Abstract
Objective
Despite the proven benefits of adjuvant endocrine therapy, adherence to oral endocrine therapy in breast cancer treatment is a substantial problem. The aim of this study was to assess adherence to adjuvant endocrine therapy by women in China for the first 5 years, and to identify its influencing factors.
Methods
Stratified sampling method was adopted to select 1875 cases of breast cancer patients for cross‐sectional telephone follow‐up. Compliance to medications was assessed using the Morisky Medication Adherence Scale. Status of endocrine therapy was assessed using nine additional questions. Binomial regression was used when assessing the factors associated with persistence, multinomial regression models were used to assess factors associated with compliance.
Results
Of 888 patients who started adjuvant endocrine therapy, 769(86.6%) persisted and 119 (13.4%) discontinued. 760 patients who completed Morisky Medication Adherence Scale, the compliance was 7.4% low, 42% medium, and 50.6% high. The type of medication, duration of medication and side effects had an impact both on persistence and compliance. Age, history of radiotherapy and caregivers only had an impact on persistence.
Conclusions
Medication adherence was affected by many factors. Special attention and interventions should be given to women taking tamoxifen in the 2nd to 3rd year of medication, and aromatase inhibitors in the 1st to 2nd year. Further prospective design studies are needed to explore effective measures to improve medication adherence of women with breast cancer treated by endocrine therapy.
Keywords: adherence, breast cancer, compliance, endocrine therapy, oncology, persistence
Of 888 patients who started adjuvant endocrine therapy, 769(86.6%) persisted and 119 (13.4%) discontinued. 760 patients who completed MMAS, the compliance was 7.4% low, 42% medium, and 50.6% high. Adherence was affected by many factors. Special attention and interventions should be given to women taking tamoxifen in the 2nd to 3rd year of medication, and AIs in the 1st to 2nd year.

1. BACKGROUND
Breast cancer (BrCa) is the most common cancer among women worldwide. 1 , 2 GLOBOCAN 2018 showed that there would be about 2.1 million newly diagnosed female breast cancer cases in 2018, accounting for almost one in four cancer cases among women. The disease is the most frequently diagnosed cancer in the vast majority of the countries (83.24%) and is also the leading cause of cancer‐related death in over 100 countries. 3 Approximately two‐thirds of breast cancer patients test positive for the estrogen receptor (ER) and/or progesterone receptor (PR). 4 For this majority of patients, adjuvant endocrine therapy (AET) such as tamoxifen (TAM) and aromatase inhibitors (AIs) have proven clinical benefit. AET can significantly reduce recurrence and mortality in BrCa women who are hormone receptor‐positive. 5 , 6 , 7 Updated clinical practice guidelines recommend extending AET use from 5 to 10 years. 4 , 8 Despite the radical difference made by AET in BrCa outcomes, up to 50% of women do not adhere to prescribed regimens 9 , 10 and 31%‐73% of women are nonpersistent with AET. 11 , 12 , 13 Taking < 80% of AET doses has been associated with a 20% increased mortality risk. 14 Adherence to oral endocrine therapy in BrCa treatment is a substantial problem. 15 Therefore, identifying possible reasons for nonpersistence and nonadherence in women with BrCa is vital.
The World Health Organization (WHO) defines adherence as “the extent to which a person's behavior—taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”. 16
Medication adherence is defined as a composite of compliance (how well physician's orders are followed) and persistence (how long an individual continues on prescribed therapy). 17
The aim of this study was to assess adherence to AET by women in China for the first five years and to identify its influencing factors, so as to provide improvement measures to ensure the efficacy of endocrine therapy in the future.
2. METHODS
2.1. Participants
A total of 9128 cases of BrCa were admitted from January 2013 to December 2017 from a university cancer hospital in Shenyang, China. Stratified random sampling method was adopted to select 375 cases of breast cancer patients each year; a total of 1875 cases were screened to meet the following inclusion and exclusion criteria.
Inclusion criteria included positive for the estrogen receptor (ER) and/or progesterone receptor (PR); newly diagnosed with breast cancer; receiving oral endocrine therapy drugs; women above 18 years old; and volunteered participation in the survey. Exclusion criteria included patients with a history of other malignant tumors or patients with distant metastasis.
2.2. Measures
Demographic & clinical variables were obtained through clinical record review: the tumor characteristics noted included stage and previous treatments; patient information at diagnosis included common epidemiological characteristics (age, height, weight, ethnicity, marital status, and medical insurance.) and Charlson morbidity score. Self‐reported demographic data (employment status, occupation, smoking, drinking, and medical payment methods.) were also included. Body mass index (BMI) and Charlson morbidity score 18 at diagnosis were calculated for each woman.
Compliance to medications was assessed using the four‐item Morisky Medication Adherence Scale (MMAS); 19 the MMAS showed good psychometric properties (Cronbach's alpha of 0.61) in the original validation study. The answer of the questionnaire include "yes" and "no". When the answer is "yes", the score is 0, and "no" is 1. The higher the score, the worse the compliance, with 0 indicating high compliance, 1‐2 indicating medium compliance, and 3‐4 indicating low compliance. Chinese researchers translated and revised MMAS into Chinese version, and we tested the internal consistency before use, Cronbach's alpha of the modified questionnaire was 0.724.
