Abstract
Adrenoleukodystrophy (ALD) is a rare X-linked disorder of peroxisomal oxidation due to mutations in ABCD1. It is a progressive condition with a variable clinical spectrum that includes primary adrenal insufficiency, myelopathy, and cerebral ALD. Adrenal insufficiency affects over 80% of ALD patients. Cerebral ALD affects one-third of boys under the age of 12 and progresses to total disability and death without treatment. Hematopoietic stem cell transplantation (HSCT) remains the only disease-modifying therapy if completed in the early stages of cerebral ALD, but it does not affect the course of adrenal insufficiency. It has significant associated morbidity and mortality. A recent gene therapy clinical trial for ALD reported short-term MRI and neurological outcomes comparable to historical patients treated with HSCT without the associated adverse side effects. In addition, over a dozen states have started newborn screening (NBS) for ALD, with the number of states expecting to double in 2020. Genetic testing of NBS-positive neonates has identified novel variants of unknown significance, providing further opportunity for genetic characterization but also uncertainty in the monitoring and therapy of subclinical and/or mild adrenal insufficiency or cerebral involvement. As more individuals with ALD are identified at birth, it remains uncertain if availability of matched donors, transplant (and, potentially, gene therapy) centers, and specialists may affect the timely treatment of these individuals. As these promising gene therapy trials and NBS transform the clinical management and outcomes of ALD, there will be an increasing need for the endocrine management of presymptomatic and subclinical adrenal insufficiency. (Endocrine Reviews 41: 1 – 17, 2020)
Keywords: adrenoleukodystrophy, hematopoietic stem cell transplant, newborn screening, gene therapy
Graphical Abstract
Graphical Abstract.
Essentials Points
Adrenoleukodystrophy (ALD) is a rare X-linked disorder of peroxisomal oxidation due to mutations in the ABCD1 gene, and primary adrenal insufficiency is often the initial manifestation.
The clinical spectrum of ALD is variable, and the cerebral variant of ALD, which occurs in one-third of affected boys under the age of 12, leads to disability and death without treatment.
Hematopoietic stem cell transplantation (HSCT) remains the only disease-modifying therapy for ALD, with significant morbidity and mortality.
Gene therapy trials with autologous hematopoietic stem cell transplant have shown short-term central nervous system disease stabilization in ALD without the morbidity and mortality of HSCT.
Newborn screening for ALD started in New York State in 2014 and is expected to expand to more than 20 states by the year 2020.
Further advances in prenatal genetic diagnosis and other forms of gene therapy have the potential to modify the clinical course of ALD.
Further experience will inform guidelines for endocrinologists to manage ALD patients with presymptomatic adrenal insufficiency.
Adrenoleukodystrophy (ALD) is a rare X-linked peroxisomal disorder with multiple distinct and often progressive manifestations that cannot be predicted at birth. The disorder is caused by mutations in the ABCD1 gene, which lead to high levels of very long-chain fatty acids (VLCFA) in the plasma that accumulate in the white matter of the brain, spinal cord, and adrenal cortex (1–3). Clinically, boys and men with ALD present with notable variation in outcomes, ranging from isolated adrenal insufficiency to rapidly evolving and fatal neurological dysfunction and chronic disability in patients reaching adulthood (4). To date, over 800 unique disease-causing mutations have been catalogued with no correlation to phenotypes (4, 5). Despite advancements in understanding the genetics and molecular pathogenesis of the disease, hematopoietic stem cell transplantation (HSCT) remains the only disease-modifying therapy in the early stages of cerebral disease (6). However, HSCT is limited by matched donor availability and associated with significant morbidity and mortality (7–9).
The recent institution of newborn screening (NBS) for ALD now allows for the detection of disease prior to the development of clinical manifestations and offers the opportunity for further genetic characterization and phenotyping (10–12). Gene therapy combined with autologous HSCT is emerging as a promising new treatment for childhood cerebral ALD (CCALD) (13). These advances in ALD screening and therapy are beginning to transform the clinical management and outcomes of ALD, but many uncertainties remain, including clinical monitoring for adrenal insufficiency and brain involvement prior to clinical manifestations, optimal timing of treatment, and clinical care for women with ALD. In this review, we discuss the major milestones in gene therapy and NBS and how these advancements have the potential to transform the endocrinologist’s practice for ALD.
Clinical Features
Adrenal insufficiency
Primary adrenal insufficiency is a major clinical phenotype in ALD, with an estimated lifetime prevalence of over 80% (14, 15) (Table 1). The earliest manifestations appear to be subclinical abnormalities of glucocorticoid secretion that can occur as early as 5 weeks of life (14, 16). Adrenal insufficiency is the initial manifestation of ALD in 38% of cases (15). While the percentage of cases of isolated primary adrenal insufficiency in boys attributable to ALD is not known, ALD has been reported to account for between 4% to 35% of cases of idiopathic primary adrenal insufficiency in which an autoimmune work-up was unrevealing (17–19). Because of this, all boys must be tested for ALD upon diagnosis of adrenal insufficiency if the cause is otherwise not clear, as subsequently discussed.
Table 1.
Clinical features of adrenoleukodystrophy.
| Pre-symptomatic Screening | Presentation | Treatments | |
|---|---|---|---|
| Adrenal insufficiency | • ACTH and cortisol • At diagnosis of ALD • <2 years: 3–4 months • ≥2 years: 4–6 months • PRA and electrolytes • After diagnosis of glucocorticoid deficiency, every 6 months |
• Subclinical abnormalities of glucocorticoid secretion as early as 5 weeks of life • Peak incidence between 3–10 years • Approximately half of patients do not develop mineralocorticoid deficiency • Lifetime prevalence >80% |
• Chronic glucocorticoid replacement therapy • Chronic mineralocorticoid replacement, if needed • Stress-dose steroids for acute physiologic stress • No current curative therapy |
| Myelopathy | • Annual clinical neurologic assessment | • Onset between 20–40 years (median of 28 years) • Peripheral neuropathy is often the first manifestation • Primary manifestation is spinal cord dysfunction • 27–63% develop cerebral involvement, 10–20% associated with rapid neurologic decline • Detected by spinal cord atrophy on T2-weighted MRI |
• Supportive care • Does not appear to be impacted by a history of HSCT for cerebral ALD • No curative therapy |
| Cerebral ALD | • Brain MRI • 12–36 months, annually • 3–10 years, every 6 months • 10–18 years, annually |
• Onset between 4–12 years, with peak age at 7 years • Affects one-third of boys with ALD • First manifestation is asymptomatic lesions on brain MRI followed by learning and behavioral problems • If untreated, universally progressive with rapid neurological decline and total disability by 6 months to 2 years, and death within 5–10 years after diagnosis |
• HSCTPros: • Arrests progression of neurologic disease in early stage • Improved survival outcomes (5-year, 95% transplanted vs. 54% untransplanted) Cons: • Not effective in advanced cerebral ALD • Requires matched stem cell donor • Risk of acute mortality, failure of engraftment, and GVHD • Gene therapy (in clinical trials)Pros: • Arrests progression of neurologic disease • Brain MRI and neurological outcomes in the short term are comparable to HSCT • No risk of GVHD Cons: • Risk of failure of engraftment • Theoretical risk of insertional oncogenesis • Unknown long-term outcomes |
| Women with ALD | • None | • Onset typically 30 years and later • Myelopathy similar to men • Neuropathic pain (generally not present in males) in 20% who are younger than 40 years and 90% older than 60 years • Conventional imaging does not show abnormalities, but spinal cord volume not yet assessed • Milder and slower progression compared to men |
• Supportive care • No approved treatments |
Abbreviations: ALD, adrenoleukodystrophy; PRA, plasma renin activity; HSCT, hematopoietic stem-cell transplant.
The mechanism of adrenal gland injury in ALD is not well understood (6). Very long-chain fatty acids are known to accumulate in the zona fasciculata and reticularis of the adrenal cortex, and the chronic accumulation of VLCFA is thought to lead to cytotoxic effects and ultimately apoptosis with atrophy of the adrenal cortex (1, 20, 21). Clinically, the loss of adrenal function in ALD is gradual, with progressive elevations in adrenocorticotropin (ACTH) prior to the development of an overtly abnormal cortisol response to a cosyntropin stimulation test and endocrine symptoms (6, 14, 16). The risk of developing adrenal insufficiency varies throughout the lifetime, and the peak incidence occurs during the first decade of life between 3 and 10 years of age (14, 15). Treatment is chronic glucocorticoid replacement therapy with stress-dose steroids for acute physiologic stressors.
Mineralocorticoid function often remains intact in boys and men with primary adrenal insufficiency due to ALD, which corresponds with the relative sparing of the zona glomerulosa from VLCFA accumulation (6, 15, 22). In a natural history study of adrenal insufficiency, approximately half of patients required daily mineralocorticoid replacement therapy compared to over 90% who required daily glucocorticoid replacement therapy, and in those with mineralocorticoid deficiency, replacement therapy occurred later than glucocorticoid replacement (15). Thus, initiation of mineralocorticoid replacement therapy has been recommended only after clinical and/or biochemical evidence of mineralocorticoid deficiency. As mineralocorticoid deficiency can cause serious or even fatal volume depletion and/or hyperkalemia, patients and their families should be educated in its symptoms and clinicians should be vigilant for its signs (postural hypotension) and laboratory abnormalities (hyperkalemia, hyponatremia, hyperreninemia). In addition, primary adrenal insufficiency—regardless of the underlying etiology—has been associated with psychological morbidity and a lower quality of life, which may confound the neurological assessment in patients with ALD (23, 24).
