Abstract
Background and Aims:
Brachial plexus (BP) blocks continue to be described with reference to anatomical landmarks (Interscalene and Supraclavicular), even after the introduction of ultrasound which enables us to directly identify the roots, trunks and divisions of the BP. The aim of this study was to describe a novel injection technique targeting trunks of BP and to determine the minimum effective local anaesthetic volume (MELAV) required to produce BP block with this approach.
Methods:
Twenty-one male patients in the age group 20–40 years, undergoing elective forearm bony procedures received an ultrasound-guided truncal injection BP block. MELAV50 was determined using the Dixon and Mood up-and-down method. Initial volume of local anaesthetic (LA; 50:50 mixture of bupivacaine 0.5% and lignocaine 2% with 5 μg/ml epinephrine) injected was 6 ml in each trunk, which was varied by 1 ml/trunk for each consecutive patient according to the response of the previous patient. The MELAV50, MELAV95 and MELAV99 were calculated using Probit transformation and logistic regression.
Results:
Out of the 21 patients, 13 patients had a successful block. The MELAV50, MELAV95 and MELAV99 were 7.41, 10.47 and 12 ml, respectively. Eight patients in whom block failed had sparing in the ulnar and median nerve territories.
Conclusion:
Trunks of the brachial plexus can be identified and targeted for the injection of local anaesthetics. The MELAV50 and MELAV95 required for ultrasound-guided truncal injection brachial plexus block were 7.4 and 10.4 ml, respectively.
Key words: Brachial plexus block, minimum effective volume, truncal injection
INTRODUCTION
Interscalene and supraclavicular brachial plexus blocks (BPB) are commonly performed to provide anaesthesia for upper limb surgery.[1,2] Before ultrasound (US), surface landmarks such as interscalene groove and subclavian artery formed anatomical reference points to guide the needle to the brachial plexus (BP) elements. With US, we can now directly observe neural elements (NE) and inject local anaesthetic (LA) around it. The NE are seen as round hypoechoic structures (traffic-light sign) in interscalene groove and as bunch-of-grapes at the supraclavicular fossa.[3,4] Since correlation between gross anatomy, sonoanatomy and microanatomy was not established, investigators were not able to describe their injection targets with respect to BP elements as root injections, truncal injections or injections at the level of divisions. Instead, injection techniques were described as interscalene, low interscalene, centre-cluster, corner-pocket, targeted-intracluster and multipoint-subfascial injections for interscalene and supraclavicular blocks.[5,6,7,8] This lack of corroborative knowledge and understanding of sonoanatomy of injection targets contributed significantly to gross difference in the minimum effective local anaesthetic (MELAV) required to produce successful supraclavicular and interscalene BPB, even under ultrasound-guidance (USG), between authors.[9,10,11,12,13] With present understanding of sonoanatomy of injection targets, we are now able to observe roots, roots joining to form trunks and trunks, dividing into divisions as distinct from each other. This study was designed to describe a novel injection technique targeting trunks of BP and to find the MELAV required to produce complete conduction blockade of all five terminal nerves of BP for upper limb anaesthesia.
METHODS
This study was done at a tertiary care teaching university hospital between april 2018 and april 2019. The study was approved by Institutional Human Ethics Committee (P.G Dissertation/2017/05/79) and registered with Clinical Trial Registry of India (CTRI/2018/03/012271). All procedures done in the study followed the ethical guidelines of the Declaration of Helsinki. Consecutive twenty-one American Society of Anesthesiologist physical status 1/2 male patients, between 20 and 40 years, undergoing elective upper extremity bony surgery at or below elbow were recruited [Figure 1]. Patients were excluded if they refused to participate, BMI >25 kg/m2, history of allergy to LA, evidence of coagulopathy, neurological deficit, infection at supraclavicular fossa and if the US targets were not clearly visible.
Figure 1.