Status and persistence of endocrine therapy assessed using nine additional questions designed by the authors included: Are you still taking endocrine therapy medications? When did you begin endocrine therapy? What's the name of your endocrine medication? whether switched the medicine? Have you ever missed to take any medicine? If so, why and when? How many days (or times) do you miss medication per month? (no missed dose, 1‐5 missed doses, and more than 6 missed doses) Do you have any discomfort with this medicine? Do you know the side effects of endocrine medicine? Do you need others' care?
Nonpersistence: Women were considered discontinuers if they reported they were no longer using TAM or AIs and their self‐reported duration of use was <5 years after breast cancer diagnosis. Women who reported 5 or more years of TAM and/or AIs use (ie, completers), or who reported current TAM or AIs use (ie, continuers) no matter whether or not they switched medication at the time of the survey (even if they reported <5 years of use) were classified as women who did not discontinue.
2.3. Data collection
This was a cross‐sectional, observational study conducted between July 2018 and September 2018. Our study was approved by the ethics committee at Liaoning Cancer Hospital. The author (Xu Hui) conducted a telephone follow‐up demonstration before the start of follow‐up, and unified instructions and a list of follow‐up procedures. Two graduate students (Xiujie Zhang and Daqiu Wang) who had follow‐up experience received training regarding the survey instrument and data collection methods. They completed the telephone follow‐up after passing the training. Participants had adequate information regarding the purpose of the research. They had the right of free choice, enabling them to voluntarily consent or decline participation in the research. Confidentiality was maintained. Eligible participants completed a telephone follow‐up survey on factors associated with endocrine therapy. Each contact number was dialed at different times on different dates for three consecutive times. If no connection could be made for three times, the telephone follow‐up was considered as a failure.
2.4. Statistical analysis
Data were analyzed using IBM SPSS Statistics (version 23). Statistical description includes frequency, percentage, P50 (P25‐P75), etc. Mann‐Whitney U test was used for comparison between the two groups. Kruskal‐Wallis H test (Nemenyi test) was used for comparison of multiple groups, and Nemenyi test was used for further pairwise comparison. χ 2 test or Fisher's exact test was used for comparison of quantitative data. Binomial regression was used when assessing the factors associated with persistence, multinomial regression models were used to assess factors associated with compliance. First, univariate analysis of variables that may affect patients' adherence with endocrine therapy was conducted. The variables with significant differences in univariate analysis were further analyzed by multivariate Logistic regression analysis to explore the influencing factors of patient compliance. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for each characteristic.
3. RESULTS
3.1. Sample characteristics
A total of 1227 patients with hormone receptor‐positive breast cancer met the patient selection criteria. Of those, 339 patients were not included in the analysis due to the following reasons: died (N = 43), lost to follow‐up (N = 145), refused to follow‐up (N = 68) and did not take or begin endocrine therapy (N = 83). Thus, we report results for 888 patients; the patient selection criteria are shown in Figure 1. The median age of all patients aged was 54.52 years (range, 27‐89 years), 357 patients took TAM and 531 patients took AIs, the characteristics are shown in Table 1.
Figure 1.
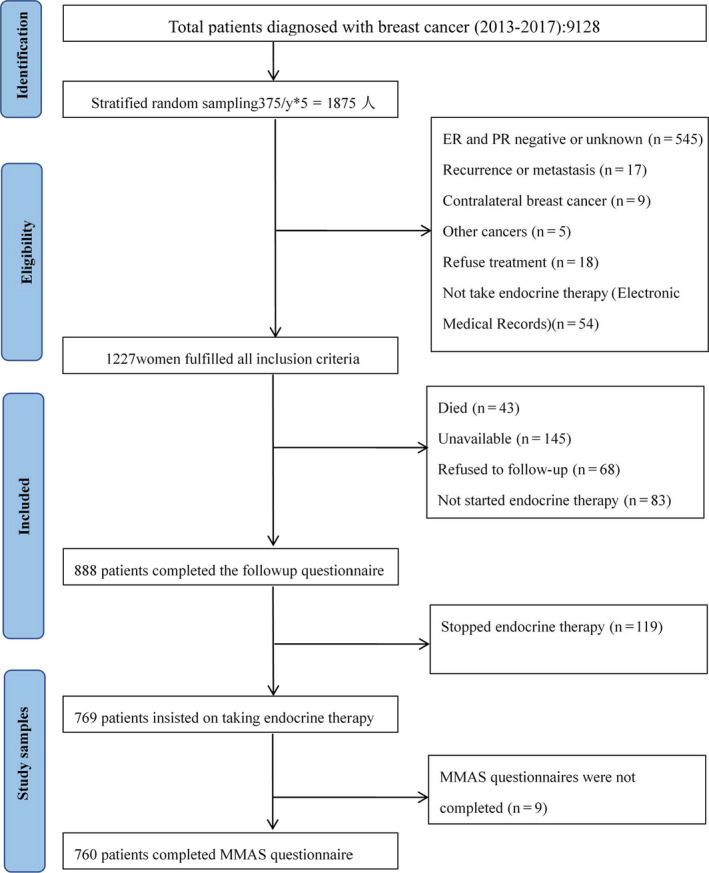
Flowchart of study sample selection
Table 1.
