Abstract
Follicle development is the most crucial step toward female fertility and is controlled mainly by follicle-stimulating hormone (FSH). In ovarian granulosa cells (GCs), FSH activates protein kinase A by increasing 3′,5′-cyclic adenosine 5′-monophosphate (cAMP). Since cAMP signaling is impinged in part by salt-inducible kinases (SIKs), we examined the role of SIKs on the regulation of FSH actions. Here, we report that SIKs are essential for normal ovarian function and female fertility. All SIK isoforms are expressed in human and rodent GCs at different levels (SIK3>SIK2>SIK1). Pharmacological inhibition of SIK activity potentiated the stimulatory effect of FSH on markers of GC differentiation in mouse, rat, and human GCs and estradiol production in rat GCs. In humans, SIK inhibition strongly enhanced FSH actions in GCs of patients with normal or abnormal ovarian function. The knockdown of SIK2, but not SIK1 or SIK3, synergized with FSH on the induction of markers of GC differentiation. SIK inhibition boosted gonadotropin-induced GC differentiation in vivo, while the genomic knockout of SIK2 led to a significant increase in the number of ovulated oocytes. Conversely, SIK3 knockout females were infertile, FSH insensitive, and had abnormal folliculogenesis. These findings reveal novel roles for SIKs in the regulation of GC differentiation and female fertility, and contribute to our understanding of the mechanisms regulated by FSH. Furthermore, these data suggest that specific pharmacological modulation of SIK2 activity could be of benefit to treat ovulatory defects in humans and to increase the propagation of endangered species and farm mammals.
Keywords: ovary, granulosa cells, SIKs, fertility, steroidogenesis
In humans, infertility affects approximately 15% of couples (1). Moreover, women postponing maternity face the natural limits of their reproductive system (2). A frequent and increasing problem restricting fertility is anovulation (3); as a result, the population of women undergoing ovulation induction and controlled ovarian stimulation has expanded worldwide (4). Therefore, controlling negative regulators of ovulation could reduce the burden of infertility.
The somatic cells of the ovarian follicle, namely the granulosa cells (GCs), play a vital role in the coordination of folliculogenesis by integrating the oocyte, theca cells, and pituitary signals. The differentiation of GCs from the preantral stage into mural and cumulus cells of the preovulatory follicle and later the luteinization of mural cells are essential for the coordination of ovulation, fertilization, and uterine receptivity. As nurse cells, cumulus GCs are necessary for oocyte maturation and survival (5). As endocrine cells, mural GCs contribute to the coordination of ovulation by producing large amounts of estradiol, particularly toward the middle of the ovarian cycle (6). Antral follicle development, GC differentiation, and estradiol production depend on the activity of follicle-stimulating hormone (FSH). Consequently, FSH is the primary hormone used to stimulate follicle growth in women undergoing assisted reproductive technology. However, an important clinical problem is that the response of patients to FSH varies widely, ranging from strong to poor (7). A poor or inadequate response to FSH results in fewer oocytes and lower pregnancy rates (8). Currently, the most common clinical approach to improving pregnancy rates is to try higher doses of FSH (9), even though this approach has no clear advantage and could lead to ovarian hyperstimulation (10, 11). There is, therefore, an urgent need to identify limiting factors that, when inhibited, could enhance the response of GCs to FSH.
In females, FSH targets exclusively ovarian GCs (12). Thus, we investigated factors that might negatively regulate FSH signaling in GCs. FSH activates receptor-associated Gα proteins, which stimulate adenylate cyclase activity and the production of 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) (13). Analysis of the literature for potential factors that might regulate FSH signaling revealed a subfamily of AMP-activated protein kinases, salt-inducible kinases (SIKs), as plausible candidates. SIKs, whose name is derived from findings showing that the expression of SIK1 (the first member described) is upregulated by dietary salt intake in the adrenal gland (14), are serine/threonine kinases. Their major biological role is to control gene expression in response to extracellular cues that increase intracellular levels of cAMP (15, 16). The physiological significance of SIKs in cAMP signaling is exemplified by their regulation of several cAMP-centered systems. For instance, in macrophages, SIKs oppose cAMP signaling stimulated by prostaglandin E2 (17). In osteocytes, inhibition of SIKs mimics the effects of parathyroid hormone, which is known to activate cAMP signaling (18). In hepatocytes, glucagon-cAMP/protein kinase A (PKA) induction of gluconeogenic genes is accompanied by inactivation of SIK activity (19). In melanocytes, SIK inhibition strongly induces melanin synthesis (darker pigmentation), which is known to be controlled by cAMP (20). Finally, in adrenal cells, SIK activity inhibition is sufficient to increase the expression of steroidogenic genes including steroidogenic acute regulator (Stard1, most commonly referred to as StAR), CYP11a1 (most commonly referred to as P450scc), and lipoprotein receptors (21-26).
