Abstract
Background and Purpose:
Sex differences in stroke incidence over time were previously reported from the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS). We aimed to determine if these differences continued through 2015 and if they were driven by particular age groups.
Methods:
Within the GCNKSS population of 1.3 million, incident (first-ever) strokes among residents ≥20 years old were ascertained at all local hospitals during five periods: 7/93-6/94 and calendar years 1999, 2005, 2010, and 2015. Out-of-hospital cases were sampled. Sex-specific incidence rates per 100,000 were adjusted for age and race, and then standardized to the 2010 U.S. Census. Trends over time by sex were compared (overall and age-stratified). Sex-specific case fatality rates were also reported. Bonferroni corrections were applied for multiple comparisons.
Results:
Over the five study periods, there were 9733 incident strokes (56.3% female). For women, there were 229 (95%CI 215-242) per 100,000 incident strokes in 1993/4 and 174 (95%CI 163-185) in 2015 (p <.05), compared with 282 (95% CI 263-301) in 1993/4 to 211 (95%CI 198-225) in 2015 (p<.05) in men. Incidence rates decreased between first and last study periods in both sexes for IS but not for ICH or SAH. Significant decreases in stroke incidence occurred between first and last study periods for both sexes in the 65-84 age group and men only in the ≥85 age group; stroke incidence increased for men only in the 20-44 age group.
Conclusions:
Overall stroke incidence decreased from the early 1990s to 2015 for both sexes. Future studies should continue close surveillance of sex differences in the 20-44 and ≥85 age groups, and future stroke prevention strategies should target strokes in the young and middle age groups as well as ICH.
Keywords: stroke, stroke in young adults, sex-specific, sex, ischemic stroke, cerebrovascular disease/stroke, intracranial hemorrhage
INTRODUCTION
Stroke remains a leading cause of disability and death for both women and men worldwide.1 There are, however, more strokes and stroke deaths in women than men,1–3 thought to be largely related to a longer life expectancy for women.4 It has been established that the female to male stroke risk increases over the course of the lifespan, which may be in part due to the protective effects of endogenous female hormones in pre-menopausal women and the lack of such protective effects later in life.5,6 In some studies, stroke incidence among women surpasses that of men for those over 80 years old.1,4,7,8
There are conflicting data regarding whether stroke incidence over time has been declining equally in women and men. Our previous work demonstrated that the incidence of overall stroke and ischemic stroke (IS) decreased significantly between 1993 and 2010 in men but not women.9 Similar sex differences were noted over the same time period for transient ischemic attacks (TIAs).10 In contrast, data from the Atherosclerosis Risk in Communities Study (ARIC), a prospective cohort study, demonstrated a decrease in stroke incidence through 2011 among both women and men, and a more recent ARIC study through 2017 showed similar decreases in stroke incidence among adults over 65 years old.11 More data on stroke incidence extending past 2010 is needed to help resolve these apparent discrepancies.
Knowledge gaps also exist with regard to how temporal trends in stroke incidence vary with age. Multiple previous studies in the United States have demonstrated that stroke incidence among young patients is not declining11,12 or is even increasing over time,13,14 conflicting with findings from outside the United States15 and illuminating important opportunities for additional work in this area.
Overall, we lack both knowledge and updated data regarding the combined effects of age and sex on stroke incidence over time. The objectives of this paper were to utilize data from the GCNKSS to 1) Investigate whether our previous findings of sex differences in stroke incidence over time continued through 2015; and 2) Describe the sex-specific epidemiology of stroke by age over time.
METHODS
Data from the GCNKSS can be obtained with permission from the principal investigators of the study.
Study population
The GCNKSS is a population-based study that ascertains stroke events occurring in a population of 1.3 million people living in a 5-county region of southern Ohio and Northern Kentucky. The study population has been shown to be representative of the United States with regard to black race, age, education, and income.
Case ascertainment
Incident (first-ever) strokes occurring in residents ≥ 20 years old living within the GCNKSS population were ascertained at all local hospitals during five periods: July 1993 through June 1994 and calendar years 1999, 2005, 2010, and 2015. Out-of-hospital cases included those identified in coroners’ offices, hospital and public health care clinics, and also were identified in a random sample of physicians’ offices. For the current analysis, in contrast to previously published incidence rates from the GCNKSS, nursing home cases were not included in incidence rates for any study period because the 2015 out-of-hospital sampling did not include nursing homes. Potential cases were identified by trained study nurses based on ICD codes for stroke (ICD9 430-436 and ICD10 160-169). Each case was adjudicated by trained study physicians using medical record review and standardized case report forms. To be confirmed as a stroke, symptoms must be sudden in onset, localize to a specific area of the brain, and persist for ≥ 24 hours. TIAs were excluded. This definition does not require performance of MRI,16 and patients with symptoms < 24 hours but with an MRI consistent with acute ischemic stroke were also excluded to allow for comparisons across the 22 year time period. Strokes were further categorized as ischemic stroke (IS), intracerebral hemorrhage (ICH), or subarachnoid hemorrhage (SAH) as previously described.17–19 Methods used to screen and adjudicate stroke cases were consistent over all study periods.