Patient demographics and tumor characteristics
| Characteristics | N | % |
|---|---|---|
| Age at diagnosis (y) | ||
| Median (interquartile range) | 54 | 47‐62 |
| ≤39 | 57 | 6.4 |
| 40‐49 | 249 | 28 |
| 50‐59 | 290 | 32.7 |
| ≥60 | 292 | 32.9 |
| Marital status | ||
| Married/partner | 878 | 98.9 |
| Single/divorced | 10 | 1.1 |
| Ethnicity | ||
| Han | 823 | 92.7 |
| Minorities | 65 | 7.3 |
| Smoker | ||
| Never | 876 | 98.6 |
| Current | 12 | 1.4 |
| Drinker | ||
| Never | 846 | 95.3 |
| Current | 42 | 4.7 |
| Employment | ||
| Yes | 311 | 35 |
| No | 531 | 59.8 |
| On work disability | 46 | 5.2 |
| Occupation | ||
| Retiree | 277 | 31.2 |
| Worker | 90 | 10.1 |
| Farmer | 143 | 16.1 |
| Office staff | 84 | 9.5 |
| Professionals | 83 | 9.3 |
| No | 119 | 13.4 |
| Others | 92 | 10.4 |
| Medical insurance | ||
| UB insurance | 310 | 34.9 |
| RC insurance | 257 | 28.9 |
| No insurance | 159 | 17.9 |
| Others | 162 | 18.2 |
| Medication type | ||
| AIs | 531 | 59.8 |
| TAM | 357 | 40.2 |
| Understanding side effectsb | ||
| Yes | 589 | 66.3 |
| No | 202 | 22.7 |
| Caretakers | ||
| No | 426 | 48 |
| Yes | 462 | 52 |
| BMI (kg/m2) | ||
| Mean (SD) | 24.8 | 10.1 |
| <18.5 | 21 | 2.4 |
| 18.5‐24.99 | 498 | 56.1 |
| 25‐29.99 | 316 | 35.6 |
| ≥30 | 53 | 6 |
| Radiotherapy | ||
| Yes | 302 | 34 |
| No | 586 | 66 |
| Chemotherapy | ||
| Yes | 745 | 83.9 |
| No | 143 | 16.1 |
| HER2/neu | ||
| Negative | 785 | 88.4 |
| Positive | 103 | 11.6 |
| K167 | ||
| <30% | 524 | 59 |
| ≥30% | 364 | 41 |
| Charlson morbidity score | ||
| 0 | 700 | 78.8 |
| 1 | 143 | 16.1 |
| ≥2 | 45 | 5.1 |
| pT | ||
| 1 | 258 | 29.1 |
| 2 | 549 | 61.8 |
| 3 | 29 | 3.3 |
| 4 | 18 | 2 |
| Unknown | 34 | 3.8 |
| pN | ||
| Positive | 363 | 40.9 |
| Negative | 499 | 56.2 |
| No axillary surgery | 26 | 2.9 |
| Duration of medication(M) | ||
| 1‐12 | 205 | 23.1 |
| 13‐24 | 209 | 23.5 |
| 25‐36 | 160 | 18 |
| 37‐48 | 168 | 18.9 |
| ≥49 | 146 | 16.4 |
| No. of side effects | ||
| 0 | 412 | 46.4 |
| 1 | 315 | 35.5 |
| ≥2 | 140 | 15.8 |
| Unknown | 21 | 2.4 |
Abbreviations: RC insurance, Rural Cooperative Medical Insurance; UB insurance, Urban Residents Basic Medical Insurance.
Fisher's exact probability method was used.
The variable has a missing value.
3.2. Persistence with endocrine therapy
Of 888 patients who started adjuvant endocrine therapy, 769 patients (86.6%) were classified as persistent, 119 patients (13.4%) were considered nonpersistent. The non‐persistence rate from the first year to the fifth year was 5.9%, 9.1%, 15.5%, 16.9% and 22.6%, respectively. Patients taking TAM for 2‐3 years showed obviously non‐persistence, while the non‐persistence rate of the AIs group was obviously increased at the 1‐2 year, the results are shown in Figure 2.
Figure 2.
