also suggest beneficial effects of lowering serum urate concentrations with febuxostat and/or allopurinol on kidney function in patients with gout and CKD stage 2.159,160 In contrast, 3 studies (in patients with diabetic nephropathy,164 immunoglobulin A nephropathy,165 and stage 3 CKD166) reported no change in eGFR with allopurinol/febuxostat. Of note, these studies were either limited in duration (12 weeks)164,166 or enrolled patients with stable CKD.165 Interestingly, beneficial effects on BP165,166 and albuminuria164,166 were still observed.
Acute Kidney Injury
Several studies have investigated whether lowering serum urate concentrations may prevent AKI. One clinical trial of hyperuricemic patients undergoing cardiac surgery reported that urate lowering with rasburicase resulted in lower concentrations of the kidney tubular injury marker urine neutrophil-associated lipocalin but no difference in postoperative serum creatinine concentrations.167 In 2 trials comparing hydration to hydration plus allopurinol in the prevention of radiocontrast nephropathy, none of the 169 participants receiving allopurinol with hydration developed AKI (defined as worsening of serum creatinine by >25%) compared to 35 of the 170 (20%) participants receiving saline solution alone.168,169
Insulin Resistance and Metabolic Syndrome
Urate-lowering therapy in hyperuricemic patients has been reported to improve insulin resistance or fasting glucose concentrations.170–172 A double-blind crossover trial that randomly assigned patients to benzbromarone or placebo found that patients with heart failure and hyperuricemia showed an improvement in insulin resistance (as assessed by Homeostatic Model Assessment of Insulin Resistance [HOMA-IR]) after 8 weeks.170 In contrast, another study found that allopurinol attenuated the increase in BP resulting from a high-fructose diet, but did not improve insulin resistance.173
Finally, one study randomly assigned patients with type 2 diabetes and asymptomatic hyperuricemia (n = 176) to allopurinol or placebo for 3 years. The allopurinol-treated group had lower SBP and diastolic BP, less worsening of HOMA-IR and serum triglyceride concentration, lower albuminuria, higher eGFR, and fewer cases of new-onset diabetic nephropathy (defined as urine albumin excretion > 200 μg/min[ 4.9% vs 10%).161
Additional Issues With Use of XORIs
XORIs are ideal urate-lowering agents because they block production and will reduce both intra- and extracellular urate. In contrast, uricosuric agents may block urate uptake into cells,38,44 but will not block intracellular urate production, such as occurs during fructose metabolism. Because most of the cardiovascular and kidney effects of urate are thought to be mediated by intracellular urate, XORIs are thought to be superior to uricosurics in blocking urate’s biological effects. Nevertheless, interpretation of studies using XORIs are confounded because the conversion of hypoxanthine and xanthine to urate by XOR results in the production of oxidants. Thus, blocking XOR also reduces oxidative stress that may be independent of urate. One study reported that XORIs could improve endothelial dysfunction, whereas probenecid could not,174 and other studies also suggest that XOR may also be induced by oxidants generated from other sources (such as from mitochondria or NADPH oxidase) to amplify local oxidative stress.175 Nevertheless, the benefit of XOR inhibition on fat accumulation in cultured hepatocytes can be blocked by adding urate back to the incubation mixture.39 Uricosuric agents have also been reported to improve BP and insulin resistance in 2 studies.149,170
Summary
When considering clinical trials of urate-lowering therapy in hypertension, it appears that the effects of urate-lowering therapy on BP are most likely to be observed among patients with hyperuricemia (especially if serum urate is >8 mg/dL [>476 μM]) when baseline SBP is >130 mm Hg and GFR is normal. Likewise, when considering trials of urate-lowering therapy and effects on CKD progression, it appears there may be benefits in patients with hyperuricemia and in longer duration trials sufficient to see non–hemodynamically-mediated changes in eGFR.