Statistical Analysis
The raw total number of events was reported overall and in each study period by sex. Demographic variables (age, race, modified Rankin Score (mRS)) over the five study periods were reported by sex using a generalized linear model. Baseline mRS was determined by trained study nurses using all information available from extensive medical record review; further details can be found in the supplement (please see https://www.ahajournals.org/journal/str). Weighted percentages were reported for categorical variables, and the mean and weighted standard error were reported for continuous variables. The interaction of sex by study period was also assessed.
Sex-specific weighted incidence rates and 95% confidence intervals (CI) per 100,000 were reported along with raw frequencies for each of the 5 study periods. Rates included all inpatient events as well as out-of-hospital ascertained events (including the weighted sample of events from physicians’ offices) and were adjusted for age and race, then standardized to the 2010 U.S. Census population. To compare trends over time period by sex, age and race adjusted sex-specific incidence rates were compared between the first (1993/4) and last (2015) study periods. Comparisons were made for all strokes and for individual stroke subtypes. A Bonferroni correction was applied for multiple comparisons.
Next, sex-specific incidence rates for all strokes were reported as stratified by age (20-44, 45-64, 65-84, and ≥ 85 years old), adjusted for age and race. Female/male rate ratios were also presented. For each of the four age categories, sex-specific comparisons of incidence of all strokes between first and last study periods was compared. Again, a Bonferroni correction was applied for multiple comparisons.
Case Fatality
The definition of case-fatality was death from any cause occurring in a 30-day window following incident stroke. Deaths were confirmed for all study periods using The National Death Index that provides dates of death, cause of death codes, state location at time of death, and corresponding death certificate numbers. Age- and race-adjusted weighted 30-day case fatality rates by sex and study period were reported for all strokes and individual stroke subtypes (IS, ICH, SAH) as frequencies and weighted proportions with 95% CI. Sex-specific differences in change in case fatality over time were tested with the inclusion of sex by study period product terms in the general linear model.
This study was approved by institutional review boards at all participating hospitals, and informed consent was waived. Statistical analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC).
RESULTS
Across all study periods, there were 9733 incident strokes: 5483 (56.4%) female, mean age 71.8 for women and 67.5 for men. Baseline characteristics are displayed in Table 1. The proportion of black patients increased between the first and last study periods in both women and men (18.7% and 17.0% in 1993/4 to 23.0% and 23.0% in 2015, respectively). The proportion of patients who were independent at baseline (defined as mRS 0 or 1) was 66.8% vs. 79.1% for women vs. men in 1993/4 and 46.8% vs. 67.2% in 2015, (p<0.0001 for sex by study period product term).
Table 1:
Baseline Characteristics of Incident Stroke Cases by Sex and Time Period in the Greater Cincinnati Northern Kentucky Stroke Study
| Year | Overall | 1993/94 | 1999 | 2005 | 2010 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males |
| N | 5477 | 4256 | 1105 | 813 | 1171 | 834 | 1055 | 826 | 1099 | 838 | 1047 | 945 |
| Age (Weighted Mean (SEM) in years | 71.8 (0.40) | 67.5 (0.74) | 72.9 (0.16) | 68.8 (0.56) | 73.3 (0.39) | 70.3 (0.66) | 71.1 (1.05) | 66.5 (0.82) | 70.3 (0.07) | 65.8 (0.29) | 71.1 (0.48) | 65.9 (0.31) |
| Black Race N (%) |
1122 (20.5) | 819 (19.2) | 207 (18.7) | 138 (17.0) | 210 (17.9) | 134 (16.1) | 229 (21.7) | 158 (19.1) | 235 (21.4) | 172 (20.5) | 241 (23.0) | 217 (23.0) |
| Baseline mRS 0 or 1 N (%) |
2925 (54.6) | 2951 (70.7) | 697 (66.8) | 603 (79.1) | 703 (61.9) | 608 (74.7) | 554 (52.6) | 567 (69.2) | 487 (44.4) | 540 (64.6) | 484 (46.8) | 633 (67.2) |
Age: Study period by sex product term, p=0.37. P<0.0001 for year.
Race: Study period by sex product term, p=0.49. P<0.0001 for year.
