Abstract
Purpose of review:
In this review article we evaluate sex differences in the natural history of NAFLD and highlight distinct risk profiles of women with NAFLD, as well as unique treatment considerations and research gaps.
Summary of findings:
Reproductive factors, such as menopausal status should be considered when evaluating NAFLD risk in women, as well as additional reproductive risk factors such as age at menarche, presence of polycystic ovary syndrome, and gestational diabetes. Women do appear to have lower risk for hepatocellular carcinoma from NASH, as well as lower mortality from NASH cirrhosis than men, although among women, NASH is now the leading indication for liver transplant. Data on sex differences in biomarker development and clinical trials are lacking, and researchers should be encouraged to evaluate biomarker performance by sex, and specifically report clinical trial endpoints in women.
Keywords: Nonalcoholic fatty liver, hormones, sex differences, women
NAFLD epidemiology:
Nonalcoholic fatty liver (NAFLD) is the most common cause of chronic liver disease in the United States, and now a leading cause of cirrhosis, liver cancer, and need for liver transplantation.1-3 The public health implications of this condition are thus vast. Like other forms of chronic liver disease, there are distinct sex differences in the epidemiology and natural history of NAFLD, as well as unique clinical considerations when caring for the rising number of women with NAFLD.
While the global prevalence of NAFLD is ~ 25%1, most studies report a higher prevalence of imaging-confirmed NAFLD in men.4 Interestingly, there may be a contribution of race/ethnicity to these estimates, as sex differences are most notable among individuals of white race, with more similar NAFLD prevalence reported in men and women of black race and Hispanic ethnicity.5 There is also a growing awareness of the effect of menopausal status on NAFLD prevalence, which is not routinely reported in clinical studies. In one study from Hong Kong the prevalence of NAFLD identified on magnetic resonance spectroscopy (MRS) was 37% in men and 23% in women, though starting at age 50 years, NAFLD prevalence continued to increase in women, in contrast to stable estimates in men.6 When adjusted for age and metabolic risk factors in that study, male sex was not associated with NAFLD. Similarly, in a large study from Asia, the prevalence of ultrasound-diagnosed NAFLD was higher in men when dichotomized by age less than 50 years, however estimates reversed after age 50, with NAFLD detected in 28% of women and 21% of men in the older group (p<0.05).7 These data are consistent with another age-stratified study from China including over 25,000 patients which noted similar NAFLD estimates in men and women over the age of 60 years.8
Data on NAFLD incidence are heterogeneous, though with adjustment for age and metabolic risk factors, most estimates appear similar in men and women.9-12 A recent community-based cohort from the U.S evaluated NAFLD incidence over a 20 year follow-up and found the magnitude and pattern of rise in NAFLD incidence to be similar in men (49/100,000 person years to 317/100,000 person years) and women (72/100,000 person years to 341/100,000 person years).13 Another U.S. study using the Framingham Cohort accounted for menopausal status and found that incident NAFLD was similar in men and post-menopausal women, and lowest among pre-menopausal women.14 These data mirror results from a Japanese study which found incident NAFLD to be twice as high in menopausal as compared to pre-menopausal women.15 Taken together, these data support efforts to consider both chronologic and reproductive aging when evaluating NAFLD risk in women.