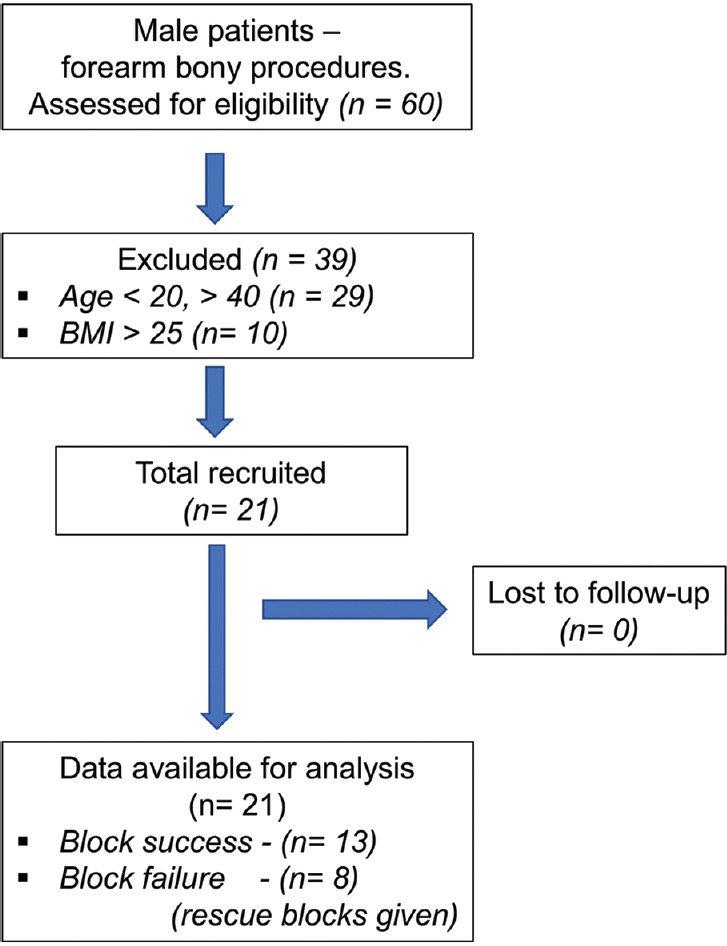
Flow chart depicting patient recruitment
Written informed consent was obtained during the pre-anaesthetic visit. All patients were premedicated as per institutional protocol the night before surgery. All blocks were performed in anaesthetic procedure room. An 18-gauge intravenous access and routine monitoring (electrocardiogram, arterial oxygen saturation and non-invasive blood pressure) were established prior to block.
Patients were placed in lateral position (injured arm non-dependent) with a thin pillow under the head and shoulder pulled down as much as possible to maximally expose the supraclavicular fossa. Performer sat at the head end and imaging screen was positioned in front, close to the back of the patient. High-frequency broadband linear-array transducer (HFL50, 15–6 MHz) of X-Porte Ultrasound system (FUJIFILM Sono Site, Inc., Bothell, USA) was used for scanning.
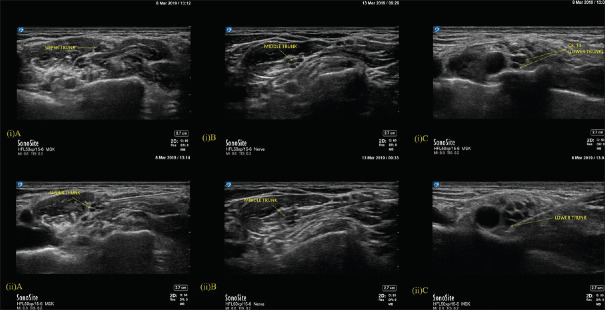
The probe was moved from midline to the lateral side of neck serially to identify the nerve roots as dark hypoechoic circles at the interscalene groove. Transverse process (TP) with prominent anterior tubercle (Chassaignac tubercle) was first identified as C6 vertebra. Cephalad to this, C5 nerve root was identified emerging from the bifid C5 TP. C5 and C6 roots were traced caudad to see them joining to form the upper-trunk (UT) [Figure 2(i)A]. C6 root often became bi-fascicular or multi-fascicular before joining C5. Nerve root emerging out of the TP with only a posterior tubercle was identified as C7. Soon after emerging, it became multi-fascicular to form the middle-trunk (MT) [Figure 2(i)B]. On tracing caudad, the MT was seen lying over the first rib, immediately below the anterior and posterior divisions of UT. Lower-trunk (LT) was located at the corner pocket after eliminating components of UT and MT. In most cases, C8 root was observed over proximal part of the first rib, which appears as a flat structure without any tubercles. T1 root popped up into the corner pocket from inner surface of 1st rib to join C8 and form the LT [Figure 2(i)C].