Angiotensin-converting enzyme (ACE) inhibitors and other blockers of the RAAS may impact the association between urate and kidney outcomes, with experimental studies and clinical studies of humans suggesting that hyperuricemia affects both blood pressure and kidney function in part by activation of the RAAS.41,55,96 In this regard, withdrawal of allopurinol in one study of patients with CKD resulted in worsening of BP and more rapid CKD progression only in patients who were not concurrently treated with ACE inhibitors.176 In addition to the possibility that lowering urate concentrations might down-regulate the RAAS, the angiotensin receptor blocker losartan can also lower serum urate concentration by increasing urine urate excretion. Hence, studies in which agents that block the RAAS are used may obscure effects of urate-lowering therapy and vice versa. Because RAAS blockade is commonly used in CKD, a trial of urate-lowering therapy may be primarily addressing whether decreasing serum urate concentrations provides benefit above and beyond that provided by RAAS inhibitors.
The safety of urate-lowering therapies must be considered. In rare cases, allopurinol can cause a hyper-sensitivity syndrome that resembles Stevens-Johnson syndrome, especially in persons with the HLA-B*58 serotype.177 There has also been some concern that use of febuxostat may be associated with increased cardiovascular risk compared to allopurinol, resulting in a recent US Food and Drug Administration alert.178,179 Uricosuric agents may increase the risk for kidney stones; in phase 3 trials, lesinurad caused transient increases in serum creatinine concentrations when used at high doses, and without concomitant XORIs.180
In summary, although pilot clinical trials of urate-lowering therapy suggest potential benefits in the treatment of hypertension and prevention of kidney disease and CVD, they have been limited in size and power and have generally used intermediate or surrogate end points. Comparisons of urate-lowering therapy and placebo on top of standard therapies (including RAAS inhibitors) are underway (Table 3) or planned (Box 3) in several adequately powered trials with hard cardiovascular and/or kidney end points. In particular, the Preventing Early Renal Function Loss (PERL) Consortium is randomly assigning 400 adults with type 1 diabetes, mild to moderate CKD with albuminuria, and serum urate concentrations ≥ 4.5 mg/dL to allopurinol or placebo, with allopurinol titrated to reduce serum urate concentrations to <4.5 mg/dL. The primary outcome of this 3-year intervention trial is GFR measured using iohexol, assessed 2 months after intervention washout to diminish the influence of possible hemodynamic effects.181
Table 3.
Summary of Randomized Clinical Trials in the Field of Serum Urate and Cardiovascular Diseases
| CV field | Intervention | Primary Outcomes | ID No. and Status |
|---|---|---|---|
| BP control | Febuxostat vs allopurinol | Clinic BP and ABPM | NCT01701622a; terminated (unable to enroll participants) |
| Coronary endothelial dysfunction | Febuxostat vs placebo | Coronary flow | NCT01763996a; completed |
| BP control | Febuxostat vs placebo | ABPM | NCT01496469a; completed |
| Exercise tolerance in chronic angina | Febuxostat vs placebo | ETT | NCT01549977a; terminated |
| Vascular structure and function (FORWARD) | Febuxostat vs allopurinol | Carotid-femoral PWV | EudraCT 2014–5567-33; enrollment closed |
| New-onset metabolic syndrome (FAST) | Febuxostat vs placebo | Insulin resistance and features of metabolic syndrome | NCT01654276a; ongoing |
| BP and CV complications (CARES) | Febuxostat vs allopurinol | MACE | NCT01101035a; ongoing |
| Treatment of CHD (ALL-HEARTY) | Allopurionl vs placebo | MACE | EudraCT 2013–003559-39; Ongoing |
| Cerebrovascular protection (XILO-FIST) | Allopurinol vs placebo | White matter protection | NCT02122718a; starting Recruitment |
| Major CV diseases (FREED) | Febuxostat vs placebo | MACE | NCT01984749a; ongoing |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; CHD, coronary heart disease; CV, cardiovascular; ETT, exercise tolerance testing; MACE, major adverse cardiovascular events; PWV, pulse wave velocity.
Box 3. Potential RCTs Assessing Urate-Lowering for Kidney Disease and Cardiovascular Disease Benefits.