NASH and NASH Fibrosis in women:
NAFLD prevalence appears to be overall greater in men, although most studies16-19, though not all20,21, demonstrate an increased association of female sex with biopsy-confirmed NASH, as well as more severe NASH fibrosis in women. In a study from the NASH CRN including 1266 participants, the presence of definitive NASH on liver biopsy was similar (30% men, 33% women) with a slightly higher proportion of women having advanced fibrosis (12% men vs 15% women).22 In a subsequent study from the NASH CRN, the authors adjusted for demographic and metabolic co-factors, and then found female sex to be associated with a nearly two-fold higher odds of having NASH, as well as more than 1.5-fold higher odds of advanced fibrosis.17
Liver-related outcomes in NAFLD:
Among individuals with advanced disease, there are limited data describing the natural history of liver-related events by sex. In a large multi-national study of patients with at least bridging NASH fibrosis, female sex was associated with lower risk of death or need for transplant, after adjusting for metabolic cofactors and markers of liver disease severity.23 Consistent with general data on hepatocellular carcinoma24 there is also a lower risk of hepatocellular carcinoma in women than men with advanced NASH fibrosis.23,25 Interestingly, a study from Japan found that male patients developed HCC from NASH at less advanced stage of fibrosis than female patients, and the prevalence of cirrhosis in patients with HCC was significantly lower in men than women (39% vs 70%).26 Survival in patients with HCC is greater in women and men27, and data from NAFLD-HCC suggests that women are more likely to undergo screening and present with smaller tumors.28 While overall mortality and HCC risk is lower in women with NAFLD, there remains a high risk for liver-related complications in women, including need for liver transplant. A recent U.S. study using the United Network of Organ Sharing (UNOS) database found NASH to now be the leading indication for liver transplantation among women, and second leading indication in men.3 This study also found a marked rise in female registrants with NASH-HCC on the waitlist from 2004 to 2016.3 The development of de novo or recurrent NAFLD after liver transplant is also a growing problem, which is closely tied to post transplant metabolic syndrome. Interestingly, recurrent NAFLD after transplant appears to disproportionately affect women29, which may have long-term implications for patient and graft survival.29,30
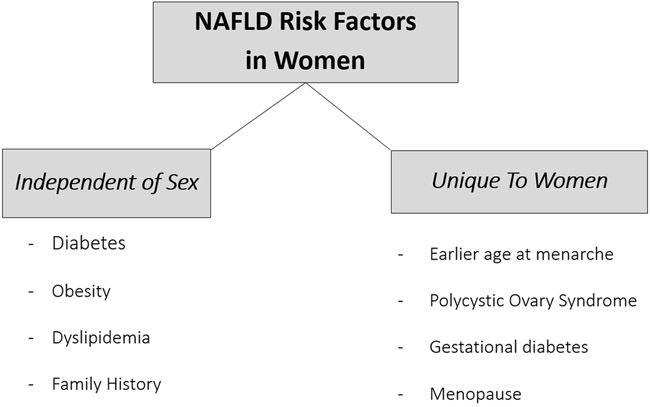
Risk factors for NAFLD/NASH in women (Figure 1):
Figure 1.
NAFLD risk factors in women
Menopausal status
While older age is an established risk factor for NAFLD in men and women, menopausal status does appear to promote NAFLD, independent of chronologic aging.31-33 The menopausal transition is associated with decreased energy expenditure and increase in visceral adiposity34- the latter being a key risk factor for insulin resistance and NAFLD. Even in younger women, earlier onset of menopause confers a higher risk NAFLD. In a large case control study from Europe, oophorectomy prior to age 50 years was associated with a 50% increased risk of NAFLD.35 A study including 488 postmenopausal women with NAFLD also found that premature menopause, and time since menopause, were associated with risk for histologically-confirmed NASH, independent of age, race, and metabolic co-factors.36
Beyond the protective metabolic effects of estrogen, there may also be more direct antifibrotic effects of estrogen. Hepatic stellate cells (HSC) are the primary cells involved in hepatic fibrogenesis, and incubation of HSCs from rats with estradiol has been shown to reduce collagen production, a key component of scar tissue.37 Oopherectomy in that study resulted in worsening fibrosis in animal models, which subsequently improved upon estrogen replacement.37 In another animal study of HSCs, estrogen replacement resulted in reduced reactive oxygen species, decreased transforming growth factor-beta1 (TGF-beta1) expression, and decreased activation of mitogen activated protein kinase (MAPK) pathways, features of hepatic inflammation and fibrosis.38 These mechanistic findings are supported by clinical studies, as earlier age at menopause, as well as longer duration of estrogen deficiency are associated with more severe liver fibrosis in women.36 Likewise in one study that did show a higher prevalence of hepatic fibrosis in men with NASH, findings dissipated when fibrosis severity was compared between men and post-menopausal women.39
Body composition
The primary drivers of NAFLD in men and women include components of the metabolic syndrome, such as insulin resistance/diabetes, dyslipidemia, and obesity, although the relative contribution of fat distribution in particular may differ by sex. Consistent with the increased risk of NAFLD after menopause, the risk of NASH fibrosis associated with differential fat distribution in men, pre-, and post-menopausal women have been observed. Data from the NASH CRN found that after adjusting for energy balance, post-menopausal women who preferentially stored fat in peripheral adipose depots, with smaller abdominal girths, were less likely to have severe hepatic fibrosis, similar to findings in men. In contrast, among premenopausal women, larger peripheral fat stores over increased abdominal girth was associated with fibrosis severity.40 In a more recent study evaluating body composition and NAFLD risk, imaging quantified fat and muscle stores were compared between men and women. Central adiposity was associated with prevalent NAFLD in men and women, although only in men was central adiposity associated with noninvasive measurements of hepatic fibrosis.41 A recent study in Chinese patients evaluated skeletal muscle to visceral fat ratios (SVR) and found that after adjusting for metabolic comorbidities, lower SVR was only associated with NAFLD risk in women, which the authors postulated could relate to greater functional impairment in women, leading to NAFLD as a manifestation of sarcopenic obesity.42 Thus, differential body compensation may contribute to risk for hepatic steatosis and fibrosis in men and women.