Figure 2.
(i) Upper trunk and Middle trunk are seen at the interscalene groove and Lower trunk of the brachial plexus is seen at supraclavicular fossa. A-Upper trunk, B-Middle trunk, C-Lower trunk. (ii) Image depicting the local anaesthetic injection (Needle tip position) in the Upper trunk, Middle trunk and Lower trunk of the brachial plexus. A-Upper trunk, B-Middle trunk, C-Lower trunk
All blocks were performed by one of two authors (TS or RS) experienced in truncal identification and injections. A 25-gauge Quinke spinal needle (BD™) was connected through a 100-cm pressure monitoring line to a 0.5 ml-graded 10 ml luer-lock syringe. Needle insertion was in-plane or out-of-plane according to convenience, sonoanatomy and ergonomics achieved with the individual patient. Needle tip was positioned in the hyperechoic connective tissues (CT) between hypoechoic NE, well below the outermost hyperechoic line (epineurium) in all trunks. UT was accessed through the space between C5-C6 roots [Figure 2(ii)A]. MT was accessed either at 3 o'clock or 9 o'clock position [Figure 2(ii)B]. LT was approached at the corner pocket well above first rib either at 9 o'clock position close to the artery through out-of-plane or at 3 o'clock position, through in-plane approach [Figure 2(ii)C]. Therefore, injections were spread across interscalene groove and supraclavicular area according to the target trunk accessed.
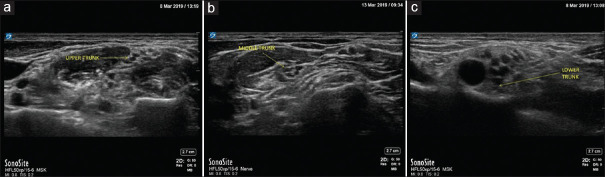
Equal mixture of 2% lignocaine with adrenaline 1: 200000 and 0.5% bupivacaine was used. First patient received 18 ml (6 ml/trunk) of LA. At each injection point, 0.5 ml of LA was used to hydrolocate and ensure that needle tip was not inside any of the hypoechoic circles (intra-fascicular) but within the hyperechoic CT matrix. In case paraesthesia was complained, needle tip was repositioned before LA injection. Remaining volume was injected in 0.5 ml aliquots every 1–2 s to prevent building up of hydrostatic pressure and seeping of LA outside the trunk. We injected UT and MT before LT to delineate their components at the supraclavicular fossa, thereby enabling clear identification of LT at the corner pocket, which was the most challenging step in truncal injection BPB [Figure 3a-c]. Dixon and Mood up-and-down study design was followed.[14] LA volume for subsequent patient was determined by success or failure of block performed in the previous patient. Drug volume was increased by 3 ml (1 ml/trunk) in case of block failure and decreased by 3 ml in case of block success. This was continued till 5 up-downs were observed. Block failure was managed by performing rescue blocks at axilla.
Figure 3.
Image depicting the Upper trunk, Middle trunk and Lower trunks of the brachial plexus post local anaesthetic injection. (a) Upper trunk, (b) Middle trunk, (c)Lower trunk
Final needle removal time was noted as Block Time. Block assessment was done at 10-min intervals by an independent observer who was blinded to LA volume. Sensory blockade was assessed on a three-point qualitative scale: Grade 0, presence of cold and touch; Grade 1, loss of cold but not touch; Grade 2, loss of both cold and touch. All five terminal nerves of BP were assessed [Axillary nerve (AN)-lower lateral deltoid, Musculocutaneous nerve (MCN) - lateral forearm, Median nerve (MN)-tip of middle finger, Ulnar nerve (UN)-tip of little finger and Radial nerve (RN)-anatomical snuff box]. Motor blockade was assessed using a similar 3-point qualitative scale: 0-normal motor function (Power 4/5, 5/5), 1-decreased motor function (Power 3/5, 2/5), 2-no motor power (Power 0/5, 1/5). AN motor function was assessed during abduction of shoulder beyond 30°, MCN-elbow flexion, MN-thumb opposition, UN-thumb adduction and RN-thumb abduction, respectively. Hence, individual nerve conduction blockade score can range from minimum of 0 to maximum of 4 (2 + 2; sensory + motor) and all five nerves put together, total composite score for conduction blockade can range from 0 to 20. A score of 20/20 at 30 min after block-time was considered successful. Patients were sedated using intravenous midazolam 1 mg and fentanyl 1–2 μg/kg. Block was considered inadequate if patients complained of pain or requirement of fentanyl was >2 μg/kg anytime during intraoperative period.