Baseline mRS: Study period by sex product term, p<.0001, P<0.0001 for year
Sex-specific stroke incidence rates in each of the five study periods are presented in Table 2. Including all strokes, for women, there were 229 (95%CI 215-242) per 100,000 incident strokes in 1993/4 and 174 (95%CI 163-185) in 2015 (p < 0.05), compared with 282 (95% CI 263-301) in 1993/4 to 211 (95%CI 198-225) in 2015 (p<0.05) for men. For IS, incidence rates decreased between first and last study periods in both women and men (Table 2). For ICH and SAH, there were no statistically significant differences in either sex between the first and last study periods.
Table 2:
Stroke Incidence Rates per 100,000 over Time by Sex in the Greater Cincinnati Northern Kentucky Stroke Study
| 1993/94 | 1999 | 2005 | 2010 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of stroke | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males |
| All | 229* (215, 242) [n=1105] | 282* (263, 301) [n=813] | 265 (251, 279) [n=1171] | 303 (283, 323) [n=834] | 220 (207, 232) [n=1055] | 245 (229, 261) [n=826] | 210 (198, 222) [n=1099] | 205 (191, 219) [n=838] | 174 (163, 185) [n=1047] | 211 (198, 225) [n=945] |
| Female/male rate ratio | 0.81 (0.74, 0.88) | 0.87 (0.80, 0.95) | 0.89 (0.82, 0.97) | 1.03 (0.93, 1.12) | 0.84 (0.93, 1.12) | |||||
| Ischemic Stroke | 202* (189, 214) [n=972] | 254* (236, 272) [n=719] | 218 (206, 231) [n=1003] | 270 (251, 288) [n=746] | 178 (167, 189) [n=924] | 220 (205, 235) [n=725] | 183 (175, 197) [n=943] | 178 (165, 191) [n=719] | 151 141, 161) [n=910] | 182 (170, 195) [n=822] |
| Female/Male rate ratio | 0.79 (0.72, 0.87) | 0.81 (0.74, 0.88) | 0.81 (0.73, 0.89) | 1.03 (0.93, 1.13) | 0.83 (0.75, 0.91)) | |||||
| Intracranial hemorrhage | 27 (23, 32) [n=140] | 33 (27, 40) [n=110] | 35 (30, 40) [n=167] | 37 (30, 43) [n=131] | 31 (26, 36) [n=171] | 38 (32, 44) [n=145] | 28 (23, 32) [n=158] | 34 (28, 39) [n=143] | 30 (25, 34) [n=183] | 32 (27, 38) [n=149] |
| Female/Male risk ratio | 0.82 (0.61, 1.03) | 0.96 (0.74, 1.18) | 0.81 (0.63, 0.99) | 0.81 (0.63, 1.00) | 0.91 (0.71, 1.12) | |||||
| Subarachnoid hemorrhage | 12 (9, 15) [n=58] | 8 (5, 12) [n=27] | 14 (11, 18) [n=71] | 6 (4, 8) [n=24] | 13 (10, 16) [n=67] | 6 (3, 8) [n=24] | 11 (8, 14) [n=59] | 5 (3, 8) [n=25] | 8 (6, 11) [n=48] | 11 (8, 14) [n=40] |
| Female/Male risk ratio | 1.44 (0.74, 2.13 ) | 2.39 (1.26, 3.52) | 2.15 (1.13, 3.17) | 2.00 (1.05, 2.95) | 0.79 (0.47. 1.10) | |||||
Incidence rates (95% CI) are per 100,000 and adjusted by age and race using standardization to the 2010 United States population. The unweighted number of strokes are shown in square brackets as [n=].
Adjusted p-value< 0.05 for comparison between 1993/94 and 2015 using Bonferroni correction
Note: Incidence rates and the unweighted numbers shown are type specific, and “All” includes any type of stroke; Ischemic, ICH, SAH or Unknown. The rate for “All Strokes” was estimated using only those stroke patients with no prior stroke of any type, whereas rates by stroke subtype are estimated using only individuals who have not had a previous stroke of that specific stroke subtype.
Sex-specific stroke incidence rates, stratified by study period within each of the four age categories, are presented in Table 3, Figure 1, and Figure 2. In women 20 to 44 years old, incidence remained relatively stable from 1993/4 to 2015 (20 to 26 per 100,000, p>0.05), while incidence in men increased from 15 to 31 per 100,000 (p<0.05). For the 45-64 age group, rates were stable between 1993/4 and 2015 in both women and men. Among individuals 65-84 years old, incidence decreased between first and last study periods in both male (810 (95%CI 737-883) to 546 (95%CI 493-600)) and female (713 (95%CI 658-769) to 482 (95%CI 437-527)) participants and appeared to drive the majority of the decrease in strokes overall. Finally, among individuals at least 85 years of age, incidence per 100,000 decreased significantly in men ((1858 (95%CI 1458-2258) to 1146 (95%CI 920-1372)), p<0.05). In women at least 85 years old, though, the decrease was not statistically significant ((1619 (95%CI 1406-1831) to 1406 (95%CI 1230-1583)), p>0.05).
