Abstract
Background and Aims:
Gastroparesis despite standard fasting in diabetic patients may increase the aspiration risk. This study aimed to compare fasting gastric volume (GV) of diabetic with non-diabetic patients scheduled for elective surgery using USG.
Methods:
This prospective observational study included 53 diabetic and 50 non-diabetic patients aged >18 years, American Society of Anesthesiologists' physical status I-III having similar fasting intervals. Before induction, using standard gastric scanning protocol, qualitative and quantitative assessments of gastric antrum in supine and right lateral decubitus (RLD) positions were performed with a curved array probe. USG grade, cross-sectional area (CSA) of the antrum and GV were calculated. The gastric antrum was classified as Grade 0, 1 or 2, signifying empty antrum, fluid in RLD position only and antral fluid in both supine and RLD positions, respectively.
Results:
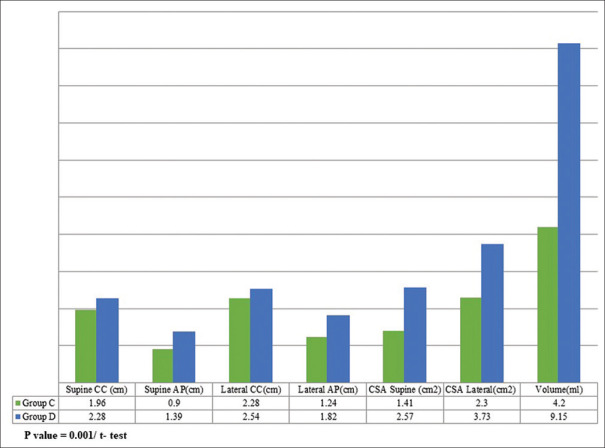
In supine position, CC and AP diameters were 1.96 ± 0.41 cm and 0.9 ± 0.57 cm in control group and 2.28 ± 0.50 cm and 1.39 ± 0.44 cm in diabetic group, respectively. In RLD, CC was 2.28 ± 0.57 cm and AP was 1.24 ± 0.42 cm in control group as compared to CC 2.54 ± 0.56 cm and AP 1.82 ± 0.56 cm in diabetic group. The CSA of 2.57 ± 1.19 cm2 and 3.73 ± 1.61 cm2 in diabetic were significantly higher (P = 0.001) than 1.41 ± 0.55 cm2 and 2.30 ± 1.18 cm2 of control, in supine and RLD positions, respectively. GV was 4.20 ± 22.26 ml in control group and 9.15 ± 25.70 ml in diabetic group.
Conclusion:
Diabetic patients have higher gastric antral cross-sectional area and gastric volumes as observed by gastric ultrasound than the non-diabetic patients.
Key words: Diabetes mellitus, fasting, gastroparesis, gastric volume, ultrasound
INTRODUCTION
Aspiration of gastric contents during perioperative period is a grave complication with significant morbidity and mortality.[1] Diabetic patients have a higher incidence of autonomic dysfunction, causing gastropathy. They are known to have gastroparesis and the consequent delayed gastric emptying which predisposes them to an increased risk of aspiration than the general population.[2]
Currently, there is no consensus on what constitutes an adequate fasting interval in diabetic patients. European Society of Anaesthesiology (ESA) 2011 fasting guidelines state that diabetic patients can follow the same guidelines as healthy adults,[3] While American Society of Anesthesiologists (ASA) in 2017 fasting guidelines mentioned that the standard eight hours fasting may not apply or may need to be modified for patients with coexisting diseases or conditions that can affect gastric emptying or fluid volume.[4]
Ultrasound is widely available and has been proven to be a reliable, bedside assessment tool for real-time evaluation of gastric contents.[5,6,7,8,9] As diabetic patients are prone to have an inadequately empty stomach even after an adequate fasting, USG can be used prior to induction for screening the fasting gastric volume (GV) of diabetic patients and see if it is more than the recommended safe limit. There is no published literature evidence documenting a significant difference in real-time fasting gastric volume between the healthy and diabetic patients after following the same fasting guidelines. In the present study, ultrasonography (USG) was used to compare the fasting GV in diabetic and non-diabetic patients scheduled for elective surgery.