Age at menarche
Over the past 30 years there has been a decreasing median age at menarche, which is closely tied to increasing rates of obesity in children and adolescents.43 The exact mechanisms responsible for this phenomenon have not been fully elucidated although longitudinal data support the theory that obesity often precedes menarche.44,45 Leptin, a key cell signaling hormone involved in regulation of appetite, is also higher in obese girls, and a threshold level appears necessary for onset of regular menses.43,46 Data linking obesity and menarche are consistent with findings from studies in NAFLD, in which earlier age at menarche is associated with increased risk of NAFLD in adult women.47-49 Importantly, weight gain and/or BMI have been identified as important mediators of this relationship, supporting at least a partially causative role of obesity in the pathway from earlier onset menarche and NAFLD in women.
Polycystic ovary syndrome (PCOS)
PCOS is the most common endocrinopathy in pre-menopausal women and is marked by irregular menses and the majority of these women also have elevated androgens. PCOS is a recognized at-risk group for NAFLD,50,51 with 40-55% of women with PCOS having imaging-confirmed disease.52 Recent data in reproductive-aged women biopsy confirmed NAFLD also show an increased risk of NASH fibrosis in women with PCOS, independent of age and BMI.53 The risk of NAFLD/NASH in PCOS likely relates to their high prevalence of metabolic comorbidities. Moreover, higher testosterone levels are also associated with prevalent NAFLD in women, independent of comprehensive metabolic co-factors.54 A case control study of women with hyperandrogenic PCOS and the less common PCOS phenotype without high androgens found hyperandrogenic PCOS to be associated with MR-quantified hepatic steatosis, independent of visceral adiposity and insulin resistance.55 Thus, PCOS does appear to increase risk for both NAFLD, as well as more histologically significant disease in these young women. Screening recommendations for NAFLD in PCOS have not yet been established, though it is reasonable to consider screening liver tests in women with PCOS, and consideration of ultrasound assessment for NAFLD in those with concomitant high-risk metabolic features, such as type 2 diabetes.
Gestational diabetes (GDM)
There has been a rise in the prevalence of gestational diabetes in U.S. women over the past thirty years56, thought to relate to the growing number of pregnancies in overweight and obese women.56 Data from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort have shown GDM to be associated with risk of prevalent NAFLD in women during midlife, independent of other metabolic covariates.57 Interestingly, duration of breastfeeding has been shown to lower the risk of developing type 2 diabetes in women with GDM.58 Likewise, another study from the CARDIA cohort also found that longer duration of breastfeeding was protective against prevalent NAFLD in women during midlife.59 Thus, while GDM appears to increase NAFLD risk, breastfeeding may offer a unique modifiable intervention for young women at risk for NAFLD.