The reported capacity of SIKs to regulate cAMP signaling led to the hypothesis that SIKs influence the response of GCs to FSH. In this report, we describe the expression of all the members of the SIK family in rodent and human GCs and reveal that SIK2 represses FSH actions and folliculogenesis while SIK3 supports ovarian function.
Materials and Methods
Cell cultures
Human cells were collected as previously described (27-29) from the follicular aspirates of women undergoing in vitro fertilization treatment at the Fertility Centers of Illinois, under Institutional Review Board approval. Informed consent was obtained from all women. In a subset of patients, we collected additional information to compare the effect of SIK inhibition in patients with different etiologies. Cells were treated with human recombinant FSH (Serono) with or without specific inhibitors of SIKs: HG-9-91-01, MRT67307, or compound C, all obtained from Tocris (Bristol, UK). Cells from each patient were cultured separately; thus, each data point reflects a result obtained from an individual patient. Rat and mouse GCs were isolated from immature animals treated with estradiol for 3 days and cultured as described previously (30-32). Rat and mouse experiments were approved by the Biologic Resources Laboratory of the University of Illinois at Chicago.
In vivo treatment with SIK inhibitors
Mice between 21 and 23 days of age were injected with YKL-05-099 (10 mg/kg intraperitoneally) suspended in phosphate-buffered saline (PBS). YKL-05-099 is stable in mouse liver microsomes for >2 hours (33), thus we reasoned that a 2-hour SIK inhibitor pretreatment concurrent with FSH treatment would have a maximal inhibitory impact on SIK activity. Therefore, 2 hours later, mice were injected again with the same dose of YKL-05-099 along with 4 IU of equine chorionic gonadotropin (eCG, Sigma, San Louis, MO), which has FSH activity. In this experiment, we used a reduced dose of 4 IU of eCG to determine if SIK inhibition enhances FSH action, as a higher eCG dose could saturate the response and mask any effects attributable to the absence of SIK activity. Controls were injected with 4 IU of eCG alone. GCs were collected 48 hours after eCG.
RNA isolation and quantification
Total ribonucleic acid (RNA) was isolated using TRIzol (Invitrogen) and reverse-transcribed using anchored oligo-dT primers (IDT, Coralville, IA) and Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen, Carlsbad, CA). Intron-spanning primers were used to amplify the gene of interest (GOI) along with a standard curve containing serial dilutions of the complementary deoxyribonucleic acid (cDNA) of the GOI. For each sample, the number of copies per microliter of cDNA was computed for each GIO, and the ribosomal protein L19 mRNA (Rpl19). The expression of each GOI is reported as the ratio between the number of copies of the GOI and Rpl19.
Promoter reporter assays
The CYP19ov-Luc reporter was generated by cloning the +1 to –320 region of the human CYP19A1 ovarian promoter followed by the firefly luciferase cDNA. Lentivirus containing this construct was generated as previously described (34). Empty plasmids were used as controls. Cells were infected with lentivirus and after overnight incubation treated as described above for 48 hours. Luciferase activity was determined in 50 μL of lysates and expressed as relative to renilla luciferase, as previously described (34).
Immunoblotting
Cells were harvested in ice-cold RIPA lysis buffer supplemented with protease inhibitors. Western blotting was performed using antibodies against CYP19A1 (35), and beta-actin (36)
Estradiol measurement
Undiluted cell culture medium was used for estradiol level determinations by enzyme-linked immunosorbent assay (37).
Immunohistochemistry and ovarian histology
Rat ovaries were embedded in paraffin to prepare 5-μm sections, which were stained using primary antibodies diluted in PBS: SIK1 (38), SIK2 (39), SIK3 (40). Antibodies were detected using Vectastain Elite (Vector Laboratories, Burlingame, CA) and counterstained with Gill’s hematoxylin. The same antibodies were used for immunofluorescence staining of cultured GCs, with the exception that visualization was performed using Cy3-conjugated streptavidin (Jackson, West Grove, PA) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen). Histological studies of ovarian sections were performed after hematoxylin and eosin staining.