RCT #1: General Population
Population: Patients with asymptomatic hyperuricemia (≥6 mg/dL), HTN, and additional CV or CKD risk
Intervention: XOI fixed or titrated dose, probenecid or lesinurad fixed or titrated dose, placebo
Outcomes: GFR slope, BP change (no. of medications), 30% decline in eGFR; CV outcomes; AEs; urine ACR
Duration: 5 y with a priori–defined longer term posttreatment follow-up
RCT #2: CKD Population
Population: Asymptomatic hyperuricemia (≥6 mg/dL), HTN, and eGFR < 60 mL/min/1.73 m2 with albuminuria or eGFR < 45 mL/min/1.73 m2 regardless of albuminuria Intervention: Allopurinol/febuxostat fixed or titrated dose, placebo
Outcomes: 30% decline in eGFR; composite of ESRD, kidney failure death, or 50% decline in eGFR; CV outcomes; AEs; urine ACR
Duration: 4 y with a priori–defined longer term posttreatment follow-up
RCT #3: AKI Risk Population
Population: Patients at risk for AKI (planned major CV surgery)
Intervention: Allopurinol/febuxostat fixed dose preprocedure for several weeks
Outcomes: AKIN stage 3, AKIN stage 1
Abbreviations: ACR, albumin-creatinine ratio; AE, adverse event; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; (e) GFR, (estimated) glomerular filtration rate; ESRD, end-stage renal disease; HTN, hypertension; RCT, randomized clinical trial; XOI, xanthine oxidase inhibitor.
Conclusions
Though hyperuricemia was considered a potential cause of hypertension by Mahomed in the 1870s, after 140 years, the potential causal role of urate in kidney disease and hypertension is still hotly debated. Hyperuricemia is a biomarker for kidney and cardiovascular risk, but serum urate concentration also increases as GFR decreases. Although serum urate concentration is a strong independent risk marker for incident CKD and hypertension, Mendelian randomization studies do not support urate as a causal factor in these conditions. At this time, in concordance with a recent Cochrane review,182 and given potential toxicities of current treatments, we cannot recommend routine treatment of hyperuricemia in persons with hypertension, kidney disease, or metabolic syndrome/type 2 diabetes. Rather, we must await the results of well-designed and adequately powered clinical trials.
Acknowledgements:
The authors recognize the following conference participants, whose contributions to breakout groups and to the lively discussion throughout the conference rendered the manuscript more substantive: Angelo Chamorro, David Cherney, Hyon Choi, Jesse Dawson, Alessandro Doria, Lawrence Edwards, Ahsan Ejaz, Mehdi Fini, John Forman, Eric Gaucher, Diana Jalal, Duk-Hee Kang, Masanari Kuwabara, Jose Luno, Magdalena Madero, Marilda Mazzali, Takahiko Nakagawa, Alan C. Pao, Roberto Pontremoli, Carlos A. Roncal, Kenneth G. Saag, Mark Stewart Segal, Jasvinder Singh, Janet Snell-Bergeon, Robert Terkeltaub, Adrienne Tin, and Saroja Voruganti. The members of the Symposium also acknowledge the important contributions of Dr Christopher King, who died prematurely after the symposium, and thank Dr Saroja Voruganti for critical review of the manuscript and Dr Eli Stahl for contributions to Table 2.
Support: We thank Takeda Pharmaceuticals, Inc for funding the symposium; of note, Takeda did not participate in the discussions and had no influence on the published proceedings.
Financial Disclosure: Dr Johnson is an inventor on patents related to lowering uric acid as it relates to BP, insulin resistance, and diabetic kidney disease and has equity in XORT Therapeutics, Inc and Colorado Research Partners LLC, which are startup companies interested in developing novel xanthine oxidase inhibitors and fructokinase inhibitors, respectively. Dr Johnson has also received honoraria from Danone and Astra Zeneca and is on the Scientific Board of Kibow, Inc. Dr Borghi received honoraria from Menarini Int, Takeda, Teejin, and Astrellas Drug Company and is on the Scientific Board of Menarini Int and Grunenthal. Dr Bakris is the principal investigator of the FIDELIO trial (Bayer) and on the steering committee of 2 other renal outcome trials, SONAR (AbbVie) and CREDENCE (Janssen), and 1 resistant hypertension trial, CALM-2 (Vascular Dynamics) and is a consultant for Merck and Relypsa. Dr Chertow has consulted for Astra Zeneca (formerly Ardea Biosciences). Dr Sanchez Lozada receives research support from Danone Research and Kibow Biotech, Inc and is a member of Colorado Research Partners, LLC. Dr Mount has involvement with UpToDate (hypouricemia card), Horizon Pharma (consultant), Kowa Pharmaceuticals (consultant), and Astra Zeneca (grant support). Dr Moe did consultations for AbbVie, Allena, Ardelyx, Genzyme-Sanofi, Relypsa, and Tricida. Dr Merriman has received research funding and has consulted with Ardea Biosciences and Ironwood Pharmaceuticals.