METHODS
The study was approved by the Institutional Ethics Committee (NK/1603/MD/10019-20) and was registered with Clinical Trials Registry of India having registration number CTRI/2015/08/006129. The study was conducted as per the principles of the Declaration of Helsinki. After receiving written informed consent, patients of both sexes, aged >18 years of ASA grade I to III and posted for elective surgery were enrolled for the study. Patients on medication for upper gastrointestinal tract (GIT) symptoms, chronic kidney disease, hypothyroidism, connective tissue disease affecting GIT motility, current smoking history, on anti-depressant medication, previous oesophageal or abdominal surgery, obese patients, pregnant patients, and patients with nasogastric tube in situ were excluded from the study.
Patients were divided into two groups based on their history of diabetes mellitus (DM) and were labelled as group D (diabetic) and group C (control). This was a convenient sampling based on the status of DM. Patients having DM were assessed for the duration of diabetes, medication history, glycaemic control and gastropathy symptoms.
The fasting status was assessed, and the duration of fasting interval noted. USG was done prior to induction of anaesthesia by a person blinded to the patient's diabetic status. A curved array, low-frequency (2-5 MHz, 60 mm) transducer providing a scan depth up to 30 cm and a Micromaxx (C60e, Bothell, WA, USA)™ sonosite machine was used. Patients were scanned in the supine position followed by right lateral decubitus (RLD) position. The sonographic appearance of the gastric antrum was classified as Grade 0,1 or 2, signifying empty antrum, fluid detected in RLD position only and antral fluid in both supine and RLD positions, respectively, based on the appearance in both the positions as defined by Perlas et al.[10] Cross-sectional area (CSA) was calculated by using two perpendicular diameters—anteroposterior (AP) and craniocaudal (CC) and the formula for area of an ellipse:
CSA = (AP × CC × π)/4.[11]
The gastric volume was calculated using the previously validated formula:
GV (ml) = 27.0 + 14.6 × right-lat CSA − 1.28 × age.[12]
The sample size was calculated based on the model for case-control studies using methods of Kelsey, Fleiss, and Fleiss with a continuity correction, assuming a 25% incidence of gastroparesis in diabetics.[13,14,15] With a prediction of 90% power and an alpha error 0.05, 53 diabetic and 50 non-diabetic subjects were enrolled in the study. Age, height, weight, BMI, fasting interval, CSA of antrum and gastric volumes are presented as mean ± SD and analysed using unpaired Student t-test. Visibility of antrum with grading in USG findings is represented as frequencies or percentage. Normality of the continuous data was checked applying the Kolmogorov-Smirnov test. Ultrasound grades were analysed with Chi-square test. Data analysis was done using SPSS Version 22.0 (IBM, USA) and Microsoft Excel 2010 (Microsoft, USA). All tests were two-tailed with 95% confidence interval and level of significance at 5% (P < 0.05).
RESULTS
A total of 50 patients were included in group C and 53 patients were in group D. The demographic data of the two groups is presented in Table 1. 35 (66.1%) patients were non-insulin dependent (NIDDM) and 18 (33.9%) patients were insulin-dependent (IDDM) in group D. The mean glycosylated haemoglobin (HbA1c) was 7.64% with a range of 5.6 to 11.7%. The mean duration of DM was 6.6 years with an inter-quartile range (IQR25-75) of 1–10 years. The maximum duration of DM was 30 years in our study group. Gastropathy symptoms like abdominal distension and postprandial fullness were present in 29 out of 53 diabetic patients (54.7%). The average fasting intervals were 11.00 ± 2.02 h in group C and 10.87 ± 1.34 h in group D (P = 0.740).
Table 1.
