Abstract
Background
Zika-exposed infants with microcephaly (proportional or disproportional) and those who are small for gestational age without microcephaly should be closely followed, particularly their growth trajectories. They are at high risk of adverse outcomes in the first year of life.
Antenatal Zika virus (ZIKV) exposure may lead to adverse infant outcomes including microcephaly and being small for gestational age (SGA). ZIKV-exposed infants with a diagnosis of microcephaly (proportional [PM] or disproportional [DM]) or SGA at birth were evaluated with anthropometric measurements and health outcomes.
Methods
Infants had laboratory-confirmed ZIKV exposure in Brazil. PM, DM, or SGA classification was based on head circumference and weight. First-year growth parameters and clinical outcomes were recorded with analyses performed.
Results
Among the 156 ZIKV-exposed infants, 14 (9.0%) were SGA, 13 (8.3%) PM, 13 (8.3%) DM, and 116 (74.4%) were neither SGA nor had microcephaly (NSNM). High rates of any neurologic, ophthalmologic, and hearing abnormalities were observed for PM (100%), DM (100%), and SGA (42.9%) vs NSNM infants (18.3%; P <.001); odds ratio [OR], 3.4 (95% confidence interval [CI], 1.1–10.7) for SGA vs NSNM. Neuroimaging abnormalities were seen in 100% of PM and DM and in 42.9% of SGA vs NSNM infants 16%; (P <.001); OR 3.9 (95% CI, 1.2–12.8) for SGA vs NSNM. Growth rates by z score, particularly for microcephaly infants, were poor after birth but showed improvement beyond 4 months of life.
Conclusions
ZIKV-exposed infants with microcephaly (PM and DM) had similarly high rates of adverse outcomes but showed improvement in growth measurements beyond 4 months of life. While SGA infants had fewer adverse outcomes compared with microcephaly infants, notable adverse outcomes were observed in some; their odds of having adverse outcomes were 3 to 4 times greater compared to NSNM infants.
Zika-exposed infants with microcephaly, irrespective of being proportional or disproportional, and those who are small for gestational age without microcephaly should be closely followed, particularly their growth trajectories. They are at high risk of adverse outcomes in the first year of life.
Keywords: Zika, congenital Zika syndrome, microcephaly, proportional microcephaly, small for gestational age (SGA)
(See the Major Article by Meder et al on pages 2607–15.)
In utero Zika virus (ZIKV) exposure may lead to a spectrum of central nervous system (CNS) infant abnormalities. Congenital ZIKV syndrome (CZS) has been used to describe ZIKV-exposed infants with devastating manifestations including severe microcephaly, other brain and ocular abnormalities, contractures, and severe neurologic impairment [1]; the vast majority are in Brazil [2–4].
CZS infants with microcephaly were the primary focus during the global ZIKV epidemic, with less attention placed on other markers of infant growth and development or their relationship to microcephaly. No prior investigations have focused on outcomes for small for gestational age (SGA) infants, which is defined as an infant whose weight is less than −1.28 standard deviations (SDs) for gender and gestational age. This unstudied relationship of SGA and microcephaly may be of importance because it allows for a distinction between microcephaly that is either proportional or disproportional. Infants with disproportional microcephaly (DM) have microcephaly but are not SGA (head circumference and weight at birth are not proportional). Infants with proportional microcephaly (PM) have microcephaly and are also SGA (head circumference and weight at birth are proportional).
The purpose of the study was to evaluate ZIKV-exposed infants with either a diagnosis of microcephaly or SGA at birth and evaluate anthropometric measurements and health outcomes during the first year of life, particularly the relationships between SGA, proportional microcephaly, and disproportional microcephaly and infant health outcomes.
METHODS
Study Population
The study was conducted at the Fernandes Figueira Institute (IFF), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Brazil. The IFF maternity and children’s hospital is a referral center for high-risk pregnancies and pediatrics, including infectious diseases such as ZIKV. This was a retrospective analysis focused on ZIKV-exposed infants with microcephaly and/or SGA at birth followed at IFF. All infants with laboratory-confirmed ZIKV exposure during pregnancy (positive maternal and/or infant ZIKV polymerase chain reaction [PCR]) and early infant outcomes available for review from IFF were included in this study (see the Supplementary Materials for details). Review board approvals for the study’s retrospective review of medical records were obtained at the IFF/FIOCRUZ and the University of California–Los Angeles (see the Supplementary Materials for details).
Study Procedures
Laboratory confirmation of ZIKV infection via real-time reverse-transcriptase PCR (RT-PCR) assays with the ZIKV QuantiTect Probe RT-PCR kit (Qiagen) was performed on mothers during pregnancy and infants after birth, primarily from serum and urine specimens. As part of the enrollment criteria, all infants had laboratory-confirmed ZIKV exposure during pregnancy (positive maternal and/or infant ZIKV PCR). More specifically, all infants fulfilled at least 1 of the following criteria for enrollment: had mothers with documented positive ZIKV PCR during pregnancy from serum, urine, amniotic fluid, placenta, or breast milk or had themselves a documented positive ZIKV PCR at birth from serum, urine, or cerebrospinal fluid (Supplementary Methods; Supplementary Table 1). We used the real-time RT-PCR protocol described by Lanciotti et al [5]. Zika MAC-ELISA (IgM antibody capture enzyme-linked immunosorbent assay), provided by the Centers for Disease Control and Prevention, was also performed on all available infant serum specimens but was not used as an inclusionary criteria in this analysis; only positive PCR results were considered for selection of the 156 infants who participated in this analysis [6–9] (see Supplementary Methods for detail). Both tests were performed at the FIOCRUZ IFF.