Footnotes
The other authors declare that they have no relevant financial interests.
References
- 1.Kim KM, Henderson GN, Ouyang X, et al. A sensitive and specific liquid chromatography-tandem mass spectrometry method for the determination of intracellular and extracellular uric acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(22):2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kratzer JT, Lanaspa MA, Murphy MN, et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci U S A. 2014;111(10):3763–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–653. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25(1): 3–8. [DOI] [PubMed] [Google Scholar]
- 5.Merriman TR. Population heterogeneity in the genetic control of serum urate. Semin Nephrol. 2011;31(5):420–425. [DOI] [PubMed] [Google Scholar]
- 6.Gondouin B, Jourde-Chiche N, Sallee M, et al. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron. 2015;131(3):167–174. [DOI] [PubMed] [Google Scholar]
- 7.Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–345. [DOI] [PubMed] [Google Scholar]
- 8.Coady MJ, Chang MH, Charron FM, et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557(pt 3):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivas SR, Gopal E, Zhuang L, et al. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2). Biochem J. 2005;392(pt 3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfinger S, Klinenberg E Jr, Seegmiller JE. Renal retention of uric acid induced by infusion of beta-hydroxybutyrate and acetoacetate. N Engl J Med. 1965;272:351–355. [DOI] [PubMed] [Google Scholar]
- 11.Gibson HV, Doisy EA. A note on the effect of some organic acids upon the uric acid excretion of man. J Biol Chem. 1923;55:605–610. [Google Scholar]
- 12.Gershon SL, Fox IH. Pharmacologic effects of nicotinic acid on human purine metabolism. J Lab Clin Med. 1974;84(2): 179–186. [PubMed] [Google Scholar]
- 13.Shapiro M, Hyde L. Hyperuricemia due to pyrazinamide. Am J Med. 1957;23(4):596–599. [DOI] [PubMed] [Google Scholar]
- 14.Guggino SE, Aronson PS. Paradoxical effects of pyrazinoate and nicotinate on urate transport in dog renal microvillus membranes. J Clin Invest. 1985;76(2):543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–452. [DOI] [PubMed] [Google Scholar]
- 16.Bahn A, Hagos Y, Reuter S, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem. 2008;283(24):16332–16341. [DOI] [PubMed] [Google Scholar]
- 17.Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007;18(2): 430–439. [DOI] [PubMed] [Google Scholar]
- 18.Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24(5–6):455–463. [DOI] [PubMed] [Google Scholar]
- 19.Anzai N, Ichida K, Jutabha P, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40): 26834–26838. [DOI] [PubMed] [Google Scholar]
- 20.Eraly SA, Vallon V, Rieg T, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genom. 2008;33(2):180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Aubel RA, Smeets PH, Van Den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288(2):F327–F333. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo H, Takada T, Ichida K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. [DOI] [PubMed] [Google Scholar]
- 23.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iharada M, Miyaji T, Fujimoto T, et al. Type 1 sodium-dependent phosphate transporter (SLC17A1 protein) is a Cl(−)-dependent urate exporter. J Biol Chem. 2010;285(34):26107–26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jutabha P, Kanai Y, Hosoyamada M, et al. Identification of a novel voltage-driven organic anion transporter present at apical membrane of renal proximal tubule. J Biol Chem. 2003;278(30):27930–27938. [DOI] [PubMed] [Google Scholar]
- 26.Jutabha P, Anzai N, Kitamura K, et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem. 2010;285(45):35123–35132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBosch BJ, Kluth O, Fujiwara H, Schurmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichida K, Matsuo H, Takada T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford AJ, Riumallo JA, Youn VR, Scrimshaw NS. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J Nutr. 1976;106(3):428–434. [Google Scholar]
- 31.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justicia C, Salas-Perdomo A, Perez-de-Puig I, et al. Uric acid is protective after cerebral ischemia/reperfusion in hyperglycemic mice. Transl Stroke Res. 2017;8(3):294–305. [DOI] [PubMed] [Google Scholar]