Cerebral ALD
In childhood, cerebral ALD presents between 4 and 12 years of age, with a peak age at onset of around 7 years, affecting approximately one-third of boys with X-ALD (25) (Table 1). Cerebral ALD is rare after 15 years of age and almost never occurs before 2 years of age. Early on, the disease is a purely radiographic finding, as lesions on brain MRI far precede clinical manifestations. Affected boys subsequently develop learning and behavior problems. This first stage is followed by neurologic deterioration that includes increasing cognitive and behavioral abnormalities, cortical blindness, central deafness, and the development of quadriparesis. Very rarely, visual function is relatively preserved despite advanced central nervous system (CNS) involvement. Approximately 20% of affected boys have seizures, which may be the first manifestation. Although the rate of deterioration can be variable, rapid progression is common, with total disability developing by 6 months to 2 years and death within 5 to 10 years of diagnosis.
Childhood cerebral ALD is characterized by inflammatory demyelination that spreads throughout the supratentorial and infratentorial white matter. The occipitoparietal regions are usually affected first, with progression toward the frontal or temporal lobes. Arcuate fibers are generally spared, except in chronic cases. Lesions sometimes involve the brainstem, especially the pons. While the spinal cord is usually spared, lesions in the bilateral corticospinal tract can occur, which are difficult to distinguish from adrenomyeloneuropathy (AMN).
Myelopathy
Adrenomyeloneuropathy typically presents in adult males between 20 and 40 years of age, with a median age at onset of 28 years (Table 1). The primary manifestation is spinal cord dysfunction, manifested by progressive stiffness and weakness of the legs (spastic paraparesis), sensory ataxia, abnormal sphincter control, and sexual dysfunction. Symptoms of peripheral neuropathy, often unnoticed until the onset of significant paraparesis, are sometimes the first manifestation of AMN. Rarely, erectile dysfunction precedes motor abnormalities. Adrenomyeloneuropathy occasionally presents as a progressive cerebellar disorder. Adrenal insufficiency is often present at the time of AMN diagnosis and may precede AMN symptoms by decades.
Brain MRI is usually normal but spinal cord atrophy can be detected by conventional T2-weighted MRI sequences. While the total cord area is reduced by 26% to 40% at all tested levels, spinal cord thickness does not appear to correlate directly with the extent of patient disability. Physiologic and advanced imaging techniques (eg, MR fractional anisotropy) confirm the presence of sensorimotor abnormalities in the dorsal columns extending rostrally into the brainstem and internal capsule, which do correlate with overall disease severity. MR spectroscopy demonstrates reduced N-acetylaspartate in the corticospinal projection fibers, suggesting axonal dysfunction.
In adulthood, cerebral involvement usually occurs after the onset of AMN symptoms. In long-term follow-up studies, 27% to 63% of patients with AMN develop symptoms of cerebral involvement (eg, cognitive decline, behavioral abnormalities, visual loss, impaired auditory discrimination, or seizures) and 37% to 41% develop cerebral demyelination on brain MRI (26, 27). In 10% to 20% of adult males, cerebral involvement is accompanied by contrast enhancement and rapid neurologic decline, with serious cognitive and behavioral disturbances that may lead to complete disability and early death.
A natural history study of MRI in adult males with cerebral ALD found that 75% of patients with MRI lesions have corticospinal tract involvement, and 50% of these patients show lesion progression (28). While lesion progression in adults is slower on average compared with children, it is still progressive and, in some cases, devastating.
Clinical manifestations in women with ALD
From 30 years onwards, female heterozygotes often develop a myelopathy similar to men (29, 30) (Table 1). Affected women typically present with gait difficulties, bladder problems, fecal incontinence, and mild spasticity—evidence of myelopathy. At times, sensory symptoms point to peripheral nerve involvement. Neuropathic pain, an important symptom in female AMN that is not generally present in males, occurs in less than 20% of women younger than 40 years but almost 90% of women older than 60 years (31). Conventional imaging shows no abnormalities, but spinal cord volume has not been analyzed in women. Although clearly debilitating, the disease tends to be milder and its progression slower in women than in men. However, the natural history of AMN in both men and women has not been well defined. Very rarely, cerebral ALD occurs in women, but this is thought to develop only if 2 ABCD1 alleles are mutated or are in the presence of complete X-chromosome inactivation (also known as lyonization) (32).
Diagnosis of ALD
Measurement of very long chain fatty acids
In patients suspected of having ALD, the measurement of very long chain fatty acids (VCLFA) in blood is diagnostic, with high specificity and sensitivity (33). The assay includes 3 VLCFA parameters: C26:0, the ratio of C26:0 to docosanoic acid (C22:0), and the ratio of tetracosanoic acid (C24:0) to C22:0. These assay components were based on the original 1981 study that demonstrated patients with ALD had significantly higher C26:0 levels and C26:0 to C22:0 and C24:0 to C22:0 ratios compared to controls (2). In a study of 1792 patients with peroxisomal disorders, including over 1000 males with ALD, high VLCFA levels were present in all cases. Although nonfasting samples and hemolysis may cause equivocal or false positive results, false negatives and positives in males are rare and have been limited to a few case reports (33, 34). Samples should be collected after a 4 to 14 hour fast. False positives have been reported in individuals on a ketogenic diet for treatment of epilepsy, some individuals with liver insufficiency, and 1 patient with diabetic ketoacidosis (35, 36). Thus, it is possible that alterations in diet, liver function, and/or metabolic states, such as acute physiologic stress (possibly including primary adrenal insufficiency not due to ALD), can lead to false elevations in VLCFA. In such cases, VLCFA should be repeated at an experienced laboratory, such as the Kennedy Krieger Institute, after resolution of the acute stressor. In addition, the measurement of VLCFA does not distinguish between ALD and other peroxisomal disorders. Though ALD is distinct in its absence of other dysmorphic features and its typical onset during later childhood (compared to early onset during infancy in other peroxisomal disorders), confirmatory genetic testing should be completed, as further discussed below. Importantly, 15% of women with ALD have normal VLCFA levels; thus, any woman with symptoms of myelopathy with or without a family history of ALD should undergo genetic testing (33).
Genetic testing
Adrenoleukodystrophy is an X-linked disorder, and all patients with ALD harbor a mutation in the ABCD1 gene (3). For elevated VLCFA levels or abnormal ratios of VLCFA, genetic testing should be performed to confirm the diagnosis of ALD. After genetic confirmation in the proband, targeted testing for the identified mutation in immediate and extended family members should be offered to those at risk of developing ALD (33, 37). To date, more than 800 nonrecurrent ABCD1 mutations have been described in the X-ALD database, of which 367 (49%) are missense mutations (38). Most of the described mutations lead to an absence of detectable ABCD1 protein expression using immunohistochemistry. While large deletions (3%), frameshifts (24%), amino acid insertions/deletions (6%), and nonsense mutations (12%) generate truncated proteins, resulting in no detectable expression of ABCD1, missense mutations often lead to unstable ABCD1, with decreased or absent expression detected by immunofluorescence microscopy or western blotting. In a case of defective translation initiation due to a 26-base pair deletion, it was suggested that, despite the presence of an out-of-frame start codon, an alternative internal translation initiation at amino acid position 66 permitted low-level expression of truncated ABCD1 (39). Approximately half of the mutations are private, nonrecurrent mutations. The reported de novo mutation rate ranges from 5% to as high as 19% (40).
All clinical phenotypes of X-ALD can occur within the same nuclear family, even with mutations that cause complete loss of the ABCD1 protein. All reported cases of translation initiation mutations in ABCD1 have presented with an AMN-only phenotype (39). In some kindreds with mutations that result in an AMN-only phenotype, AMN is fully penetrant in female carriers. However, except for rare examples, no correlation exists between ABCD1 mutation and X-ALD phenotype.
Newborn screening
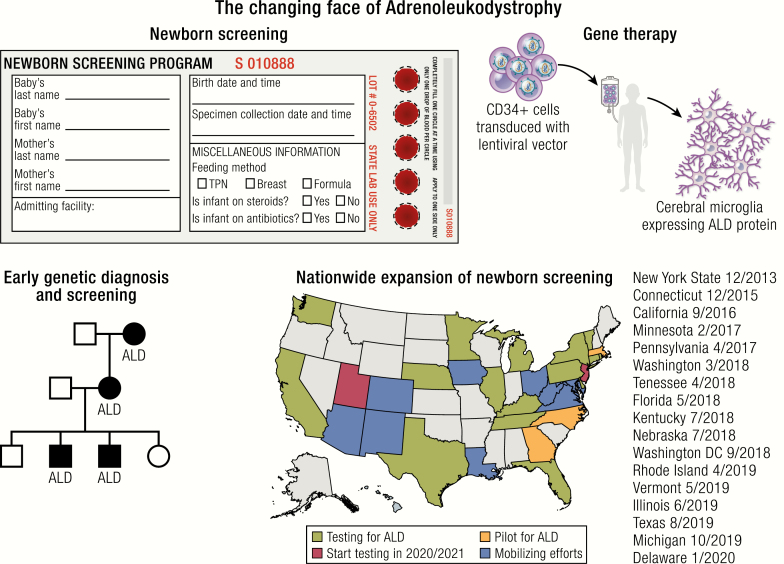
As survival and clinical outcomes are superior if treatment is offered in the early stages of CCALD, ALD was nominated to be added to the U.S. Recommended Uniform Screening Panel for NBS in 2012. Following improvements in a high throughput screening assay and patient and family advocacy, over a dozen states in the United States are actively screening for ALD. Globally, the Netherlands is the only other country that is actively screening for ALD through the Screening for ALD in the Netherlands (SCAN) pilot study, a sex-specific newborn screen for boys only. Given the advancements in ALD NBS and treatment, NBS for ALD will likely be initiated in additional counties in the near future (38). Three U.S. states, New York, Minnesota, and North Carolina, have reported their experience with notable insights on screening methods and infrastructure of follow-up testing, incidence of disease, and opportunities for genetic and phenotypic characterization of ALD (10–12, 41).
