Abstract
Stroke is the leading cause of long-term disability with no current treatment addressing post-stroke disability. The complex pathophysiology of stroke and the brain’s limited potential for regeneration prevents sufficient endogenous repair for complete recovery. While engineered materials provide an exciting opportunity to augment endogenous repair in conjunction with other therapies that address post-stroke disability, much of the preclinical work in this arena is still in its infancy. Biomaterials can be used to enhance drug- or stem cell-sustained and targeted delivery. Moreover, materials can act as extracellular matrix-mimics and augment a pro-repair environment by addressing astrogliosis, inflammation, neurogenesis, axonal sprouting, and angiogenesis. Lastly, there is a growing need to elucidate stroke repair mechanisms to identify novel targets to inform material design for brain repair after stroke.
Ischemic stroke
Stroke is the second leading cause of morbidity in the world and remains the leading cause of severe long-term disability[1]. Stroke affects approximately 795,000 people in the US alone at a rate of one every 40 seconds[1]. The total annual cost of stroke is over $34 billion and is projected to grow to over $184 billion by 2030[1,2]. This significant economic burden can be primarily attributed to direct medical costs related to stroke, while the remaining 30% of the cost is attributed to the indirect costs, such as diminishing productivity as a result of stroke-related disability[1]. Stroke is the result of sudden interrupted or reduced blood flow to the brain, depriving brain tissue of oxygen and nutrients. Ischemic stroke accounts for 87% of all strokes[1] and occurs when blood vessels occlusion leads to local oxygen deprivation, formation of an infarct that is associated with physical and cognitive disabilities[3–5]. The remaining 13% of stroke cases are hemorrhagic[1].
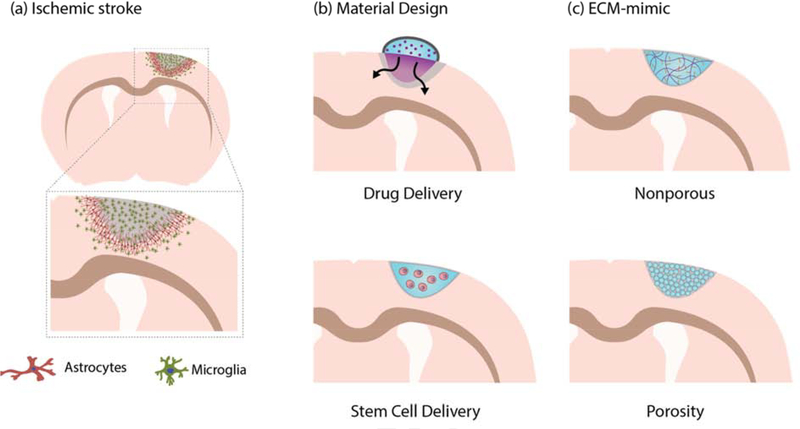
Stroke is characterized by complex pathophysiology, which begins with a sudden energy deficit and hypoxic conditions results in necrosis of neurons and glia that forms the stroke infarct[4]. Moreover, dying neurons uncontrollably release glutamate that causes further cell death by cellular excitoxicity. The blood-brain barrier (BBB) breaks down and triggers immune cell infiltration, release of free radicals and proteases. Substantial cell death and BBB compromise activates microglia, the local inflammatory cells, to express extracellular matrix (ECM)-degrading enzymes (i.e. matrix metalloproteases (MMP), hyaluronidase) that further degrades the brain tissue ECM and compromises its mechanical and biochemical integrity. The rapid cell death and ECM degradation leads to the formation of a stroke cavity. In order to limit growing stroke infarct and matrix degradation, astrocytes undergo astrogliosis where they form a glial scar that compartmentalizes the lesion (Figure 1a). While this prevents the stroke infarct from growing, it may limit regeneration as glial scar thickness positively correlates with stroke severity[6].
Figure 1.
Overview of ischemic stroke and materials for brain repair after stroke. (a) showing ischemic stroke pathophysiology in the form of a coronal section anterior to the bregma, specifically highlighting astrogliosis and neuroinflammation as shown by astrocyte scarring and increase in microglia, respectively. (b) Materials can be used as a vehicle in conjunction with other therapeutics to sustain local delivery of drugs (i.e. small molecules and proteins) or support stem cell transplant survival. (c) In addition to employing materials as vehicles, materials can also present instructive cues as an extracellular matrix (ECM) analog. Materials can be engineered to be nonporous or porous to elicit desired cellular and tissue response.
There are currently two FDA-approved ischemic stroke interventions that are used within the acute time window, tissue plasminogen activator (tPA) [7] and endovascular thrombectomy[8]. Both interventions focus on restoring blood flow by removing blood clot within the first few hours of stroke onset to prevent further infarct damage. Intravenously administered tPA enzymatically degrades the thrombus while endovascular thrombectomy is a surgical procedure to mechanically remove the thrombus in large vessels. Although the availability of these treatments has been effective for reperfusion, both these interventions have a very limited time window and they do not address the long-term neurological deficit resulting from stroke[7,8]. After the acute phase, the only available therapy to minimize stroke-related disability is rehabilitation Low-intensity training can begin as early as 72 hours after poststroke, followed by additional rehabilitation programs up to 2 months after stroke[9]. Unfortunately, the extent of recovery from rehabilitation is challenging to predict and may not result in sufficient functional recovery that warrants an independent standard of living[10,11]. Stroke’s encouraging decreasing mortality leads to more survivor living with stroke-related disabilities[1,2,12] and therefore, new treatments that address poststroke disability are critically needed to improve patient quality of life.