Post-operatively, when patient perceived pain, injection acetaminophen 1 g and ketorolac 30 mg was given IV and continued at regular intervals. At the end of 24 h, patients were directly questioned for symptoms suggestive of persistent paraesthesia or dysesthesia. Any report of persistent sensory-motor deficit at follow-up visit with surgeon, 1-week after surgery, was also communicated to the research team. Study ended when 5 up and downs were noted. MELAV50, MELAV90 and MELAV95 were estimated by Probit analysis and logistic regression.
RESULTS
Twenty-one male patients satisfying inclusion criteria completed the study. Physical characteristics of study population are presented in Table 1.
Table 1.
Physical characteristics of the study population
| Physical characteristics | Data |
|---|---|
| Age in years* | 30±7 |
| BMI kg/m2* | 23±2 |
| ASA 1:2 | 11:10 |
| Type of surgery | |
| Both bone fracture | 9 |
| Radius fracture | 8 |
| Ulna fracture | 4 |
| Duration of surgeries in min* | 154±15 |
*indicates mean±SD
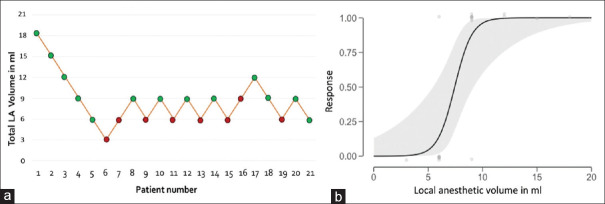
Out of 21 patients, 13 had a successful block. Five up-downs were observed between 6 and 9 ml of LA [Figure 4a]. The dose-response curve is shown in Figure 4b. Through Probit transformation and logistic regression analysis, MELAV50, MELAV95 and MELAV99 were calculated as 7.41 ml, 10.47 ml and 12.02 ml, respectively. Minimum LA volume administered was 3 ml. Even though the 3-ml volume produced a total composite score of 20/20 at 30 min, block was considered a failure as patient complained of pain after 2 h of block time requiring skin infiltration to complete surgery. In all 8 patients in whom block failed, sparing was noted in both MN and UN territories. Even in patients with successful block, MN and UN took significantly longer time for complete conduction blockade when compared to AN, MCN and RN [Table 2]. Paraesthesia on needling the MT was encountered in 1/20 patients and in LT in 3/20 patients. No other complications were noted. None of the patients reported back with persistent sensory or motor changes at 1-week follow-up.
Figure 4.
(a) Graph showing the sequence of successful and unsuccessful blocks performed. Y-axis: Total LA volume used for the three trunks. (b) Dose-Response curve (X–axis: volume of LA in ml, Y-axis: response to a particular dose)
Table 2.
Mean time taken for complete sensory and motor blockade of the five terminal nerves of the brachial plexus in patients with successful block
| Time in minutes (mean±SD) to Complete Conduction Blockade | Mean time to complete conduction blockade in MCN/AN/RN combined Vs. MN and UN combined | P | |
|---|---|---|---|
| Musculocutaneous nerve | 11.2±3.23 | 12.9±2.1 | |
| Axillary nerve | 12.3±4.3 | 0.0001 | |
| Radial nerve | 15.4±7.1 | ||
| Median nerve | 16.9±8.9 | 17.5±0.8 | |
| Ulnar nerve | 18.1±8. 0 |
MCN – Musculocutaneous nerve, AN – Axillary nerve, RN – Radial nerve, MN – Median nerve, UN – Ulnar nerve
DISCUSSION
In this study, we have described an injection technique targeting trunks of BP and found the MELAV to produce an effective BPB to be 7.41 ml for 50% patients and 10.47 ml for 95% patients. Previously, authors who described the trunks during interscalene block studies misinterpreted 'traffic light sign' at the interscalene groove as the three trunks. Their outcome did not include assessment of MN, UN and RN to demonstrate MT and LT blockade.[11,13,15] Selective UT blocks have been described as case reports and randomized trials for analgesia during shoulder surgeries but MT and LT blocks have not been described.[16,17] To the best of our knowledge, this is the first clinical report describing the identification of trunks and targeting LA injection of all three trunks to produce complete BPB for surgical anaesthesia.