Table 3:
Incidence Rates for All Strokes, per 100,000, by Sex and Age Group and by Time Period
| N | Overall Incidence (95% CI) | N | Incidence in Females (95% CI) | N | Incidence in Males (95% CI) | Rate ratio Females/Males (95% CI) | |
|---|---|---|---|---|---|---|---|
| 20-44 years old | |||||||
| 1993/4 | 87 | 17* (14, 21) | 52 | 20 (14, 25) | 35 | 15* (10, 20) | 1.32 (0.75, 1.89) |
| 1999 | 111 | 22 (18, 27) | 61 | 24 (18, 30) | 50 | 21 (15, 27) | 1.12 (0.70, 1.54) |
| 2005 | 140 | 31 (26, 36) | 81 | 35 (27, 42) | 59 | 27 (20, 33) | 1.30 (0.87, 1.74) |
| 2010 | 119 | 26 (21, 31) | 65 | 27 (21, 34) | 54 | 25 (18, 31) | 1.11 (0.71, 1.51) |
| 2015 | 132 | 28 (23, 33) | 62 | 26 (19, 32) | 70 | 31 (24, 38) | 0.82 0.54, 1.10) |
| 45-64 years old | |||||||
| 1993/4 | 412 | 165 (149, 181) | 192 | 144 (123, 164) | 220 | 187 (162, 211) | 0.77 (0.62, 0.92) |
| 1999 | 456 | 161 (146, 175) | 218 | 144 (125, 164) | 238 | 177 (154, 199) | 0.82 (0.67, 0.97) |
| 2005 | 570 | 169 (155, 182) | 256 | 144 (126, 161) | 314 | 195 (173, 217) | 0.74 (0.61, 0.86) |
| 2010 | 657 | 175 (161, 188) | 316 | 161 (143, 179) | 341 | 189 (1769, 209) | 0.85 (0.72, 0.98) |
| 2015 | 652 | 171 (158, 184) | 284 | 142 (125, 158) | 368 | 201 (181, 222) | 0.70 (0.66, 0.81) |
| 65-84 years old | |||||||
| 1993/4 | 1111 | 757* (712, 802) | 637 | 713* (658, 769) | 474 | 810* (737, 883) | 0.88 (0.78, 0.99) |
| 1999 | 1082 | 751 (706, 796) | 622 | 723 (666, 780) | 460 | 784 (713, 856) | 0.92 (0.81, 1.03) |
| 2005 | 878 | 618 (577, 659) | 499 | 592 (540, 645) | 379 | 647 (582, 712) | 0.92 (0.79, 1.04) |
| 2010 | 813 | 553 (515, 591) | 462 | 553 (502, 604) | 351 | 554 (496, 612) | 1.00 (0.86, 1.14) |
| 2015 | 861 | 510 (476, 545) | 453 | 482 (437, 527) | 408 | 546 (493, 600) | 0.88 (0.76, 1.00) |
| ≥ 85 years old | |||||||
| 1993/4 | 308 | 1696* (1502, 1890) | 224 | 1619 (1406, 1831) | 84 | 1858* (1458, 2258) | 0.87 (0.65, 1.09) |
| 1999 | 356 | 1751 (1566, 1937) | 270 | 1785 (1571, 1999) | 86 | 1677 (1321, 2033) | 1.06 (0.80, 1.32) |
| 2005 | 293 | 1258 (1112, 1403) | 219 | 1317 (1141, 1492) | 74 | 1134 (874, 1394) | 1.16 (0.85, 1.47) |
| 2010 | 348 | 1446 (1293, 1599) | 256 | 1519 (1332, 1706) | 92 | 1287 (1024, 1551) | 1.18 (0.90, 1.46) |
| 2015 | 347 | 1322 (1182, 1462) | 248 | 1406 (1230, 1583) | 99 | 1146 (920, 1372) | 1.23 (0.94, 1.51) |
Rates include inpatient and out-of-hospital ascertained and are standardized to the 2010 United States population, adjusted by sex, race and age (for overall incidence rates), or race and age (for sex-specific rates). Columns with the heading N represent unweighted frequency of stroke events.
P<0.05 for comparison between first and last study periods.