Clinical studies for stroke that focus on neuroprotection and not reperfusion has been conducted since the 1990s. Clinical studies can be grouped into interventions addressing neuroprotection, neurogenesis, inflammation, excitoxicity and oxidative stress[13–15]. Unfortunately, nearly all fail to demonstrate clinical efficacy with a third of these studies indicated false positives while the majority reported neutral results. This underscores the need for better preclinical experimental design and more consistent endpoints between animal studies and human clinical trials[16]. The goal of ongoing research on brain repair after stroke is to enhance recovery of functional brain tissue and ultimately reduce loss of function. Strategies of promoting repair poststroke has focused on the delivery of drugs in the form of small molecules, antibodies, and proteins to limit further brain damage and regenerate brain tissue. Additionally, preclinical stem cells therapies have also shown promise as they promoted recovery. For instance, the use of mesenchymal stem cells to promote recovery after stroke[17]. Unfortunately, systemic drug therapies face the challenge of spatial and temporal control of delivery. With stroke, the BBB serves as an additional drug localization challenge to systemic delivery before renal clearance. On the other hand, poor survival of cell transplants has limited its potential to enhance brain repair after stroke.
To combat challenges with systemic intravenous delivery, current approaches on drug delivery to central nervous system (CNS) across BBB show two main strategies: improving systemic delivery or developing local delivery. Improving systemic delivery ranges from modifying chemical properties for improved solubility and membrane penetration, exploiting cellular processes such as transcytosis, to engineered viral and non-viral nanoparticles[18,19]. For example, intravenous nanoparticle delivery of brain-derived neurotrophic factors (BDNF) has shown to increase its influx into the brain vs native BDNF[20]. However, it’s still challenging to sustain drug concentration in the brain for efficacy while minimizing systemic exposure. In contrast, there are other administration techniques that are more invasive but directly targets regions of interest within the CNS, such as intracerebral, intraventricular or intrathecal. With the additional risk of a more invasive delivery, local delivery methods avoid the BBB and results in high drug concentration in CNS while minimizing systemic exposure. However, drug clearance is still a problem that demands multiple invasive bolus injections to sustain drug concentration [18]. Local delivery of stem cells has seen success in animal models and several clinical trials are now underway to assess safety and efficacy towards reduced stroke disability [21,22], however, cell death is still a major concern.
Biomaterials offer a promising treatment option together with local delivery to prevent systemic exposure and sustain drug release in the brain (Figure 1b). For instance, a hyaluronan-methylcellulose composite hydrogel implanted epi-cortically has showed sustained delivery of peptide and protein therapies in stroked rats [23,24] (Figure 2c). Other studies have shown that hydrogels can provide a platform for local drug delivery with enhanced spatial and temporal control, working in conjunction with small molecule, peptide, protein and stem cell therapies [25,26]. In the following sections, we present an alternative approach to promote recovery after stroke: the use of materials locally to support the repairing peri-infarct and the necrotic infarct tissue.
Figure 2.
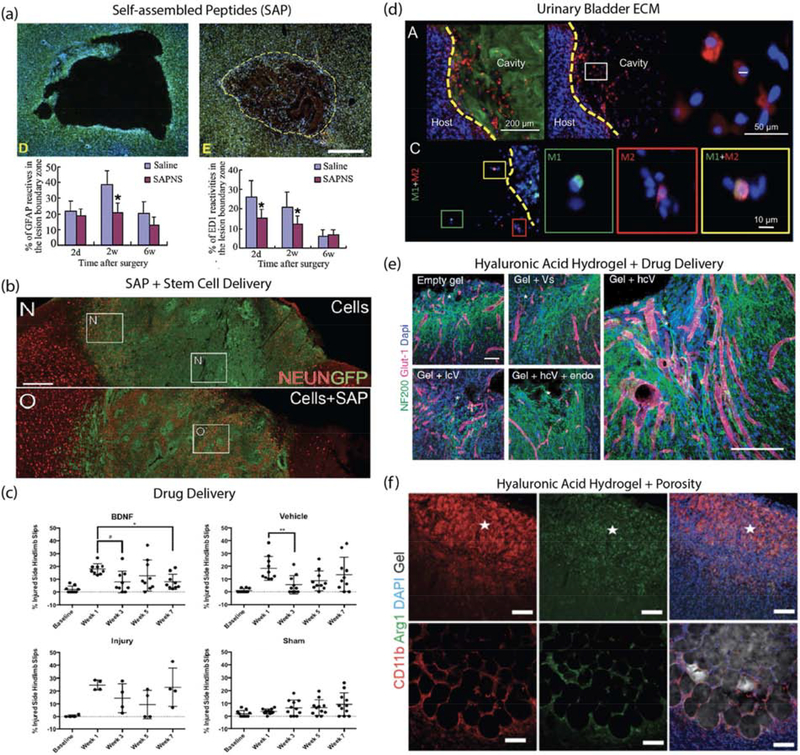
Material examples for brain repair after stroke. (a) RADA16 self-assembled peptide nanofiber scaffold (SAPNS): Nissl and DAPI stained images of saline-injected lesion and SAPNS-injected lesion as well as quantification of astrocytes (GFAP positive) and macrophages (ED1 positive) in the lesion boundary zone at 2 days, 2 weeks and 6 weeks after treatment. Source: Adapted from [44]. (b) IKVAV self-assembled peptide (SAP) and human cortical progenitor: NeuN stained images comparing GFP-labelled human cortical progenitors in stem cell delivery vs stem cell+SAP. Image shows increased proportion of NeuN-positive cells in SAP group. Source: Adapted from [36]. (c) BDNF-loaded hyaluronan-methylcellulose and PLGA nanoparticle scaffold composite: Behavioral studies after local BDNF delivery via material vehicle showed improvement in hindlimb recovery following stroke injury. Source: Adapted from [23]. (d) Urinary bladder matrix-ECM hydrogel: Immunostained images showing ECM hydrogel (Collagen I), cell nucleus (DAPI), macrophage/microglia (Iba-1), M1-like phenotype (CD86) and M2-like phenotype (CD206). Quantification of results showed that ECM hydrogel modulated neuroinflammation. Source: Adapted from [35]. (e) Hyaluronic acid and clustered VEGF on heparin nanoparticle hydrogel: Immunostained images of neurons (NF200 positive) and vessels (Glut-1 positive) in stroke infarct showed gel + high cluster VEGF delivery resulted in enhanced angiogenesis, neurogenesis and axonal sprouting. Source: Adapted from [41]. (f) Microporous annealed particles (MAP) hydrogel: Immunostained images of sham and MAP-treated stroke lesions showing gel, cell nucleus (DAPI), macrophage/microglia (CD11b) and pro-repair phenotype (Arg1). Quantification showed material modulated neuroinflammation by increasing pro-repair macrophage/microglia in the stroke infarct. Source: Adapted from [38].