ZIKV-exposed infants were evaluated from March 2016 to June 2017. Infant outcomes including head circumference (HC), weight, length, and clinical exams were documented by IFF pediatric infectious diseases specialists at all infant follow-up visits. Infants were evaluated by pediatric neurologists and geneticists at the time of birth. Preterm birth was defined as gestational age <37 weeks. Neuroimaging was performed on infants after birth. Transfontanelle ultrasound (TFUS) was done on infants, and those with abnormalities on TFUS or physical exam had computerized tomography (CT) or magnetic resonance brain imaging (MRI) performed. Infants were evaluated by a pediatric ophthalmologist at birth and every 3 months and had hearing evaluations including brainstem auditory evoked response tests.
Microcephaly and SGA Definitions
Ballard assessment was done on infants at the time of birth to confirm gestational age. Microcephaly was defined as HC z score of less than −2 for gestational age and gender at the time of birth. Severe microcephaly was defined as a HC z score of less than −3 for gestational age and gender at the time of birth. SGA was defined as a weight z score of less than −1.28 for gestational age and gender at the time of birth. This group included only those infants who were SGA without microcephaly at birth. PM was defined by both microcephaly and SGA at birth. DM was defined by only microcephaly but not SGA at birth. (The Supplementary Materials include CZS, abnormal neuroimaging, fundoscopic exam, hearing, and morphologic exam definitions).
We used Intergrowth 21st online software to calculate z scores for all birth measurements (weight, height, HC) based on gestational age and gender [10]. Growth curves were created by calculating postnatal values of weight, height, and HC z scores. For full-term infants, postnatal values were determined based on World Health Organization (WHO) growth standards software [11]. As recommended by Intergrowth 21st, for preterm infants, postnatal z scores were calculated using Intergrowth 21st software up until postmenstrual age (gestational age + postnatal age) of 64 weeks. Beyond this age, the WHO software was used to calculate postnatal z scores for growth measurements.
Statistical Analyses
The χ2 test of association was used to examine the association between categorical variables and infant groups (PM, DM, SGA, and neither SGA nor microcephaly [NSNM]). Analysis of variance was conducted to study the difference in means of continuous variables among infant groups, and 95% confidence intervals (CIs) were reported to compare the difference in means of HC, length, and weight measurements at birth. Bonferroni adjustment was used when multiple pairwise comparisons between the infant groups were performed. The association between the grouped clinical outcomes and infant groups (SGA vs NSNM) were examined using bivariate and multivariable logistic regression. A stepwise model selection that included all clinically important variables was used to construct the final multivariable logistic models.
The change in growth measurement z scores for each infant group over time was modeled as the dependent variable of a mixed effect piecewise regression model for repeated measurements assuming a compound symmetry covariance structure. Several different mixed effect piecewise regression models were explored with different knots from 2 to 7 months in order to select the best fitting model. Using the Bayesian information criterion and prior knowledge, we selected the model with a knot at 4 months [12]. Due to variability in the infants’ follow-up schedules, time was included in the model as a continuous variable. Independent covariates in each model include time, infant group, and the interaction between time and infant group. To determine if the change in the expected mean z scores from 0 to 4 and/or from 4 to 12 months was significantly different, we conducted pairwise comparisons between the slope of the lines before and after 4 months among the 3 infant groups. We examined the difference in the slopes at 4 months within each infant group. Statistical analyses were conducted using SAS, version 9.4 (Cary, NC).
RESULTS
A total of 156 infants with laboratory-confirmed in utero ZIKV exposure were evaluated for microcephaly and/or SGA at birth. A total of 145 infants were born to mothers with ZIKV-positive PCR testing in pregnancy, and the other 11 infants had positive ZIKV PCRs from specimens collected after birth. There were 98 (62.8%) ZIKV-exposed infants who were asymptomatic at birth and during follow-up, whereas 22 (14.1%) were symptomatic with mild to moderate neurologic symptoms, and 36 (23.1%) had findings consistent with CZS.
Among the 156 ZIKV-exposed infants, 116 (74.4%) had NSNM at birth, whereas 14 (9.0%) had SGA without microcephaly, 13 (8.3%) had PM, and 13 (8.3%) had DM. All PM and DM infants were identified as CZS; 4 (28.6%) SGA infants were CZS, and 2 (14.3%) were symptomatic with additional neurologic findings.
Of the 40 infants with either microcephaly (PM, DM) or SGA without microcephaly, 24 (60%) demonstrated laboratory confirmation of ZIKV infection with either a positive infant ZIKV PCR and/or positive serum ZIKV IgM. For the PM infants, 10 (76.9%) had laboratory-confirmed ZIKV infection in contrast to 6 (46.2%) with DM, 8 (57.1%) with isolated SGA, and 39 (33.6%) with NSNM (Table 1).
Table 1.