On March 31, 2013, Aidan’s Law, named after a New York State resident with ALD, was passed and mandated the New York State Newborn Screening Program to begin screening for ALD. In December 2013, New York became the first state to start NBS for ALD with the following 3-tier algorithm: the first tier is tandem mass spectrometry (MS/MS) of C26:0-lysophosphatidylcholine (LPS); the second tier is a confirmatory HPLC-MS/MS; and the third tier is Sanger DNA sequencing of the ABCD1 gene (11). All newborn males with an identified ABCD1 mutation undergo confirmatory VLCFA analysis in an independent laboratory. Parental testing of both affected male and female newborns is offered through the NBS program. All 3 tiers of testing are generally completed prior to the referral to clinical specialists (10, 11).
In the first 18 months, 365 000 infants were screened and 13 males and 13 females were found to have mutations in the ABCD1 gene (11). Family members of the affected males and females were offered testing, but these data were not available at the time of the initial report. Based on these data, implementing NBS for all 4 000 000 annual births in the United States would prevent an estimated 18 deaths by 15 years of age (11). The New York State experience and the potential for improved outcomes with early disease detection and treatment were instrumental in the addition of ALD to the federal recommended uniform screening panel in February 2016. Individual states had the option to choose when and how they implement the screening and, as a result, various models of screening and clinical care have emerged.
Minnesota become the fourth state to add ALD to NBS in 2017 and recently reported their extensive experience with follow-up and testing of family members of identified male and female infants (12). The screening test is a single liquid chromatography (LC)-MS/MS of C26:0 LPS. Following a positive screen, referral to a specialty center with a genetic counselor and a pediatric neurologist consultation would be recommended. At the initial clinical visit, a multigenerational family history and confirmation of the diagnosis with VLCFA serum analysis and ABCD1 genetic testing would be obtained. Families were further counseled and provided information regarding testing for at-risk family members.
In the first 12 months, the Minnesota NBS program identified positive screens in 9 males and 5 females out of a total of 67 835 newborn infants. All 14 cases were confirmed to have ALD by serum VLCFA analysis (12). The majority of infants (11/14) did not have a known family history of ALD and had available subsequent familial diagnostic work-up. Of the families who underwent screening, 17/32 male family members were subsequently diagnosed with ALD, and 24 females were confirmed to be heterozygous for ALD, which yielded a total of 41 new diagnoses, an average of 3.7 family diagnoses per infant. Following familial testing, only 1 case was confirmed to have occurred de novo (12).
Genetic testing of newborns with positive screens and testing of their family members have identified novel variants of unknown significance, providing opportunities for further genetic characterization. Out of the 14 infants with positive newborn screens, all but 2 cases had an identified mutation in the ABCD1 gene identified on postnatal genetic testing. One female infant had a known family history of ALD and was found to carry the familial variant on prenatal amniocentesis. One male infant who did not have an identified mutation on next generation sequencing or deletion/duplication analysis had reduced presence of the ALDP protein on immunoblot, consistent with the diagnosis of ALD (12). This individual’s older brother, mother, and maternal male first cousin were also noted to have elevated VLCFA, consistent with an X-linked inheritance pattern. This is the only published case of an ALD diagnosis without an ABCD1 gene variant, possibly due to a noncoding variant or structural defect, although targeted sequencing might have revealed a pathogenic mutation. Out of the remaining 12 individuals, 4 had variants of unknown significance, and 3 of these were reclassified as likely being pathogenic following a segregation analysis in family members; the remaining variant was not reclassified, as it is unknown if segregation studies were performed (12). As newborn screening for ALD continues to expand in the United States, the reporting of mutations to variant databases and follow-up classification of variants based on phenotypic outcomes in affected families will be crucial to understanding the pathogenicity of new variants.
North Carolina launched a pilot study for ALD NBS in 2018 and recently reported the first cases of presumed false-positive results for ALD (41). The pilot study used HPLC-MS/MS of C24:0-LPS and C26:0-LPS as first-tier screening. All positive screens underwent confirmatory Sanger DNA sequencing of the ABCD1 gene and simultaneous outpatient referral to a genetic counselor, who facilitated a physical examination and VLCFA confirmatory testing with a primary care physician. If VLCFA testing was abnormally elevated, the infant was referred to a genetics clinic for further evaluation.
Over 6 months, 52 301 infants were screened, and 3 male and 3 female infants were found to have elevated VLCFA and mutations in the ABCD1 gene diagnostic of ALD. Notably, 3 infants (all female) tested positive on initial NBS but had normal VCLFA results and no variant identified in the ABCD1 gene on follow-up testing. These were considered false-positive screens, and no further follow-up was recommended. Of those infants who were confirmed to have ALD, carrier testing for the mother and male siblings was offered but only 2 out of the 6 families pursued recommended testing during the pilot study. This may be attributed to an incomplete understanding of the implications of an ALD diagnosis and/or the cost of genetic testing, which was not included in the state’s pilot study (41).
Based on the experience of New York State’s, Minnesota’s, and North Carolina’s NBS for ALD, the birth prevalence of ALD in Minnesota is strikingly high, with 1 in 3878 male infants affected, which is more than 5 times the estimated incidence of 1 in 21 000 males based on VLCFA testing from Kennedy Krieger Institute and Mayo Medical Laboratories, 4 times the birth prevalence of 1 in 16 074 detected by NBS in New York State, and double the prevalence of 1 in 8814 males in North Carolina (10, 12, 41–43). One potential explanation for the differences in birth prevalence of ALD in the 3 states is the cutoff level of C26:0-LPC used to define an abnormal value. In Minnesota, all C26:0-LPC levels detected by LS-MS/MS that are ≥ 0.30 μmol/L and any borderline C26:0-LPC levels that test ≥ 0.16 μmol/L twice are considered positive screens (12). In comparison, the New York State NBS program defines all C26:0-LPC cutoff levels detected by the first-tier MS/MS method of ≥ 0.40 μmol/L and second-tier testing by HPLC-MS/MS ≥ 0.24 μmol/L twice as positive screens (42). As all positive screens in Minnesota were confirmed to have ALD, it is likely that states with higher cutoff values are missing cases of ALD; data on false-negative rates are not yet available given the recent implementation of NBS (12). In contrast, North Carolina uses the lowest C26:0-LPS cutoff level detected by HPLC-MS/MS as a positive screen (≥ 0.15 μmol/L alone or ≥ 0.08 μmol/L in conjunction with an elevated C24:0-LPC level of ≥ 0.175 μmol/L), which likely accounts for the cases of false-positive screens not reported in the other 2 states (41). As the C26:0-LPS cutoff level used to define a positive screen is lower in North Carolina than in Minnesota, a higher birth prevalence of ALD might be expected in North Carolina, which is not the case. Thus, it is also possible that Minnesota has a unique population structure, including ALD founder mutations and/or bottlenecks, and it remains to be seen whether these findings indicate that the prevalence of ALD varies widely in different regions of the United States. Another factor that may contribute to the discrepancy between Minnesota’s and North Carolina’s birth prevalence and historical estimates is mild and/or subclinical disease that previously would have gone undetected with routine clinical care, such that the phenotypic spectrum of ALD extends to include a substantial group of either asymptomatic or mildly affected males who will now be detected by more selective screening measures. This will become clearer over time, especially as patients with milder elevations in VLCFA who have new genetic variants of unknown significance are evaluated as they age.
In 2019, over a dozen states (California, Connecticut, Florida, Illinois, Kentucky, Michigan, Minnesota, Nebraska, New York, Pennsylvania, Rhode Island, Tennessee, Texas, Vermont, Washington) were actively screening for ALD, with an additional 15 states (Arizona, Colorado, Delaware, Georgia, Iowa, Louisiana, Maryland, Massachusetts, New Jersey, New Mexico, North Carolina, Ohio, Utah, Virginia, West Virginia) starting testing or mobilizing efforts (38). Newborn screening is expected to result in improved clinical outcomes based on modeling from the New York State NBS outcomes, but the cost effectiveness of adding ALD to NBS in the United States is not yet known given its very recent implementation, differences in funding for confirmation and familial testing between states, and the unknown costs of future FDA-approved gene therapies (11). Outside of the United States, a 2018 model based on the UK National Health Service Newborn Blood Spot Screening Programme projected that screening boys for ALD in the U.K. would reduce overall lifetime costs driven by reductions in social and educational costs and increased quality-adjusted life-years for boys with cerebral ALD (44). Although the cost effectiveness modeling from the U.K. is promising, the findings may not be transferrable to other nations due to differences in the healthcare and social structure. As NBS continues to expand in the United States and other countries, optimization of testing methodology, including defining C26:0-LPS cutoff values, establishing follow-up protocols with multidisciplinary care teams, modeling the public health economic impact, and reporting genetic and phenotypic information will be critical for advancing our understanding of the spectrum of disease in ALD and meeting the medical care needs of this growing patient population.
Prenatal diagnosis of ALD
As NBS will increase identification of women with ALD, prenatal diagnosis of ALD will likely become an area of active investigation. Currently, amniocentesis may be performed for prenatal genetic diagnoses for at-risk fetuses, but it is an invasive procedure associated with the risk of miscarriage (45, 46). Prior to conception, preimplantation genetic diagnosis may also be considered. Limited prior case reports have used sex-selection and polymorphic markers around the ABCD1 gene to select unaffected embryos with variable success rates (47–50). Further investigations may improve pregnancy rates, and preimplantation genetic diagnosis may be offered to affected families. Noninvasive prenatal testing (NIPT) has been traditionally used to identify fetal aneuploidies, but recent advancements have allowed for the diagnosis of single gene disorders, such as achondroplasia, congenital adrenal hyperplasia, and some cases of sickle cell disease (51, 52). Adrenoleukodystrophy may be a candidate disease for NIPT in the near future.