Demographic Profile
| Parameter assessed | Group C (mean±SD) (n=50) | Group D (mean±SD) (n=53) | Pa |
|---|---|---|---|
| Age (years) | 44.06±13.99 | 56.55±8.95 | 0.01 |
| Sex (F/M) | 30/20 | 29/24 | 0.588 |
| ASA | |||
| 1 | 42 | 0 | 0.001 |
| 2 | 8 | 51 | |
| 3 | 0 | 2 | |
| Weight (Kg) | 61.82±8.81 | 66.15±11.03 | 0.031 |
| Height (m) | 1.63±0.08 | 1.64±0.09 | 0.515 |
| BMIb | 23.12±3.17 | 24.31±3.24 | 0.063 |
| Fasting interval (h) | 11.00±2.02 | 10.87±1.34 | 0.740 |
aCalculated using t-test/Chi-square test. bBMI – Body Mass Index
Differences in ultrasound grading between the groups are presented in Table 2. The measurement of AP and CC diameters is depicted in Figure 1. Comparison of diameters, CSA and volume in both the groups in supine as well as RLD position is presented in Figure 2. In supine position, CC and AP diameters were 1.96 ± 0.41 cm and 0.9 ± 0.57 cm in group C and 2.28 ± 0.50 cm and 1.39 ± 0.44 cm in group D, respectively. In RLD, the CC diameter was 2.28 ± 0.57 cm and the AP diameter was 1.24 ± 0.42 cm in group C. However, in group D, the CC diameter was 2.54 ± 0.56 cm and AP diameter was 1.82 ± 0.56 cm. The calculated CSA-supine was 1.41 ± 0.55 cm2 in group C and 2.57 ± 1.19 cm2 in group D. The CSA-lateral obtained in group C was 2.30 ± 1.18 cm2 and 3.73 ± 1.61 cm2 in group D (P = 0.001). The mean volumes calculated were 4.20 ± 22.26 ml in control subjects as compared to 9.15 ± 25.70 ml in diabetic patients (P = 0.001) [Figure 2].
Table 2.
Comparison of USG Grade
| Ultrasound Grade | Group C (n=50) | Group D (n=53) | Pa |
|---|---|---|---|
| 0 | 18 (36%) | 14 (26.4%) | 0.294 |
| 1 | 22 (44%) | 20 (37.7%) | 0.518 |
| 2 | 10 (20%) | 19 (35.8%) | 0.074 |
| Total | 50 | 53 | 0.199 |
aCalculated using Chi-square test. USG – Ultrasonography
Figure 1.
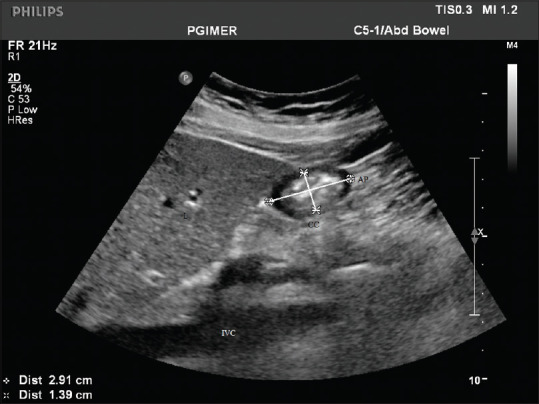
Craniocaudal (CC) and anteroposterior (AP) diameters for calculating the cross-sectional area (CSA). liver (L) on the right, inferior vena cava (IVC) below
Figure 2.