Figure 1:
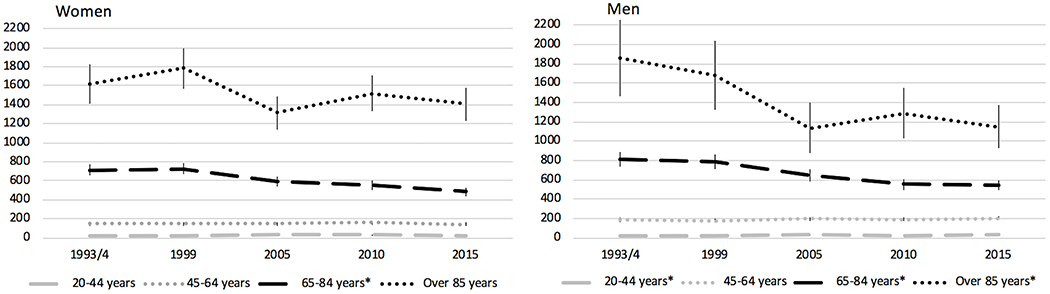
Incidence Rates of All Strokes per 100,000 by Sex and Age Group over Time
Incidence rates are per 100,000 with 95% CI and have been adjusted for age and race, standardized to 2010 U.S. Census population
*p<0.05 for change in incidence between 1993/4 and 2015
Figure 2:
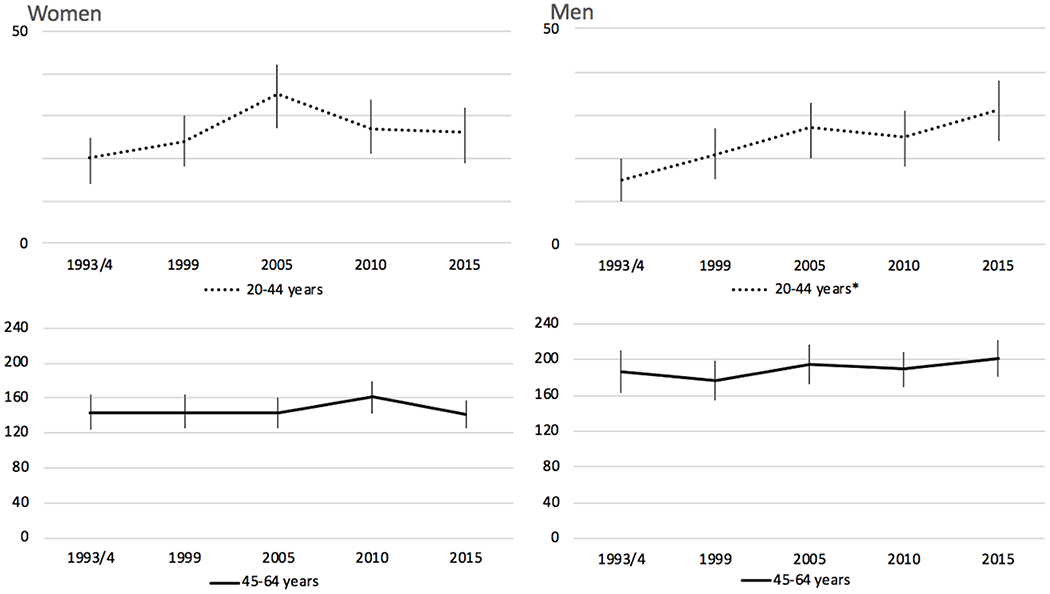
Incidence Rates of All Strokes per 100,000 by Sex in 20-44 and 45-64 Years Categories
Incidence rates are per 100,000 with 95% CI and have been adjusted for age and race, standardized to 2010 U.S. Census population
*p<0.05 for change in incidence between 1993/4 and 2015.
Age, race, and sex adjusted case-fatality rates (for the overall study population), and age and race adjusted (stratified by sex) rates are reported in Table 4 for all five study periods. Case fatality rates for all strokes were similar by sex in the first four study periods (p>0.05); in 2015, case fatality for women was 12.8% (95% CI 10.5, 15.6) compared with 9.2% (95%CI 7.3, 11.6) for men (p<0.05). A change in case fatality by sex over time was corroborated by a sex by year product term (p<0.05). Sex differences in case fatality rates for ICH did not reach statistical significance. Case fatality rates for SAH appear to be decreasing for both sexes between 1993/4 and 2015 (39.6% to 15.4% for women, 32.3% to 16.0% for men, respectively).