- 33.Hooper DC, Spitsin S, Kean RB, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalo-myelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95(2):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imaram W, Gersch C, Kim KM, Johnson RJ, Henderson GN, Angerhofer A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic Biol Med. 2010;49(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patricio ES, Prado FM, da Silva RP, et al. Chemical characterization of urate hydroperoxide, a pro-oxidant intermediate generated by urate oxidation in inflammatory and photoinduced processes. Chem Res Toxicol. 2015;28(8):1556–1566. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–521. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lym-phocytes to transplanted cells and in an autoimmune diabetes model. J Immunol. 2006;176(7):3905–3908. [DOI] [PubMed] [Google Scholar]
- 38.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–C596. [DOI] [PubMed] [Google Scholar]
- 39.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287(48): 40732–40744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275. [DOI] [PubMed] [Google Scholar]
- 41.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. [PubMed] [Google Scholar]
- 42.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20(3): 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12): 2888–2897. [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. [DOI] [PubMed] [Google Scholar]
- 45.Xiao J, Zhang XL, Fu C, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med. 2015;35(5):1347–1354. [DOI] [PubMed] [Google Scholar]
- 46.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3–4): e71–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;292(1):F116–F122. [DOI] [PubMed] [Google Scholar]
- 50.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60(9):1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8608. [PubMed] [Google Scholar]
- 52.Lanaspa MA, Cicerchi C, Garcia G, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7(11):e48801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. [DOI] [PubMed] [Google Scholar]
- 55.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4): F1134–F1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Lozada LG, Tapia E, Lopez-Molina R, et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol. 2007;292(4): F1238–F1244. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Lozada LG, Tapia E, Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa T, Mazzali M, Kang DH, et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003;23(1): 2–7. [DOI] [PubMed] [Google Scholar]
- 61.Johnson RJ, Segal MS, Srinivas T, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez-Lozada LG, Tapia E, Soto V, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol Dial Transplant. 2008;23(4): 1179–1185. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Lozada LG, Tapia E, Vila-Casado C, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283(5):F1105–F1110. [DOI] [PubMed] [Google Scholar]
- 64.Sathisha KR, Gopal S, Rangappa KS. Antihyperuricemic effects of thiadiazolopyrimidin-5-one analogues in oxonate treated rats. Eur J Pharmacol. 2016;776:99–105. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez-Lozada LG, Tapia E, Soto V, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108(4):69–78. [DOI] [PubMed] [Google Scholar]
- 66.Mazzali M, Kim YG, Suga S, et al. Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation. 2001;71(7):900–905. [DOI] [PubMed] [Google Scholar]
- 67.Kim HS, Lim SW, Jin L, Jin J, Chung BH, Yang CW. The protective effect of febuxostat on chronic tacrolimus-induced nephrotoxicity in rats. Nephron. 2017;135(1):61–71. [DOI] [PubMed] [Google Scholar]
- 68.Mazali FC, Johnson RJ, Mazzali M. Use of uric acid-lowering agents limits experimental cyclosporine nephropathy. Nephron Exp Nephrol. 2012;120(1):e12–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HJ, Jeong KH, Kim YG, et al. Febuxostat ameliorates diabetic renal injury in a streptozotocin-induced diabetic rat model. Am J Nephrol. 2014;40(1):56–63. [DOI] [PubMed] [Google Scholar]
- 70.Komers R, Xu B, Schneider J, Oyama TT. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes. Br J Pharmacol. 2016;173(17):2573–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S-M, Lee S-H, Kim Y-G, et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol. 2015;308(9):F993–F1003. [DOI] [PubMed] [Google Scholar]
- 72.Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297(2). F481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22(7):1333–1340. [DOI] [PubMed] [Google Scholar]
- 74.Gois P, Canale D, Volpini RA, et al. Allopurinol attenuates rhabdomyolysis-associated acute kidney injury: renal and muscular protection. Free Radic Biol Med. 2016;101:176–189. [DOI] [PubMed] [Google Scholar]
- 75.Omori H, Kawada N, Inoue K, et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin Exp Nephrol. 2012;16(4):549–556. [DOI] [PubMed] [Google Scholar]
- 76.Tsuda H, Kawada N, Kaimori J-y, et al. Febuxostat suppressed renal ischemia-reperfusion injury via reduced oxidative stress. Biochem Biophys Res Commun. 2012;427(2):266–272. [DOI] [PubMed] [Google Scholar]