Comparison of diameter, cross-sectional area (CSA) and gastric volume (GV) in control and diabetic patients
DISCUSSION
Diabetes has often been considered a high-risk state posing a serious challenge to the anaesthesiologist in many aspects. One of the feared complications is pulmonary aspiration as diabetic patients are considered as possible full stomach due to autonomic gastropathy.[15,16] Camilleri et al. observed that delayed gastric emptying was the major highlight of DM.[2]
Putte et al. did a retrospective cohort study in 538 patients and found that a standard fasting interval does not ensure adequate emptying even in healthy individuals. They found fasting gastric volume more than the safe limit in 32 patients which lead to a change in the plan of anaesthetic induction.[17]
Diabetic patients have always been thought of as high-risk patients but no study has been carried out in real time to correctly stratify the fasting volume status of the diabetic patient and delineate the actual GV using gastric ultrasound. With the advent of enhanced recovery after surgery (ERAS) protocols and liberal fasting guidelines, USG may be useful in our daily perioperative practice to assess the GV in patients with diabetes. This study compared the fasting GV of diabetic with non-diabetic patients posted for elective surgery by performing USG of the gastric antrum.
Eight patients in group C were ASA 2 physical status as they had controlled hypertension. There was a difference in weight of the patients in two groups as diabetic patients presenting had a higher weight even with controlled blood sugar levels. However, there was no statistically significant difference in the BMI (P = 0.063) in both the groups.
Forty patients (80%) of control group and 34 patients (64.1%) of the total 53 diabetic patients had grade 0 and 1 antrum representing safe volumes.[11,12] There was no statistically significant difference in USG grade in both the groups (P value = 0.199; Chi-square test). However, there was a higher incidence of USG grade 2 in diabetic patients (n = 19, 35.8%) as compared to the control group (n = 10, 20%). However, the incidence did not achieve a statistically significant difference (P = 0.074).
Darwiche et al. compared the gastric emptying rate (GER) in 33 diabetic and non-diabetic volunteers using USG after ingestion of a semi-solid meal. Measurements of the gastric antrum were taken in the supine position in 19 healthy subjects and 14 patients with IDDM and clinically suspected delayed gastric emptying. It was found that diabetic patients showed significantly wider median values of postprandial antral area after 90 min. The median value of GER in these diabetic patients was 29% as compared to 63% of the healthy subjects. Their study showed a significant difference in GER between healthy subjects and patients with IDDM,[18] which is similar to the present study.
Gustafsson et al. have advocated preoperative carbohydrate loading in diabetic patients and deduced that diabetic patients do not show delayed gastric emptying using paracetamol tracer techniques. However, the sample size is small to generalize it for the diabetic group.[19] Moreover, the difference in measurement technique makes comparison with the present study difficult.
Our study group constituted type 2 diabetes patients. Though gastroparesis has been reported more in type 1 DM, it is also seen in type 2 DM even with the patients being asymptomatic.[13] Chiu et al. compared the gastric antral area in type 2 diabetic patients and healthy controls after ingestion of a meal in 11 type 2 diabetic patients and health volunteers each. All underwent transabdominal ultrasound for gastric motility and visual analogue scales. They found that the gastric emptying was significantly slower in diabetic patients, who also had lesser antral contractions than controls (46.3 min versus 20.8 min, respectively).[20] Their study showed a similar finding to the current study.
There were negative values derived in the calculation of GV. This was seen in patients having a lesser CSA leading to negative value. Previous studies evaluating the gastric volume with the formula derived by Perlas et al. have elicited negative values as well. Thus, when the stomach is empty, small values of RLD CSA give a negative volume value, which only indicates an empty state.[12]
The mean diameters and CSA calculated in supine and RLD positions had a statistically significant difference with a higher value observed in the diabetic group (P value = 0.001). Quantitative analysis of GV showed a higher value in diabetic patients. This is consistent with the previous studies suggesting a delayed gastric emptying in diabetic patients.[18,20] However, these studies have been done after ingestion of a solid meal or fluid to assess the gastric emptying and not the fasting gastric residual volumes.
Even though there was no statistically significant difference in the USG grading of both the groups, the quantitative data showed a higher value in diabetic group. This could be due to objective error in qualitative grading. Hence, a qualitative screening should be followed by a volume computation for risk stratification of such patients. The standard formula for quantitative estimation of GV by Perlas et al. was chosen in this study as it shows the highest correlation with the visually guided suctioning of gastric contents (r = 0.86) and USG grade. The negative values derived from this formula signify an empty stomach.[12] Though the quantitative data showed a statistically significant value in diabetic group, the maximum value of the volume obtained was 67.26 ml. This could be due to patients having higher age in our study.