Table 4:
GCNKSS Thirty-Day Case-Fatality Rates, adjusted for age and race (95% CI) after Incident Stroke, by Sex and Stroke Subtype
| Year | 1993/94 | 1999 | 2005 | 2010 | 2015 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Females N (%) [95% CI] |
Males N (%) [95% CI] |
Females N (%) [95% CI] |
Males N (%) [95% CI] |
Females N (%) [95% CI] |
Males N (%) [95% CI] |
Females N (%) [95% CI] |
Males N (%) [95% CI] |
Females N (%) [95% CI] |
Males N (%) [95% CI] |
| All strokes* | 181 (13.1) [10.8,15.8] | 119 (12.7) [10.2,15.7] | 202 (12.5) [10.4,15.0] | 136 (14.7) [12.0,18.0] | 179 (13.0) [10.8,15.6] | 124 (13.0) [10.6,16.0] | 193 (13.3) [11.1,16.0] | 108 (12.3) [10.0,15.2] | 181 (12.8)** [10.5,15.6] | 95 (9.2) [7.3, 11.6] |
| Ischemic | 128 (8.3) [6.4, 10.8] | 85 (9.0) [6.8, 10.8] | 132 (9.0) [7.1, 11.5] | 88 (9.0) [6.9, 11.8] | 117 (8.7) [6.8, 11.2] | 73 (8.1) [6.2, 10.7] | 123 (8.6) [6.7, 11.0] | 66 (7.9) [6.0, 10.6] | 115 (8.6) [6.6, 11.2] | 53 (5.6) [4.1, 7.6] |
| ICH | 51 (34.3) [24.8,47.5] | 38 (35.1) [24.8,49.6] | 70 (34.4) [26.0,45.6] | 53 (42.1) [31.1,56.9] | 76 (38.8) [29.1,51.6] | 62 (40.7) [30.0,55.3] | 69 (37.1) [27.7,49.7] | 53 (36.7) [27.7,49.6) | 88 (37.1) [28.5,48.2] | 47 (29.1) [21.1,40.0] |
| SAH | 25 (39.6) [24.4,64.2] | 9 (32.3) [15.4,67.6] | 24 (32.4) [20.0,52.5] | 7 (29.3) [13.0,64.5] | 20 (26.2) [15.8,43.7] | 5 (18.1) [7.1,46.4] | 17 (23.7) (13.1,42.7] | 4 (15.6) [5.5,44.6] | 8 (15.4) [7.2,33.0] | 9 (16.0) [6.9, 39.7] |
All strokes: sex by year product term p=0.04, adjusting for race and age
P-values for sex difference < 0.05
DISCUSSION
In summary, the incidence of both all strokes and IS among women and men decreased between 1993/4 and 2015 in the GCNKSS, a nationally representative population-based study. This contrasts with previously published GCNKSS data through 2010 that demonstrated a significant decrease over time among men but not women.9 Similar to our previous findings, incidence rates of ICH and SAH did not show evidence of change over the study period, though these data may be limited by power. In the current study, we also report statistically significant sex differences in stroke incidence over time within specific age categories, particularly in the youngest and oldest age groups, a contribution to the current knowledge gap in stroke epidemiology.
Our finding that, in the total study population, both all strokes and IS decreased over time in both sexes is consistent with recent data from other epidemiologic studies such as ARIC.11 In contrast to the ARIC data that only included adults > 65 years old,11 though, our study included adults 20 years of age and older, adding important data to the current literature regarding recent changes in stroke incidence across the lifespan. Data from other epidemiologic studies, including Framingham, REGARDS, and Oxford Vascular data, have not reported stroke incidence trends over time past 2004.20,21
The lack of a statistically significant change in ICH or SAH over time is notable but must be interpreted with caution in the setting of small sample sizes. Possible explanations for stable ICH over time despite better control of risk factors like hypertension include higher rates of ICH related to anticoagulation for atrial fibrillation22 or increasing statin use. Further population-based investigations of temporal trends in risk factor prevalence and severity (including hypertension) by stroke subtype are needed to better understand these differences by stroke subtype.
Our findings of a decrease in stroke incidence among women between 1993/4 and 2015, but not between 1993/4 and 2010,9 demonstrate a potential lag in the decrease of stroke incidence in women. The earlier decline in men vs. women could be due to unexplained variability but could also be related to an increased awareness of stroke risk in women1 on the part of both patients and physicians, sex differences in the way stroke risk factors are changing over time, or even competing risks. For example, some data indicate that deaths from ischemic heart disease are increasing in women but decreasing in men,23 which has the potential to affect stroke incidence by sex over time. These findings emphasize the need for ongoing surveillance of temporal changes in stroke incidence by sex to continue to characterize these epidemiologic data.
We also demonstrated critical sex differences in case fatality. Specifically, 2015 was the first study period in which overall stroke case fatality rates in women surpassed men, despite adjustment for age. This is a novel finding which requires further investigation to determine contributing factors. Potential factors include the sex difference in stroke risk in the ≥85 group, as this age category that may drive the overall case fatality. Though our case fatality rates are age-adjusted, it is possible that sex differences in survival to age 85 may be contributing to differences in case fatality as well. Another possible contributor is a changing population of stroke cases over time (i.e., women with greater functional impairment at baseline), which is supported by our results showing a decreasing proportion of participants who are functionally independent at baseline. Though only speculative, possible explanations for more patients with functional impairment at baseline across study periods include more patients (especially women) surviving to older ages with greater disability. In addition, it is possible that advances in stroke care have resulted in more elderly patients with baseline functional impairments and stroke symptoms being evaluated in acute settings like the emergency department. A changing epidemiology of stroke in men over time (more strokes in the young, fewer in the old), as demonstrated by our findings in this study, could also be contributing to our findings of sex differences in case fatality over time. We also report a change in case fatality rates for SAH over time for both sexes, which may be related to improved stroke care and/or improved identification methods for SAH,24 though these data are limited by small numbers.