Surveillance and Treatment
Surveillance of asymptomatic individuals
Asymptomatic infants and family members identified on a newborn screen are typically referred to a multidisciplinary team that includes a geneticist, neurologist, and endocrinologist. Based on clinical experience, routine screening guidelines for neurological and adrenal disease have been recommended (Table 1, Fig. 1). Brain MRIs should be obtained annually starting at 12 months of age and obtained more frequently every 6 months from the ages of 3–10 years, when the risk of developing CCALD is highest (10). Although the peak incidence of adrenal insufficiency occurs between 3–10 years of life, adrenal insufficiency can be the initial manifestation of ALD as early as infancy. Given the potential life-threatening consequences of untreated adrenal insufficiency, laboratory screening (ACTH and cortisol) for glucocorticoid deficiency should be obtained at diagnosis of ALD in infancy and every 3–6 months, the frequency of which is dependent on the age (Fig. 1) (53). Early diagnosis of infants and toddlers with asymptomatic ALD is crucial to providing potentially life-saving anticipatory guidance on the use of stress-dose steroids with physiological stress, including acute illness, trauma, and/or surgery.
Figure 1.
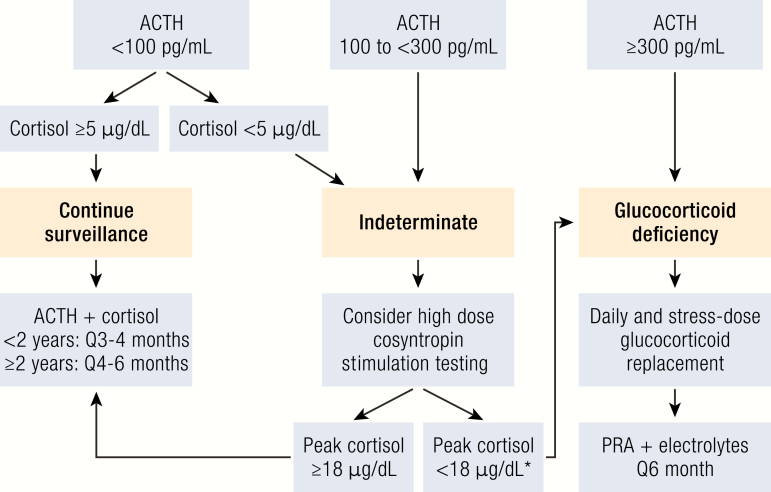
Recommended endocrine surveillance for presymptomatic infants and children with ALD. Infants identified through NBS should have an early morning ACTH and cortisol level at the time of diagnosis to screen for glucocorticoid deficiency, which usually precedes mineralocorticoid deficiency (10, 53). Any ACTH level of > 100 pg/mL and/or any morning cortisol level < 5 ug/dL is considered abnormal and warrants further diagnostic evaluation (53). *In the case of a stimulated peak cortisol level of < 18 μg/dL, stress dose steroids should be started, but daily glucocorticoid replacement can be considered as clinically indicated. Abbreviation: PRA, plasma renin activity.
Diagnosis and treatment of adrenal insufficiency
ACTH levels should always be interpreted in the context of a simultaneous cortisol level, the clinical presentation, and the overall ACTH trend, if available. For instance, an elevated ACTH level could be appropriate secondary to acute physiologic stress and normal if there is an adequate cortisol response. However, a trend of a gradual elevation in ACTH levels with declining cortisol levels can suggest evolving adrenal insufficiency. Clinically, the monitoring of skin pigmentation can be a helpful indicator of integrated ACTH levels over time. Any elevated ACTH level ≥ 300 pg/mL with an inappropriate cortisol response < 18 μg/mL is diagnostic of glucocorticoid deficiency, and chronic glucocorticoid replacement and stress-dose steroids should be initiated.
It remains unknown when and how to treat early, subclinical manifestations of adrenal involvement with indeterminate testing. The decision to add chronic glucocorticoid treatment in this situation may commit patients to secondary adrenal insufficiency before they develop primary adrenal insufficiency. Alternatively, patients with subclinical abnormalities may benefit from stress-dose steroids in the event of physiologic stress, without needing chronic glucocorticoid replacement therapy. Existing ALD adrenal surveillance guidelines consider any cortisol level < 5 μg/mL and/or ACTH level between 100 and 300 pg/mL as indeterminate and recommend follow-up with a high-dose cosyntropin stimulation test (53). Data on the normal ranges of a stimulated cortisol level in children are limited by study size and the use of different cortisol assays. Thus, existing recommendations are based on these limited studies in children and recent data in adults analyzing modern LC-MS/MS and immunoassays (53, 54). A stimulated peak cortisol < 18 μg/mL is considered consistent with glucocorticoid deficiency, and stress-dose steroids should be started with a discussion of initiating chronic glucocorticoid replacement as clinically indicated.
As mineralocorticoid deficiency is less common and generally presents after glucocorticoid deficiency, evaluation with plasma renin activity and electrolytes is recommended every 6 months starting after diagnosis of glucocorticoid deficiency (53). Given the possible life-threatening consequences of untreated mineralocorticoid deficiency and nonspecific presenting symptoms in infants and young children, clinicians should be vigilant in regular clinical assessments and have a low threshold for laboratory evaluation.
Allogenic hematopoietic stem cell transplantation
Allogeneic HSCT can arrest the progression of the neurologic disease when performed in the early stages of CCALD (Table 1, Fig. 2). However, the precise mechanism by which allogeneic HSCT arrests CCALD progression is not clear. The survival advantage of transplantation compared to no transplant in patients with early stage CCALD was demonstrated in a retrospective analysis (60). The projected 5-year survival in the transplanted group was 95% in comparison to 54% in the untransplanted group. This and multiple subsequent investigations highlighted the importance of patient selection to the potential success of allogeneic transplant in CCALD. While there are no universally accepted standard criteria for HSCT in boys with ALD, the general criteria are a genetically and/or clinically confirmed diagnosis of ALD and the presence of cerebral disease that is not advanced, based on neurological symptoms and findings on brain MRI.
Figure 2.
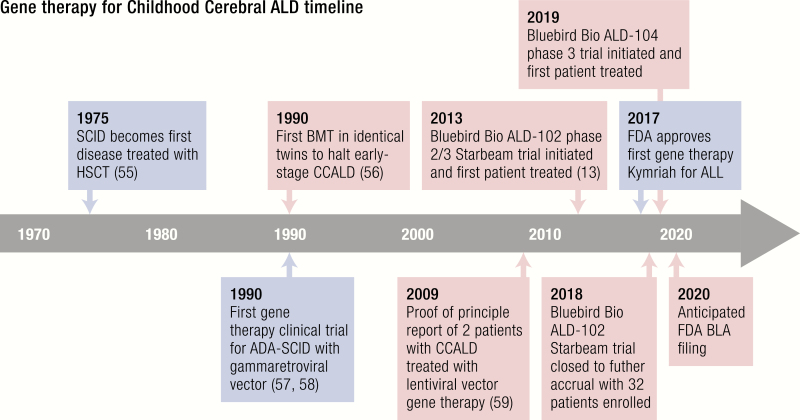
Key milestones and developments in BMT/HSCT and gene therapy that led to their use to treat CCALD. General milestones are highlighted in blue, and those specific for the treatment of ALD are highlighted in red. Abbreviations: ADA, adenosine deaminase; ALL, acute lymphoblastic leukemia; BLA, biologics license application; BMT, bone marrow transplant; SCID, severe combined immunodeficiency; other abbreviations noted in the text.
Hematopoietic stem cell transplantation is not effective in patients who have advanced CCALD. The ALD-specific Neurologic Function Scale (NFS) and the Loes MRI severity score are used to help determine the suitability of a patient for transplant. The NFS is a 25-point, ALD-specific tool that assesses the severity of neurologic dysfunction by assigning scores to 15 different disabilities. Lower scores indicate fewer symptoms and higher scores indicate a more significant disability. The NFS score can be used to guide the recommendation for HSCT, but there is no score that absolutely determines the decision for HSCT. The Loes MRI severity score is a 34-point scale that assigns a score to an MRI based on the extent of white matter lesions (61), with higher scores indicating more significant ALD involvement. MRI gadolinium contrast enhancement is used to indicate the presence of the inflammatory process, and there is an association between the presence of contrast enhancement on T1-weighted MRI and cerebral ALD progression (62). To be considered for HSCT, patients must have evidence of cerebral disease on brain MRI with the presence of gadolinium contrast enhancement around a consistent lesion, indicating a minimum Loes MRI score of 1. The upper limit of the Loes MRI score is debated and often depends on the clinical scenario.
Survival and major functional disability (MFD)-free survival following transplant are superior in patients with lower NFS and Loes scores. A comparison of the overall survival of patients transplanted with MRI Loes scores < 10 and those with scores ≥ 10 showed survival approaching 90% in the cohort, with Loes scores < 10 contrasted with approximately 15% in the group with higher scores (63). Similar differences were observed when the overall survival of patients with MRI Loes scores < 9, and 0 to 1 neurologic deficits (92% 5-year overall survival), were compared to patients with Loes scores ≥ 9 and ≥ 2 neurologic deficits (45% 5-year overall survival) (64). Additionally, the disease severity impacts not only survival, but also the long-term function status of patients. A recent multicenter analysis of the overall survival and major functional disability-free survival in patients with advanced and those with early stage cerebral disease showed an overall survival of 90% at 5 years in patients with advanced disease; however, MFD-free survival was 10% at the same time point. In patients transplanted with early stage disease, the overall survival at 5 years from a CCALD diagnosis was 94% and the MFD-free survival was 91% (9).