There are limitations in our study. Our patients were type 2 DM patients. The control group population was slightly younger than the diabetic group. There was a variation of diet among the patients which can influence the gastric emptying. The mean fasting interval was around ten hours. In daily clinical practice, it is difficult to exactly control the fasting interval in the preoperative period. Surgery itself is a stress factor and its influence on gastric motility has not been evaluated by studies so far. An already published reference standard was chosen for quantitative analysis. Further studies are required in diabetic patients to correctly stratify the fasting volumes.
CONCLUSION
This prospective case-control study of 103 patients suggests that diabetic patients have higher gastric antral cross-sectional area and gastric volumes as observed by gastric ultrasound than the non-diabetic patients signifying delayed gastric emptying. While the qualitative grading may be used for screening, quantitative assessment provides a more reliable estimate of gastric volume.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient/participant consent forms. In the form, the patients/participants have given their consent for their images and other clinical information to be reported in the journal. The patients/participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Robinson M, Davidson A. Aspiration under anaesthesia: Risk assessment and decision-making. Contin Edu Anaesth Crit Care Pain. 2013;14:171–5. [Google Scholar]
- 2.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 4.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–93. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–92. doi: 10.1097/ALN.0b013e31820dee48. [DOI] [PubMed] [Google Scholar]
- 6.Cubillos J, Tse C, Chan VW, Perlas A. Bedside ultrasound assessment of gastric content: an observational study. Can J Anaesth. 2012;59:416–23. doi: 10.1007/s12630-011-9661-9. [DOI] [PubMed] [Google Scholar]
- 7.Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–9. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 8.Perlas A, Arzola C, Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: A narrative review. Can J Anesth. 2018;65:437–48. doi: 10.1007/s12630-017-1031-9. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Deo A, Raman P. Effectiveness of standard fasting guidelines as assessed by gastric ultrasound examination: A clinical audit. Indian J Anaesth. 2018;62:747–52. doi: 10.4103/ija.IJA_54_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–7. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 11.Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 12.Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 13.Intagliata N, Koch KL. Gastroparesis in type 2 diabetes mellitus: Prevalence, etiology, diagnosis, and treatment. Curr Gastroenterol Rep. 2007;9:270–9. doi: 10.1007/s11894-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 14.Parkman HP, Fass R, Foxx-Orenstein AE. Treatment of patients with diabetic gastroparesis. Gastroenterol Hepatol. 2010;6:1–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnasamy S, Abell TL. Diabetic gastroparesis: principles and current trends in management. Diab Ther. 2018;9:1–42. doi: 10.1007/s13300-018-0454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moningi S, Nikhar S, Ramachandran G. Autonomic disturbances in diabetes: Assessment and anaesthetic implications. Indian J Anaesth. 2018;62:575–83. doi: 10.4103/ija.IJA_224_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: A retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118:363–71. doi: 10.1093/bja/aew435. [DOI] [PubMed] [Google Scholar]
- 18.Darwiche G, Almer LO, Bjorgell O, Cederholm C, Nilsson P. Measurement of gastric emptying by standardized real-time ultrasonography in healthy subjects and diabetic patients. J Ultrasound Med. 1999;18:673–82. doi: 10.7863/jum.1999.18.10.673. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson UO, Nygren J, Thorell A, Soop M, Hellstrom PM, Ljungqvist O, et al. Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52:946–51. doi: 10.1111/j.1399-6576.2008.01599.x. [DOI] [PubMed] [Google Scholar]
- 20.Chiu YC, Kuo MC, Rayner CK, Chen JF, Wu KL, Chou YP, et al. Decreased gastric motility in type II diabetic patients. BioMed Res Int. 2014;2014:894087. doi: 10.1155/2014/894087. [DOI] [PMC free article] [PubMed] [Google Scholar]