Serum biomarkers of NAFLD/NASH:
While liver biopsy is considered the most definitive test for identifying NASH, efforts to avoid invasive testing have resulted in emerging biomarkers with reasonable accuracy for detecting NAFLD and associated fibrosis.60 However, there is variability regarding inclusion of sex as a component of various scoring systems. For the diagnosis of NAFLD, the Hepatic Steatosis index61 and SteatoTest (proprietary)62 have area under the received operator curves (AUROCs) of ~ 0.79-0.81 and either include sex61 or provide sex adjusted risk.62 The Fatty Liver Index63, NAFLD liver fat score64, and NAFLD ridge score65 (AUROCs of 0.84-0.87) do not incorporate sex as a component of their algorithms. NASH biomarkers have had less success, with markers of apoptosis, adipokines, and hepatic inflammation having limited accuracy and/or lack of validation for diagnostic purposes, and have not been separately evaluated in men and women.60 One scoring system, called the NASH Diagnostics Panel (AUROC of 0.81) does include sex as a component although was primarily developed in bariatric populations and warrants validation in non-bariatric populations.66 Markers of hepatic fibrosis are more commonly used in clinical practice and include measures such as APRI, Fibrosis-4 index, NAFLD fibrosis score, AST:ALT ratio, and BARD.60 These tests have AUROCS ranging from 0.6-0.82 to detect advanced fibrosis, and include components that are readily available from clinical data, although none include sex. Whether biomarkers of NAFLD, NASH, or fibrosis perform differently in men and women has not been evaluated, and represents an important area of research. As biomarker development remains an active area of NAFLD research, investigators should be encouraged to evaluate and report performance characteristics by sex.
A few studies have specifically evaluated NAFLD biomarkers within female cohorts. In a cross-sectional study from China, a iron:ferritin ratio was evaluated as measure of dietary patterns in women, given its prior association with central adiposity. This ratio was used to reflect higher meat and fruit consumption, and lower refined carbohydrates. A higher iron:ferritin ratio was predictive of more muscle mass, less visceral adiposity, and severe hepatic steatosis in younger, but not in middle-aged women, which the authors postulated could relate to increased central obesity with aging and its associated alteration of iron levels.67 Another recent study evaluated the association of two protein as biomarkers of NAFLD - the retinol binding protein 4 (RBP4) which has been studied as a biomarker of obesity and insulin resistance as well as galectin-3 binding protein (LGALS3BP) which has been implicated in systemic inflammation and immune response. Both proteins were found to be upregulated in post-menopausal women with NAFLD as compared to those without NAFLD, suggesting their potential role as a NAFLD biomarker within postmenopausal populations.68 Another study evaluated the performance of alpha-ketoglutarate, a metabolite involved in regulating energy homeostasis, to predict NAFLD in morbidly obese women. While this marker could not distinguish between simple steatosis and NASH, it had an AUROC for detecting steatosis of 0.76 in morbidly obese women, which improved to an AUROC of 0.89 with additional inclusion of AST and ALT.69
Unique treatment considerations:
Given their distinct hormonal profiles, drugs targeting sex hormones could potentially offer unique treatment targets in women in NAFLD. For example, data supporting the benefits of estrogen on metabolic health and liver fibrosis do raise the question of estrogen modulation as a novel treatment modality for NAFLD in women. A randomized controlled trial of HRT in women with diabetes and presumed NAFLD showed improvement in elevated liver enzymes among HRT users, supporting the potential protective effects of exogenous estrogen on hepatic inflammation.70 The past 40 years have been marked by controversy regarding HRT safety in post-menopausal women.71 A sentinel publication from the Women’s Health Initiative included primarily women over age 60 years and reported increased risk of coronary artery disease and breast cancer risk in HRT users.72 However, additional studies as well as subsequent age-stratified analyses from the WHI have not identified such increased risk in the younger age group between 50-59 years, and even found potential protective effects of HRT in this age strata on risk of heart disease and mortality.71,73,74 Thus, recommendations for HRT for treatment of menopausal symptoms now embrace a more individualized approach that considers each woman’s risk-benefit ratio.71 With a growing number of women again using these agents, there may be an opportunity to further evaluate the potential benefits of estrogen replacement on liver health. Whether shorter duration of HRT may offer more sustained effects on NAFLD in post-menopausal women also warrants exploration.