There are drawbacks to allogeneic HSCT. In addition to the lack of efficacy in advanced disease, transplantation does not reverse neurologic findings present at the time of HSCT and does not stabilize cerebral disease for 3 to 24 months after stem cell infusion. Symptoms can progress during this time. This makes the early identification of potential HSCT candidates essential. Transplant is ineffective for the adrenal manifestations of disease and is not felt to impact the development of adult onset AMN.
Transplantation requires the identification of a stem cell donor. If an acceptable human leukocyte antigen (HLA)-matched related donor is not available, then an unrelated donor or cord blood unit must be found. This process can take weeks and, in some circumstances, an acceptable unrelated stem cell donor may not be identified. Allogeneic transplantation comes with risks of acute mortality (~10% at day 100 from transplant) and late complications, a 5% risk failure of donor cell engraftment, and graft-versus-host disease (GVHD) (10–40% risk of acute GVHD and 20% risk of chronic GVHD) (8, 64–67). Given the significant risk of morbidity and mortality with HSCT, patients with ALD must also meet institutional criteria for organ functioning, infectious disease status, and performance status. In summary, allogeneic HSCT is an effective therapy for patients who have early stage CCALD, but it comes with significant short- and long-term risk.
Gene therapy
Transplantation of autologous, genetically modified hematopoietic stem/progenitor cells (HSPCs), a modality of ex vivo gene therapy administration, was proposed and is being extensively explored as an alternative to HSCT in several monogenic conditions worldwide, with a greater number of trials opened and patients treated in the United States and Europe (68). This treatment strategy, based on autologous cells, allows prompt identification of a stem cell source for transplant in every patient and overcomes the most severe immunological limitation of allogeneic HSCT represented by GVHD. In this setting, gene transfer is used to deliver a normal copy of the disease-causing gene (generally as a cDNA) to HSPCs, thus replacing the allogeneic cells carrying 2 normal alleles of the same gene with autologous HSPCs corrected in their genetic defect. The use of integrating vectors allows for the long-term persistence of the therapeutic gene into the HSPC genome and the transmission of its correction or expression to the mature progeny of the transplanted, genetically modified cells, potentially allowing for a permanent correction of the disease phenotype. γ-retroviral vectors (γ-RVs), derived from the Moloney leukemia virus, were employed as first-generation integrating vectors for HSPC gene transfer. In particular, γ-RVs were used with success for HSPC gene therapy in some primary immunodeficiencies, which resulted in robust clinical benefits but also in severe side effects due to genotoxicity (57, 58) (Fig. 2). Indeed, γ-RV integration occurs preferentially in active regulatory elements, that is, promoters and enhancers, of genes with key regulatory functions in cell commitment and cell identity. This, along with predisposing conditions related to the primary disease, caused oncogenic transformation of transduced cell clones, leading to leukemia or myelodysplastic disease in a number of the treated patients (57, 58). Based on these observations, an extensive effort was made to design a new generation of integrating vectors depleted of transacting sequences and with a neutral integration pattern into the genome. These included HIV-derived lentiviral vectors (LVs) that have been extensively employed in HSPC gene therapy applications in several monogenic conditions, including CCALD, as a safe and effective alternative to γ-RVs (69–71). In self-inactivating (SIN) configuration, all the original viral genes and regulatory elements are removed from LVs, and transgene expression is driven by an exogenous promoter, usually of human origin, placed in an internal position (Fig. 3). Lentiviral vectors used for HSPC gene transfer are usually produced as single-stranded RNA particles pseudotyped with the envelope protein-g of the vesicular stomatitis virus (VSVg).
Figure 3.
Lenti-D LV encoding ALD protein used in the Bluebird Starbeam ALD-102 study. Lenti-D LVV is a replication defective, SIN, third-generation HIV-1 based LVV pseudotyped with glycoprotein (G) of the vesicular stomatitis virus (VSV-G), carrying human ABCD1 cDNA-derived sequences that encode the normal human ALDP. The vector sequences encoding the normal ALDP are under control of the MND promoter, which has been shown to drive expression of the transgene in HSCs. The vector is similar to that used in Cartier et al (CG1711 hALD) (59) in regard to the promotor used and ALDP cDNA but differs in elements of the vector backbone, including removal of the WPRE. Abbreviations: cPPT/FLAP, central polypurine tract; ΔU3, promoter/enhancer-deleted unique 3’; MND, myeloproliferative sarcoma virus enhancer with negative control region deleted, dl587rev primer-binding site substituted; ppt, 3’ poly purine tract; Ψ+, packaging signal; R, repeat; RRE, rev response element; U5, unique 5’ site. (Reproduced with permission from Eichler F, Duncan C, Musolino PL, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med. 2017;377 (17):1630–8.).
The ex vivo gene therapy procedure in CCALD, as well as in other neurometabolic applications/conditions, includes the infusion of gene-modified HSPC after the administration of a busulfan-based myeloablative conditioning regime intended to facilitate the engraftment of the transplanted HSPCs in the hematopoietic and CNS (72). Indeed, multiple preclinical studies have demonstrated that the administration of a myeloablative regimen with CNS-penetrating agents, such as busulfan, before cell transplantation is instrumental to the achievement of a critical myeloid engraftment in the brain, which is essential for the HSPC-derived myeloid cells to exert a therapeutic effect in CCALD (72–74).
Childhood cerebral ALD is one of the first neurologic disorders treated by the HSC gene therapy approach. Promising results reported in 2009 by Cartier and colleagues were obtained in 2 patients treated in the first clinical trial of HSC gene therapy for ALD in France that targeted the transfer of the ABCD1 cDNA (Fig. 2) (59). Similar to what has been observed following allogeneic HSCT, clinical and neuroradiological disease stabilization was obtained in the 2 boys treated in the first study, who had progressive demyelination but no HLA-matched or cord blood donors. Cerebral demyelination, as measured by MRI imaging and quantitated by the Loes score arrested after 14 and 16 months, respectively, in the 2 patients, without neurological signs of disease progression up to the last reported follow-up. From a gene therapy standpoint, these patients demonstrated the presence of polyclonal hematopoietic reconstitution by the genetically modified cells, with approximately 9% to 14% of multilineage gene marking. After these encouraging results, a larger, multicenter phase II/III clinical trial was launched in 2013 (Fig. 4) (ClinicalTrials.gov #NCT01896102). Patients at early stages of the disease received autologous HSPCs in which the ABCD1 cDNA was inserted by LV-mediated gene transfer upon the administration of a conditioning regime based on myeloablative busulfan and cyclophosphamide. Preliminary results were recently published on 17 boys affected by CCALD who received this treatment (13). Post-treatment brain MRI and neurological outcomes at 29.4 months mean follow-up are comparable to historical patients treated with allogeneic HSCT and confirmed the benefit observed in the initial gene therapy study (Table 1). Importantly, 15/17 patients are alive and free of major functional disability. Transplantation was well tolerated. No medicinal product-related adverse events were reported. One death was registered from rapid disease progression, and another after study withdrawal from HSCT-related complications. The transduced cell infusion resulted in a polyclonal, benign hematopoietic reconstitution with multilineage gene marking and no molecular evidence of clonal dominance, insertional oncogenesis, or replication competent virus. This study has now met its target accrual of 32 subjects and has been closed to additional accruals, and it is in the data analysis stage, with the most recent results reported in abstract form (75). These results granted the Breakthrough Therapy designation to the gene therapy product (Lenti-D) from the American Food and Drug Administration (FDA) in May 2018 for the treatment of patients with CCALD. Recruiting for a following phase III trial has been recently opened across the United States and Europe (Fig. 2) (January 2019, NCT03852498). Overall, these studies support the therapeutic value of HSPC-based ex vivo gene therapy in CCALD and encourage its transferability to other similar neurodegenerative diseases.
Figure 4.
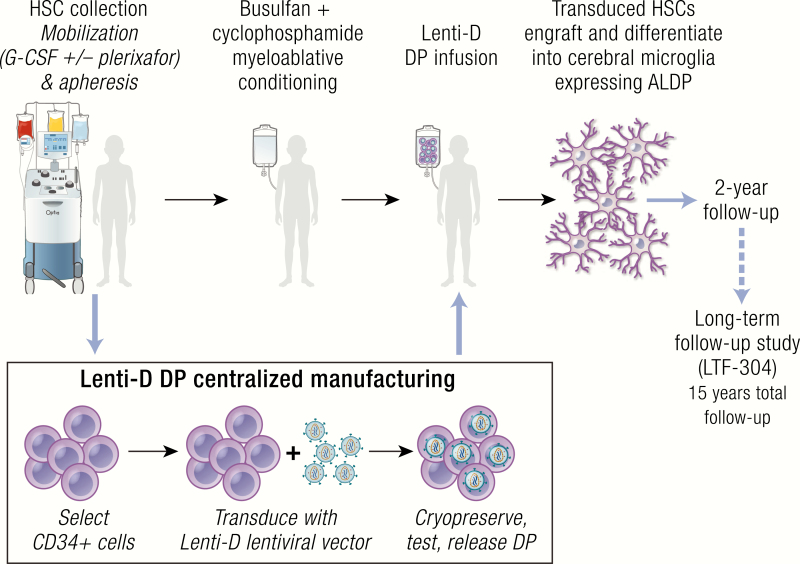
Treatment protocol for the gene therapy study utilized in the Bluebird Starbeam ALD-102 trial. Abbreviations: ALDP, adrenoleukodystrophy protein; DP, drug product; HSC, hematopoietic stem cell; LTF, long-term follow-up. (Reproduced with permission from Sevin, C. Phase 2/3 trial to assess the safety and efficacy of lenti-D hematopoietic stem cell gene therapy for cerebral adrenoleukodystrophy. Presented at: European Society of Paediatric Neurology, Megaron Athens International Conference Centre, Athens, Greece, September 2019. Oral Abstract Presentation [Abstract OC036]).