As MELAV is affected by age and degree of noxious stimulus (soft tissue/bony procedure), we recruited only male patients between age group 20 and 40 years with a BMI of < 25 kg/m2, undergoing bony forearm procedures.[8,18] The final needle tip position (extra-fascial/sub-fascial/targeted-intracluster) and definition of block success (analgesia/surgical anaesthesia/complete conduction blockade) are important factors influencing observed results. Hence, to reduce inter-performer bias, all blocks were performed only by one of two investigators, experienced in Truncal identification and interfascicular needle placement. Complete conduction block of core fibres (composite score of 20/20), as assessed at distal most innervation areas of the terminal nerves were alone considered successful. We started with 18 ml of LA, due to our previous experience of 100% success with 20 ml LA volume.[8] We used combination of LA to take advantage of quick onset of lignocaine and to have the prolonged block effect of bupivacaine, especially at the lower volumes, where the longer latency period (bupivacaine) and shorter duration of surgical anaesthesia (lignocaine) would be challenging to manage during a forearm bony procedure. Similar combination of LA had been studied earlier for MELAV during supraclavicular block.[10] Rice andMcMahon have earlier described the advantage of using sharper needles over the blunt needles (300–450) during intraneural injections.[19] Since subepineural needle placement is an intraneural injection, we have used a 25-gauge Quinke spinal needle to take advantage of the smaller, sharp and short (200) bevel cutting-edge needle tip.[20]
We attribute the low MELAV95 (10.47 ml) in our study when compared to the previous studies to the unique anatomical organisation of trunks in terms of ratio between NE and CT.[9,10] At level of trunks, BP elements are enclosed by a well-defined epineurium (outermost hyperechoic line) and contain large size fascicles (hypoechoic circles) bathed in CT matrix (hyperechoic elements between the fascicles).[21] Microsurgical literature has demonstrated that number of fascicles and CT proportionately increase from proximal to distal part of BP. Average number of fascicles increase from 8, 10 and 11 for UT, MT and LT to 15, 13 and 18 for lateral, medial and posterior cord, respectively.[22] Similarly, perineural CT increases from 5% to 28% at roots to 53% to 58% at trunk and together with epineural CT it makes up to 90%, 78%, 73% and 86% for AN, RN, MN and UN, respectively.[22] The increase in CT from roots to peripheral nerves in turn increases the diffusion distance for LA to reach the axons inside the fascicles, thereby delaying onset of block.
With similar dose finding methodology, Tran et al. and Duggan et al. reported an MELAV90 of 32 ml and 42 ml, respectively, during USG supraclavicular BP block.[9,10] This high volume is possibly due to the single-point and double-point injection at supraclavicular level. Both real-time scanning and microanatomy literature demonstrate that NE at this level are spread across a wider area and injecting LA at one or two points may not deliver drug across all NE at the same time. Hence, effective conduction blockade in these studies was largely dependent on LA volume (30–40 ml). When LA is delivered at multiple-points as in our present study as well as in the report by Song et al., the function of volume in spreading drug across all NE is taken over by the injection technique,[23] hence, the decrease in MELAV.
AN, MCN and RN were completely blocked in all 21 patients irrespective of volume, while UN and MN were incompletely blocked at low volumes. Even though MN receives fascicles from all 5 nerve roots, we consider LT (C8 and T1) to be the cause for the incomplete blockade of MN because it paralleled UN sparing and the UN receives fascicles mostly from LT. On the other hand, RN which also receives contribution from all three trunks was completely blocked even at low volumes. This could be because RN is formed from posterior divisions of individual trunks, which split away early from the bulk of the trunk.[24] C8-T1 NE contributing to RN are thereby located peripherally in LT and consequently amenable to block. Hence topographically, nerve fibres of MN and UN that supply intrinsic muscles of the hands would travel in the core of any given fascicle. Hence, when LA is deposited in between fascicles in any given trunk, core fibres of the fascicles are likely to get blocked at the last. Our assumption was further strengthened by the observation that complete conduction blockade occurred early in AN, MCN and RN when compared to MN and UN even in patients with successful block [Table 2].