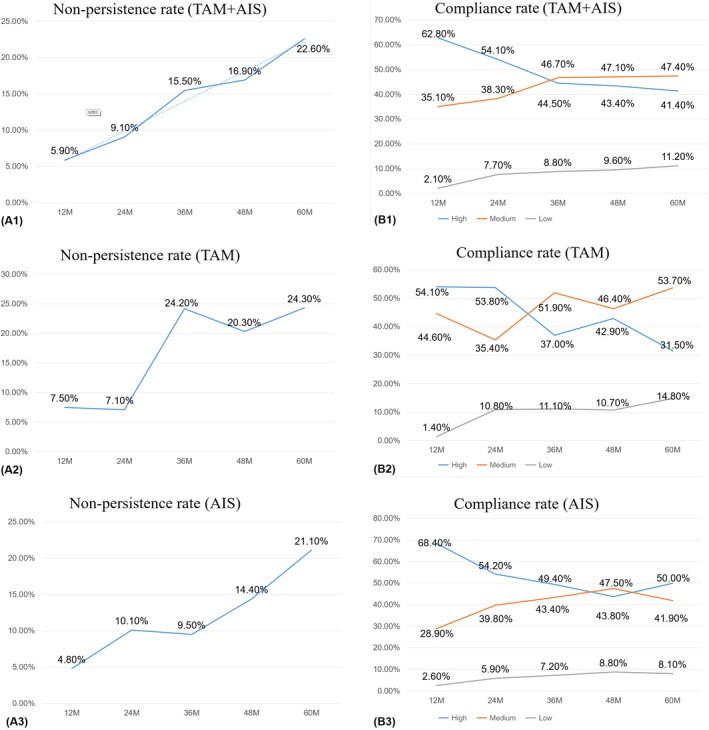
Nonpersistence rate and compliance rate curve
3.3. Compliance with endocrine therapy
Of the 769 patients who insisted on taking the medicine, 422 (54.88%) reported consistently taking their medication, with no missed doses, patients who missed less than five doses per month were 263 (34.2%). MMAS assessed compliance among 760 patients who completed the questionnaire, the distribution of compliance for these women was 7.4% low, 42% medium, and 50.7% high. The rate of high compliance ranged from 41.4% to 62.8% at 1 to 5 years, and the rate of low compliance ranged from 2.1% to 11.2%. Compliance fluctuated at 2 to 3 years, the results are shown in Figure 2.
3.4. Characteristics associated with persistence and compliance
The analysis showed that the type of medication, duration of medication and side effects had an impact both on persistence and compliance to endocrine therapy for breast cancer. Age, history of radiotherapy, and presence of caregivers only had an impact on persistence to medication. BMI as a continuous variable had an effect on medication compliance, but as ranked data, the effect on medication compliance was not statistically significant. The multivariate analysis of influencing factors results is shown in Tables 2 and 3. Univariate analysis of influencing factors is shown in Additional file 1 and Additional file 2.
Table 2.
Analysis of influencing factors of medication persistence
| Characteristics | Persistence (N = 769) | Nonpersistence (N = 119) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| OR (95%CI) | P‐value | OR (95%CI) | P‐value | |||
| Age at diagnosis (y) | ||||||
| ≤39 | 53 (6.9) | 4 (3.4) | 1.00 | .027 | 1.00 | <.001 |
| 40‐49 | 219 (28.5) | 30 (25.2) | 1.82 (0.61‐5.38) | .282 | 1.52 (0.50‐4.61) | .464 |
| 50‐59 | 258 (33.6) | 32 (26.9) | 1.64 (0.056‐4.84) | .368 | 1.86 (0.61‐5.66) | .275 |
| ≥60 | 239 (31.1) | 53 (44.4) | 2.94(1.02‐8.47) | .046 | 4.83 (1.54‐15.18) | .007 |
| Radiotherapy | ||||||
| Yes | 271 (35.2) | 31 (26.1) | 1.00 | |||
| No | 498 (64.8) | 88 (73.9) | 1.55 (1.00‐2.39) | .050 | ||
| Duration of medication(M) | ||||||
| 1‐12 | 192 (25.0) | 13 (10.9) | 1.00 | <.001 | 1.00 | .002 |
| 13‐24 | 190 (24.7) | 19 (16.0) | 1.48 (0.71‐3.08) | .297 | 1.51 (0.71‐3.17) | .283 |
| 25‐36 | 135 (17.6) | 25 (21.0) | 2.74 (1.35‐5.54) | .005 | 2.58 (1.26‐5.29) | .010 |
| 37‐48 | 139 (18.1) | 29 (24.4) | 3.08 (1.55‐6.14) | .001 | 2.86 (1.42‐5.79) | .003 |
| ≥49 | 113 (14.7) | 33 (27.7) | 4.31 (2.18‐8.54) | <.001 | 3.60 (1.79‐7.21) | <.001 |
| Medication type | ||||||
| AIs | 472 (61.4) | 59 (49.6) | 1.00 | 1.00 | ||
| TAM | 297 (38.6) | 60 (50.4) | 1.62 (1.10‐2.38) | .015 | 2.91 (1.74‐4.85) | <.001 |
| No. of side effects | ||||||
| 0 | 366 (47.6) | 46 (38.7) | 1.00 | .028 | 1.00 | .026 |
| 1 | 267 (34.7) | 48 (40.3) | 1.43(0.93‐2.21) | .106 | 1.45 (0.92‐2.27) | .107 |
| ≥2 | 122 (15.9) | 18 (15.1) | 1.17 (0.66‐2.10) | .589 | 1.29 (0.71‐2.37) | .406 |
| Unknown | 14 (1.8) | 7 (5.9) | 3.98 (1.53‐10.37) | .005 | 4.37 (1.60‐11.91) | .004 |
| Caretakers | ||||||
| No | 349 (45.4) | 77 (64.7) | 1.00 | |||
| Yes | 420 (54.6) | 42 (35.3) | 0.45 (0.30‐0.68) | <.001 | ||
Table 3.