Theoretical treatment targets for ALD
Advances in gene therapy for other monogenic diseases may identify candidate treatments for ALD. The FDA recently approved 2 antisense oligonucleotide agents for spinal muscular atrophy and Duchenne muscular dystrophy. Antisense oligonucleotides are single-stranded oligodeoxynucledotides that can modulate gene expression or pre-mRNA splicing and can be used to exclude or include particular exons (76). In the case of ALD, antisense oligonucleotides could target specific mutations to reverse a known splicing defect, establish a normal reading frame after a frameshift mutation, or exclude pathogenic variants altogether. It could also be employed to target ELOVL1, the single elongase catalyzing the synthesis of both saturated VLCFA (C26:0) and monounsaturated VLCFA (C26:1). Gene editing is another gene therapy method under investigation that allows permanent modifications to specific DNA segments through the use of guide RNA sequences and endonucleases. This strategy is currently in trial for sickle cell disease and could have the potential to edit out pathogenic mutations in the ABCD1 gene for ALD. An additional theoretical therapy is a targeted viral vector that could deliver a normal copy of the ABCD1 gene to steroidogenic cells to prevent or halt adrenal disease and to neuronal or microglial cells to prevent or halt the cerebral or myelopathy phenotypes.
Despite the success of gene therapy for ALD in hematopoietic stem cells, the precursor to the microglial cells implicated in the pathogenesis of cerebral ALD, gene therapy for adrenal disease in ALD faces additional challenges (77). Successful gene therapy for adrenal disease would require identification of a viable steroidogenic precursor cell and successful delivery of a normal copy of the ABCD1 gene to that cell, or the in vivo editing of the mutant ABCD1 gene using a CRISPR-assisted gene editor or other novel method (78, 79). Currently, the etiology and regulation of steroidogenic stem cells remain an active area of investigation (80). While gene therapy for adrenal disease in ALD is not likely to occur in the immediate future, it remains a possibility as the regulation of stem and progenitor cells in the adrenal cortex continues to be characterized.
Metabolic intervention and lipid modulation
Lorenzo’s oil is a combination of erucic and oleic acid that is taken orally (81). While it lowers levels of plasma VLCFAs in ALD patients, it is not clear that conversion to cerebral disease differs on Lorenzo’s oil, as other studies of untreated boys reported similar conversion rates as those reported on Lorenzo’s oil. Further, Lorenzo’s oil does not arrest progression of cerebral disease once brain demyelination has set in. A randomized, double-blind, placebo-controlled, crossover trial comparing lovastatin (40 mg once daily) with placebo concluded that lovastatin led to a small, nonspecific decrease in C24:0 and C26:0 in plasma and red blood cells due to a decrease in the level of LDL cholesterol (82).
As oxidative stress and mitochondrial failure contributes to axonal degeneration, key regulators of mitochondrial function, such as sirtuin 1 (SIRT1), have been examined and found to be impaired in X-ALD (83). Using resveratrol treatment and transgenic overexpression of SIRT1 in the Abcd1 knockout mouse, redox homeostasis and mitochondrial respiration could be restored. Similarly, pioglitazone, an agonist of peroxisome proliferator-activated receptor gamma (PPARγ), may restore mitochondrial function and neutralize damage due to oxidative stress (84). Whether improvements in axonal degeneration and associated locomotor disabilities seen in Abcd1 knockout mice treated with either SIRT1 or pioglitazone translates to humans has yet to be examined. Several drugs currently in medical practice are known to upregulate ABCD2, which could compensate for ABCD1 deficiency. Valproic acid is known to be a histone deacetylase inhibitor and thereby induces expression of ABCD2 (85). Similarly, metformin induces ABCD2 via AMP-activated protein kinase α1 (AMPKα1) and can rescue mitochondrial dysfunction and inhibit the proinflammatory response (86). Metformin lowers VLCFA levels, improves mitochondrial function and ameliorates inflammatory gene expression in ALD patient-derived cells. Furthermore, metformin together with lovastatin attenuates T-cell autoimmunity and neurodegeneration in experimental autoimmune encephalitis. Other preclinical studies are examining novel ways to enhance ABCD2 expression through activation of its functional thyroid hormone response element or via liver X receptor (LXR) antagonism.
Future Considerations
As more individuals with ALD are identified at birth, many uncertainties remain regarding the infrastructure of follow-up and subspecialty clinical care, the monitoring and therapy of asymptomatic or mild disease, the medical management of women with ALD, and the potential adverse effects of earlier treatment.
Timely biochemical and genetic testing following a positive NBS is paramount for diagnosis and follow-up testing for affected family members. Since ALD was added to the federal recommended uniform screening panel in 2016, individual states have been tasked with the responsibility of implementing testing and follow-up procedures. The optimal model of care for confirmation testing and clinical follow-up remains to be determined. Possible models include the NBS program, primary care providers, or subspecialty care centers. For example, the New York State NBS program performs the confirmatory genetic testing and offers testing to family members prior to referral to subspecialists (10). However, this model requires substantial state funding and resources, which may not be feasible in other states. In the early stages of NBS implementation in Minnesota, primary care providers were requested to order confirmatory VLCFA analysis and coordinate the referral process if ALD was confirmed. Because this initial process took over a month in numerous cases and the newborn screen was noted to be highly reliable, Minnesota’s protocol was updated to immediate referral to a genetic counselor, who subsequently discussed the diagnosis, obtained a family history, and coordinated confirmatory VLCFA and genetic analysis for the newborn and their family (12). Due to the volume of referrals, complexity of genetic testing with insurance coverage, and difficulty interpreting genetic results, the University of Minnesota Medical Center established a multidisciplinary ALD clinic to meet the needs of newborns identified by NBS and their family members (12). In contrast, only 2 out of the 6 families with an infant diagnosed with ALD in the North Carolina pilot study pursued recommended familial testing, which may reflect challenges in patient education and the coordination and/or costs of care (41). States that are initiating NBS for the condition should anticipate more cases than predicted, based on prior incidence estimates, and proactively establish a timely protocol of diagnostic confirmation and subspecialty referral for counseling and evaluation of family members.
The higher incidence of ALD detected by newborn screen and reporting of novel variants of unknown significance suggest that ALD may have been underdiagnosed previously, particularly in the cases of mild or subclinical disease. While follow-up and monitoring of these potential cases provides an opportunity for both expansion of genetic and phenotypic characterization of the disorder, the optimal timing of screening tests for neurological and adrenal disease is unknown. This uncertainly can lead to significant family stress and anxiety regarding the inability to predict the phenotype and need for future treatment. For example, definitive diagnosis of neurological disease in the absence of brain imaging abnormalities is not well defined. Further, the diagnosis of early primary adrenal insufficiency that might not require chronic glucocorticoid treatment but would benefit from the prescription of stress doses of steroids is unclear. As noted previously, the decision to add chronic glucocorticoid treatment might commit patients to secondary adrenal insufficiency before they develop primary adrenal insufficiency. In New York State, mild abnormalities have been detected in glucocorticoid secretion as early as 5 weeks of life (16). When and how to treat these borderline results in asymptomatic infants remains to be determined. Initial recommendations for laboratory screening of adrenal function and brain MRI screening for cerebral disease have been proposed that will likely be modified with additional follow-up data (10, 53) (Table 1, Fig. 1).
The identification of presymptomatic infants allows for earlier timing of treatment in relation to adrenal and cerebral disease. As more presymptomatic individuals are identified by newborn screening, the need for bone marrow transplant (BMT) centers with matched donors and potentially gene therapy centers for BMT is anticipated to increase. These ongoing needs in this growing patient population raise the concern about the availability of subspecialty centers and matched donors to provide timely treatment to these individuals.
Currently, HSCT is offered after the detection of cerebral disease by brain MRI. Given the severity of the cerebral phenotype and advancements in the safety of BMT, treatment might even be considered in the presymptomatic period, especially regarding gene therapy with autologous stem cell transplantation. The risk of morbidity and mortality associated with HSCT must be balanced with the potential benefits of early treatment. In addition, little is known about the effect of treatment on the development of myelopathy in later life. While HSCT therapy does not prevent the development of myelopathy in adulthood, additional follow-up studies are needed to determine if there is a disease-modifying effect on the phenotype (87). The risks of gene therapy with autologous HSCT are likely to be far lower than traditional heterologous HSCT, due to the milder conditioning regimens needed and the avoidance of GVHD. This treatment might therefore be ideal for presymptomatic patients with ALD. However, although the initial results of the Starbeam study are hopeful (13), more time is needed to determine the long-term efficacy of this therapy. Of note, there has been no published follow-up of 2 patients who received similar gene therapy with autologous HSCT 10 years ago (59).
Clinical management of women with ALD who are identified on NBS has not been established. In New York State, Minnesota, and North Carolina, the ALD status for female infants is disclosed to facilitate testing and timely diagnosis of at-risk family members. However, routine disclosure at birth can be controversial given ALD in females is considered to be an adult-onset disorder without current treatment options. Given the high prevalence of symptoms in women with ALD, appropriate clinical monitoring for myelopathy and consideration of treatment for select cases will likely be an active area of investigation and discussion.