- 77.Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014;16(2):400,1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rock KL, Kataoka H, Lai J-J. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2012;9(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanbay M, Solak Y, Afsar B, et al. Serum uric acid and risk for acute kidney injury following contrast. Angiology. 2017;68(2): 132–144. [DOI] [PubMed] [Google Scholar]
- 80.Roncal-Jimenez C, Garcia-Trabanino R, Barregard L, et al. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on Mesoamerican nephropathy. Am J Kidney Dis. 2016;67(1):20–30. [DOI] [PubMed] [Google Scholar]
- 81.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. [DOI] [PubMed] [Google Scholar]
- 82.Choi YJ, Shin HS, Choi HS, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94(10): 1114–1125. [DOI] [PubMed] [Google Scholar]
- 83.Cicerchi C, Li N, Kratzer J, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28(8):3339–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Z, Hong Q, Zhang X, et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal. 2017;15(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–F631. [DOI] [PubMed] [Google Scholar]
- 87.Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens. 2008;17(5):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Preitner F, Pimentel A, Metref S, et al. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int. 2015;87(5):940–947. [DOI] [PubMed] [Google Scholar]
- 89.Johnson RJ, Andrews P. The fat gene: a genetic mutation in prehistoric apes may underlie today’s pandemic of obesity and diabetes. Sci Am. 2015;313(4):64–69. [Google Scholar]
- 90.Borghi C, Cicero AFG. Serum uric acid and cardiometabolic disease: another brick in the wall? Hypertension. 2017;69(6): 1011–1013. [DOI] [PubMed] [Google Scholar]
- 91.Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33(9): 1729–1741; discussion 1741. [DOI] [PubMed] [Google Scholar]
- 92.Feig DI, Madero M, Jalal DI, Sanchez-Lozada LG, Johnson RJ. Uric acid and the origins of hypertension. J Pediatr. 2013;162(5):896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang W, Sun K, Yang Y, Zhang H, Hu FB, Hui R. Plasma uric acid and hypertension in a Chinese community: prospective study and meta-analysis. Clin Chem. 2009;55(11):2026–2034. [DOI] [PubMed] [Google Scholar]
- 95.Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension. 2005;45(1):18–20. [DOI] [PubMed] [Google Scholar]
- 96.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004;286(4):F606–F616. [DOI] [PubMed] [Google Scholar]
- 98.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lv Q, Meng XF, He FF, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2): e56864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480. [DOI] [PubMed] [Google Scholar]
- 102.Johnson RJ, Nakagawa T, Jalal D, Sanchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28(9): 2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One. 2014;9(6):e100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Cosmo S, Viazzi F, Pacilli A, et al. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10(11): 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61(1):134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lapsia V, Johnson RJ, Dass B, et al. Elevated uric acid increases the risk for acute kidney injury. Am J Med. 2012;125(3):302.e309–302.e317. [DOI] [PubMed] [Google Scholar]
- 110.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–1190. [DOI] [PubMed] [Google Scholar]
- 111.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62(2): 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wheeler JG, Juzwishin KD, Eiriksdottir G, Gudnason V, Danesh J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med. 2005;2(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tangri N, Weiner DE. Uric acid, CKD, and cardiovascular disease: confounders, culprits, and circles. Am J Kidney Dis. 2010;56(2):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuwabara M, Niwa K, Hisatome I, et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five-year Japanese cohort study. Hypertension. 2017;69(6):1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999–2002. Am J Kidney Dis. 2014;64(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia Puig J, Mateos Anton F, Munoz Sanz A, et al. Renal handling of uric acid in normal subjects by means of the pyrazinamide and probenecid tests. Nephron. 1983;35(3): 183–186. [DOI] [PubMed] [Google Scholar]
- 117.Kottgen A, Albrecht E, Teumer A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okada Y, Sim X, Go MJ, et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet. 2012;44(8):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anzai N, Miyazaki H, Noshiro R, et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C-terminal. J Biol Chem. 2004;279(44):45942–45950. [DOI] [PubMed] [Google Scholar]