Knockout mice
The generation of the genomic knockout SIK1, SIK2, or SIK3 in mice has been described previously (20, 41). For ovulation studies, 25- to 27-day-old knockout mice and wild-type mice were injected intraperitoneally with 7.5 IU of eCG to stimulate follicular development maximally, followed 48 hours later by the injection of 7.5 IU of human chorionic gonadotropin (hCG), which stimulates ovulation. Ovulated cumulus–oocyte complexes were collected from oviducts 17 hours after hCG injection and counted. For breeding studies, knockout or control females were mated with fertile males for 6 months. Cages were inspected daily, and parturition dates and litter sizes recorded. The National Institute of Biomedical Innovation of Japan and The University of Illinois approved all experiments with knockout mice.
Statistics
Data were analyzed using Prism 6 (San Diego, CA). Differences between 2 groups were determined by Student’s t-test. For multiple groups, 1-way analysis of variance (ANOVA) was used, and differences between individual means were determined by the Tukey test. Data are represented as mean ± standard error of the mean (SEM). Significant differences were recognized at P < .05.
Results
Expression of SIK isoforms in GCs and the ovary
Since the expression of SIKs in GCs has not been previously investigated, we first quantified the mRNA levels of SIK isoforms in rat and human GCs. The mRNA for all isoforms was detected in the GCs of both species, although the relative expression of Sik1 and Sik2 mRNAs was lower than the expression of Sik3 (Fig. 1A). Western blot analyses demonstrated the presence of all SIK isoforms in rat GCs and showed that treatment with FSH does not affect the expression levels of these kinases (Fig. 1B). The expression of SIKs in rat GCs was further visualized using immunofluorescence, which showed a strong signal for SIK2 and SIK3, while the SIK1 signal was significantly lower (Fig. 1C).
Figure 1.
Expression of SIKs in rat and human GCs. (A) SIK1, SIK2, and SIK3 mRNA levels in rat and human GCs expressed as relative expression (RE) to ribosomal L19 protein (Rpl19). Bars represent mean ± SEM, N ≥ 10. (B) Representative blots of SIK1, SIK2, and SIK3 protein levels in rat GCs treated with vehicle (C) or FSH (50 ng/mL) for 48 hours, N = 3. BACT, β-actin; MM, protein molecular marker in kDa. (C) Representative immunofluorescent imaging of SIK1, SIK2, and SIK3 (green) in cultured primary rat GCs (N = 3). Nuclei were stained with DAPI (blue).
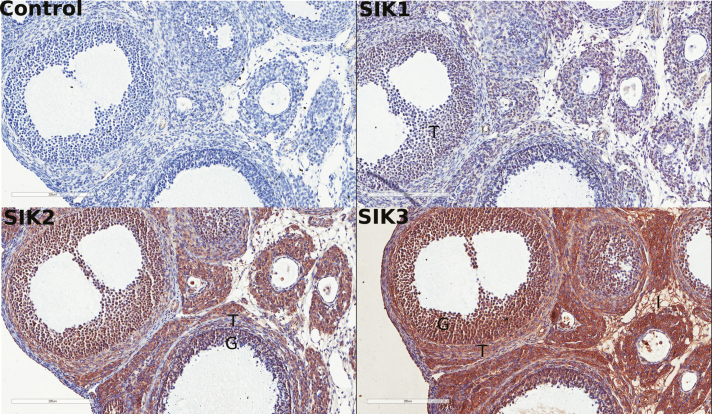
Immunohistochemical studies of rat ovaries showed a robust signal for SIK2 and SIK3 proteins while the SIK1 protein signal was substantially lower (Fig. 2), mirroring our observations at the mRNA level in rat GCs. These studies also showed that GCs express both SIK2 and SIK3, while the interstitial tissue and the theca cells express mostly SIK3 (Fig. 2).
Figure 2.
Expression of SIKs in the rat ovary. Representative stains of SIK1, SIK2, and SIK3 (brown) in rat ovaries. Slides were counterstained with hematoxylin (blue), N = 5.