Analysis of influencing factors of medication compliance
| Characteristics | Control group N (%) | Comparison group N (%) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P‐value | OR (95%CI) | P‐value | ||||
| Medium compliance | Duration of medication(M) | ||||||
| 1 −12 | 118 (30.6) | 65 (20.4) | 1.00 | 1.00 | |||
| 13‐24 | 100 (26.0) | 70 (21.9) | 1.27 (0.83‐1.95) | .275 | 1.28 (0.83‐1.97) | .272 | |
| 25‐36 | 60 (15.6) | 64 (20.1) | 1.94 (1.22‐3.08) | .005 | 1.91 (1.19‐3.06) | .007 | |
| 37‐48 | 59 (15.3) | 65 (20.4) | 2.00 (1.26‐3.18) | .003 | 1.96 (1.22‐3.14) | .005 | |
| ≥49 | 48 (12.5) | 55 (17.2) | 2.08 (1.27‐3.40) | .003 | 2.01 (1.22‐3.3) | .006 | |
| Medication type | |||||||
| AIs | 249 (64.7) | 180 (56.4) | 1.00 | 1.00 | |||
| TAM | 136 (35.3) | 139 (43.6) | 1.41 (1.04‐1.92) | .026 | 1.41 (1.03‐1.92) | .034 | |
| No. of side effects | |||||||
| 0 | 202 (52.5) | 133 (41.7) | 1.00 | 1.00 | |||
| 1 | 127 (33.0) | 131 (41.1) | 1.54 (1.11‐2.14) | .007 | 1.57 (1.12‐2.19) | .008 | |
| ≥2 | 55 (14.3) | 54 (16.9) | 1.25 (0.81‐1.95) | .072 | 1.24 (0.8‐1.95) | .337 | |
| Unknown | 1 (0.3) | 1 (0.3) | 5.94 (0.66‐53.73) | .768 | 4.14 (0.45‐38.2) | .211 | |
| BMI | 385 (100.0) | 319 (100.0) | 0.96 (0.93‐1.01) | .033 | 0.97 (0.93‐1.01) | .123 | |
| Low compliance | Duration of medication (M) | ||||||
| 1‐12 | 118 (30.6) | 4 (7.1) | 1.00 | 1.00 | |||
| 13‐24 | 100 (26.0) | 14 (25.0) | 4.13 (1.32‐12.95) | .015 | 4.43 (1.4‐14.04) | .012 | |
| 25‐36 | 60 (15.6) | 12 (21.4) | 5.90 (1.83‐19.08) | .003 | 6.20 (1.89‐20.32) | .003 | |
| 37‐48 | 59 (15.3) | 13 (23.2) | 6.50 (2.03‐20.81) | .002 | 7.04 (2.17‐22.84) | .001 | |
| ≥49 | 48 (12.5) | 13 (23.2) | 7.99 (2.48‐25.74) | <.001 | 7.80 (2.39‐25.45) | .001 | |
| Medication type | |||||||
| AIs | 249 (64.7) | 28 (50.0) | 1.00 | 1.00 | |||
| TAM | 136 (35.3) | 28 (50.0) | 1.83 (1.04‐3.22) | .036 | 1.78 (0.99‐3.20) | .054 | |
| No. of side effects | |||||||
| 0 | 202 (52.5) | 16 (28.6) | 1.00 | 1.00 | |||
| 1 | 127 (33.0) | 20 (35.7) | 1.75 (0.88‐3.50) | .052 | 1.82 (0.90‐3.68) | .096 | |
| ≥2 | 55 (14.3) | 20 (35.7) | 4.04 (1.99‐8.21) | <.001 | 4.06 (1.96‐8.41) | <.001 | |
| Unknown | 1 (0.3) | 0 | — | — | — | — | |
| BMI | 385 (100.0) | 56 (100.0) | 0.94 (0.87‐1.01) | .034 | 0.95 (0.88‐1.03) | .186 | |
4. DISCUSSION
4.1. Adherence status to AET in Chinese women with early breast cancer
Despite the proven benefits of AET, patient persistence on and compliance to tamoxifen and AIs are suboptimal. Persistence with adjuvant endocrine therapy steadily declined over time in women receiving adjuvant endocrine therapy for early breast cancer in China. Women taking tamoxifen in the 2nd to 3rd year of medication and AIs in the 1st to 2nd year showed the greatest decrease.