Zika Virus (ZIKV)–Exposed Infant Cohort Characteristics at Birth Including Infant/Maternal Laboratory Results and Maternal Health/Pregnancy Issues by Infant ZIKV Group
| Characteristic | Total | NSNM | PM | DM | Isolated SGA | P Value |
|---|---|---|---|---|---|---|
| Number of participants (%) | 156 | 116 (74.4) | 13 (8.3) | 13 (8.3) | 14 (9) | … |
| Gender | ||||||
| Male | 76 (48.7%) | 55 (47.4%) | 6 (46.2%) | 7 (53.9%) | 8 (57.1%) | .911 |
| Female | 80 (51.3%) | 61 (52.6%) | 7 (53.9%) | 6 (46.2%) | 6 (42.9%) | … |
| Gestational age (days) | ||||||
| Mean (SD) | 268.8 (±14.4) | 270.2 (±12.5) | 263.3 (±24.9) | 268.2 (±16.6) | 263.8 (±14.5) | .197 |
| Birth HC (cm) | ||||||
| Mean (SD) | 33.5 (±3.1) | 34.8 (±1.8) | 27.1 (±2.8) | 29.1 (±1.6) | 32.6 (±2.2) | … |
| Mean difference (95% CI)c PM–DM | −2.0 (−3.9, −0.2) | … | … | … | … | .031 |
| Mean z score (SD) | 0.2 (±2.1) | 1.1 (±1.1) | −4.0 (±1.0) | −3.3 (±0.6) | −0.4 (±1.2) | … |
| Mean difference (95% CI) z scorec (PM–DM) | −0.7 (−1.4, −0.1) | … | … | … | … | .028 |
| Birth weight (kg) | ||||||
| Mean (SD) | 3.0 (±0.7) | 3.3 (±0.5) | 2.1 (±0.7) | 2.9 (±0.4) | 2.2 (±0.4) | … |
| Mean difference (95% CI)c SGA–PM | 0.1 (–0.3, 0.5) | … | … | … | … | .679 |
| Mean z score (SD) | −0.1 (±1.2) | 0.40 (±0.9) | −2.2 (±0.6) | −0.3 (±0.7) | −1.9 (±0.4) | … |
| Mean difference (95% CI) z scorec SGA–PM |
0.3 (−0.2, 0.7) | … | … | … | … | .217 |
| Birth length (cm) | ||||||
| Mean (SD) | 48.3 (±3.7) | 49.0 (±2.8) | 44.1 (±5.7) | 49.3 (±2.9) | 45.3 (±4.8) | <.001 |
| Mean difference (95% CI)d | ||||||
| SGA–DM | −3.9 (−7.4, −0.5) | … | … | … | … | … |
| SGA–PM | 1.2 (−2.2, 4.7) | … | … | … | … | … |
| PM–DM | −5.2 (−8.7, −1.6) | … | … | … | … | … |
| Mean z score (SD) | −0.1 (±1.7) | 0.1 (±1.6) | −1.8 (±1.1) | 0.50 (±1.5) | −1.3 (±1.6) | <.001 |
| Mean difference (95% CI) z scored | ||||||
| SGA–DM | −1.8 (−3.4, −0.2) | … | … | … | … | … |
| SGA–PM | 0.5 (−1.1, 2.1) | … | … | … | … | … |
| PM–DM | −2.3 (−3.9, −0.6) | … | … | … | … | … |
| Infant ZIKV laboratory results | ||||||
| Any laboratory-confirmed infant ZIKV (+PCR and/or +IgM) | ||||||
| Yes | 63 (40.4%) | 39 (33.6%) | 10 (76.9%) | 6 (46.2%) | 8 (57.1%) | … |
| No | 93 (59.6%) | 77 (66.4%) | 3 (23.1%) | 7 (53.8%) | 6 (42.9%) | … |
| Infant ZIKV IgM positive | ||||||
| Yes | 42 (26.9%) | 25 (21.6%) | 5 (38.5%) | 5 (38.5%) | 7 (50%) | … |
| Noe | 56 (35.9%) | 44 (37.9%) | 3 (23.1%) | 5 (38.5%) | 4 (28.6%) | … |
| Any infant ZIKV PCR positive | ||||||
| Yes | 35 (22.4%) | 18 (15.5%) | 10 (76.9%) | 4 (30.8%) | 3 (21.4%) | … |
| Nof | 121 (77.6%) | 98 (84.5%) | 3 (23.1%) | 9 (69.2%) | 11 (78.6%) | … |
| Any maternal PCR positive | ||||||
| Yes | 145 (92.9%) | 112 (96.6%) | 8 (61.5%) | 12 (92.3%) | 13 (92.9%) | … |
| Nog | 11(7.1%) | 4 (3.4%) | 5 (38.5%) | 1 (7.7%) | 1 (7.1%) | … |
| Timing of maternal ZIKV infection | ||||||
| First trimester | 48 | 27 (56.3%) | 8 (16.7%) | 10 (20.8%) | 3 (6.3%) | … |
| Second trimester | 68 | 58 (85.3%) | 0 (0%) | 2 (2.9%) | 8 (11.8%) | … |
| Third trimester | 32 | 30 (93.8%) | 0 (0%) | 0 (0%) | 2 (6.3%) | … |
| History of prior maternal health issues and/or pregnancy issuesh | ||||||
| Yes | 10 (6.4%) | 1 (0.9%) | 2 (15.4%) | 1 (7.7%) | 6 (42.9%) | <.001 |
| No | 146 (93.6%) | 115 (99.1%) | 11 (84.6%) | 12 (92.3%) | 8 (57.1%) | |
| Other congenital infectionsi | ||||||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | 0 0%) | 0 (0%) | NA |
| No | 156 (100%) | 116 (100%) | 13 (100%) | 0 (100%) | 14 (100%) | |
| Maternal drug use during pregnancy | ||||||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| No | 156 (100%) | 116 (100%) | 13 (100%) | 13 (100%) | 14 (100%) | |
Due to infant classification criteria, only specific groups in which the infants were not grouped based on the outcome were compared for statistically significant differences: HC z score was compared among DM and PM infants; weight z score was compared among PM and SGA infants; and length z score was compared among all groups. For maternal/infant ZIKV lab testing, note that some infants did not receive testing after birth (IgM or ZIKV PCR) because they were referred from outside hospitals. Thus, some columns may not add up to 100%. For maternal/infant PCRs, some mother–infant pairs were PCR-positive from both maternal and infant specimens. The Fisher exact test was used for P values for maternal health/pregnancy issues. For maternal trimester of infection, trimester of ZIKV infection was not available for all mothers.