Pharmacological inhibition of SIK activity enhances FSH actions in rats and humans
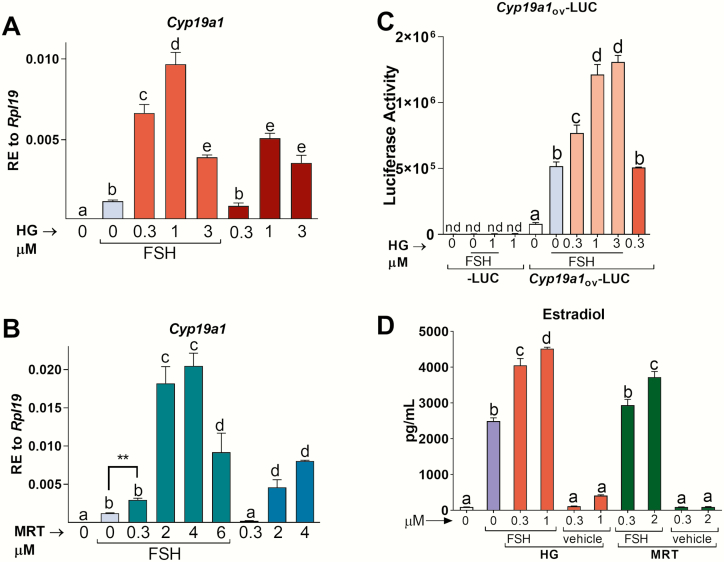
To test whether SIKs regulate GC function, we ablated their activity using HG-9-91-01 (HG), a SIK inhibitor whose potency and specificity toward SIKs has been extensively characterized (42). FSH actions center on the stimulation of the Cyp19a1 gene, which encodes for the estrogen-producing enzyme CYP19A1 (most commonly called aromatase) (43). Therefore, we used the expression of Cyp19a1 to evaluate the effects of SIK inhibition on FSH actions. In rat GCs, SIK inhibition potentiated FSH stimulation of aromatase in a concentration-dependent manner (Fig. 3A); however, a stronger potentiation was observed with the lower concentrations (0.3 and 1 µM) than with the higher concentration (3 µM) of HG. In the absence of FSH, treatment with 1 or 3 µM HG also increased Cyp19a1 mRNA levels. Comparable findings were seen using MRT67307 (MRT), another SIK inhibitor (Fig. 3B).
Figure 3.
SIK inhibition enhances FSH actions in primary rat GCs. Rat GCs were treated with vehicle, FSH, FSH plus (A) HG-9-91-01 (HG) or (B) MRT67307 (MRT), or SIK inhibitors alone. Cyp19a1 mRNA levels were quantified 48 hours later and expressed as relative expression (RE) to Rpl19. (C) Lentivirus was used to deliver an empty luciferase reporter or a reporter carrying the ovarian Cyp19a1 promoter. Cells were treated with vehicle, FSH, FSH plus HG, or HG alone 48 h after the addition of the virus. Luciferase activity was quantified 48 h after the initiation of treatments. (D) Rat GCs were treated with vehicle, FSH, FSH plus HG or MRT, or SIK inhibitors alone. Estradiol concentration in the media was determined by enzyme-linked immunosorbent assay 48 hours after the initiation of treatments. Different letters represent significant differences between groups, 1-way ANOVA followed by Tukey, P < .05, N ≥ 5. **P = .0012, t-test. nd, non-detectable.
To determine whether the effect of SIK inhibition on Cyp19a1 mRNA levels is mediated by an increase in transcription, rat GCs were infected with a reporter controlled by the proximal promoter of the Cyp19a1 gene. Luciferase activity was detectable but low in the absence of FSH, while FSH stimulated reporter activity by 20-fold (Fig. 3C). Cotreatment with the SIK inhibitor HG enhanced the stimulatory effect of FSH on aromatase promoter activity in a concentration-dependent manner. No activity was observed in cells infected with an empty reporter. Treatment with HG alone increased Cyp19a1 promoter activity.
CYP19A1 drives the production of estradiol, a steroid hormone playing a central role in the regulation of all aspects of female reproductive activity. Consequently, we examined the effect of SIK inhibition on estradiol production by rat GCs. Inhibition of SIK activity using either HG or MRT potentiated the stimulation of estradiol production by FSH in a concentration-dependent manner (Fig. 3D).