Biomaterials for brain repair
It is important to note that like other tissues, the brain attempts to repair itself after an ischemic event as evident by increase in endogenous neuroplasticity [27]. However, this enhanced plasticity is transient and does not always result in sufficient recovery after stroke. In addition, neurons have limited self-repair capability[28]. Nevertheless, the fact that pro-repair pathways are activated after stroke offer an opportunity to augment their outcomes. We propose that hydrogel materials, when placed in the affected area, can offer a platform to augment these endogenous repair pathways through retention of expressed proteins or delivery of complementary activators/inhibitors. So far biomaterials have been designed to address the key aspects of stoke pathophysiology that are believed to prevent a pro-repair environment or that need to promote further repair, inflammation, astrogliosis, angiogenesis, neurogenesis and axonal sprouting.
Unlike previous examples where materials were strictly utilized as drug delivery vehicles, material scaffolds can additionally serve as ECM placeholders when delivered locally to the stroke infarct (Figure 1c). Materials acting as matrix-analog can promote recovery after stroke by providing temporary ECM within the compromised stroke infarct ECM. Around the stroke infarct, astrocytes have been shown to both promote and obstruct stroke recovery. Astrogliosis, or formation of an astrocytic scar surrounding the stroke cavity, is a reactive neurotoxic astrocyte phenotype that severely limits stroke recovery and loses its ability to promote neuronal survival, axonal sprouting and synaptogenesis[29]. However, the scar or peri-infarct region is also where pro-repair pathways are activated. Thus, placing a hydrogel ECM material in the infarct core, also places it next to the region where pro-repair pathways are activated, and endogenous repair is occurring.
Inflammation
Normally, the CNS is an immune-privileged site that is separated from peripheral immune system by the BBB. However, ischemic injury activates microglia, the resident immune cells of the CNS and they start exhibiting differential activated phenotypes that are analogous to the simplified M1 pro-inflammatory and M2 pro-healing phenotypes of macrophages [4]. Classically activated (M1) microglia release proinflammatory cytokines, ROS, and nitrous oxide that induces reactive astrocytes, BBB breakdown and extravasation of peripheral immune cells [29]. However, selective ablation of microglia is also detrimental as alternatively activated (M2) microglia are anti-inflammatory and promote brain repair by increased production of anti-inflammatory cytokines and neurotrophic factors [30]. Multiple studies have shown that the degree of microglia activation, timing and degree of cytokine or factor expression can result in ether positive or negative outcome after stroke [4,31,32]. Therefore, immunomodulation seeking to enhance brain repair after stroke requires powerful spatiotemporal control. Injection of materials into the stroke cavity has demonstrated that relatively simple materials can have a dramatic effect on the number of macrophage/microglia both in the stroke and peri-infarct areas. Injection of in-situ gelling hyaluronic acid (HA) hydrogels [33], assembling ECM derived materials [34,35] and peptide-based gels [36] into the stroke core, lower the number of microglia and in some cases switch the phenotype of macrophages from M1 to M2 even without the explicit delivery of anti-inflammatory agents. In the case of hyaluronic acid hydrogels, mechanical properties are important as materials that are >1000Pa were found to be pro-inflammatory [37]. For natural ECM derived materials like urinary bladder matrix, lower concentration or a less stiff scaffold is important to modulate macrophage and microglia number as well as M1 to M2 transition[34,35]. Further, simple changes to the hydrogel microstructure have also shown reduction of macrophage number in the infarct and peri-infarct tissue. While a nanoporous hyaluronic acid gel reduces the number of microphage/microglia in the peri-infarct area, hyaluronic acid microporous annealed particles (MAP) reduces this number in both the infarct and peri-infarct tissue and does so to a greater extent [33]. Further, porous hyaluronic acid MAP hydrogels with average pore diameter of 15–20 μm have been shown to reduce the number of macrophage/microglia early by decreasing the number of new macrophage/microglia infiltration after hydrogel injection and maintaining the lower level over time [38]. Lastly, the same porous MAP hydrogels are able to switch the phenotype of macrophage/microglia that are located within the hydrogel towards Arginase 1 (Arg1)-expressing [38] (Figure 2f). These results agree with other reports that porosity and spatial confinement can modulate the phenotype of macrophages [39,40]. Thus, biocompatible injectable hydrogels, in the context of stroke, can act as anti-inflammatory agents themselves even without the delivery of additional factors.