Abbreviations: CI, confidence interval; DM, disproportional microcephaly; HC, head circumference; IgM, immunoglobulin M; NA, not applicable; NSNM, neither SGA nor microcephaly; PCR, polymerase chain reaction; PM, proportional microcephaly; SD, standard deviation; SGA, small for gestational age; ZIKV, Zika virus.
aFor infant growth measurements, P value performing a χ 2test of association.
bFor infant growth measurements, P value performing 1-way analysis of variance.
cFor infant growth measurements, 95% CI using a t test.
dFor infant growth measurements, pairwise comparison of 95% CIs with Bonferroni adjustment.
eIgM tested and negative.
fPCR not done or unknown as opposed to negative PCR.
gPCR not done or unknown as opposed to negative PCR.
hFor maternal health issues/pregnancy complications, pregnancy complications included hypothyroidism, obesity, premature rupture of membranes, preeclampsia/eclampsia, and hypertension.
iFor maternal health issues/pregnancy complications, there was 1 case of maternal cytomegalovirus (CMV) IgM positive in the first and second trimester, but the DM infant did not have congenital CMV.
Significant differences were noted among groups with regard to gestational age at ZIKV infection (P < .001). For PM infants, all maternal ZIKV infections occurred during the first trimester; 83.3% occurred in the first trimester for DM infants. For SGA infants, the majority of ZIKV maternal infections occurred in the second trimester (61.5%) and among NSNM infants in the second or third trimesters (77%; Table 2).
Table 2.
Zika Virus (ZIKV)–Exposed Infant Group and Trimester of ZIKV Infection
| Trimester of ZIKV Infectiona | |||||
|---|---|---|---|---|---|
| Infant Group | Infants with Laboratory-Confirmed ZIKV | First | Second | Third | P Valueb,c |
| Neither SGA nor microcephaly, N = 115 | 27 (56.3%) | 58 (85.3%) | 30 (93.8%) | ||
| Yes | 16 (59.3%) | 14(24.1%) | 8 (26.7%) | .004 | |
| No | 11 (40.7%) | 44(75.9%) | 22 (73.3%) | ||
| Proportional microcephaly, N = 8 | 8 (16.7%) | 0 (0%) | 0 (0%) | ||
| Yes | 5 (62.5%) | 0 (0%) | 0 (0%) | .231 | |
| No | 3 (37.5%) | 0 (0%) | 0 (0%) | ||
| Disproportional microcephaly, N = 12 | 10 (20.8%) | 2 (2.9%) | 0 (0%) | ||
| Yes | 6 (60.0%) | 0 (0%) | 0 (0%) | .315 | |
| No | 4 (40.0%) | 2 (100%) | 0 (0%) | ||
| SGA, N = 13 | 3 (6.3%) | 8 (11.8%) | 2 (6.3%) | ||
| Yes | 2 (66.7%) | 4 (50.0%) | 1 (50.0%) | >.999 | |
| No | 1 (33.3%) | 4 (50.0%) | 1 (50.0%) | ||
| Total | 48 | 68 | 32 | ||
| Yes | 29 (60.4%) | 18 (26.5%) | 9 (28.1%) | ||
| No | 19 (39.6%) | 50 (73.5%) | 23 (71.9%) | <.001 | |
Abbreviations: SGA, small for gestational age; ZIKA, Zika virus.
aTrimester of infection was unknown for 8 infants
bFisher exact test examining association between laboratory-confirmed ZIKV and trimester of infection within each infant group.
cFischer exact test was used to determine P value.
Birth Cohort Characteristics and Growth Measurements
Mean gestational age was 268.8 days for all infants, with a similar gestational age at birth noted among groups. Mean HC at birth was 34.8 cm for NSNM infants, with lower mean HCs for the PM (27.1 cm), DM (29.1 cm), and SGA (32.6 cm) groups. Comparison of the mean HCs for infants with PM and DM revealed that PM infants had statistically significantly smaller mean HCs compared to DM infants (P = .031). The mean birth weight for NSNM infants was 3.3 kg and was similarly low for the PM (2.1 kg) and SGA (2.2 kg) groups, in contrast to 2.9 kg for the DM group (Table 1).
First-year Growth Curve Evaluations
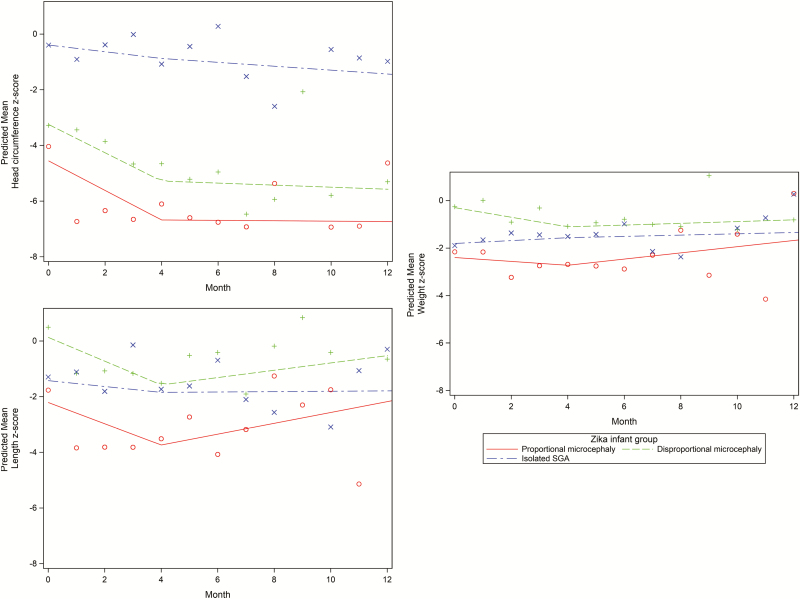
Infant growth measurements including HC, weight, and length were taken at birth and during monthly follow-up visits during the first 7 months of life, with additional measurements taken until 13 months. Mean z scores for infant length, weight, and HC from birth to 13 months are shown in Figure 1. To further elucidate growth changes, piecewise regression (prediction) lines of mean z scores were created for HC, weight, and length for each infant group.
