Abstract
Background
Performing cranial imaging prior to lumbar punctures (LPs) in patients with suspected central nervous system (CNS) infections has been associated with delayed treatments and poor outcomes. Various guidelines provide different criteria for cranial imaging prior to LP.
Methods
We describe the use of cranial imaging in a cohort of adult patients with suspected CNS infections, and evaluated adherence to the recommendations made in the Infectious Disease Society of America (IDSA), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Swedish, and Dutch guidelines. We also analyzed the association between cranial imaging and the time between emergency department entrance and intravenous antibiotic administration.
Results
From 2012–2015, 203 patients with suspected CNS infections were included, of whom 56 (27%) were diagnosed with CNS infections and 16 were diagnosed with bacterial meningitis (8%). Cranial imaging, in all cases computed tomography (CT), was performed in 130 patients (64%) and led to the deferral of LPs in 7 (5%). Criteria by the IDSA, ESCMID, Swedish, and Dutch guidelines showed indications for imaging in 64%, 39%, 39%, and 40% of patients, respectively. The times between emergency department arrivals and the start of antibiotic therapy between patients with and without CT before LP were similar (median 134 [interquartile range (IQR) 58–292] vs. 141 minutes [IQR 52–227], respectively; Mann-Whitney U P = .74).
Conclusions
A cranial CT prior to LP was done in the majority of patients with a suspected CNS infection, irrespective of guideline indications. The ESCMID, Swedish, and Dutch guidelines were more restrictive in advising imaging, compared to the IDSA guidelines. Performing cranial imaging prior to LP was not associated with treatment delays in this Dutch cohort study.
Keywords: lumbar puncture, guidelines, cranial imaging, CNS infections
In a study of 203 patients with suspected central nervous system infections, cranial computed tomography was done prior to lumbar puncture in the majority of patients, irrespective of guideline indications, and was not associated with treatment delays.
Patients with suspected central nervous system (CNS) infections need prompt diagnostic work-ups and initiation of treatment [1]. Once an initial patient evaluation has been completed, including collecting a medical history and performing a physical examination, lumbar puncture (LP) is the diagnostic procedure of choice if the diagnoses of bacterial meningitis and viral encephalitis cannot be ruled out [1, 2]. In view of the urgent nature of cerebrospinal fluid (CSF) testing, an issue physicians are faced with in an emergency department setting is whether neuroimaging is required before LP. Cranial imaging is performed to rule out diagnoses of space-occupying lesions, which cause substantial brain shifts and, potentially, could lead to the compression of vital brains structures, triggered by the increased pressure gradient caused by LP [1, 2].
Guideline recommendations for cranial imaging prior to LP are based on a prospective study including 301 adult patients with suspected meningitis [3–5]. In this study, 10 characteristics were identified as associated with abnormal computed tomography (CT) of the brain: age over 60 years; altered mental status; gaze or facial palsy; abnormal language or inability to answer 2 questions or follow 2 commands; immunocompromise; history of central nervous system disease; seizure in past week; visual field abnormalities; arm and leg drifts. If none of these features are present, there is a negative predictive value of 97% for an intracranial abnormality, confirming that clinical features can be used to identify patients who are unlikely to have abnormal findings on brain CT scans. However, in the recent years, there has been a discussion about whether these criteria indeed should be used in clinical practice, based on their predictive value, to detect contraindications for LP [6]. The potential trade-offs of using these items are a high proportion of patients with CT scans prior to LPs, the potential treatment delays caused by CT scans, and the association between these delays and clinical outcomes [7–10].
We aimed to describe the use of cranial imaging in a cohort of patients with suspected CNS infections, and evaluated adherence to the recommendations made in bacterial meningitis guidelines published by the Infectious Disease Society of America (IDSA; 2004) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID; 2016) [3, 4]. Furthermore, we evaluated recommendations in the Swedish national guideline (2013), as it was recently described to result in less cranial imaging and, thereby, potentially, improved prognoses [11], and the Dutch national guideline (2012), which was the criteria most likely to be used by the physicians in the study period. We also analyzed the association between cranial imaging and time between emergency department entrance and intravenous antibiotic administration.