In addition to regulating macrophage/microglia, modulation of the astrocytic scar and astrocyte phenotype can be achieved by injectable hydrogel injections into the stroke cavity. As mentioned, reactive astrocytes fail to contribute to repair processes such as to promote neuronal survival, axonal sprouting and synaptogenesis[29]. The same materials that reduce macrophage/microglia also reduce the thickness of the astrocytic scar [33,38,41] and in some cases have been shown to modulate astrocyte phenotype [38]. Although explicit modulation of the astrocyte phenotype has not been reported for all studies injecting hydrogel materials, the fact that a porous hyaluronic acid hydrogel has demonstrated that astrocytes in the infarct and peri-infarct areas switch their phenotype to a less reactive state (lower pERK, s100-beta, and C3 expression) [38] suggests that this is an outcome that can be engineered into a biomaterial.
Although the material itself can act as an anti-inflammatory agent, further immunomodulation can be achieved with the incorporation of anti-inflammatory agents. For example, the introduction of heparin, a known anti-inflammatory agent [42,43], further reduces the number of macrophage/microglia and the thickness of the astrocytic scar [41]. A self-assembled peptide (SAP) RADA16 (RADARADARADARADA) nanofiber scaffold was shown to reduce astrogliosis and macrophage/microglia numbers in a traumatic brain injury (TBI) model [44] (Figure 2a). RADA16 SAP scaffold was then used to transplant activated astrocytes and human umbilical cord mesenchymal stem cells (hUC-MSC) along with BDNF peptide as their previous in vitro studied improved neuronal differentiation of hUC-MSC [45].
There are other pro-repair approaches to modulate inflammation. Angiogenic agents such as vascular endothelial growth factor (VEGF) and angiopoietin 1 (Ang1) delivered in HA-PLGA scaffolds decreased astrocyte and microglia [46]. Epi-cortical delivery of hyaluronan methylcellulose hydrogel and poly(lactic-co-glycolic acid) (PLGA) microparticles loaded with cyclosporin A, an FDA-approved immunosuppressant, has been shown to sustain cyclosporin A release in the brain while also increasing the numbers of proliferating cells [24]. It would be interesting to also look at the treatment’s effects on astrogliosis and inflammation since cyclosporin A is a known immunosuppressant. There are also small molecules being studied for modulating inflammation in acute stroke. The antibiotic minocycline was delivered systematically in acute stroke and decreased astrogliosis and reactive microglia [47]. Melanin has been shown to have potential as a radical scavenger and delivered in a polyethylene glycol (PEG) nanoparticle; its systematic delivery reduces astrogliosis and activated macrophages[48]. In addition to delivery of small molecules, peptides and proteins in the context of materials, stem cell with material delivery approaches have been shown to reduce astrogliosis and immunoreactive microglia and macrophages; for example, epidural delivery of neural progenitor cell (NPC) loaded in commercial fibrin glue (Beriplast) [49].
Neurogenesis/axonal sprouting
Stroke results in necrosis and apoptosis of neurons within the infarct that leads to neurological deficit[4]. Damaged neuronal circuitry requires enhanced neuroplasticity so new or existing neurons can form new connections. Similar to inflammation and angiogenesis (below) hydrogel biomaterials can promote axonal sprouting in the peri-infarct region even when not loaded with neurogenic or angiogenic factors. Thus, hydrogel materials can modulate the post-stroke environment in favor of repair processes that extend beyond inflammation. In the case of axonal sprouting, microstructure plays a critical role. Hyaluronic acid MAP gels improved axonal sprouting in both infarct and peri-infarct region compared to the same non-porous hydrogel or sham wounds [38]. Biodegradation of urinary bladder matrix-derived ECM hydrogels also led to higher neurogenesis in ECM hydrogel [35].