Testosterone appears to have a sexually dimorphic association with NAFLD in men in women, with higher levels increasing NAFLD risk in women while testosterone deficiency appears to be associated with risk of NAFLD75 and histologically-confirmed NASH in men.76 Among women, the association of testosterone with NAFLD is seen across baseline levels, including in women without “excess” or high androgen levels.77 Visceral adiposity, and to a lesser degree, hypertriglyceridemia, were found to be significant mediators of the association between testosterone and NAFLD, suggesting a particularly important role of VAT in this relationship. These findings are consistent with data from the Study of Women's Health Across the Nation (SWAN) cohort showing bioavailable testosterone to increase risk for visceral adiposity in women across the menopausal transition, and thus its important role in regional fat distribution.78 Likewise, a randomized controlled trial in postmenopausal women without known liver disease found a significant increase in VAT among exogenous testosterone users.79 Thus targeting testosterone could potentially provide another novel hormonal target for NAFLD in women. Spironolactone, a commonly used diuretic, is also a competitive antagonist of testosterone receptors and used to treat symptoms of high testosterone such as hirsutism in women.80 In mouse models of NAFLD, spironolactone improves hepatic triglycerides, serum lipid levels, as well as NASH histology.81 Spironolactone also induces hepatocyte expression of lipogenic enzymes, supporting a potential direct role of testosterone receptors within hepatocytes on worsening lipid metabolism.81 In a study of women with PCOS without known NAFLD, 12 months of spironolactone improved insulin resistance and serum lipid profiles.82Given its specific anti-testosterone effects, spironolactone is being studied as a potential therapeutic option for women with NASH.83
In existing NAFLD clinical trials, differential treatment response by sex remains largely unexplored, as few studies have evaluated differences in study outcomes in women and men. In a randomized controlled trial of vitamin D supplementation for NAFLD, vitamin D was only found to lower malondialdehyde, a serum marker of oxidative stress, but not alter cholesterol measures or other inflammatory markers.84 In a post-hoc analysis the authors then evaluated sex differences in study endpoints and found that vitamin D lowered C-reactive protein as well as low density lipoprotein in women with NAFLD, but not in men, supporting additional beneficial effects of vitamin D in women with NAFLD that would not have been identified without specific assessment by sex.85 Within the cardiovascular and diabetes literature there are well described sex differences in response to drug therapy, as well as differences in target drug doses and side effect profile. These findings span a range of pharmacotherapies including medications for dyslipidemia, obesity, and glucose control.86,87 Such differences may relate to distinct drug absorption, distribution, and drug metabolism in women. Given overlapping pathophysiology of cardio-metabolic treatments and NAFLD, it stands to reason that differential response to NAFLD therapies may too be observed, and clinical trials should be encouraged to evaluate and report sex-specific outcomes.
Lifestyle interventions, including dietary modification and exercise, remain the cornerstone of NAFLD treatment, and such interventions may differ by sex. A sentinel study by Vilar-Gomez and colleagues evaluated change in NASH histology on paired biopsies in response to weight loss following a 12-month lifestyle intervention. Among individuals losing 7-10% of body weight, female sex was associated with lower probability of NASH improvement or resolution, although most patients, independent of sex achieved these endpoints with at least 10% total body weight loss.88 These data suggest potential need for greater weight loss targets in women, although how results may differ by menopausal status is not known. Regarding diet, there has been debate regarding the direct effects of fructose on NAFLD risk, though diets high in fructose do contribute to excess calories. In a small study of healthy young men and women with short term high fructose intake, endogenous glucose production, alanine aminotransferase levels and fasting insulin increased in men, but not significantly altered in female participants.89 Several large, population-based studies have also reported differential dietary patterns in men and women, with greater fruit and vegetable consumption, lower meat consumption, though more added sugars in diets of women.90,91 A study specifically evaluating the association of such dietary patterns with NAFLD risk identified an increased risk with greater soda and meat consumption, regardless of sex.92
Conclusions:
In conclusion, there are distinct sex differences in the epidemiology and natural history of NAFLD. While men overall have a higher prevalence of NAFLD, when considering menopausal status, NAFLD prevalence appears more similar between men and post-menopausal women. Many studies also note higher risk for NASH and advanced fibrosis in women. Among women, NAFLD is now the leading indication for liver transplant. The management of women with NAFLD should also consider their unique risk profiles, including hormonal milieu and reproductive factors. Data on biomarker performance by sex are needed, such that prediction models specific evaluate performance in women. Moreover, clinical trials should be encouraged to evaluate and report study endpoints in female participants, and also consider therapeutic targets that uniquely contribute to NAFLD progression in women.