METHODS
We included consecutive adult patients with suspected CNS infections who underwent CSF examinations in the Academic Medical Centre in Amsterdam, The Netherlands, from February 2012 through May 2015. The methods have been described previously [12]. In short, all patients 16 years or older presenting to the emergency department or admitted to the hospital with the suspicion of an infection of the CNS who underwent a CSF examination were included in this study. The study was set up for a prospective cohort, but to assure no episodes were missed, an overview of all CSF samples analyzed by the hospital laboratory was conducted, and all episodes fulfilling the inclusion criteria were also included [12]. Data collection was planned before the index test and reference standard were performed; therefore, this study is considered as a prospective study according to the Standards for Reporting of Diagnostic Accuracy Studies criteria. Exclusion criteria were suspected infection within 1 month of neurosurgery or a traumatic brain injury, or implanted neurosurgical devices. Online case record forms were used to collect data on each patient’s medical history, clinical characteristics on presentation, and results of laboratory investigations and radiological imaging.
The clinical diagnosis upon discharge by the treating physician was used to categorize patients in 1 of 5 diagnostic categories, consisting of (1) CNS infection; (2) nervous system inflammation without infection; (3) noninfectious, noninflammatory neurological disorder; (4) non-neurological infection; and (5) other systemic disorder. For the category of CNS infections, we made subcategories consisting of bacterial meningitis, viral meningitis, and other neurological infections. All patients with CNS infections who had no definitive diagnosis based on microbiological testing were categorized by 2 independent investigators, with good interrater agreement (kappa 0.76) [12]. Outcomes were scored using the Glasgow Outcome Scale score, a validated outcome score ranging from 1 (death) to 5 (minor or no disability) [13].
For the current analysis, we restricted the population of this study to patients who were evaluated in the emergency department for suspected CNS infections. Furthermore, we excluded patients transferred from other hospitals, or those with no acute indication for LP while in the emergency department. Radiology data were collected for all patients, and abnormalities were recorded as diagnosed by the on-call radiologist. We scored all items included using the IDSA, ESCMID, Swedish, and Dutch guidelines, and used proxy markers when the definitions were not identical. For instance, for the score “loss of all reactions” in the Swedish guideline, we used a Glasgow Coma Scale score of E1M1V1. We contacted the corresponding authors of recent articles on the Swedish guidelines to clarify the used definitions [7, 14].
Statistical testing was performed using the Chi-squared (χ 2) test for dichotomous data and the Mann-Whitney U (MWU) test for continuous data. A P value below 0.05 was considered statistically significant. All patient data were rendered anonymous and the study was carried out in accordance with Dutch privacy legislation. The study was approved by the medical ethical committee of the Academic Medical Centre, Amsterdam, The Netherlands.
RESULTS
Between February 2012 through May 2015, 238 episodes of suspected CNS infections were evaluated in 238 patients (Supplementary Figure 1). In 35 of 238 patients (14.7%) the clinician judged that performing an LP in the emergency department was not indicated, and an LP was performed later, during hospital admission. Of the remaining 203 patients, 114 (56.2%; Table 1) were women and the median age was 44 years (interquartile range [IQR] 29–59). Final diagnoses of CNS infections were made in 56 patients (27.6%), consisting of bacterial meningitis in 16 patients (7.9%), viral meningitis in 24 (11.8%), and other CNS infections in 16 (7.9%). Of the 16 bacterial meningitis cases, 11 were confirmed by culture, and the diagnoses were made in the remaining cases on clinical grounds (elevated CSF white cell count and protein, and high blood C-reactive protein concentration and/or leukocytosis). Other CNS infections consisted of viral encephalitis (3 patients); tuberculous (2 patients); parasitic, fungal, or leptospirosis meningitis (each 2 patients); cerebral toxoplasmosis (2 patients); progressive multifocal leukoencephalopathy (2 patients); and human immunodeficiency virus meningitis (1 patient). Those patients with no CNS infection were diagnosed with inflammatory CNS disease (n = 11; 5.4%), non-neurologic infectious disease (n = 63; 32.5%), noninfectious neurological disease (n = 66; 32.5%), or other systemic disease (n = 3; 1.5%).
Table 1.