To more robustly enhance neuroplasticity post stroke, introduction of bioactive factors that can modulate neuroplasticity processes into hydrogel biomaterials is an obvious choice. There have been a number of efforts to deliver BDNF after stroke. BDNF has been linked to neuroprotection, synaptic plasticity, neurogenesis and axonal sprouting as well as functional recovery poststroke in patients and serum levels of BDNF have been proposed as a biomarker for rehabilitation [50]. BDNF delivery via PEG crosslinked hyaluronic acid hydrogel into the stroke core promotes axonal sprouting, neurogenesis and motor recovery after stroke in young mice [51]. In contrast, in aged mice hydrogel mediated delivery of BDNF resulted in a modest motor recovery; however, the co-delivery BDNF and ampakine, an attenuator of AMPA receptor currents, showed significant motor improvement[52]. Further, BDNF was linked to the critical window for recovery poststroke in aged mice [53]. Similar to BNDF, other bioactive factors (proteins and small molecules) have been delivered to attempt to tip the balance towards neuroplasticity and repair. For example, delivery of anti-NogoA antibody neutralizes neurite outgrowth inhibitor (Nogo-A) and promotes axonal sprouting, angiogenesis and behavioral recovery after stroke without affecting inflammation or astrogliosis [54,55]. Administration of FDA-approved HIV antiviral drug Maraviroc antagonizes C-C chemokine receptor type 5 (CCR5) and improves axonal sprouting and neuroplasticity after stroke [56].
Given that multiple factors are likely required to promote sufficient neuroplasticity and the formation of functional circuits and that the source of new neurons (neural progenitors) is likely limited in the adult brain, stem cell delivery has been extensively studied to promote recovery after stroke. Stem cells can be the source of the complex milieu of signals needed for repair, the source of new neurons or support cells, or both. Though not in clinical trials yet, delivery of stem cells within hydrogel biomaterials has shown promise in pre-clinical models. Local delivery of stem cells in a material scaffold has been reported to retain cells within the stroke cavity and maintain their survival to a greater extent compared to cell delivery alone [49,57,58]. Hydrogels can be engineered to optimize stem cell survival and differentiation after delivery in the stroke core. For example, the composition of hyaluronic acid hydrogels containing bone morphogenic protein 4 (BMP-4), BDNF, and peptides was optimized to promote induced pluripotent stem cell-NPC survival and differentiation towards neurons or astrocytes depending on composition [59]. Further, a self-assembled peptide hydrogel was shown to promote NPC survival, neuronal differentiation, decrease atrophy and promote motor function [36] (Figure 2b). Though the concept of stem cell delivery from hydrogel scaffolds for stroke treatment has begun to be studied, much work remains to define what key features of materials lead to survival, differentiation and host engraftment.
Angiogenesis
Experimental and clinical studies have shown that stroke-induced enhanced angiogenesis correlates to improved survival and behavioral recovery [60]. Thus, efforts to increase angiogenesis in the infarct and peri-infarct regions has been explored in the context of materials and stroke. Similar to inflammation and axonal sprouting materials can, even in the absence of angiogenic factors, act to promote angiogenesis. In general, materials that substantially decrease inflammation and scar thickness, lead to improved angiogenesis in the peri-infarct space but not the infarct area. As with neuroplasticity, delivery of angiogenic factors have been investigated to further promote vessel formation. In this context, VEGF has been extensively studied in the context of stroke recovery reaching clinical studies. However, VEGF is yet to be an FDA-approved therapy. We believe that the lack of success with VEGF and other pro-angiogenic therapies is the way in which these therapies are delivered, as soluble factors or overexpressed proteins. In this context, VEGF delivery can further cause brain damage by increasing BBB breakdown, promoting edema and inducing disorganized and immature vessel formation[61]. Hydrogel materials can be used to modulate delivery of angiogenic factors at more appropriate doses and in the right context (to mediate co-signaling events). For example, immobilizing VEGF onto nanoparticles to display VEGF in clusters and delivering these from a material that also lowered inflammation and decreased the glial scar showed functional improvement after stroke [41] (Figure 2e). This material was able to generate a coordinated pro-repair environment that led to vascularization and axonal sprouting of the stroke core. Other examples, of VEGF delivery from materials show that sustained delivery of VEGF from nanoparticles, while promoting beta-1 integrin binding lead to reduced leaky vessels. These studies demonstrated that proper delivery of VEGF in the right ECM context can lead to non-leaky vessel formation, which has been a hallmark of VEGF induced angiogenesis. A study comparing soluble VEGF with immobilized VEGF and found that immobilized VEGF on microparticles led to prolonged VEGFR-2 and protein kinase B (Akt) phosphorylation as well as higher outgrowth endothelial progenitor cell survival [62]. Also as mentioned above, VEGF and Ang1 was delivered via a hyaluronic acid-PLGA scaffold and showed improved angiogenesis[46]. There are likely other features of biomaterials that can be exploited to result in effective pro-angiogenic signaling that can lead to functional revascularization rather than vascular permeability.
Elucidating stroke repair mechanisms
Given the complexity of post stroke repair and the many different cell types that need to act in concert to lead to functional improvement, new therapeutic targets must be identified. Thus far, most biomaterial approaches have focused on old targets to reduce inflammation, promote angiogenesis and neurogenesis.