Table 1:
Unique Considerations in Women with NAFLD
| NAFLD Characteristic |
Women vs Men | Comments |
|---|---|---|
| NAFLD prevalence | Overall M > W | - Lowest prevalence in pre-menopausal women - Prevalence similar in men and post-menopausal |
| NASH and Fibrosis | W > M | Greater risk of NASH and advanced fibrosis observed in most studies |
| Biomarker performance | ??? |
RESEARCH NEEDS: - Evaluation of biomarker performance and development in women |
| Liver-related complications | Mortality: M>W HCC: M>W LT for NASH: M>W |
- Among women, NAFLD is now the #1 indication for liver transplant - Rising number of women being listed for transplant for HCC due to NASH |
| Response to NAFLD treatment | ??? |
RESEARCH NEEDS: - Whether differential response to NAFLD treatment by sex - Considering unique hormonal targets for NAFLD in women (i.e. estrogen replacement, anti-androgen therapy) |
Footnotes
Conflict of Interest
Liyun Yuan and Ani Kardashian each declare no potential conflicts of interest.
Monika Sarkar is a site PI for a pharmaceutical sponsored drug trial in women with NAFLD and PCOS for Zydus Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES:
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Noureddin M, Vipani A, Bresee C, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. The American journal of gastroenterology. 2018;113(11):1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonardo A, Nascimbeni F, Ballestri S, et al. Sex Differences in NAFLD: State of the Art and Identification of Research Gaps. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–415. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YJ, Li YY, Nie YQ, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World journal of gastroenterology : WJG. 2007;13(47):6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Xu M, Hu Z, Hultstrom M, Lai E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur J Gastroenterol Hepatol. 2014;26(9):1015–1021. [DOI] [PubMed] [Google Scholar]
- 9.Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. Journal of hepatology. 2012;56(5):1145–1151. [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Wong GL, Yeung DK, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. Journal of hepatology. 2015;62(1):182–189. [DOI] [PubMed] [Google Scholar]
- 11.Sung KC, Kim BS, Cho YK, et al. Predicting incident fatty liver using simple cardio-metabolic risk factors at baseline. BMC gastroenterology. 2012;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. The American journal of gastroenterology. 2013;108(8):1299–1304. [DOI] [PubMed] [Google Scholar]
- 13.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67(5):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long MT, Pedley A, Massaro JM, et al. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: The Framingham Heart Study. Liver international : official journal of the International Association for the Study of the Liver. 2018;38(8):1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World journal of gastroenterology : WJG. 2012;18(3):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapper EB, Krajewski K, Lai M, et al. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol Rep (Oxf). 2014;2(4):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55(3):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turola E, Petta S, Vanni E, et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis Model Mech. 2015;8(9):1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh DK, Sakhuja P, Malhotra V, Gondal R, Sarin SK. Independent predictors of steatohepatitis and fibrosis in Asian Indian patients with non-alcoholic steatohepatitis. Digestive diseases and sciences. 2008;53(7):1967–1976. [DOI] [PubMed] [Google Scholar]
- 20.Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(11):1224–1229, 1229 e1221–1222. [DOI] [PubMed] [Google Scholar]
- 21.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. Journal of hepatology. 2009;51(2):371–379. [DOI] [PubMed] [Google Scholar]
- 22.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52(3):913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155(2):443–457 e417. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. [DOI] [PubMed] [Google Scholar]