Characteristics of Included Patients
| Characteristic | n/N (%) |
|---|---|
| Median age (IQR), yearsa | 44 (29–59) |
| Female sex | 114/203 (56.2%) |
| Symptoms <24 hours | 86/200 (43.0%) |
| Previously diagnosed infection | 24/203 (11.8%) |
| Otitis/sinusitis | 15/203 (7.4%) |
| Pneumonia | 11/203 (5.4%) |
| Immunocompromised state | |
| Cancer | 17/201 (8.5%) |
| Diabetes | 22/203 (10.8%) |
| Alcoholism | 6/203 (3.0%) |
| Immunosuppressive treatment | 24/203 (11.8%) |
| HIV positivity | 20/203 (9.9%) |
| Presenting symptoms | |
| Headache | 145/203 (71.4%) |
| Seizures | 22/203 (10.8%) |
| Clinical signs | |
| Fever >38°C | 89/203 (43.8%) |
| GCS scorea (IQR) | 15 (14–15) |
| Altered mental status, GCS <14 | 50/203 (24.6%) |
| Coma, GCS <8 | 10/203 (4.9%) |
| Neck stiffness | 38/203 (18.7%) |
| Focal neurologic deficits | 39/203 (19.2%) |
| Papilledema | 2/52 (3.8%) |
Abbreviations: GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; IQR, interquartile range.
aAge and GCS score were available for all 203 patients.
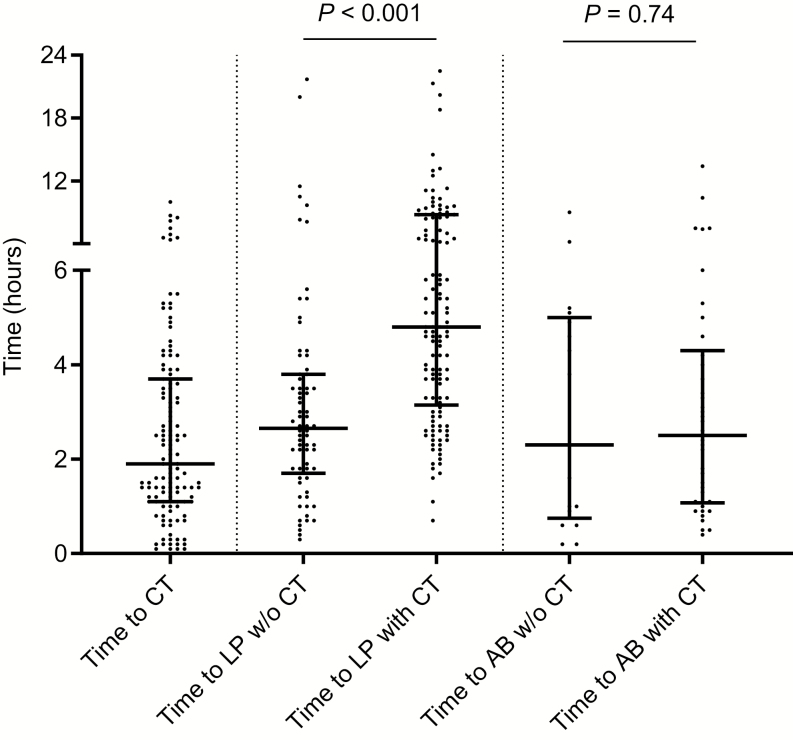
CT was performed prior to LP in 130 of 203 (64.0%) patients. The median time between emergency room arrival and CT was 115 minutes (IQR 66–220 min; Figure 1). Cranial CT scans showed abnormalities in 70 of the 130 patients (53.8%: Table 2): most commonly, old vascular lesions were seen in 21 (16.2%), leukoaraoisis in 19 (14.6%), new hypodense lesions in 5 (3.8%), and mass lesions in 4 (3.1%). CT abnormalities were related to the current illness in 19 patients (14.6%).
Figure 1.
Time from entry at emergency room to cranial computed tomography (CT), lumbar puncture (LP), and antibiotic treatment (AB). Cranial CT scans were performed in 120 patients, LPs were performed in all 203 patients, and ABs were started in the emergency room in 72 patients. The bars indicate the medians, the lines and whiskers indicate the interquartile ranges, and P values were calculated with the Mann-Whitney U test. Abbreviations: AB, antibiotic treatment; CT, computed tomography; LP, lumbar puncture; w/o, without.
Table 2.