Figure 1.
Piecewise regression lines for head circumference, weight, and length z scores over 12 months for proportional microcephaly, disproportional microcephaly, and Zika virus–infected infants, knot at 4 months. The figure shows mean z scores and piecewise regression (prediction) lines of mean z scores for head circumference, weight, and length during the first year of life for each of the 3 ZIKV-exposed infant groups (PM, DM, SGA). Abbreviations: DM, disproportional; PM, proportional; SGA, small for gestational age.
Prior to 4 months of age, the rate of HC z score changes (slope) was not significantly different among PM and DM infants but was significantly different between SGA and PM infants (P = .002) and between SGA and DM infants (P = .005). At 4 months, HC z scores improved with significant changes noted in the slope for PM (P < .001) and DM (P = .002) infants. No significant changes were noted in HC growth (slope) for the SGA group after 4 months of age. The rate of weight z score changes (slope) showed significant differences among SGA and DM infants until 4 months of age (P = .01). Improvement in infant weight z scores was also significant for PM (P = .04) and DM (P = .04) groups at 4 months. In contrast, no significant differences were observed in the rate of length z score changes (slope) among the 3 groups in the period before or after 4 months of age. However, at 4 months, the change in length z score (slope) showed significant improvement for PM and DM infants (PM, P = .005; DM, P = .007; Figure 1).
First-year Clinical Outcomes
Significant differences were observed in the frequency of adverse outcomes among ZIKV-exposed infant groups. Rates of seizures were significantly different among groups, with seizures more frequent in PM (84.6%) and DM (84.6%) groups in comparison to the SGA (21.4%) and NSNM (4.3%) groups (P < .001). Rates of dysphagia were significantly higher among PM (53.8%) and DM (38.5%) compared to SGA (14.4%) and NSNM (1.7%) infants (P < .001). Ten percent of infants (PM, DM, SGA) required placement of ventriculo-peritoneal shunts compared to only 1.7% of NSNM infants; 12.5% required gastric tube placement, which was not necessary for NSNM infants (Table 3).
Table 3.
Frequency of Adverse Infant Outcomes by Zika Virus Infant Groups
| NSNM | Proportional Microcephaly | Disproportional Microcephaly | SGA | Odds Ratioa,b (95% Confidence Interval); P Value for SGA vs NSNM Groups | ||
|---|---|---|---|---|---|---|
| Type of Infant Outcome | n = 116 | n = 13 | n = 13 | n = 14 | P Value | |
| NICU (days) | 2.1 (4.7) | 21.8 (30.0) | 9.8 (11.9) | 28.8 (78.9) | <.001 | … |
| Mean (standard deviation) rangec | (0 –30) | (0–100) | (0–47) | (0–300) | ||
| Median (interquartile range) | 0 (0 –0) | 4.0 (0 –35.0) | 8.0 (5.0–10.0) | 0 (0.0–18.0) | ||
| NICU stay | ||||||
| Yes | 25 (21.7%) | 7 (53.9%) | 10 (76.9%) | 6 (42.9%) | <.001 | … |
| No | 90 (78.3%) | 6 (46.2%) | 3 (23.1%) | 8 (57.1%) | ||
| TFUS | ||||||
| Abnormal | 5 (5.0%) | 11 (100%) | 11 (100%) | 5 (35.7%) | <.001 | … |
| Normal | 106 (95.0%) | 0 (0%) | 0 (0%) | 9 (64.3%) | ||
| Head CT scan | ||||||
| Abnormal | 7 (63.6 %) | 13 (100%) | 13 (100%) | 5 (83.3%) | .010 | … |
| Normal | 4 (36.4%) | 0 (0%) | 0 (0%) | 1 (16.7%) | ||
| Head MRI scan | ||||||
| Abnormal | 18 (60%) | 4 (100%) | 9 (100%) | 1 (50%) | .056 | … |
| Normal | 12 (40%) | 0 (0%) | 0 (0%) | 1 (50%) | ||
| Ophthalmologic exam | ||||||
| Abnormal | 6 (5.2%) | 10 (77.9%) | 9 (69.2%) | 4 (28.6%) | <.001 | … |
| Normal | 109 (94.8%) | 3 (23.1%) | 4 (30.8%) | 10 (71.4%) | ||
| Hearing exam | ||||||
| Abnormal | 2 (1.9%) | 4 (33.3%) | 3 (23.1%) | 1 (7.7%) | <.001 | … |
| Normal | 103 (98.1%) | 8 (66.7%) | 10 (76.9%) | 12 (92.3%) | ||
| Morphologic evaluation | ||||||
| Abnormal | 4 (3.5%) | 13 (100%) | 13 (100%) | 4 (28.6%) | <.001 | … |
| Normal | 112 (96.5%) | 0 (0%) | 0 (0%) | 10 (71.4%) | ||
| Neurologic evaluation | ||||||
| Abnormal | 13 (11.2%) | 13 (100%) | 13 (100%) | 6 (42.9%) | <.001 | … |
| Normal | 103 (88.8%) | 0 (0%) | 0 (0%) | 8 (57.1%) | ||
| Infectionsd | ||||||
| Yes | 43 (37.1%) | 6 (46.2%) | 1 (7.7%) | 2 (14.3%) | .045 | … |
| No | 73 (62.9%) | 7 (53.8%) | 12 (92.3%) | 12 (85.7%) | ||
| Seizures | ||||||
| Yes | 5 (4.3%) | 11 (84.6%) | 11 (84.6%) | 3 (21.4%) | <.001 | … |
| No | 111 (95.7%) | 2 (15.4%) | 2 (15.4%) | 11 (78.6%) | ||
| Dysphagia | ||||||
| Yes | 2 (1.7%) | 7 (53.8%) | 5 (38.5%) | 2 (14.4%) | <.001 | … |
| No | 114 (98.3%) | 6 (46.2%) | 8 (61.5%) | 12 (85.7%) | ||