- 25.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. [DOI] [PubMed] [Google Scholar]
- 26.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(5):428–433; quiz e450. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Hanna DL, Usher J, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2014;120(23):3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan P, Mara K, Izzy M, et al. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation. 2019;103(1):e14–e21. [DOI] [PubMed] [Google Scholar]
- 30.Gitto S, de Maria N, di Benedetto F, et al. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. 2018;30(7):766–773. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Jeon WK, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. Journal of gastroenterology and hepatology. 2006;21(1 Pt 1):138–143. [DOI] [PubMed] [Google Scholar]
- 32.Florentino GS, Cotrim HP, Vilar CP, Florentino AV, Guimaraes GM, Barreto VS. Nonalcoholic fatty liver disease in menopausal women. Arq Gastroenterol. 2013;50(3):180–185. [DOI] [PubMed] [Google Scholar]
- 33.Volzke H, Schwarz S, Baumeister SE, et al. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56(4):594–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florio AA, Graubard BI, Yang B, et al. Oophorectomy and risk of non-alcoholic fatty liver disease and primary liver cancer in the Clinical Practice Research Datalink. Eur J Epidemiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klair JS, Yang JD, Abdelmalek MF, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29(3):719–727 [DOI] [PubMed] [Google Scholar]
- 38.Itagaki T, Shimizu I, Cheng X, et al. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54(12):1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki A, Abdelmalek MF, Unalp-Arida A, et al. Regional anthropometric measures and hepatic fibrosis in patients with nonalcoholic Fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(12):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen VL, Wright AP, Halligan B, et al. Body Composition and Genetic Lipodystrophy Risk Score Associate With Nonalcoholic Fatty Liver Disease and Liver Fibrosis. Hepatol Commun. 2019;3(8):1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su X, Xu J, Zheng C. The relationship between non-alcoholic fatty liver and skeletal muscle mass to visceral fat area ratio in women with type 2 diabetes. BMC Endocr Disord. 2019;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121 Suppl 3:S208–217. [DOI] [PubMed] [Google Scholar]
- 44.Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC. Weight status in young girls and the onset of puberty. Pediatrics. 2007;119(3):e624–630. [DOI] [PubMed] [Google Scholar]
- 45.Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics. 2003;111(4 Pt 1):815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. The Journal of clinical endocrinology and metabolism. 1997;82(9):2849–2855. [DOI] [PubMed] [Google Scholar]
- 47.Ryu S, Chang Y, Choi Y, et al. Age at menarche and non-alcoholic fatty liver disease. Journal of hepatology. 2015;62(5):1164–1170. [DOI] [PubMed] [Google Scholar]
- 48.Yi KH, Hwang JS, Lim SW, Lee JA, Kim DH, Lim JS. Early menarche is associated with non-alcoholic fatty liver disease in adulthood. Pediatr Int. 2017;59(12):1270–1275. [DOI] [PubMed] [Google Scholar]
- 49.Mueller NT, Pereira MA, Demerath EW, et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: The CARDIA study. Obesity. 2015;23(2):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 51.Rocha ALL, Faria LC, Guimaraes TCM, et al. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta-analysis. J Endocrinol Invest. 2017;40(12): 1279–1288. [DOI] [PubMed] [Google Scholar]
- 52.Cerda C, Perez-Ayuso RM, Riquelme A, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Journal of hepatology. 2007;47(3):412–417. [DOI] [PubMed] [Google Scholar]
- • 53.Sarkar M,Terrault N, Chan W, et al. WOMEN WITH POLYCYSTIC OVARY SYNDROME (PCOS) HAVE MORE ADVANCED NASH FIBROSIS AND EARLIER ONSET DISEASE. American Association for the Study of Liver Diseases; 2019; Boston, MA.Higher levels of testosterone are associated with increased odds of prevalent NAFLD in women during midlife, and may represent a potential novel target for NAFLD therapeutics in women.