Cranial Computed Tomography Results in Emergency Department in 130 Patients With Suspected Central Nervous System Infections
| Characteristic | n (%) |
|---|---|
| No abnormalities | 60 (46.2%) |
| Old vascular lesions | 21 (16.2%) |
| Leukoaraiosis | 19 (14.6%) |
| Old traumatic/surgery lesions | 7 (5.4%) |
| Cerebral atrophy | 6 (4.6%) |
| New hypodense lesionsa | 5 (3.8%) |
| Space-occupying lesionsb | 4 (3.1%) |
| Generalized edema | 2 (1.5%) |
| Arachnoidal cyst | 2 (1.5%) |
| Subdural hematoma/hygroma | 2 (1.5%) |
| Intracerebral hemorrhage | 1 (0.8%) |
| Obliteration basal cisterns or full-aspect posterior fossac | 2 (1.5%) |
Abbreviation: MRI, magnetic resonance imaging.
aFinal diagnoses, after MRI and follow-up, were progressive multifocal leukencephalopathy (n = 2), viral encephalitis (n = 1), astrocytoma (n = 1), and old vascular lesion/no space–occupying lesion (n = 1).
bFinal diagnoses, after MRI and follow-up, were meningeoma (n = 2) and toxoplasmosis (n = 2).
cFinal diagnosis, after MRI and follow-up, was normal aspect brain on MRI (n = 2).
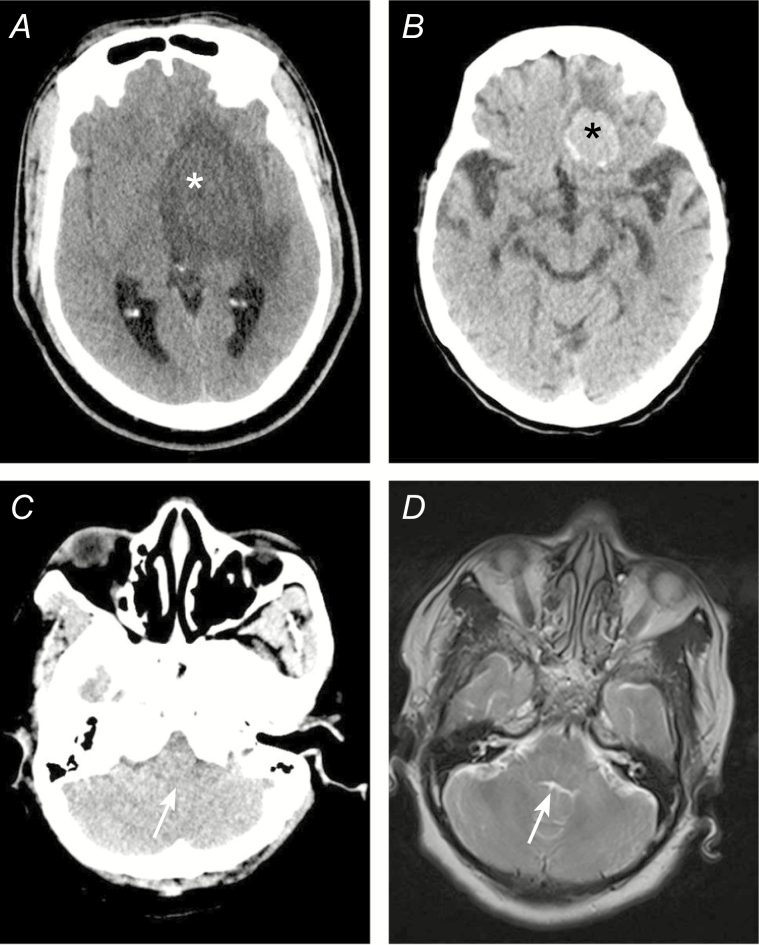
LPs were not performed because of CT results in 7 patients (5.4%; Figure 2). In 5 of these 7 patients (71%), artifacts precluded the adequate evaluation of the brain stem, fourth ventricle, and subarachnoid cisterns (CSF spaces) in the posterior cranial fossa. Subsequent magnetic resonance imaging was performed in these patients within 24 h, showing no contra-indications for LPs. Mass lesions with substantial brain shifts were seen on cranial CT scans in 2 remaining patients (29%); both were diagnosed with cerebral toxoplasmosis–complicating human immunodeficiency virus infections. CT scans were not performed before LPs in 73 patients (36.0%), and none of these patients deteriorated after their LP. The outcome was favorable in 71 of 73 patients (97.3%); 2 patients had a Glasgow Outcome Scale score of 4.
Figure 2.