| Gastrostomy tube | ||||||
| Yes | 0 (0%) | 2 (15.4%) | 1 (7.7%) | 2 (14.3%) | .001 | … |
| No | 116 (100%) | 11 (84.6%) | 12 (92.3%) | 12 (85.7%) | ||
| Ventriculoperitoneal shunt | ||||||
| Yes | 2 (1.7%) | 1 (7.7%) | 1 (7.7%) | 2 (14.3%) | .051 | … |
| No | 114 (98.3%) | 12 (92.3%) | 12 (92.3%) | 12 (85.7%) | ||
| Grouped infant outcomes | ||||||
| Any abnormal neuroimaging (TFUS, head CT, head MRI) | ||||||
| Yes | 17 (16.0%) | 13 (100%) | 13 (100%) | 6 (42.9%) | <.001 | 3.9 (1.2–12.8)e; P = .023 |
| No | 89 (84.0%) | 0 (0%) | 0 (0%) | 8 (57.1%) | ||
| Any abnormal ophthalmologic, hearing, or neurologic exam | ||||||
| Yes | 21(18.3%) | 13 (100%) | 13 (100%) | 6 (42.9%) | <.001 | 3.4 (1.1–10.7)e; P = .041 |
| No | 94 (81.7%) | 0 (0%) | 0 (0%) | 8 (57.1%) | ||
| Any abnormality except NICU stay or infection | ||||||
| Yes | 33 (28.5%) | 13 (100%) | 13 (100%) | 6 (42.9%) | <.001 | 1.9 (0.6–5.9)e; P = .272 |
| No | 83(71.5%) | 0 (0%) | 0 (0%) | 8 (57.1%) | ||
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; NICU, neonatal intensive care unit; NSNM, neither SGA nor microcephaly; SGA, small for gestational age; TFUS, transfontanelle ultrasound.
aOdds ratio (OR) comparing SGA and NSNM groups only (reference group NSNM).
bRefer to Supplementary Table 3 for multivariate logistic regression with adjusted ORs for grouped infant outcomes shown above adjusted for infant gender, prematurity, maternal health and pregnancy issues, and infection.
cPlease note that regarding NICU stay (days) for the SGA group, mean length of stay (days) was longest because 1 SGA infant with congenital Zika syndrome was never discharged from the hospital following 300 days. Further details are available in Supplementary Table 2 for infant 3.
dInfections (including pneumonias, urinary tract infections, skin infections, and bronchiolitis) among Zika virus–exposed infants primarily occurred beyond the immediate postnatal period during follow-up.
eFischer exact test was performed to determine P values comparing the 4 infant groups.
Morphologic clinical evaluation was frequently abnormal in PM (100%), DM (100%), and SGA (28.7%) infants compared to NSNM infants (3.5%), with significant differences noted among groups (P < .001). Similarly, neurologic evaluation was abnormal for PM (100%), DM (100%), and SGA (42.9%) groups compared to NSNM infants (11.2%; P < .001). Differences existed in the rates of any neurologic, ophthalmologic, or hearing abnormalities among PM (100%), DM (100%), and SGA (42.9%) groups compared to NSNM infants (18.3%; P < .001), with differences also seen when evaluated independently for each of the 4 groups. Significant differences among groups existed in the rates of neuroimaging abnormalities, including any abnormality seen on TFUS, CT, or MRI; 100% of PM and DM infants had abnormalities in contrast to 42.9% of SGA and 16% of NSNM infants (P <.001). When isolated SGA and NSNM infants were compared directly, the odds of having abnormal neuroimaging were nearly 4 times greater (odds ratio [OR], 3.9; 95% CI, 1.2–12.8) and the odds of having an abnormal ophthalmologic, hearing, or neurologic exam (OR, 3.4; 95% CI, 1.1–10.7) were more than 3 times greater for SGA infants compared to NSNM infants. These findings remained even after controlling for infant gender, prematurity (<37 weeks), maternal health and pregnancy issues, and infant infections (Table 3; Supplementary Table 3). Significant differences among infant groups were also noted with respect to rates of infections, neonatal intensive care unit (NICU) admissions, and mean number of days spent in the NICU.
DISCUSSION
We are the first to investigate differences in anthropomorphic measurements and outcomes for in utero ZIKV-exposed infants with respect to categorization at birth as PM, DM, or SGA. PM and DM infants had similarly high rates of adverse outcomes and were primarily exposed to ZIKV infection during the first trimester compared to SGA and NSNM infants who were primarily exposed in later trimesters. While infants with SGA without microcephaly had lower rates of adverse infant outcomes compared to those with microcephaly, notable adverse outcomes were observed in a subset of infants. The odds of adverse outcomes for SGA infants were 3 to nearly 4 times greater in comparison to NSNM infants. Mean z score growth measures for microcephaly infants (PM, DM) were poor after birth but began to show significant improvement beyond 4 months of life.