- 120.Tin A, Woodward OM, Kao WH, et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum Mol Genet. 2011;20(20): 4056–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ichida K, Hosoyamada M, Hisatome I, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15(1):164–173. [DOI] [PubMed] [Google Scholar]
- 122.Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dinour D, Gray NK, Campbell S, et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phipps-Green AJ, Merriman ME, Topless R, et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Ann Rheum Dis. 2016;75(1):124–130. [DOI] [PubMed] [Google Scholar]
- 125.Urano W, Taniguchi A, Inoue E, et al. Effect of genetic polymorphisms on development of gout. J Rheumatol. 2013;40(8): 1374–1378. [DOI] [PubMed] [Google Scholar]
- 126.Robinson PC, Choi HK, Do R, Merriman TR. Insight into rheumatological cause and effect through the use of Mendelian randomization. Nat Rev Rheumatol. 2016;12(8):486–496. [DOI] [PubMed] [Google Scholar]
- 127.Yang Q, Kottgen A, Dehghan A, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sluijs I, Holmes MV, van der Schouw YT, et al. A Mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes. 2015;64(8):3028–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pfister R, Barnes D, Luben R, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54(10): 2561–2569. [DOI] [PubMed] [Google Scholar]
- 130.Hughes K, Flynn T, de Zoysa J, Dalbeth N, Merriman TR. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014;85(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keenan T, Zhao W, Rasheed A, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol. 2016;67(4): 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Preitner F, Bonny O, Laverriere A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106(36):15501–15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Topless RK, Flynn TJ, Cadzow M, et al. Association of SLC2A9 genotype with phenotypic variability of serum urate in premenopausal women. Front Genet. 2015;6:313,1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Batt C, Phipps-Green AJ, Black MA, et al. Sugar-sweetened beverage consumption: a risk factor for prevalent gout with SLC2A9 genotype-specific effects on serum urate and risk of gout. Ann Rheum Dis. 2014;73(12):2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scheepers LE, Wei FF, Stolarz-Skrzypek K, et al. Xanthine oxidase gene variants and their association with blood pressure and incident hypertension: a population study. J Hypertens. 2016;34(11):2147–2154. [DOI] [PubMed] [Google Scholar]
- 136.Yang J, Kamide K, Kokubo Y, et al. Associations of hypertension and its complications with variations in the xanthine dehydrogenase gene. Hypertens Res. 2008;31(5):931–940. [DOI] [PubMed] [Google Scholar]
- 137.Kleber ME, Delgado G, Grammer TB, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Testa A, Mallamaci F, Leonardis D, et al. Synergism between asymmetric dimethylarginine (ADMA) and a genetic marker of uric acid in CKD progression. Nutr Metab Cardiovasc Dis. 2015;25(2):167–172. [DOI] [PubMed] [Google Scholar]
- 139.Voruganti VS, Laston S, Haack K, et al. Serum uric acid concentrations and SLC2A9 genetic variation in Hispanic children: the Viva La Familia Study. Am J Clin Nutr. 2015;101(4): 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voruganti VS, Nath SD, Cole SA, et al. Genetics of variation in serum uric acid and cardiovascular risk factors in Mexican Americans. J Clin Endocrinol Metab. 2009;94(2):632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun X, Zhang R, Jiang F, et al. Common variants related to serum uric acid concentrations are associated with glucose metabolism and insulin secretion in a Chinese population. PLoS One. 2015;10(1):e0116714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shafiu M, Johnson RJ, Turner ST, et al. Urate transporter gene SLC22A12 polymorphisms associated with obesity and metabolic syndrome in Caucasians with hypertension. Kidney Blood Press Res. 2012;35(6):477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parsa A, Brown E, Weir MR, et al. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int. 2012;81(5): 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mallamaci F, Testa A, Leonardis D, et al. A genetic marker of uric acid level, carotid atherosclerosis, and arterial stiffness: a family-based study. Am J Kidney Dis. 2015;65(2):294–302. [DOI] [PubMed] [Google Scholar]
- 145.Mallamaci F, Testa A, Leonardis D, et al. A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens. 2014;32(8):1621–1628. [DOI] [PubMed] [Google Scholar]
- 146.Voruganti VS, Franceschini N, Haack K, et al. Replication of the effect of SLC2A9 genetic variation on serum uric acid levels in American Indians. Eur J Hum Genet. 2014;22(7):938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palmer TM, Nordestgaard BG, Benn M, et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pattaro C, Teumer A, Gorski M, et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun. 2016;7:10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5): 1148–1156. [DOI] [PubMed] [Google Scholar]