The nonpersistence rate of 13.4% in our study was similar to that of Japan (12%). 20 Our discontinuation rate of 22.6% at the fifth year is lower than that of most previous reports, which estimated discontinuation rates ranging from 31% to 73%. 11 , 12 , 21 , 22 Higher than an USA study of 538 women with 18% non‐persistent at 5 years, 23 similar to the NSABP B‐14 trial, whose discontinuation rates were 23% in both the placebo group and the experimental group for 60 months. 24 Our study population included patients from one university cancer hospital, where all women had continuous medical care; considering the different calculation methods of discontinuation rate, this study only calculated the patients who simply stopped using the drug, and did not calculate the switch drug replacement; all these factors may help explain our low discontinuation rate.
Compliance is defined as the consistency of taking protocol treatment; a patient was compliant if she took at least 80% of the pills during each month. The results of this study showed that 89.09% of the patients self‐reported taking more than 80% of their doctor's prescription pills each month. A systematic review identified 29 studies of adjuvant endocrine treatment adherence: compliance ranged from 41% to 72%. 13 Several studies have suggested that information and support improve adherence, lending credence to this possibility. 25 , 26 The mean medication compliance score of breast cancer patients was 0.853, and 50.6% of patients had high compliance. It is similar to the research of Kesmodel et al which respectively showed 50% of 100 cases of early breast cancer with high compliance. 27
4.2. Analysis of influencing factors associated with adherence
4.2.1. Type of medication
The type of medication is another factor affecting adherence to endocrine therapy in this study. Women who used tamoxifen had significantly increased odds of nonpersistence compared with women who used AIs. As in several prior reports, women who discontinued endocrine therapy were more likely to have used tamoxifen compared with AIs. 12 , 23 Lack of awareness of tamoxifen is an important factor affecting compliance. 28 Misunderstanding and fear of side effects of tamoxifen also affect medication compliance. A Los Angeles study also points to inadequate information about side effects as a factor in the persistence of medication. 29 Other studies have shown that depression is associated with early discontinuation of tamoxifen. 30 It is suggested that special attention should be paid to the patients taking tamoxifen in the future research and clinical practice, and effective methods should be adopted to ensure that the side effects of patients can be targeted intervention and the knowledge related to endocrine drugs can be timely acquired.
4.2.2. Duration of medication
Analyses showed that the 2nd to 3rd year of endocrine therapy was the most apparent period of the increase of medication interruption rate and decrease of compliance rate in breast cancer patients. Fontein et al also found that patients with adjuvant endocrine therapy had a high interruption rate after 2.5 years of treatment. 31 After the basic treatment, patients began to take endocrine therapy drugs, which were convenient to take and had fewer side effects than radiotherapy and chemotherapy. The patients were willing to stick to the medication rather than go back to the original process. As treatment continued, side effects began to appear. Some patients think they are in good physical condition, but their perception is more about the troubles and intolerable side effects brought by the treatment. 28 Patients' awareness and attention to endocrine therapy decreased, their belief in taking medicine gradually decreased, and their fear of tumor recurrence and metastasis also decreased. 32 , 33 At this point, their compliance with the medication becomes worse. This may explain why endocrine therapy produces the first peak of recurrence in 2‐3 years.
4.2.3. Side effects
Among the 888 patients followed‐up, 442 (49.8%) reported side effects, and 139 patients had more than two side effects. Of the 119 patients who discontinued the medication, 55 (46.22%) reported side effects, and 30.25% discontinued the medication as a result of side effects. Of the 769 patients who took the medication consistently, 50.2% reported side effects, and 69.9% of patients with low compliance had at least one side effect. The rate of side effects in this study was similar to a New Zealand study in which 52.5% of patients had side effects and 30.9% of patients stopped taking drugs due to side effects. 22 A survey of women in Detroit and Los Angeles who started endocrine therapy showed that of those who stopped early, 40% stopped for side effects and 25% cited worry about risks. 29 Another Chinese study showed that 36% of patients discontinued endocrine therapy because of its side effects, another 14% stopped treatment after reading the package insert about side effects rather than after having experienced by themselves. 34 The adverse effects may be unbearable, life threatening, or decrease quality of life. 28 Side effects are the main factors affecting compliance to adjuvant endocrine therapy. 35 , 36 , 37
4.2.4. Other factors
The results of this study showed that older than 60 years and younger than 39 years patients were more likely to be non‐persistent, which was similar to the results of several studies. 13 , 35 , 38 , 39 , 40 Younger women may be more likely to make a risk‐benefit assessment rejecting AET because AET is associated with side effects. In addition, tamoxifen is a SERMS drug, and young women may prioritize fertility issues, while older women may consider comorbidities, life expectancy, and quality of life. 28 , 41
A univariate analysis found that whether a patient had been treated with radiation had an effect on medication persistence. However, further multivariate analysis showed that radiotherapy history was not one of the factors influencing nonpersistence. Whether the history of radiotherapy is related to the early interruption of treatment is inconsistent with the existing studies. 20 , 31 , 34 One possible explanation is that patients who did undergo radiotherapy may be more likely to receive standard‐of‐care therapy in general. 41
The lack of caregivers during endocrine therapy affected the persistence of treatment, similar to the study results of Cluze et al 's follow‐up of 196 breast cancer patients taking tamoxifen, which found that treatment interruption was mainly due to lack of social support. 42
In this study, univariate analysis showed that body mass index (BMI) as a continuous variable had an impact on compliance, but it was not significant when BMI was changed into ranked data for analysis. There have been several studies, which associated higher BMI with lower efficacy of antihormonal treatment. 43 Being overweight or obese was a significant time dependent factor predictive for discontinuing endocrine therapy. But this was not found in our study, the reason may be that the BMI data we selected were at the time of diagnosis. As the treatment progresses, the patient's BMI may change, especially the weight gain caused by endocrine therapy; medication compliance will reduce. However, we did not collect the BMI data at that time. BMI influence on compliance needs further research.