Mean HCs for PM and DM infants were similar to those seen in prior studies of CZS infants (28.1 cm ± 1.8 cm), and mean birth weights were within the range seen in other studies of microcephaly infants (2577 g ± 260 g) [13]. One study of 87 Brazilian CZS infants found that 40% were low birth weight and 29% were SGA at birth [13]. The SGA infants also had lower HC z scores compared to those who were not SGA [13]. Although not specifically stated, it is likely that many of those infants would have been classified as PM as the majority of the infants had microcephaly [13]. Similarly, we found that PM infants had smaller HC z scores compared to DM infants.
Few previous studies have investigated growth rates of ZIKV-exposed infants, and none have made distinctions between PM, DM, and SGA groups. However, 1 study of 48 Brazilian infants with probable CZS did report infant growth measurements over the first 8 months; the majority (87%) had microcephaly, mostly severe [12]. Nearly 20% of those infants had birth weights ≥2 SDs below the mean (SGA based on our criteria) [12]. In that study, HC decreased to a z score of −5.5 by 4 months [12]. Likewise, for the microcephaly infants (PM, DM) in our study, decreased mean z scores for weight, length, and HC were observed, especially in the initial months after birth. Our findings were most prominent for head growth over time, which is likely due to the severe CNS damage characteristic of in utero ZIKV [12, 14]. Mean HC z scores for microcephaly infants (PM, DM) in our study similarly declined by the fourth month. Our modeled predicted lines based on microcephaly infant growth measures also suggested improvement in z score growth beyond 4 months of age.
All ZIKV-exposed infants (PM, DM, SGA) experienced high rates of health problems in comparison to NSNM infants. Not surprisingly, clinical abnormalities and adverse outcomes were most prominent in microcephalic infants compared to SGA infants. However, initial expectations that PM infants may fare better than DM infants were not validated; both groups demonstrated equally high poor-outcome rates. Microcephaly infants had extremely high rates of seizures; abnormal neurologic, morphologic, and ophthalmologic exams; and abnormal neuroimaging studies. The findings for microcephaly infants appear largely consistent with reported literature. All PM and DM patients had neuroimaging abnormalities, which is consistent with prior studies of Brazilian ZIKV microcephaly infants, where nearly all had calcifications and many had cortical malformations and ventriculomegaly [13, 15]. Eye abnormalities in microcephaly infants ranged from 69% to 77%, while others have reported 35% to 100% among infants with microcephaly and intracerebral calcifications [13, 16–22]. The microcephaly infants had higher rates of hearing abnormalities (23–33%) than previously reported (6%) [23, 24]. Chronic issues including seizures and dysphagia appeared more frequently in microcephaly infants (seizures, 85%; dysphagia, 39%–54%) compared to previous reports (seizures, 50%; dysphagia 15%) [1, 12, 25, 26].
The relatively high rates of adverse outcomes among SGA infants were also of interest and were higher than those seen among NSNM infants. No other studies of ZIKV-exposed infants have focused on SGA as a risk factor for adverse infant outcomes. In other congenital infections, little has been published about SGA as a risk factor for adverse outcomes [27–29]. Approximately 43% of SGA infants were found to have a neurologic, ophthalmologic, or hearing abnormality in comparison to only 18% of NSNM infants (OR, 3.4; 95% CI, 1.1–10.7); 21% had seizures and 43% had abnormal neuroimaging compared to only 16% of NSNM infants (OR, 3.9; 95% CI, 1.2–12.8). While these SGA infants were not microcephalic at birth, it appears that the majority of adverse outcomes can be attributed to a subset of 6 infants who were not only SGA at birth but also had other severe manifestations of congenital ZIKV infection. Of these 6 infants, laboratory confirmation of ZIKV infection was observed among 4 (67%); only 1 mother had notable prior health issues or problems during pregnancy (hypothyroidism and hypertension; see Supplementary Table 2; Table 1). For ZIKV-exposed infants, our findings suggest that SGA may be another important but less emphasized manifestation of CZS.
While the rates of abnormalities in NSNM infants was lower than for microcephaly (100%) or SGA (43%) infant groups, nearly 29% of children had some type of adverse outcome. This likely reflects CNS ZIKV complications in children without microcephaly or other forms of growth restriction. It is curious that 37% of NSNM infants had some sort of infection after birth or during follow-up, which was higher than for DM or SGA infants. This finding warrants further investigation.
Primary study limitations include the relatively small sample size, which limited the power to determine differences between growth curve changes for PM, DM, and SGA groups and generalizability of additional multivariate analyses related to maternal and infant factors. This issue impacted our study primarily beyond 7 months of age when there was decreased frequency of growth measurements for each of the 3 groups. In addition, while all infants had laboratory-confirmed ZIKV exposure, many of the SGA and/or microcephaly infants did not have laboratory-confirmed ZIKV infection at the time of birth, which included 23% of PM and 54% of DM infants. This underscores inherent limitations in reliance on laboratory diagnosis of ZIKV at birth from infant ZIKV IgM and PCR. While differences were observed in timing of maternal ZIKV infection during pregnancy, women typically were tested at the time of clinical symptoms or at the time of referral for further evaluation but did not undergo ZIKV testing in each semester. It is also important to note that our study was a retrospective review of clinical data from a referral center for ZIKV-exposed infants. Thus, frequency data does not reflect incidence data.