The Carmichael lab has focused on understanding the mechanisms of axonal sprouting poststroke [63–65]. Their research has led to a novel target that can be used when designing a material therapeutic for stroke. They’ve identified growth differentiation factor 10 (GDF10) and found that it enhanced motor recovery when locally delivered in stroke cavity using a HA-heparin hydrogel[66]. In addition, they’ve also determined that CCR5 signaling is uniquely expressed in cortical neurons poststroke and its knockdown enhanced motor recovery. It is also the first reported gene associated with enhanced recovery in humans as seen by subpopulations of stroke patients with naturally occurring loss-offunction mutation in CCR5 [67]. However, their studies solely focus on axon sprouting neurons after endogenous recovery poststroke. Next-generation transcriptomic and proteomic approaches need to be utilized to identify relevant cellular players, pathways and proteins for brain repair after stroke. This will generate novel targets that can be help inform our material design.
Conclusions
Stroke is a traumatic brain insult is the leading cause for long-term disability in adults due to the brain’s poor regenerative capacity. Current clinical treatments are reperfusion efforts that still leave patients with long-term neurological deficits. Over the years, preclinical research has identified therapeutic strategies to enhance endogenous brain repair after stroke. Unfortunately, clinical trial translational attempts have failed where systemically delivered therapeutic drugs are quickly cleared and transplanted stem cells have poor survival. More recent research in biomaterials for brain repair after stroke have showed that innovative biomaterial designs alone can reduce inflammation, glial scar formation and increase cellular infiltration to stroke infarct. With the addition of drugs and stem cells therapeutics, the combined therapeutic-loaded biomaterials strategy overcame challenges with systemic delivery and cell transplant survival, enhancing functional recovery as shown by a number of successfully examples in the field. Despite the encouraging results of biomaterial strategies in tackling brain repair after stroke, it is difficult to predict whether documented functional recovery found in rodent models can be translated in humans. This warrants a better understanding of the mechanisms of brain repair after stroke. By appreciating the mode of action of these biomaterial strategies, novel targets that can more holistically promote brain repair after stroke and result in complete functional recovery will be discovered.
Local delivery using materials should not be viewed as being at odds with systemic delivery. Materials-based local delivery can be used in conjugation with systemic delivery to utilize the advantages of both modes of delivery. For instance, systemic delivery of agents could be used to treat early stages (i.e. reperfusion and neuroprotection) and/or be used to “prime” the stroke environment for the ultimate delivery of a biomaterial to promote plasticity and recovery from stroke.
The application of biomaterial strategies for brain repair is in its nascent preclinical stages. However, the field of biomaterials to promote endogenous repair in other tissue systems such as bone and skin is substantially more developed and used clinically. Learning from these strategies and attracting a new generation of biomaterial scientist is necessary to bring the field closer to clinical translation.
Acknowledgements
KE is credited with conception and writing of the manuscript. TS provided valuable edits, suggestions and feedback. This work was supported by the National Institutes of Health (1R01NS094599). KE is a recipient of the William M. “Monty” Reichert Fellowship granted by the Biomedical Engineering Department at Duke University.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, et al. : Heart Disease and Stroke Statistics—2016 Update. 2015. [Google Scholar]
- 2.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, et al. : Forecasting the future of stroke in the united states: A policy statement from the American heart association and American stroke association. Stroke 2013, 44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 3.Barkho BZ, Zhao X: Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther 2011, 6:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing C, Arai K, Lo EH, Hommel M: Pathophysiologic cascades in ischemic stroke. Int J Stroke 2012, 7:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiss WD: The ischemic penumbra: How does tissue injury evolve? Ann N Y Acad Sci 2012, 1268:26–34. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Wu ZB, ZhuGe Q, Zheng WM, Shao B, Wang B, Sun F, Jin K: Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci 2014, 11:344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The national institute of neurological disorders and stroke rt-PA stroke study group: Tissue Plasminogen Activator for Acute Ischemic Stroke — NEJM. N Engl J Med 1995, doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. : Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med 2015, doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 9.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et al. : Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. 2016. [DOI] [PubMed]
- 10.Lin DJ, Finklestein SP, Cramer SC: New Directions in Treatments Targeting Stroke Recovery. Stroke 2018, 49:3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwakkel G, Kollen BJ, Wagenaar RC: Therapy impact on functional recovery in stroke rehabilitation. Physiotherapy 1999, 85:377–391. [Google Scholar]
- 12.Katan M, Luft A: Global Burden of Stroke. Semin Neurol 2018, 38:208–211. [DOI] [PubMed] [Google Scholar]
- 13.Reis C, Akyol O, Ho WM, Araujo C, Huang L, Applegate R, Zhang JH: Phase i and Phase II Therapies for Acute Ischemic Stroke: An Update on Currently Studied Drugs in Clinical Research. Biomed Res Int 2017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnerup J, Wersching H, Schilling M, Schäbitz WR: Analysis of early phase and subsequent phase III stroke studies of neuroprotectants: Outcomes and predictors for success. Exp Transl Stroke Med 2014, 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajah GB, Ding Y: Experimental neuroprotection in ischemic stroke: A concise review. Neurosurg Focus 2017, 42:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, Clarkson AN, Dancause N, Weiloch T, Johansen-Berg H, et al. : Enhancing the Alignment of the Preclinical and Clinical Stroke Recovery Research Pipeline: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable Translational Working Group *. Neurorehabil Neural Repair 2017, 31:699–707. [DOI] [PubMed] [Google Scholar]
- 17.Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, Hu X, Feng W: Mesenchymal Stem Cell Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research. Cell Transplant 2018, 27:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong X: Current strategies for brain drug delivery. Theranostics 2018, 8:1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ: Current approaches to enhance CNS delivery of drugs across the brain barriers. Int J Nanomedicine 2014, 9:2241–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris NM, Ritzel R, Mancini NS, Jiang Y, Yi X, Manickam DS, Banks WA, Kabanov AV, McCullough LD, Verma R: Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav 2016, 150:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marei HE, Hasan A, Rizzi R, Althani A, Afifi N, Cenciarelli C, Caceci T, Shuaib A: Potential of stem cell-based therapy for ischemic stroke. Front Neurol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borlongan CV: Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl Med 2019, 8:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermeyer JM, Tuladhar A, Payne SL, Ho E, Morshead CM, Shoichet MS: Local Delivery of Brain-Derived Neurotrophic Factor Enables Behavioral Recovery and Tissue Repair in Stroke-Injured Rats. Tissue Eng Part A 2019, 25:1175–1187.**BDNF was delivered via PLGA nanoparticles in hyaluronan-methylcellulose hydrogel epi-cortically above stroke lesion induced mice. Local and sustained delivery of BDNF increases neurplasticity and vehicle lesion size and neuron loss. Overall, treatment resulted in improved motor recovery.