Regarding sex-specific differences in stroke incidence over time stratified by age categories, trends for stroke in the youngest age group are especially concerning. In this group, stroke incidence rates increased over time in men over time but remained stable in women. The lack of decreased stroke incidence in this age group overall is consistent with prior GCNKSS data through 2005, though the current analysis adds to our knowledge by identifying important sex-specific trends.13 Other data on stroke in this age group are significantly limited as they come from administrative databases such as the National Inpatient Sample25,26 or from cohort studies.27,28 One national cohort study from the Netherlands evaluated stroke in the young over time by sex and found an overall increase in incidence over time with higher rates among women than men in the 18 to 44 age group.14 Other data report increased stroke prevalence among women compared with men in the midlife age range, suggesting that more young women than men have had strokes, but our incidence data do not reflect this.29
Though there are relatively fewer strokes in the 20 to 44 age group compared to the older age categories, stroke-related death and disability in this group have the potential to make a disproportionate impact on young adults in terms of disability-adjusted life years and health care costs over the lifetime. Thus, identification of strategies to reduce strokes in this young age group needs to be a priority, and future work should include an increased emphasis on the prevention of and management of both traditional (i.e., hypertension, diabetes) and non-traditional (i.e. substance use, trauma) risk factors in this age group with sex-specific approaches. For example, GCNKSS data through 2005 demonstrated increasing use of illicit substances prior to stroke between 1993 and 2005, with higher rates of use in men, a possible contributing factor to the sex-specific trends in the current study.30
Our finding that stroke rates in the 45-64 age group were stable from 1993/4 to 2015 is consistent data from other epidemiologic studies though 2010.12,31 Efforts should continue to better identify high risk patients (i.e., those with hypertension, diabetes, etc.), which may be feasible given the high prevalence of stroke risk factors in this age group.32
In the oldest age group, the decrease in stroke incidence over time was significant in men but not women. Though contributors to the more accentuated decline in stroke incidence among men in this age group are not clear, our findings emphasize the importance of future research on this topic, especially considering that women comprise the majority of strokes in this oldest age group and are at risk for worse functional outcomes.33 It should be noted that our findings of sex differences in the ≥ 85 age group are potentially limited by a lower life expectancy in men and thus fewer strokes, though adjustment for age may help mitigate this limitation. Regardless, improved prevention strategies (including sex-specific approaches) for women and men in this oldest age group should be considered.
Limitations
A major limitation in our study is the small number of events in some of the combined sex and age subgroups, most notably for those subjects 20-44 years old and for those in the ≥85 age group. Ability to interpret trends in the ICH and SAH rates over time is also limited due to small frequencies. Our findings are also limited by the exclusion of patients with strokes in area nursing homes who do not present to a hospital. Though not the primary objective of this study, another limitation of our analysis is the lack of ability to further stratify by race due to very small numbers in age, race, and sex subgroups. Our findings of an increasing proportion of black individuals over the five study periods, though, emphasizes the need to investigate trends over time by race in a future paper.
Conclusions
Our analysis using updated data from GCNKSS through 2015 demonstrated a decrease in incidence of all stroke and of IS over time in both women and men. Our age- and sex-stratified analyses demonstrate differences in how stroke risk by age differs by stroke subtype, sex, and over time and points to several future research priorities. Specifically, future research is needed to better understand data on trends in stroke incidence in the youngest and oldest age groups, with particular attention to sex-specific factors.
Supplementary Material
Acknowledgments
FUNDING SOURCES
The GCNKSS was funded by a grant from the National Institutes of Neurological Disorders and Stroke (NINDS) (R01 NS 30678). TEM is funded by the National Heart, Lung, and Blood Institute (K23HL140081).
DISCLOSURES: JCK, KA, CJM, HS, DW, SF, PK, MLF, JM, EM, KW, SJS, BMK, and DOK are supported by a research grant (NINDS R01NS30678). OA reports support from Founder and Equity Holder and Sense Diagnostics, Inc. JM also reports grants from Indiana University (IU) Health/IU School of Medicine, IU Clinical and Translational Sciences Institute, and Patient Centered Outcomes Research Institute outside the submitted work, medicolegal work, and is an NIH Loan Repayment recipient. SLD reports grant support from the University of Cincinnati and personal fees from Genentech. KW is on the speaker’s bureau for Portola Pharmaceuticals. EM reports funding from NINDS (K23NS113858).