CONCLUSIONS
We conducted this analysis to determine if distinctions between proportional and disproportional microcephaly and SGA at birth are important in determining the prognosis of ZIKV-exposed infants with respect to growth and adverse outcomes in the first year of life. ZIKV-exposed infants with PM, DM, and even SGA had high rates of adverse outcomes. In addition, notable rates of adverse outcomes were observed among ZIKV-exposed infants who were not microcephalic or SGA at birth. Our preliminary findings highlight the need for further long-term outcome studies for all ZIKV-exposed infants, especially those who have microcephaly (both PM and DM) or SGA at birth.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the women and infants who participated in this study.
Financial support. The work was supported by the Departamento de Ciência e Tecnologia do Ministério da Saúde do Brasil and grants from Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES/ 88887.116627/2016-01), the National Institute of Allergy and Infectious Diseases (AI28697, AI1259534-01) and the National Eye Institute (AI129847-01) of the National Institutes of Health (NIH)/National Center for Advancing Translational Science of the University of California–Los Angeles Clinical and Translational Science Institute (UL1TR001881), Thrasher Research Fund (20164370), and the UK Department for International Development.
Potential conflicts of interest. M. E. M. reports grants from CNPq 441098/2016–9, Fiocruz-PIP, Wellcome Trust, the UK Department for International Development (205377/Z/16/Z), Faperj E_18/2015TXB, and European Union’s Horizon 2020 research and innovation program under Zika-PLAN (grant agreement 734584) during the conduct of the study. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171:288–95. doi:10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. PAHO WHO. Regional Zika epidemiological update (Americas) 2016.
- 3. PAHO WHO. Zika suspected and confirmed cases reported by countries and territories in the Americas Cumulative cases, 2015–2016 Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=36813&lang=en.
- 4. PAHO WHO. Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015–12017: cumulative cases 2017.
- 5. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Zika MAC-ELISA. Vol. 2019. Atlanta, GA; CDC. [Google Scholar]
- 7. L’Huillier AG, Hamid-Allie A, Kristjanson E, et al. Evaluation of euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol 2017; 55:2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granger D, Hilgart H, Misner L, et al. Serologic testing for Zika virus: comparison of three Zika virus IgM-screening enzyme-linked immunosorbent assays and initial laboratory experiences. J Clin Microbiol 2017; 55:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer C, Pedroso C, Mendrone A Jr, et al. External quality assessment for Zika virus molecular diagnostic testing, Brazil. Emerg Infect Dis 2018; 24. doi:10.3201/eid2405.171747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.http://intergrowth21.ndog.ox.ac.uk
- 11.http://www.who.int/childgrowth/software/en
- 12. Moura da Silva AA, Ganz JS, Sousa PD, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis 2016; 22:1953–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meneses JDA, Ishigami AC, de Mello LM, et al. Lessons learned at the epicenter of Brazil’s congenital Zika epidemic: evidence from 87 confirmed cases. Clin Infect Dis 2017; 64:1302–8. [DOI] [PubMed] [Google Scholar]
- 14. Corona-Rivera JR, Corona-Rivera E, Romero-Velarde E, Hernández-Rocha J, Bobadilla-Morales L, Corona-Rivera A. Report and review of the fetal brain disruption sequence. Eur J Pediatr 2001; 160:664–7. [DOI] [PubMed] [Google Scholar]
- 15. Microcephaly Epidemic Research Group. Microcephaly in infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis 2016; 22:1090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valentine G, Marquez L, Pammi M. Zika virus-associated microcephaly and eye lesions in the newborn. J Pediatric Infect Dis Soc 2016; 5:323–8. [DOI] [PubMed] [Google Scholar]
- 17. de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol 2016; 134:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228. [DOI] [PubMed] [Google Scholar]
- 19. Ventura CV, Maia M, Travassos SB, et al. Risk factors associated with the ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol 2016; 134:912–8. [DOI] [PubMed] [Google Scholar]
- 20. Verçosa I, Carneiro P, Verçosa R, et al. The visual system in infants with microcephaly related to presumed congenital Zika syndrome. J AAPOS 2017; 21:300–304.e1. [DOI] [PubMed] [Google Scholar]
- 21. Ventura LO, Ventura CV, Lawrence L, et al. Visual impairment in children with congenital Zika syndrome. J AAPOS 2018; 22:218–22.e1. doi:10.1016/j.jaapos.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yepez JB, Murati FA, Pettito M, et al. ; Johns Hopkins Zika Center Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol 2017; 135:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leal MC, Muniz LF, Ferreira TS, et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection—Brazil, November 2015-May 2016. MMWR Morb Mortal Wkly Rep 2016; 65:917–9. [DOI] [PubMed] [Google Scholar]
- 24. Leal MC, Muniz LF, Caldas Neto SD, van der Linden V, Ramos RC. Sensorineural hearing loss in a case of congenital Zika virus. Braz J Otorhinolaryngol 2016. pii: S1808-8694(16)30127–6. doi:10.1016/j.bjorl.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. MMWR Morb Mortal Wkly Rep 2016; 65:1343–8. [DOI] [PubMed] [Google Scholar]
- 26. Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Primhak RA, Simpson RM. Screening small for gestational age babies for congenital infection. Clin Pediatr (Phila) 1982; 21:417–20. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto R, Ishii K, Shimada M, et al. Significance of maternal screening for toxoplasmosis, rubella, cytomegalovirus and herpes simplex virus infection in cases of fetal growth restriction. J Obstet Gynaecol Res 2013; 39:653–7. [DOI] [PubMed] [Google Scholar]
- 29. Simonazzi G, Curti A, Murano P, et al. Congenital cytomegalovirus infection and small for gestational age infants. Prenat Diagn 2014; 34:765–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.