4.2.5. Study limitations
This study has several limitations. First, a total of 888 cases were included in this study, and only 146 people were in their fifth year of endocrine therapy, which is a relatively small sample size compared to the first 2 years. In future studies, the sample size can be further expanded, and the prospective follow‐up investigation on the samples will continue. Second, the nonpersistence rate of 13.4% in our study was lower than most previous studies.Considering the different calculation methods of interruption rate, our study only calculated the patients who stopped the drug, and the patients who changed the drug did not calculate the interruption rate. Future research should adopt unified evaluation of discontinuation rate calculation method to obtain comparable results. Third, there was a recall bias in our study, cross‐sectional follow‐up was used to understand the medication status of patients during the first 5 years of treatment in the same period to decrease bias caused by recall bias and environmental causes. Finally, we applied nine additional questions to help us understand the entire state of the patient's current treatment, to get a sense of possible factors that might affect treatment compliance, and since it was not a formal questionnaire, we did not test these questions for consistency and feasibility.
4.2.6. Clinical implications
The results of this study found that the time of changes in nonpersistence rate and compliance rate in AET patients provided a reference for the selection of clinical intervention time. This study suggests that more attention and timely intervention should be given to patients receiving endocrine therapy before they show obvious nonpersistence, and clinicians should emphasize the importance of completing the 5‐year treatment to patients.
5. CONCLUSION
Medication adherence is affected by many factors. Special attention and interventions should be given to women taking tamoxifen for the 2nd to 3rd year of medication, and to women taking AIs for the 1st to 2nd year. Future research should adopt unified evaluation of discontinuation rate calculation method to obtain comparable results. Further prospectively designed studies are needed to explore the factors affecting medication adherence in patients undergoing endocrine therapy for breast cancer.
AUTHOR CONTRIBUTIONS
Hui Xu carried out data analysis, interpretation, and wrote the paper. Ai‐ping Wang provided guidance on study design, organized the investigation, and is the corresponding author. Feng Jin provided guidance on study design and interpretation of analysis. Xiu‐jie Zhang, Da‐qiu Wang and Shao‐fen Yu provided help with the data collection, analysis, and interpretation. All authors read and approved the final manuscript. The authors declare that there is no conflict of interest regarding the publication of this article.
Xu H, Jin F, Zhang X‐J, Wang D‐Q, Yu S‐F, Wang A‐P. Adherence status to Adjuvant Endocrine Therapy in Chinese Women with Early Breast Cancer and its influencing factors: A cross‐sectional survey. Cancer Med. 2020;9:3703–3713. 10.1002/cam4.3017
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fan L, Strasser‐Weippl K, Li J‐J, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279‐e289. [DOI] [PubMed] [Google Scholar]
- 2. Li T, Mello‐Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395‐406. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 4. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor‐positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423‐438. [DOI] [PubMed] [Google Scholar]
- 5. Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8):dju165‐dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41(3):274‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrow A, Dryden R, McCowan C, et al. A hard pill to swallow: a qualitative study of women’s experiences of adjuvant endocrine therapy for breast cancer. BMJ Open. 2014;4(6):e005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang B, Gong C, Hu X. Clinical practice guidelines in breast cancer by chinese anti‐cancer association (2015 version): interpretation of updates in terms of systemic treatment. Chin J Breast Dis. 2016;2(10):65‐70. [Google Scholar]
- 9. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early‐stage breast cancer patients. J Clin Oncol. 2010;28(27):4120‐4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Herk‐Sukel MPP, van de Poll‐Franse LV, Voogd AC, Nieuwenhuijzen GAP, Coebergh JWW, Herings RMC. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population‐based analysis. Breast Cancer Res Treat. 2010;122(3):843‐851. [DOI] [PubMed] [Google Scholar]
- 11. Ayres LR, Baldoni Ade O, Borges AP, Pereira LR. Adherence and discontinuation of oral hormonal therapy in patients with hormone receptor positive breast cancer. Int J Clin Pharm. 2014;36(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 12. Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7(4):378‐387. [DOI] [PubMed] [Google Scholar]
- 13. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winn AN, Dusetzina SB. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiol Drug Saf. 2016;25(8):953‐959. [DOI] [PubMed] [Google Scholar]