- 54.Sarkar M, Wellons M, Cedars MI, et al. Testosterone Levels in Pre-Menopausal Women are Associated With Nonalcoholic Fatty Liver Disease in Midlife. The American journal of gastroenterology. 2017; 112(5):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. The Journal of clinical endocrinology and metabolism. 2012;97(10):3709–3716. [DOI] [PubMed] [Google Scholar]
- 56.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational Diabetes Mellitus Is Strongly Associated With Non-Alcoholic Fatty Liver Disease. The American journal of gastroenterology. 2016;111(5):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunderson EP, Hurston SR, Ning X, et al. Lactation and Progression to Type 2 Diabetes Mellitus After Gestational Diabetes Mellitus: A Prospective Cohort Study. Annals of internal medicine. 2015;163(12):889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 59.Ajmera VH, Terrault NA, VanWagner LB, et al. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. Journal of hepatology. 2019;70(1):126–132.A longer duration of lactation, particularly greater than 6 months, is associated with lower odds of NAFLD in mid-life and may represent a modifiable risk factor for NAFLD
- 60.Wong VW, Adams LA, de Ledinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nature reviews Gastroenterology & hepatology. 2018;15(8):461–478. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508. [DOI] [PubMed] [Google Scholar]
- 62.Poynard T, Ratziu V, Naveau S, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC gastroenterology. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872. [DOI] [PubMed] [Google Scholar]
- 65.Keating SE, Parker HM, Hickman IJ, et al. NAFLD in clinical practice: Can simple blood and anthropometric markers be used to detect change in liver fat measured by (1) H-MRS? Liver international : official journal of the International Association for the Study of the Liver. 2017;37(12):1907–1915. [DOI] [PubMed] [Google Scholar]
- 66.Younossi ZM, Page S, Rafiq N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21(4):431–439. [DOI] [PubMed] [Google Scholar]
- 67.Sabrina N, Bai CH, Chang CC, Chien YW, Chen JR, Chang JS. Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai H, Lu S, Chen Y, et al. Serum retinol binding protein 4 and galectin-3 binding protein as novel markers for postmenopausal nonalcoholic fatty liver disease. Clin Biochem. 2018;56:95–101. [DOI] [PubMed] [Google Scholar]
- 69.Aragones G, Auguet T, Berlanga A, et al. Increased Circulating Levels of Alpha-Ketoglutarate in Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease. PloS one. 2016;11(4):e0154601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clinical endocrinology. 2006;65(1):40–44. [DOI] [PubMed] [Google Scholar]
- 71.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220–231. [DOI] [PubMed] [Google Scholar]
- 72.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 73.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women's Health Initiative. Archives of internal medicine. 2006;166(3):357–365. [DOI] [PubMed] [Google Scholar]
- 74.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. Jama. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Ann Hepatol. 2017;16(3):382–394. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar M, Yates K, Suzuki A, et al. Low Testosterone is Associated with Nonalcoholic Steatohepatitis and Fibrosis Severity in Men with Nonalcoholic Fatty Liver Disease Digestive Diseases Week; 2019; San Diego, California. [Google Scholar]
- 77.Sarkar M, Wellons M, Cedars MI, et al. Testosterone Levels in Pre-Menopausal Women are Associated With Nonalcoholic Fatty Liver Disease in Midlife. The American journal of gastroenterology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity. 2010;18(3):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. The Journal of clinical endocrinology and metabolism. 1996;81(6):2198–2203. [DOI] [PubMed] [Google Scholar]
- 80.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151(5):2040–2049. [DOI] [PubMed] [Google Scholar]
- 82.Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest. 2005;28(1):49–53. [DOI] [PubMed] [Google Scholar]
- 83.ClinicalTrials.gov Identifier: NCT03576755.
- 84.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70–80. [DOI] [PubMed] [Google Scholar]
- 85.Sharifi N, Amani R, Hajiani E, Cheraghian B. Women may respond different from men to vitamin D supplementation regarding cardiometabolic biomarkers. Exp Biol Med (Maywood). 2016;241(8):830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tamargo J, Rosano G, Walther T, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3(3):163–182. [DOI] [PubMed] [Google Scholar]
- 87.Kautzky-Willer A, Harreiter J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res Clin Pract. 2017;131:230–241. [DOI] [PubMed] [Google Scholar]
- 88.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–378 e365; quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 89.Couchepin C, Le KA, Bortolotti M, et al. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes care. 2008;31(6):1254–1256. [DOI] [PubMed] [Google Scholar]
- 90.Shiferaw B, Verrill L, Booth H, et al. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey, 2006–2007. Clin Infect Dis. 2012;54 Suppl 5:S453–457. [DOI] [PubMed] [Google Scholar]
- 91.Vitale M, Masulli M, Cocozza S, et al. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes - The TOSCA.IT study. Nutr Metab Cardiovasc Dis. 2016;26(10):879–885. [DOI] [PubMed] [Google Scholar]
- 92.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. Journal of hepatology. 2007;47(5):711–717. [DOI] [PubMed] [Google Scholar]