Examples of cranial imaging results, showing (A) space-occupying lesion consistent with cerebral toxoplasmosis left basal ganglia (white asterisk) and leading to the deferral of a lumbar puncture; (B) frontal meningioma, a slow-growing, benign tumor not related to the patient’s symptoms (black asterisk) and not interfering with a lumbar puncture; (C) posterior fossa computed tomography image showing no cerebrospinal fluid in the lower part of the fourth ventricle (white arrow); and (D) magnetic resonance imaging of patient in panel C, showing no compression of the fourth ventricle after which lumbar puncture was performed.
Out of 203 patients, 1 or more indications to perform CT before LP were present in 129 patients (63.5%) according to IDSA guidelines, 80 patients (39.4%) according to ESCMID guidelines, 78 (38.4%) according to the Swedish guidelines, and 81 (39.9%) according to the Dutch guidelines (Table 3). In the 130 patients with CT scans before LPs, the procedure was in agreement with the IDSA guidelines in 100 patients (76.9%), ESCMID guidelines in 66 patients (50.8%), Swedish guidelines in 53 (40.8%) and the Dutch guidelines in 67 patients (51.5%). Of the 73 patients without CT scans prior to LPs, a CT before LP was recommended according to IDSA guideline in 29 patients (39.7%), to ESCMID guidelines in 14 (19.2%), to Swedish guidelines in 25 (34.2%), and to Dutch guidelines in 14 (19.2%). The 2 patients for whom LPs were deferred because of mass lesions met the imaging criteria of all 4 guidelines.
Table 3.
Overview of Guideline Indications for Cranial Imaging, and Proportion of Patients Fulfilling the Criteria
| Item | IDSA 2004 | n/N (%) | ESCMID 2016 | n/N (%) | Swedish Guideline 2010 | n/N (%) | Dutch Guideline 2012 | n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Medical history | Immunocompromised statea or history of central nervous system diseasea | 65/203 (32%) | Immunocompromised stateb | 34/203 (17%) | No indication | … | Immunocompromised stateb | 34/203 (17%) |
| Symptoms | No indication | … | No indication | … | Long duration (>4 days) of cerebral symptomsc or ABM-atypical symptomsd | 50/203 (24%) | No indication | … |
| Epileptic seizures | New-onset seizures (<1 week of presentation) | 22/203 (11%) | New-onset seizures | 22/203 (11%) | New onset epileptic seizures without clinical picture of ABMc | 9/203 (4%) | New-onset seizures | 22/203 (11%) |
| Mental status | GCS score <15 | 91/203 (45%) | GCS score <10 | 17/203 (8%) | Unconsciousness plus ≥1 of the following clinical findings: rigid dilated pupils; increasing blood pressure and bradycardiae; disturbed breathing patterne; opisthotonus; and loss of all reactions | 4/203 (2%) | GCS score <10 | 17/203 (8%) |
| Cranial Nerves | Anisocoria, abnormal ocular motility, or visual fields | 8/203 (4%) | No indication | … | N. III palsy | 5/203 (2%) | No indication | … |
| Ophthalmoscopy | Papilledema | 2/52 (4%) | No indication | … | No indication | Papilledema | 2/52 (4%) | |
| Neurologic deficits | Limb paresis | 17/203 (8%) | Aphasia, gaze palsy, limb paresis, ataxia | 33/203 (16%) | Arm or leg drift | 17/203 (8%) | Aphasia, gaze palsy, limb paresis, ataxia | 33/203 (16%) |
| Total with indication CT | … | 129/203 (64%) | … | 80/203 (39%) | … | 78/203 (39%) | … | 81/203 (40%) |
Abbreviations: ABM, acute bacterial meningitis; AIDS, acquired immunodeficiency syndrome; CT, computed tomography; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; IDSA, Infectious Disease Society of America; N. III, oculomotor nerve.
aThe IDSA guideline defines immunocompromised states as “due to HIV infection or AIDS, immunosuppressive therapy, or organ transplantation.”
bThe ESCMID guideline defines “severe immunocompromised” states as untreated HIV or organ transplant patients.
cThe Swedish and Dutch guideline defines “cerebral symptoms” as including headache, impaired mental status, nausea/vomiting, and/or (focal) seizures.
dThe Swedish guideline defines the “clinical picture of acute bacterial meningitis” as a fever or signs of infection—otitis, sinusitis, pneumonia, or pharyngitis—in combination with a headache and neck stiffness.
eNot further defined in the guideline, and left to the interpretation of the physician.