Acknowledgments
The authors thank Dr Stephan Kemp and adrenoleukodystrophy.info for the use of figures, and Dr David T. Breault for his critical review of the manuscript.
Financial Support: J.Z. is supported by grant 5T32DK007699 from National Institute of Diabetes and Digestive and Kidney Diseases.
Glossary
Abbreviations
- ALD
adrenoleukodystrophy
- AMD
adrenomyeloneuropathy
- AMPKα1
AMP-activated protein kinase α1
- CCALD
childhood cerebral ALD
- CNS
central nervous system
- FDA
American Food and Drug Administration
- γ-RVs
γ-retroviral vectors
- GVHD
graft-versus-host disease
- HSCT
hematopoietic stem cell transplantation
- HSPCs
hematopoietic stem/progenitor cells
- LC
liquid chromatography
- LV
lentiviral vectors
- LXR
liver X receptor
- LPS
lysophosphatidylcholine
- MFD
major functional disability
- MRI
magnetic resonance imaging
- MS/MS
tandem mass spectrometry
- NIPT
noninvasive prenatal testing
- PPARγ
peroxisome proliferator-activated receptor gamma
- SIN
self-inactivating
- SIRT1
sirtuin 1
- VLCFA
very long-chain fatty acids
- VSVg
envelope protein-g of the vesicular stomatitis virus
Additional Information
Disclosure Summary: J.Z. and J.M. have nothing to declare. F.E. reports grant support from Bluebird Bio for the Starbeam Study and is a member of the Scientific Advisory Board of the United Leukodystrophy Foundation. C.D. reports personal fees from Blueblue Bio. A.B. is a member of the Scientific Advisory Board of Orchard Therapeutics, a member of the Medical Advisory Board for AAVP biosystems, and the Founder and Scientific Advisory Board Member of Altheia Science. D.W. reports grant support and nonfinancial support from Bluebird Bio for the Starbeam Study and outside of the study, and holds a patent related to compositions and methods to treating hemoglobinopathies (PCT/US15/27527) licensed to Bluebird Bio.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Igarashi M, Schaumburg HH, Powers J, et al. . Fatty acid abnormality in adrenoleukodystrophy. J Neurochem. 1976;26(4):851–860. [DOI] [PubMed] [Google Scholar]
- 2. Moser HW, Moser AB, Frayer KK, et al. . Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology. 1981;31(10):1241–1249. [DOI] [PubMed] [Google Scholar]
- 3. Mosser J, Douar AM, Sarde CO, et al. . Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361(6414):726–730. [DOI] [PubMed] [Google Scholar]
- 4. Wiesinger C, Eichler FS, Berger J. The genetic landscape of X-linked adrenoleukodystrophy: inheritance, mutations, modifier genes, and diagnosis. Appl Clin Genet. 2015;8:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kemp S, Pujol A, Waterham HR, et al. . ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat. 2001;18(6):499–515. [DOI] [PubMed] [Google Scholar]
- 6. Kemp S, Huffnagel IC, Linthorst GE, et al. . Adrenoleukodystrophy - neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol. 2016;12(10):606–615. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro E, Krivit W, Lockman L, et al. . Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356(9231):713–718. [DOI] [PubMed] [Google Scholar]
- 8. Miller WP, Rothman SM, Nascene D, et al. . Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118(7):1971–1978. [DOI] [PubMed] [Google Scholar]
- 9. Raymond GV, Aubourg P, Paker A, et al. . Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(3):538–548. [DOI] [PubMed] [Google Scholar]
- 10. Vogel BH, Bradley SE, Adams DJ, et al. . Newborn screening for X-linked adrenoleukodystrophy in New York State: diagnostic protocol, surveillance protocol and treatment guidelines. Mol Genet Metab. 2015;114(4):599–603. [DOI] [PubMed] [Google Scholar]
- 11. Kemper AR, Brosco J, Comeau AM, et al. . Newborn screening for X-linked adrenoleukodystrophy: evidence summary and advisory committee recommendation. Genet Med. 2017;19(1):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiens K, Berry SA, Choi H, et al. . A report on state-wide implementation of newborn screening for X-linked Adrenoleukodystrophy. Am J Med Genet A. 2019;179(7):1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eichler F, Duncan C, Musolino PL, et al. . Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubey P, Raymond GV, Moser AB, et al. . Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. 2005;146(4):528–32. [DOI] [PubMed] [Google Scholar]
- 15. Huffnagel IC, Laheji FK, Aziz-Bose R, et al. . The natural history of adrenal insufficiency in X-linked adrenoleukodystrophy: an international collaboration. J Clin Endocrinol Metab. 2019;104(1):118–126. [DOI] [PubMed] [Google Scholar]
- 16. Eng L, Regelmann MO. Early onset primary adrenal insufficiency in males with adrenoleukodystrophy: case series and literature review. J Pediatr. 2019;211:211–214. [DOI] [PubMed] [Google Scholar]
- 17. Laureti S, Aubourg P, Calcinaro F, et al. . Etiological diagnosis of primary adrenal insufficiency using an original flowchart of immune and biochemical markers. J Clin Endocrinol Metab. 1998;83(9):3163–3168. [DOI] [PubMed] [Google Scholar]
- 18. Laureti S, Casucci G, Santeusanio F, et al. . X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison’s disease in young adult male patients. J Clin Endocrinol Metab. 1996;81(2):470–4. [DOI] [PubMed] [Google Scholar]
- 19. Guran T, Buonocore F, Saka N, et al. . Rare causes of primary adrenal insufficiency: genetic and clinical characterization of a large nationwide cohort. J Clin Endocrinol Metab. 2016;101(1):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson AB, Schaumburg HH, Powers JM. Histochemical characteristics of the striated inclusions of adrenoleukodystrophy. J Histochem Cytochem. 1976;24(6):725–730. [DOI] [PubMed] [Google Scholar]
- 21. Powers JM, Schaumburg HH, Johnson AB, Raine CS. A correlative study of the adrenal cortex in adreno-leukodystrophy–evidence for a fatal intoxication with very long chain saturated fatty acids. Invest Cell Pathol. 1980;3(4):353–376. [PubMed] [Google Scholar]
- 22. Powers JM, Schaumburg HH. Adreno-leukodystrophy (sex-linked Schilder’s disease). A pathogenetic hypothesis based on ultrastructural lesions in adrenal cortex, peripheral nerve and testis. Am J Pathol. 1974;76(3):481–491. [PMC free article] [PubMed] [Google Scholar]
- 23. Tiemensma J, Andela CD, Kaptein AA, et al. . Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: cross-sectional study and review of the literature. Eur J Endocrinol. 2014;171(2):171–182. [DOI] [PubMed] [Google Scholar]
- 24. Ho W, Druce M. Quality of life in patients with adrenal disease: a systematic review. Clin Endocrinol (Oxf). 2018;89(2):119–128. [DOI] [PubMed] [Google Scholar]
- 25. Liberato AP, Mallack EJ, Aziz-Bose R, et al. . MRI brain lesions in asymptomatic boys with X-linked adrenoleukodystrophy. Neurology. 2019;92(15):e1698–e1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Beer M, Engelen M, van Geel BM. Frequent occurrence of cerebral demyelination in adrenomyeloneuropathy. Neurology. 2014;83(24):2227–2231. [DOI] [PubMed] [Google Scholar]
- 27. van Geel BM, Bezman L, Loes DJ, et al. . Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol. 2001;49(2):186–94. [DOI] [PubMed] [Google Scholar]
- 28. Eichler F, Mahmood A, Loes D, et al. . Magnetic resonance imaging detection of lesion progression in adult patients with X-linked adrenoleukodystrophy. Arch Neurol. 2007;64(5):659–664. [DOI] [PubMed] [Google Scholar]
- 29. Engelen M, Barbier M, Dijkstra IM, et al. . X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain. 2014;137(Pt 3):693–706. [DOI] [PubMed] [Google Scholar]
- 30. Habekost CT, Pereira FS, Vargas CR, et al. . Progression rate of myelopathy in X-linked adrenoleukodystrophy heterozygotes. Metab Brain Dis. 2015;30(5):1279–1284. [DOI] [PubMed] [Google Scholar]
- 31. Huffnagel IC, Dijkgraaf MGW, Janssens GE, et al. . Disease progression in women with X-linked adrenoleukodystrophy is slow. Orphanet J Rare Dis. 2019;14(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finsterer J, Lässer S, Stöphasius E. Dementia from the ABCD1 mutation c.1415-1416delAG in a female carrier. Gene. 2013;530(1):155–157. [DOI] [PubMed] [Google Scholar]
- 33. Moser AB, Kreiter N, Bezman L, et al. . Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45(1):100–110. [DOI] [PubMed] [Google Scholar]
- 34. Steinberg S, Jones R, Tiffany C, et al. . Investigational methods for peroxisomal disorders. Curr Protoc Hum Genet. 2008;58(1):17.6.1–17.6.23. [DOI] [PubMed] [Google Scholar]
- 35. Theda C, Woody RC, Naidu S, et al. . Increased very long chain fatty acids in patients on a ketogenic diet: a cause of diagnostic confusion. J Pediatr. 1993;122(5 Pt 1):724–726. [DOI] [PubMed] [Google Scholar]
- 36. Stradomska TJ, Bachański M, Pawłowska J, et al. . The impact of a ketogenic diet and liver dysfunction on serum very long-chain fatty acids levels. Lipids. 2013;48(4):405–409. [DOI] [PubMed] [Google Scholar]
- 37. Valianpour F, Selhorst JJM, van Lint LEM, et al. . Analysis of very long-chain fatty acids using electrospray ionization mass spectrometry. Mol Genet Metab. 2003;79(3):189–196. [DOI] [PubMed] [Google Scholar]
- 38. ALD info website ProMED-mail website. https://adrenoleukodystrophy.info/. Updated March 4, 2020. Accessed March 13, 2020.