The technical difficulty with LT was that unlike C5, C6 and C7, C8 and more commonly T1 nerve roots cannot be traced to the entire length from the TP to where they join to form LT. Second, LA spread across LT was not visualized clearly because of depth and attenuation of US waves by the hyperechoic superficial UT and MT. Whether MN and UN sparing at low volumes is because of inadequate drug delivery at LT due to technique-related issues or anatomical factors (monofascicular vs multifascicular/length of lower trunk exposed to LA/continuous subclavian artery pulsation) or inadequate blockade of core fibres in all three trunks needs to be answered by future studies.
Our study has the following limitations. Dose-finding studies for peripheral nerve block using Dixon up-and-down method measures the 50th quantile and its accuracy is very weak in estimating higher quantiles situated far away from the midline (ED 90/95/99).[15] Probit and logistic regression analysis are often used in conjunction with the Dixon-Mood design to estimate MELAV 90/95/99.[14] Future studies should confirm the efficacy of this MELAV95 dose and its complication profile like incidence of phrenic nerve palsy and Horner's syndrome. While the UT and MT were successfully blocked with 2 ml LA itself, the LT was spared at this volume. Chances of drug being injected within the perineurium during sub-epineural injections are being highlighted in recent cadaver studies.[25,26] Hence, a novice anaesthesiologist should develop adequate needling skills before contemplating on sub-epineural injections and executing needle choices.
CONCLUSION
The trunks of BP can be used as a potential target for LA injection. The MELAV50 and MELAV95 required for US-guided truncal injection BP block were 7.4 and 10.47 ml respectively, which is significantly lower than the existing reports for BP blocks above clavicle.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abhinaya RJ, Venkatraman R, Matheswaran P, Sivarajan G. A randomised comparative evaluation of supraclavicular and infraclavicular approaches to brachial plexus block for upper limb surgeries using both ultrasound and nerve stimulator. Indian J Anaesth. 2017;61:581–6. doi: 10.4103/ija.IJA_402_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pani N, Routray SS, Pani S, Mallik S, Pattnaik S, Pradhan A. Post-operative analgesia for shoulder arthroscopic surgeries: A comparison between inter-scalene block and shoulder block. Indian J Anaesth. 2019;63:382–7. doi: 10.4103/ija.IJA_65_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore C. Ultrasound-guided procedures in emergency medicine. Ultrasound Clin. 2011;6:277–89. [Google Scholar]
- 4.Raju PKBC, Coventry DM. Ultrasound-guided brachial plexus blocks. Contin Educ Anaesth Crit Care Pain. 2014;14:185–91. [Google Scholar]
- 5.Chan VWS, Perlas A, Rawson R, Odukoya O. Ultrasound-guided supraclavicular brachial plexus block. Anesth Analg. 2003;97:1514–7. doi: 10.1213/01.ANE.0000062519.61520.14. [DOI] [PubMed] [Google Scholar]
- 6.Soares LG, Brull R, Lai J, Chan VW. Eight ball, corner pocket: The optimal needle position for ultrasound-guided supraclavicular block. Reg Anesth Pain Med. 2007;32:94–5. doi: 10.1016/j.rapm.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Techasuk W, González A, Bernucci F, Cupido T, Finlayson R, Tran D. A Randomized comparison between double-injection and targeted intracluster-injection ultrasound-guided supraclavicular brachial plexus block. Anesth Analg. 2014;118:1363–9. doi: 10.1213/ANE.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 8.Sivashanmugam T, Ray S, Ravishankar M, Jaya V, Selvam E, Karmakar MK. Randomized comparison of extrafascial versus subfascial injection of local anesthetic during ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med. 2015;40:337–43. doi: 10.1097/AAP.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 9.Tran DQH, Dugani S, Correa JA, Dyachenko A, Alsenosy N, Finlayson RJ. Minimum effective volume of lidocaine for ultrasound-guided supraclavicular block. Reg Anesth Pain Med. 2011;36:466–9. doi: 10.1097/AAP.0b013e3182289f59. [DOI] [PubMed] [Google Scholar]