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics - 2018 update: A report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Leading Causes of Death in Males, CDC [Internet]. [cited 2017 Feb 4]; Available from: https://www.cdc.gov/men/lcod/2014/index.htm [Google Scholar]
- 3.Leading Causes of Death in Females, CDC. [Internet]. [cited 2017 Feb 4]; Available from: https://www.cdc.gov/women/lcod/ [Google Scholar]
- 4.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koellhoffer EC, McCullough LD. The Effects of Estrogen in Ischemic Stroke. Transl. Stroke Res 2013;4:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard VJ, Madsen TE, Kleindorfer D, Judd SE, Rhodes JD, Soliman EZ, et al. Sex and race differences in incident ischemic stroke and risk factors. Jama Neurol. 2019;76:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J. Cereb. Blood Flow Metab 2012;32:2100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009;40:1082–1090. [DOI] [PubMed] [Google Scholar]
- 9.Madsen TE, Khoury JC, Alwell KA, Moomaw CJ, Rademacher E, Flaherty ML, et al. Sex Specific Stroke Incidence Over Time in the Greater Cincinnati Northern Kentucky Stroke Study. Neurology. 2017;89:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen TE, Khoury JC, Alwell K, Moomaw CJ, Rademacher E, Flaherty ML, et al. Temporal Trends of Sex Differences in Transient Ischemic Attack Incidence Within a Population. J. Stroke Cerebrovasc. Dis 2019;28:28:2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in Stroke Incidence Rates in Older US Adults: An Update From the Atherosclerosis Risk in Communities (ARIC) Cohort Study. JAMA Neurol. 2019;In Press:E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koton S, Schneider ALC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in us communities, 1987 to 2011. JAMA. 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 13.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekker MS, Verhoeven JI, Vaartjes I, Van Nieuwenhuizen KM, Klijn CJM, De Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92:e2444–e2454. [DOI] [PubMed] [Google Scholar]
- 15.Wafa HA, Wolfe CDA, Rudd A, Wang Y. Long-term trends in incidence and risk factors for ischaemic stroke subtypes: Prospective population study of the South London Stroke Register. PLoS Med. 2018;15:e1002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleindorfer D, Khoury J, Alwell K, Moomaw CJ, Woo D, Flaherty ML, et al. The impact of Magnetic Resonance Imaging (MRI) on ischemic stroke detection and incidence: Minimal impact within a population-based study. BMC Neurol. 2015;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke Incidence Is Decreasing in Whites But Not in Blacks. Stroke. 2010;54:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: Preliminary results of a population-based study. Stroke. 2002;33:1190–1195. [DOI] [PubMed] [Google Scholar]
- 19.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 1998;29:415–421. [DOI] [PubMed] [Google Scholar]
- 20.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–46. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 22.Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Semin. Neurol 2010;30:565–572. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update : A report from the American Heart Association. Circulation. 2015;131:e29–e39. [DOI] [PubMed] [Google Scholar]
- 24.Mackey J, Khoury JC, Alwell K, Moomaw CJ, Kissela BM, Flaherty ML, et al. Stable incidence but declining case-fatality rates of subarachnoid hemorrhage in a population. Neurology. 2016;87:2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann. Neurol. 2011;70:713–721. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez L, Kim-Tenser MA, Sanossian N, Cen S, Wen G, He S, et al. Trends in Acute Ischemic Stroke Hospitalizations in the United States. J. Am. Heart Assoc 2016;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutten-Jacobs LA, Arntz R, Maaijwee N, Schoonderwaldt H, Dorresteijn L, Van Dijk E, et al. Long-term Mortality After Stroke. JAMA. 2013;309:1136–1144. [DOI] [PubMed] [Google Scholar]
- 28.Synhaeve NE, Arntz RM, Van Alebeek ME, Van Pamelen J, Maaijwee NAM, Rutten-Jacobs LCA, et al. Women have a poorer very long-term functional outcome after stroke among adults aged 18–50 years: the FUTURE study. J. Neurol 2016;263:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towfighi A, Markovic D, Ovbiagele B. Persistent sex disparity in midlife stroke prevalence in the United States. Cerebrovasc. Dis 2011;31:322–328. [DOI] [PubMed] [Google Scholar]
- 30.De Los Ríos F, Kleindorfer DO, Khoury J, Broderick JP, Moomaw CJ, Adeoye O, et al. Trends in substance abuse preceding stroke among young adults: A population-based study. Stroke. 2012;43:3179–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgenstern LB, Smith MA, Sánchez BN, Brown DL, Zahuranec DB, Garcia N, et al. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann. Neurol 2013;74:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic Stroke and Transient Ischemic Attack in Young Adults. JAMA Neurol. 2013;70:51–57. [DOI] [PubMed] [Google Scholar]
- 33.Gall S, Phan H, Madsen TE, Reeves M, Rist P, Jimenez M, et al. Focused Update of Sex Differences in Patient Reported Outcome Measures After Stroke. Stroke. 2018;49:531–535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