- 24.Tuladhar A, Morshead CM, Shoichet MS: Circumventing the blood–brain barrier: local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J Control release 2015, 215:1–11. [DOI] [PubMed] [Google Scholar]
- 25.Tuladhar A, Payne SL, Shoichet MS: Harnessing the potential of biomaterials for brain repair after stroke. Front Mater 2018, 5:1–25. [Google Scholar]
- 26.Nih LR, Carmichael ST, Segura T: Hydrogels for brain repair after stroke: An emerging treatment option. Curr Opin Biotechnol 2016, 40:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobkin BH, Carmichael ST: The specific requirements of neural repair trials for stroke. Neurorehabil Neural Repair 2016, 30:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Björklund A, Lindvall O: Self-repair in the brain. Nature 2000, 405:893–895. [DOI] [PubMed] [Google Scholar]
- 29.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC: Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szalay G, Martinecz B, Lénárt N, Környei Z, Orsolits B, Judák L, Császár E, Fekete R, West BL, Katona G: Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 2016, 7:11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ao L yao, Yan YY, Zhou L, Li C yuan, Li WT, Fang W rong, Li Y man: Immune Cells After Ischemic Stroke Onset: Roles, Migration, and Target Intervention. J Mol Neurosci 2018, 66:342–355. [DOI] [PubMed] [Google Scholar]
- 32.Chamorro Á, Meisel A, Planas AM, Urra X, Van De Beek D, Veltkamp R: The immunology of acute stroke. Nat Rev Neurol 2012, 8:401–410. [DOI] [PubMed] [Google Scholar]
- 33.Nih LR, Sideris E, Carmichael ST, Segura T: Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv Mater 2017, 29:1–8.**Hyaluronic acid hydrogel microparticles were annealed in situ to form microporous annealed particle (MAP) gel inside lesion of stroke-induced mice. Porous scafflold reduced astrocyte scarring, microglia infiltration to stroke cavity while also promoting NPC migration and infiltration into the scaffold.
- 34.Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, Modo M: ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate. Biomaterials 2016, 91:166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghuman H, Mauney C, Donnelly J, Massensini AR, Badylak SF, Modo M: Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater 2018, 80:66–84.**Porcine-derived urinary bladder matrix ECM was used to form injectable ECM hydrogel delivered to the lesion of MCAO mice. They found that lower ECM hydrogel concentration leads to better immunomodulation, angiogensis and neurogenesis.
- 36.Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H, McDougall S, Williams RJ, Nisbet DR, Thompson LH, et al. : Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke. Cell Rep 2017, 20:1964–1977.* Laminin-derived IKVAV peptide was used to make self-assembled peptide (SAP) scaffold loaded with progenitor cells and delivered to stroked mice. Peptide-based scaffold progenitor cell implants showed increased neuronal differentiation, and enhanced electrophysiological properties.
- 37.Lam J, Carmichael ST, Lowry WE, Segura T: Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater 2015, 4:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sideris E, Yu A, Chen J, Carmichael ST, Segura T: Hyaluronic acid particle hydrogels decrease cerebral atrophy and promote pro-reparative astrocyte/axonal infiltration in the core after ischemic stroke. bioRxiv 2019, doi: 10.1101/768291.**Hyaluronic acid MAP hydrogel was injected to stroke cavity. The authors demonstrated that their MAP gel reduced astrogliosis and modulated neuroinflammation as shown by increase in microglia/macrophage pro-repair phenotype.
- 39.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD: Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng 2014, 42:1508–1516. [DOI] [PubMed] [Google Scholar]
- 40.Jain N, Vogel V: Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater 2018, 17:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nih LR, Gojgini S, Carmichael ST, Segura T: Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater 2018, 17:642–651.** Hyaluronic acid-based hydrogel was crosslinked in situ with MMP-degradable peptide and decorated with RGD adhesion peptide, naked heparin nanoparticles and clustered VEGF on heparin nanoparticles. Their material was injected into stroke cavity induced in mice and demonstrated reduced astrogliosis and inflammation, and increased angiogensis and neuron infiltration into the stroke cavity, which were colocalized.