Empirical antibiotic treatment was started in the emergency department for 72 of 203 patients (35.4%). Antibiotic treatment was initiated for suspected sepsis in 23 patients (31.9%) and for suspected bacterial meningitis in 49 patients (68.1%). The median time between emergency department entry and antibiotic treatment was 136 minutes (IQR 57–258), and the times were similar between patients with and without CT scans (141 minutes [IQR 58–292] vs 134 minutes [IQR 52–227], respectively; MWU P = .74; Figure 1); results were also similar when limiting the analysis to those patients treated for suspected bacterial meningitis (MWU P = .34). Antibiotic treatments were started prior to CT scans in 11 of 33 (33.3%) patients with suspected bacterial meningitis, and those CT scans were before LPs. Antibiotic treatments were started within 1 hour, according to the ESCMID guideline recommendations, in 14 of 49 (28.6%) patients with suspected bacterial meningitis. The median time to LP was 157 minutes (IQR 103–228) in patients for whom CT scans were not performed, compared to 290 minutes (IQR 191–527) in those for whom CT scans were performed before LP (MWU P < .001). The time to treatment was known for 15 of the 16 patients who were eventually diagnosed with bacterial meningitis, and showed a median time to treatment of 62 minutes (IQR 32–171), which was shorter than for those treated with antibiotics who eventually received another diagnosis, albeit the difference was nonsignificant (median 136 minutes, IQR 65–278; MWU P = .09). There were 7 bacterial meningitis patients who were treated within 1 hour (46.7%), in compliance with the ESCMID guidelines.
DISCUSSION
Our data show that the majority of patients with suspected CNS infections presenting to the emergency department received cranial imaging, irrespective of guideline criteria. This is concordant with previous studies in bacterial meningitis [8, 9, 11, 15]. A potential explanation for the high rate of CT scans prior to LPs is that conditions other than CNS infections can be considered in the differential diagnoses of these patients, such as cerebral hemorrhage. Furthermore, CNS infections may present with mass lesions causing substantial brain shifts—for example, herpes simplex encephalitis or brain abscess—and physicians want to rule out this possibility prior to the LP. Such mass lesions can be detected by a cranial CT, but the risk of a substantial brain shift is very low, particularly when clinical signs of a brains shift are absent [6]. Finally, nowadays cranial CT imaging is performed extremely quickly in some settings, especially in those centers with acute stroke care infrastructure [12, 16, 17]. The availability of cranial CT results within minutes after the emergency department entrance may lower the threshold for performing a CT before LP.
The performance of a cranial CT before an LP was not associated with the delayed initiation of empirical antibiotic therapy. Previously, a retrospective analysis of 123 cases of community-acquired bacterial meningitis in patients admitted from 1990–2002 showed that having a CT scan prior to an LP was associated with an odds ratio of 5.6 for having a delay in antibiotic treatment of more than 6 hours [18]. However, in this Canadian study, a substantial proportion of patients had to be transferred from a peripheral hospital to receive a cranial CT, which was a major contributor to the delay. A more recent, retrospective, Danish study studying 358 bacterial meningitis patients who were admitted between 1998–2014 showed that a CT was associated with a late diagnosis, which was associated with a poor prognosis [10]. However, the contribution of cranial imaging to the delay in imaging was not reported, and several other factors were identified that contributed to the late diagnoses [19]. Finally, a retrospective Swedish study of bacterial meningitis patients who were identified between 2005–2012 showed that a cranial CT led to a delay in antibiotic treatment of 1.6 hours, even though 60% of patients received antibiotics prior to a CT [7]. Several explanations for the discrepancy between our results and the literature can be identified. First, we studied patients presenting to our center and excluded transferred patients. Second, in the time periods in which the previous studies were performed, cranial imaging was likely to be less readily available than in our setting, which increased the risk of a delay. Finally, all previous studies had selection biases, as they studied only confirmed bacterial meningitis cases, as opposed to all patients suspected of CNS infections, which is the population at risk and was included in our study.