- 15. Nekhlyudov L, Li L, Ross‐Degnan D, Wagner AK. Five‐year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early‐stage breast cancer. Breast Cancer Res Treat. 2011;130(2):681‐689. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . Adherence to long‐term therapies: evidence for action. Geneva; 2003. [Google Scholar]
- 17. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 19. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 20. Kuba S, Ishida M, Nakamura Y, Taguchi K, Ohno S. Persistence and discontinuation of adjuvant endocrine therapy in women with breast cancer. Breast Cancer. 2016;23(1):128‐133. [DOI] [PubMed] [Google Scholar]
- 21. Bosco‐Levy P, Jove J, Robinson P, Moore N, Fourrier‐Reglat A, Bezin J. Persistence to 5‐year hormonal breast cancer therapy: a French national population‐based study. Br J Cancer. 2016;115(8):912‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson B, Dijkstra B, Davey V, Tomlinson S, Frampton C. Adherence to adjuvant endocrine therapy in Christchurch Women with early breast cancer. Clin Oncol (R Coll Radiol). 2018;30(1):e9‐e15. [DOI] [PubMed] [Google Scholar]
- 23. Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient‐reported discontinuation of endocrine therapy and related adverse effects among women with early‐stage breast cancer. J Oncol Pract. 2012;8(6):e149‐e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher B, Costantino JP. RESPONSE: re: tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P‐1 study. J Natl Cancer Inst. 1999;91(21):1891a‐a1892. [DOI] [PubMed] [Google Scholar]
- 25. Heisig SR, Shedden‐Mora MC, von Blanckenburg P, et al. Informing women with breast cancer about endocrine therapy: effects on knowledge and adherence. Psychooncology. 2015;24(2):130‐137. [DOI] [PubMed] [Google Scholar]
- 26. Simon R, Latreille J, Matte C, Desjardins P, Bergeron E. Adherence to adjuvant endocrine therapy in estrogen receptor‐positive breast cancer patients with regular follow‐up. Can J Surg. 2014;57(1):26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kesmodel SB, Goloubeva OG, Rosenblatt PY, et al. Patient‐reported adherence to adjuvant aromatase inhibitor Therapy using the morisky medication adherence scale. Am J Clin Oncol. 2016;41:508‐512. [DOI] [PubMed] [Google Scholar]
- 28. Xu H, Zhang XJ, Wang DQ, Xu L, Wang AP. Factors influencing medication‐taking behaviour with adjuvant endocrine therapy in women with breast cancer: a qualitative systematic review. J Adv Nurs. 2020;76(2):445‐458. [DOI] [PubMed] [Google Scholar]
- 29. Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322‐328. [DOI] [PubMed] [Google Scholar]
- 31. Fontein D, Nortier J, Liefers GJ, et al. High non‐compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol. 2012;38(2):110‐117. [DOI] [PubMed] [Google Scholar]
- 32. Gatti ME, Jacobson KL, Gazmararian JA, Schmotzer B, Kripalani S. Relationships between beliefs about medications and adherence. Am J Health‐System. 2009;66(7):657‐664. [DOI] [PubMed] [Google Scholar]
- 33. Schüz B, Marx C, Wurm S, et al. Medication beliefs predict medication adherence in older adults with multiple illnesses. J Psychosom Res. 2011;70(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 34. Gao P, You L, Wu D, et al. Adherence to endocrine therapy among Chinese patients with breast cancer: current status and recommendations for improvement. Patient Prefer Adherence. 2018;12:887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brett J, Fenlon D, Boulton M, et al. Factors associated with intentional and unintentional non‐adherence to adjuvant endocrine therapy following breast cancer. Eur J Cancer Care. 2018;27(1):e12601. [DOI] [PubMed] [Google Scholar]
- 36. Chirgwin JH, Giobbie‐Hurder A, Coates AS, et al. Treatment adherence and its impact on disease‐free survival in the breast international group 1–98 trial of Tamoxifen and Letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Milata JL, Otte JL, Carpenter JS. Oral endocrine therapy nonadherence, adverse effects, decisional support, and decisional needs in women with breast cancer. Cancer Nurs. 2018;41(1):E9‐E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hershman DL, Kushi LH, Hillyer GC, et al. Psychosocial factors related to non‐persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. 2016;157(1):133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacob L, Hadji P, Kostev K. Age‐related differences in persistence in women with breast cancer treated with tamoxifen or aromatase inhibitors in Germany. J Geriatr Oncol. 2016;7(3):169‐175. [DOI] [PubMed] [Google Scholar]
- 40. Tinari N, Fanizza C, Romero M, et al. Identification of subgroups of early breast cancer patients at high risk of nonadherence to adjuvant hormone therapy: results of an Italian survey. Clin Breast Cancer. 2015;15(2):e131‐e137. [DOI] [PubMed] [Google Scholar]
- 41. Daly B, Olopade OI, Hou N, Yao K, Winchester DJ, Huo D. Evaluation of the quality of adjuvant endocrine therapy delivery for breast cancer care in the United States. JAMA Oncol. 2017;3(7):928‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cluze C, Rey D, Huiart L, et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23(4):882‐890. [DOI] [PubMed] [Google Scholar]
- 43. Gnant M, Pfeiler G, Stöger H, et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG‐6a trial. Br J Cancer. 2013;109(3):589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