- 150.Higgins P, Walters MR, Murray HM, et al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart. 2014;100(14):1085–1092. [DOI] [PubMed] [Google Scholar]
- 151.Madero M, Rodriguez Castellanos FE, Jalal D, et al. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo controlled trial. J Am Soc Hypertens. 2015;9(11):837–844. [DOI] [PubMed] [Google Scholar]
- 152.Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8): 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borgi L, McMullan C, Wohlhueter A, Curhan GC, Fisher ND, Forman JP. Effect of uric acid-lowering agents on endothelial function: a randomized, double-blind, placebo-controlled trial. Hypertension. 2017;69(2):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin J Am Soc Nephrol. 2017;12(5):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Segal MS, Srinivas TR, Mohandas R, et al. The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J Am Soc Hypertens. 2015;9(8):610–619.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1): 51–59. [DOI] [PubMed] [Google Scholar]
- 157.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66(6):945–950. [DOI] [PubMed] [Google Scholar]
- 159.Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7–13. [DOI] [PubMed] [Google Scholar]
- 160.Kim HA, Seo YI, Song YW. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci. 2014;29(8): 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu P, Chen Y, Wang B, Zhang F, Wang D, Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf). 2015;83(4): 475–482. [DOI] [PubMed] [Google Scholar]
- 162.Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543–549. [DOI] [PubMed] [Google Scholar]
- 163.Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J. 2013;77(8):2043–2049. [DOI] [PubMed] [Google Scholar]
- 164.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132. [PubMed] [Google Scholar]
- 165.Shi Y, Chen W, Jalal D, et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35(3): 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol. 2015;19(6):1044–1053. [DOI] [PubMed] [Google Scholar]
- 167.Ejaz AA, Dass B, Lingegowda V, et al. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int Urol Nephrol. 2013;45(2):449–458. [DOI] [PubMed] [Google Scholar]
- 168.Kumar S, Bhawani G, Kumari N, Murthy KSN, Lalwani V, Raju CHN. Comparative study of renal protective effects of allopurinol and N-acetyl-cysteine on contrast induced nephropathy in patients undergoing cardiac catheterization. J Clin Diagn Res. 2014;8(12):HC03–HC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Erol T, Tekin A, Katircibasi MT, et al. Efficacy of allopurinol pretreatment for prevention of contrast-induced nephropathy: a randomized controlled trial. Int J Cardiol. 2013;167(4): 1396–1399. [DOI] [PubMed] [Google Scholar]
- 170.Ogino K, Kato M, Furuse Y, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3(1):73–81. [DOI] [PubMed] [Google Scholar]
- 171.Meng J, Li Y, Yuan X, Lu Y. Effects of febuxostat on insulin resistance and expression of high-sensitivity C-reactive protein in patients with primary gout. Rheumatol Int. 2017;37(2): 299–303. [DOI] [PubMed] [Google Scholar]
- 172.Takir M, Kostek O, Ozkok A, et al. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med. 2015;63(8):924–929. [DOI] [PubMed] [Google Scholar]
- 173.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34(3):454–461. [DOI] [PubMed] [Google Scholar]
- 174.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–2516. [DOI] [PubMed] [Google Scholar]
- 175.McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–H2297. [DOI] [PubMed] [Google Scholar]
- 176.Talaat KM, El-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. [DOI] [PubMed] [Google Scholar]
- 177.Jung JW, Song WJ, Kim YS, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26(11): 3567–3572. [DOI] [PubMed] [Google Scholar]
- 178.MacDonald TM, Ford I, Nuki G, et al. Protocol of the Febuxostat versus Allopurinol Streamlined Trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia. BMJ Open. 2014;4(7):e005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.FDA. Uloric (febuxostat): Drug Safety Communication - FDA to evaluate increased risk of heart-related death. 2017. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm585281.htm. Accessed November 23, 2017.
- 180.Sanchez-Nino MD, Zheng-Lin B, Valino-Rivas L, et al. Lesinurad: what the nephrologist should know. Clin Kidney J. 2017;10(5):679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maahs DM, Caramori L, Cherney DZ, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sampson AL, Singer RF, Walters GD. Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev. 2017;10: CD009460. [DOI] [PMC free article] [PubMed] [Google Scholar]