- 42.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M: Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poterucha TJ, Libby P, Goldhaber SZ: More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost 2017, 117:437–444. [DOI] [PubMed] [Google Scholar]
- 44.Guo J, Leung KKG, Su H, Yuan Q, Wang L, Chu TH, Zhang W, Pu JKS, Ng GKP, Wong WM, et al. : Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine Nanotechnology, Biol Med 2009, 5:345–351. [DOI] [PubMed] [Google Scholar]
- 45.Shi W, Huang CJ, Xu XD, Jin GH, Huang RQ, Huang JF, Chen YN, Ju SQ, Wang Y, Shi YW, et al. : Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater 2016, 45:247–261. [DOI] [PubMed] [Google Scholar]
- 46.Ju R, Wen Y, Gou R, Wang Y, Xu Q: The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant 2014, 23:83–95. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y: Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation 2015, 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Ai K, Ji X, Askhatova D, Du R, Lu L, Shi J: Comprehensive insights into the multiantioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J Am Chem Soc 2017, 139:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee IH, Huang SS, Chuang CY, Liao KH, Chang LH, Chuang CC, Su YS, Lin HJ, Hsieh JY, Su SH, et al. : Delayed epidural transplantation of human induced pluripotent stem cell-derived neural progenitors enhances functional recovery after stroke. Sci Rep 2017, 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo W, Liu T, Li S, Wen H, Zhou F, Zafonte R, Luo X, Xu M, Black-Schaffer R, Wood LJ: The serum BDNF level offers minimum predictive value for motor function recovery after stroke. Transl Stroke Res 2019, 10:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook DJ, Nguyen C, Chun HN, L Llorente I, Chiu AS, Machnicki M, Zarembinski TI, Carmichael ST: Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab 2017, 37:1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarkson AN, Parker K, Nilsson M, Walker FR, Gowing EK: Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J Cereb Blood Flow Metab 2015, 35:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houlton J, Zhou LYY, Barwick D, Gowing EK, Clarkson AN: Stroke Induces a BDNF-Dependent Improvement in Cognitive Flexibility in Aged Mice. Neural Plast 2019, 2019:1460890.** Authors compared stroked young and aged mice and the effect of BDNF decoy, TrkB-Fc, on behavioral recocery. They found that a BDNF-dependent improvement in stroke aged mice, even outperforming sham aged mice.
- 54.Otero-Ortega L, Gómez-De Frutos MC, Laso-García F, Sánchez-Gonzalo A, Martínez-Arroyo A, Díez-Tejedor E, Gutiérrez-Fernández M: NogoA Neutralization Promotes Axonal Restoration after White Matter Injury in Subcortical Stroke. Sci Rep 2017, 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rust R, Grönnert L, Gantner C, Enzler A, Mulders G, Weber RZ, Siewert A, Limasale YDP, Meinhardt A, Maurer MA, et al. : Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc Natl Acad Sci U S A 2019, 116:14270–14279.* Neutralization of Nogo-A or one of its receptor S1PR2 by antibody treatment or knock-out improves angiogensis and neuroprotection after stroke but does not reduce inflammation and astrogliosis.
- 56.Joy MT, Ben Assayag E, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M, Abduljawad N, Kliper E, Korczyn AD, Thareja NS, et al. : CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176:1143–1157.e13.** FDA-approved HIV antiviral drug Maraviroc was systematically delivered to CNS injured mice and showed improved neural repair in both stroke and traumatic brain injury. Maraviroc works as CCR5 antagonist that increaes neuroplasticity.
- 57.Ballios BG, Cooke MJ, Donaldson L, Coles BLK, Morshead CM, Van Der Kooy D, Shoichet MS: A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports 2015, 4:1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam J, Lowry WE, Carmichael ST, Segura T: Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater 2014, 24:7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T, Carmichael ST: Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM: Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25:1794–1798. [DOI] [PubMed] [Google Scholar]
- 61.Geiseler SJ, Morland C: The janus face of VEGF in stroke. Int J Mol Sci 2018, 19:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aday S, Zoldan J, Besnier M, Carreto L, Saif J, Fernandes R, Santos T, Bernardino L, Langer R, Emanueli C: Synthetic microparticles conjugated with VEGF 165 improve the survival of endothelial progenitor cells via microRNA-17 inhibition. Nat Commun 2017, 8:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST: A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A 2012, 109:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST: An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci 2010, 13:1496–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH: Molecular, cellular and functional events in axonal sprouting after stroke. Exp Neurol 2017, 287:384–394.** Authors investigated axonal sprouting after ischemic event in mice by labeling neurons that undergo axonal sprouting using neuroanatomical tracer injections. They observed three distinct patters of axonal sprouting: reactive, reparative and unbounded.
- 66.Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters H V, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G: GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci 2015, 18:1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joy MT, Assayag E Ben, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M, Abduljawad N, Kliper E, Korczyn AD, Thareja NS: CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 2019, 176:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]