LPs were delayed by 2 hours when patients underwent CT scans prior to LPs. Previous studies in bacterial meningitis have shown that the yield of CSF cultures decreased from 94% to 88% in patients in whom CT scans were done before LPs [9]. A single-center, retrospective study from the United Kingdom including 92 patients with bacterial and viral meningitis between 2002–2004 showed that 67% of patients had a delayed time to LP due to a CT, and only 3% of cases had an LP performed within 2 hours of initiation of antibiotic treatment [20]. Culture positivity sharply decreased if the LP was performed more than 4 hours after the initiation of antibiotic treatment, and none of the patients with LPs performed more than 8 hours after beginning antibiotics had a positive culture. This, and increased costs and radiation exposure, remain arguments for restricted cranial imaging in patients with suspected CNS infections when guideline indications are not met and imaging is not indicated for other neurological conditions included in the differential diagnosis. We observed that the time to treatment in patients eventually diagnosed with bacterial meningitis was half that of the time in those receiving an alternative diagnosis (median 62 vs 136 minutes, respectively). This may indicate that physicians are able to identify those patients with a high probability of bacterial meningitis, even though this profile has not been identified by looking at individual or combined clinical and laboratory characteristics [12].
We found that the IDSA guideline differs substantially from the ESCMID, Swedish, and Dutch guidelines with regard to the proportion of patients for whom cranial imaging is advised. The IDSA guideline has the most conservative recommendations, in which 64% of our patients were advised to be scanned, compared to 39–40% for the ESCMID, Swedish, and Dutch guidelines. This is in contrast to a recent study in bacterial meningitis patients, which describing imaging indications in 65%, 32%, and 7% of patients based on the IDSA, ESCMID, and Swedish guidelines, respectively [11]. The explanation of the discrepancy between studies is a selection bias, as only confirmed bacterial meningitis cases were included in the Swedish study. Importantly, some of the criteria in the Swedish guideline are left to the interpretation of the treating physician, such as “cerebral symptoms,” “increasing blood pressure,” and “disturbed breathing patterns” [4, 7, 11, 14].
The guideline items that explain the differences in advice on imaging were an altered mental status, medical history, and “a long duration of symptoms or acute bacterial meningitis atypical symptoms.” Whereas the IDSA, ESCMID, and Dutch guidelines have altered mental status defined by Glasgow Coma Scale score cut-offs (<15, <10, and <10, respectively), the Swedish guideline advises physicians to perform imaging only when unconsciousness is combined with clinical signs of cerebral herniation. Only 2% of patients met this mental status criterion of the Swedish guideline. This did not result in an overall reduction in the proportion of patients in whom imaging was advised, because of the incorporation of the “long duration symptoms and acute bacterial meningitis atypical symptoms” item. As only 41% of bacterial meningitis patients have the classic triad of neck stiffness, fever, and altered mental status, and even fewer have the triad in the population with suspected CNS infections, many patients fulfilled this criterion [12, 21].
This study has several limitations. The most important limitation is that our study was limited to patients who underwent an LP, and patients in whom an LP was not performed due to cranial imaging were not included. Ideally, a diagnostic accuracy study on the use of CT before LP would include all patients in whom CNS infections are considered, regardless of whether an LP is performed. However, a definite diagnosis of CNS infection can not be made in patients in whom no CSF examination is performed and, therefore, such a study will not provide conclusive results. Another limitation of our study is that it is observational and not all patients received cranial imaging, which means lesions could have been missed in patients in whom the physicians choose to directly perform the LP. However, follow-ups of these patients showed that all survived and that none developed severe complications from their LP. Furthermore, several other guidelines exist for bacterial meningitis, encephalitis, and tuberculous meningitis that were not evaluated in our study [22–24]. We selected the bacterial meningitis guidelines of 2 major infectious disease societies (IDSA and ESCMID), as well as the local (Dutch) guideline and Swedish guideline: the latter because a recent publication suggested the superiority of this guideline [11]. We choose bacterial meningitis guidelines, as this disease has the highest mortality and the most urgency in diagnosis and treatment [25]. A final limitation is that our hospital is a tertiary care facility with a specific clinical and research focus on neurological infectious disease and, therefore, may not be representative of other hospitals or countries.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all the neurologists, residents, and patients who participated in the study.
Financial support. This work was supported by the Netherlands Organization for Health Research and Development (NWO-Vici grant number 918.19.627 to D. v. d. B.; and NWO-Vidi grant number 917.17.308 to M. C. B.).