- 10.Duggan E, El Beheiry H, Perlas A, Lupu M, Nuica A, Chan VW, et al. Minimum effective volume of local anesthetic for ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med. 2009;34:215–8. doi: 10.1097/AAP.0b013e31819a9542. [DOI] [PubMed] [Google Scholar]
- 11.Falcão LFR, Perez MV, de Castro I, Yamashita AM, Tardelli MA, Amaral JLG. Minimum effective volume of 0.5% bupivacaine with epinephrine in ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2013;110:450–5. doi: 10.1093/bja/aes419. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Wang JJ, Awad IT, McHardy P, Safa B, McCartney CJ. The minimal effective volume (MEAV 95) for interscalene brachial plexus block for surgical anesthesia under sedation: A prospective observational dose finding study. Can J Pain. 2017;1:8–13. doi: 10.1080/24740527.2017.1304805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier P, Vandepitte C, Ramquet C, DeCoopman M, Xu D, Hadzic A. The minimum effective anesthetic volume of 0.75% ropivacaine in ultrasound-guided interscalene brachial plexus block. Anesth Analg. 2011;113:951–5. doi: 10.1213/ANE.0b013e31822b876f. [DOI] [PubMed] [Google Scholar]
- 14.Saranteas T, Finlayson RJ, Tran DQH. Dose-Finding Methodology for Peripheral Nerve Blocks. Reg Anesth Pain Med. 2014;39:550–5. doi: 10.1097/AAP.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 15.Franco CD, Williams JM. Ultrasound-guided interscalene block: Reevaluation of the “stoplight” sign and clinical implications. Reg Anesth Pain Med. 2016;41:452–9. doi: 10.1097/AAP.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 16.Burckett-St Laurent D, Chan V, Chin KJ. Refining the ultrasound-guided interscalene brachial plexus block: The superior trunk approach. Can J Anesth Can Anesth. 2014;61:1098–102. doi: 10.1007/s12630-014-0237-3. [DOI] [PubMed] [Google Scholar]
- 17.Kang R, Jeong JS, Chin KJ, Yoo JC, Lee JH, Choi SJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology. 2019;131:1316–26. doi: 10.1097/ALN.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 18.Šarić JP, Vončina V, Paklar N, Dvorščak MB. Effects of age on onset time and duration of sensory blockade in ultrasound guided supraclavicular block. Period Biol. 2015;117:287–90. [Google Scholar]
- 19.Rice ASC, McMahon SB. Peripheral nerve injury caused by injection needles used in regional anaesthesia: Influence of bevel Configuration, studied in a rat model. Br J Anaesth. 1992;69:433–8. doi: 10.1093/bja/69.5.433. [DOI] [PubMed] [Google Scholar]
- 20.Steinfeldt T, Nimphius W, Werner T, Vassiliou T, Kill C, Karakas E, et al. Nerve injury by needle nerve perforation in regional anaesthesia: Does size matter? Br J Anaesth. 2010;104:245–53. doi: 10.1093/bja/aep366. [DOI] [PubMed] [Google Scholar]
- 21.Boezaart A, Tighe P. New trends in regional anesthesia for shoulder surgery: Avoiding devastating complications. Int J Shoulder Surg. 2010;4:1–7. doi: 10.4103/0973-6042.68410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnel F. Microscopic anatomy of the adult human brachial plexus: An anatomical and histological basis for microsurgery. Microsurgery. 1984;5:107–17. doi: 10.1002/micr.1920050302. [DOI] [PubMed] [Google Scholar]
- 23.Song JG, Jeon DG, Kang BJ, Park KK. Minimum effective volume of mepivacaine for ultrasound-guided supraclavicular block. Korean J Anesthesiol. 2013;65:37–41. doi: 10.4097/kjae.2013.65.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York, NY: Springer Berlin Heidelberg; 2014. [Google Scholar]
- 25.Gadsden J, Orebaugh S. Targeted intracluster supraclavicular brachial plexus block: Too close for comfort. Br J Anaesth. 2019;122:713–5. doi: 10.1016/j.bja.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Retter S, Szerb J, Kwofie K, Colp P, Sandeski R, Uppal V. Incidence of sub-perineural injection using a targeted intracluster supraclavicular ultrasound-guided approach in cadavers. Br J Anaesth. 2019;122:776–81. doi: 10.1016/j.bja.2019.01.006. [DOI] [PubMed] [Google Scholar]