- 39. O’Neill GN, Aoki M, Brown RH Jr. ABCD1 translation-initiator mutation demonstrates genotype-phenotype correlation for AMN. Neurology. 2001;57(11):1956–1962. [DOI] [PubMed] [Google Scholar]
- 40. Horn MA, Retterstøl L, Abdelnoor M, et al. . Adrenoleukodystrophy in Norway: high rate of de novo mutations and age-dependent penetrance. Pediatr Neurol. 2013;48(3):212–219. [DOI] [PubMed] [Google Scholar]
- 41. Lee S, Clinard K, Young SP, et al. . Evaluation of X-linked adrenoleukodystrophy newborn screening in North Carolina. JAMA Netw Open. 2020;3(1):e1920356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caggana M. Update on X-ALD in the New York Laboratory 2018. https://www.aidanhasaposse.org/uploads/7/3/6/5/73650801/new_york_ald_nbs_update_michele_caggana_sc.d._facmg.pdfggana_sc.d._facmg.pdf. Accessed November 1, 2019.
- 43. Bezman L, Moser AB, Raymond GV, et al. . Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49(4):512–517. [PubMed] [Google Scholar]
- 44. Bessey A, Chilcott JB, Leaviss J, Sutton A. Economic impact of screening for X-linked Adrenoleukodystrophy within a newborn blood spot screening programme. Orphanet J Rare Dis. 2018;13(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maier EM, Roscher AA, Kammerer S, et al. . Prenatal diagnosis of X-linked adrenoleukodystrophy combining biochemical, immunocytochemical and DNA analyses. Prenat Diagn. 1999;19(4):364–368. [PubMed] [Google Scholar]
- 46. Lan F, Wang Z, Ke L, et al. . A rapid and sensitive protocol for prenatal molecular diagnosis of X-linked adrenoleukodystrophy. Clin Chim Acta. 2010;411(23–24):1992–1997. [DOI] [PubMed] [Google Scholar]
- 47. Handyside AH, Kontogianni EH, Hardy K, et al. . Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344(6268):768–70. [DOI] [PubMed] [Google Scholar]
- 48. Gigarel N, Frydman N, Burlet P, et al. . Single cell co-amplification of polymorphic markers for the indirect preimplantation genetic diagnosis of hemophilia A, X-linked adrenoleukodystrophy, X-linked hydrocephalus and incontinentia pigmenti loci on Xq28. Hum Genet. 2004;114(3):298–305. [DOI] [PubMed] [Google Scholar]
- 49. Lledó B, Bernabeu R, Ten J, et al. . Preimplantation genetic diagnosis of X-linked adrenoleukodystrophy with gender determination using multiple displacement amplification. Fertil Steril. 2007;88(5):1327–33. [DOI] [PubMed] [Google Scholar]
- 50. Iglesias M, Ceballos P, Giménez C, et al. . Pregnancy outcome after preimplantation genetic diagnosis in an affected couple with X-linked adrenoleukodystrophy. Fertil Steril. 2008;90(5):2010.e1-3. [DOI] [PubMed] [Google Scholar]
- 51. Khattab A, Yuen T, Sun L, et al. . Noninvasive prenatal diagnosis of congenital adrenal hyperplasia. Endocr Dev. 2016;30:37–41. [DOI] [PubMed] [Google Scholar]
- 52. Skrzypek H, Hui L. Noninvasive prenatal testing for fetal aneuploidy and single gene disorders. Best Pract Res Clin Obstet Gynaecol. 2017;42:26–38. [DOI] [PubMed] [Google Scholar]
- 53. Regelmann MO, Kamboj MK, Miller BS, et al. . Adrenoleukodystrophy: guidance for adrenal surveillance in males identified by newborn screen. J Clin Endocrinol Metab. 2018;103(11):4324–4331. [DOI] [PubMed] [Google Scholar]
- 54. Ueland GÅ, Methlie P, Øksnes M, et al. . The short cosyntropin test revisited: new normal reference range using LC-MS/MS. J Clin Endocrinol Metab. 2018;103(4):1696–1703. [DOI] [PubMed] [Google Scholar]
- 55. Horowitz SD, Bach FH, Groshong T, et al. . Treatment of severe combined immunodeficiency with bone-marrow from an unrelated, mixed-leucocyte-culture-non-reactive donor. Lancet. 1975;2(7932):431–433. [DOI] [PubMed] [Google Scholar]
- 56. Aubourg P, Blanche S, Jambaque I, et al. . Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322(26):1860–1866. [DOI] [PubMed] [Google Scholar]
- 57. Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. . A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348(3):255–256. [DOI] [PubMed] [Google Scholar]
- 58. McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2004;350(9):913–922. [DOI] [PubMed] [Google Scholar]
- 59. Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. . Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. [DOI] [PubMed] [Google Scholar]
- 60. Mahmood A, Raymond GV, Dubey P, et al. . Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: a comparison study. Lancet Neurol. 2007;6(8):687–692. [DOI] [PubMed] [Google Scholar]
- 61. Loes DJ, Fatemi A, Melhem ER, et al. . Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. 2003;61(3):369–374. [DOI] [PubMed] [Google Scholar]
- 62. Melhem ER, Loes DJ, Georgiades CS, et al. . X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000;21(5):839–844. [PMC free article] [PubMed] [Google Scholar]
- 63. Orchard PJ, Blazar BR, Wagner J, et al. . Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151(4):340–436. [DOI] [PubMed] [Google Scholar]
- 64. Peters C, Charnas LR, Tan Y, et al. . Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104(3):881–888. [DOI] [PubMed] [Google Scholar]
- 65. Mitchell R, Nivison-Smith I, Anazodo A, et al. . Outcomes of haematopoietic stem cell transplantation for inherited metabolic disorders: a report from the Australian and New Zealand Children’s Haematology Oncology Group and the Australasian Bone Marrow Transplant Recipient Registry. Pediatr Transplant. 2013;17(6):582–588. [DOI] [PubMed] [Google Scholar]
- 66. Beam D, Poe MD, Provenzale JM, et al. . Outcomes of unrelated umbilical cord blood transplantation for X-linked adrenoleukodystrophy. Biol Blood Marrow Transplant. 2007;13(6):665–674. [DOI] [PubMed] [Google Scholar]
- 67. Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94(8):4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shahryari A, Saghaeian Jazi M, Mohammadi S, et al. . Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet. 2019;10:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. [DOI] [PubMed] [Google Scholar]
- 70. Kaufmann KB, Büning H, Galy A, et al. . Gene therapy on the move. EMBO Mol Med. 2013;5(11):1642–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Naldini L, Trono D, Verma IM. Lentiviral vectors, two decades later. Science. 2016;353(6304):1101–1102. [DOI] [PubMed] [Google Scholar]
- 72. Capotondo A, Milazzo R, Politi LS, et al. . Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc Natl Acad Sci U S A. 2012;109(37):15018–15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Biffi A, Aubourg P, Cartier N. Gene therapy for leukodystrophies. Hum Mol Genet. 2011;20(R1):R42–R53. [DOI] [PubMed] [Google Scholar]
- 74. Biffi A, Montini E, Lorioli L, et al. . Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. [DOI] [PubMed] [Google Scholar]
- 75. Yengi L, Parsons G, Pasackow E, et al. . Evidence that prevalent SMG6 insertions are not associated with abnormal clinical findings in patients treated with lenti-D autologous hematopoietic stem cell gene therapy for cerebral adrenoleukodystrophy. Paper presented at: Program of the 27th Annual Congress of the European Society for Gene and Cell Therapy; 2019; Barcelona, Spain, 164. (Abstract P264). [Google Scholar]
- 76. Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14(1):9–21. [DOI] [PubMed] [Google Scholar]
- 77. Markmann S, De BP, Reid J, et al. . Biology of the adrenal gland cortex obviates effective use of adeno-associated virus vectors to treat hereditary adrenal disorders. Hum Gene Ther. 2018;29(4):403–412. [DOI] [PubMed] [Google Scholar]
- 78. Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168(1–2):20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anzalone AV, Randolph PB, Davis JR, et al. . Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lerario AM, Finco I, LaPensee C, et al. . Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Front Endocrinol (Lausanne). 2017;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moser HW, Raymond GV, Lu SE, et al. . Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo’s oil. Arch Neurol. 2005;62(7):1073–1080. [DOI] [PubMed] [Google Scholar]
- 82. Engelen M, Ofman R, Dijkgraaf MG, et al. . Lovastatin in X-linked adrenoleukodystrophy. N Engl J Med. 2010;362(3):276–277. [DOI] [PubMed] [Google Scholar]
- 83. Morató L, Ruiz M, Boada J, et al. . Activation of sirtuin 1 as therapy for the peroxisomal disease adrenoleukodystrophy. Cell Death Differ. 2015;22(11):1742–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morató L, Galino J, Ruiz M, et al. . Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain. 2013;136(Pt 8):2432–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fourcade S, Ruiz M, Guilera C, et al. . Valproic acid induces antioxidant effects in X-linked adrenoleukodystrophy. Hum Mol Genet. 2010;19(10): 2005–2014. [DOI] [PubMed] [Google Scholar]
- 86. Singh J, Olle B, Suhail H, et al. . Metformin-induced mitochondrial function and ABCD2 up-regulation in X-linked adrenoleukodystrophy involves AMP-activated protein kinase. J Neurochem. 2016;138(1):86–100. [DOI] [PubMed] [Google Scholar]
- 87. van Geel BM, Poll-The BT, Verrips A, et al. . Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J Inherit Metab Dis. 2015;38(2):359–361. [DOI] [PubMed] [Google Scholar]