Potential conflicts of interest. D. v. d. B. reports a fellowship from Academic Medical Centre and a grant from the European Research Council. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brouwer MC, Thwaites GE, Tunkel AR, van de Beek D. Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet 2012; 380:1684–92. [DOI] [PubMed] [Google Scholar]
- 2. Costerus JM, Brouwer MC, van de Beek D. Technological advances and changing indications for lumbar puncture in neurological disorders. Lancet Neurol 2018; 17:268–78. [DOI] [PubMed] [Google Scholar]
- 3. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–84. [DOI] [PubMed] [Google Scholar]
- 4. van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 2016; 22(Suppl 3):S37–62. [DOI] [PubMed] [Google Scholar]
- 5. Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–33. [DOI] [PubMed] [Google Scholar]
- 6. Costerus JM, Brouwer MC, Sprengers MES, Roosendaal SD, van der Ende A, van de Beek D. Cranial computed tomography, lumbar puncture, and clinical deterioration in bacterial meningitis: a nationwide cohort study. Clin Infect Dis 2018; 67:920–6. [DOI] [PubMed] [Google Scholar]
- 7. Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–9. [DOI] [PubMed] [Google Scholar]
- 8. Salazar L, Hasbun R. Cranial imaging before lumbar puncture in adults with community-acquired meningitis: clinical utility and adherence to the Infectious Diseases Society of America guidelines. Clin Infect Dis 2017; 64:1657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costerus JM, Brouwer MC, Bijlsma MW, Tanck MW, van der Ende A, van de Beek D. Impact of an evidence-based guideline on the management of community-acquired bacterial meningitis: a prospective cohort study. Clin Microbiol Infect 2016; 22:928–33. [DOI] [PubMed] [Google Scholar]
- 10. Bodilsen J, Dalager-Pedersen M, Schønheyder HC, Nielsen H. Time to antibiotic therapy and outcome in bacterial meningitis: a Danish population-based cohort study. BMC Infect Dis 2016; 16:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glimåker M, Sjölin J, Åkesson S, Naucler P. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis 2018; 66:321–8. [DOI] [PubMed] [Google Scholar]
- 12. Khatib U, van de Beek D, Lees JA, Brouwer MC. Adults with suspected central nervous system infection: a prospective study of diagnostic accuracy. J Infect 2017; 74:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet 1976; 1:1031–4. [DOI] [PubMed] [Google Scholar]
- 14. Glimåker M, Johansson B, Bell M, et al. Early lumbar puncture in adult bacterial meningitis–rationale for revised guidelines. Scand J Infect Dis 2013; 45:657–63. [DOI] [PubMed] [Google Scholar]
- 15. van de Beek D, de Gans J, Spanjaard L, Vermeulen M, Dankert J. Antibiotic guidelines and antibiotic use in adult bacterial meningitis in The Netherlands. J Antimicrob Chemother 2002; 49:661–6. [DOI] [PubMed] [Google Scholar]
- 16. Saver JL, Goyal M, van der Lugt A, et al. ; Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke trials Collaborators. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316:1279–88. [DOI] [PubMed] [Google Scholar]
- 17. Zinkstok SM, Beenen LF, Luitse JS, Majoie CB, Nederkoorn PJ, Roos YB. Thrombolysis in stroke within 30 minutes: results of the acute brain care intervention study. PLOS One 2016; 11:e0166668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–8. [DOI] [PubMed] [Google Scholar]
- 19. Bodilsen J, Brandt CT, Sharew A, et al. Early versus late diagnosis in community-acquired bacterial meningitis: a retrospective cohort study. Clin Microbiol Infect 2018; 24:166–70. [DOI] [PubMed] [Google Scholar]
- 20. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J 2010; 27:433–8. [DOI] [PubMed] [Google Scholar]
- 21. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis 2016; 16:339–47. [DOI] [PubMed] [Google Scholar]
- 22. Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J; British Infection Society British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009; 59:167–87. [DOI] [PubMed] [Google Scholar]
- 23. McGill F, Heyderman RS, Michael BD, et al. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J Infect 2016; 72:405–38. [DOI] [PubMed] [Google Scholar]
- 24. Tunkel AR, Glaser CA, Bloch KC, et al. ; Infectious Diseases Society of America The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008; 47:303–27. [DOI] [PubMed] [Google Scholar]
- 25. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016; 2:16074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.