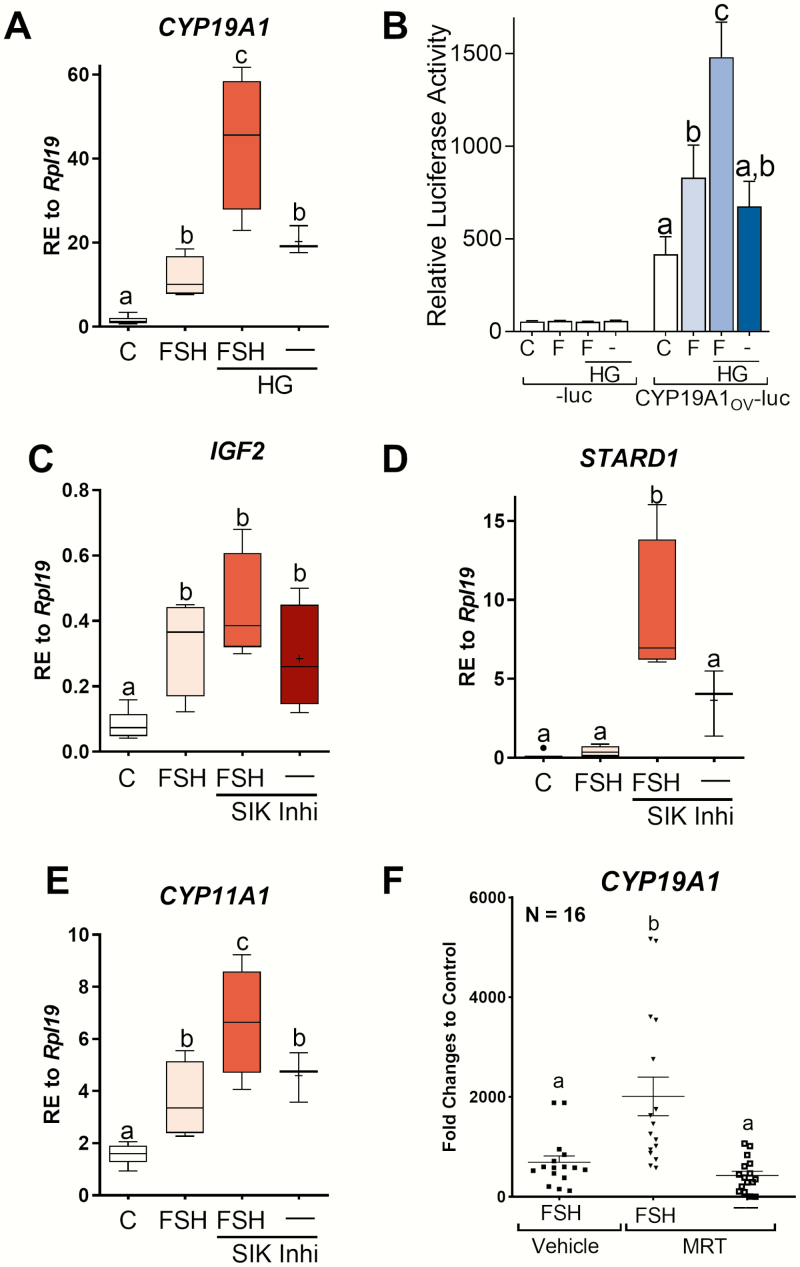
Next, we examined the effect of SIK inhibition in primary human GCs that were isolated and cultured as previously described (27-29). As in rats, SIK inhibition in human GCs potentiated FSH stimulation of CYP19A1 mRNA expression (Fig. 4A) and CYP19A1 promoter activation (Fig. 4B). We also examined the impact of SIK on the expression of insulin-like growth factor 2 (IGF2), which is expressed exclusively in human GCs and strongly stimulated by FSH (28). The addition of HG to the media enhanced the expected stimulatory effect of FSH on IGF2 mRNA expression, although the difference was not statistically significant when compared with cells treated with FSH alone (Fig. 4C). Interestingly, SIK inhibition by HG in the absence of FSH increased IGF2 mRNA levels significantly when compared with controls (Fig. 4C).
Figure 4.
SIK inhibition with HG enhances FSH actions in primary human GCs. (A and B) Aromatase mRNA and promoter activity in primary human GCs after treatment with vehicle, FSH, FSH+HG, or HG alone for 48 hours. HG was used at a 1 µM concentration. (C) IGF2, (D) STARD1, and (E) CYP11A1 relative mRNA expression in primary human GCs after treatment with vehicle, FSH, FSH+HG, or HG alone for 48 hours. Different letters represent significant differences (mean ± SEM, N ≥ 7, 1-way ANOVA followed by Tukey, P < .05). (F) Aromatase expression in primary human GCs after treatment with FSH, FSH+MRT, or MRT alone. Different letters represent significant differences (mean ± SEM, N = 16, 1-way ANOVA followed by Tukey, P < .05).
SIK inhibition also potentiated the stimulatory effect of FSH on STARD1 and CYP11A1 mRNA levels (Fig. 4D and 4E), the expression of which is known to increase after treatment of human GCs with FSH (28, 29). Moreover, as observed for CYP19A1, treatment with HG alone was enough to increase STARD1 and CYP11A1 mRNA levels.
To examine further the role of SIK in human GCs, a larger cohort of patients (n = 16) was used to study the effect of SIK activity inhibition using MRT on CYP19A1 mRNA levels. In this experiment, the combination of FSH plus MRT significantly increased CYP19A1 mRNA levels by 3-fold when compared with cells treated with FSH only (P < .0001) (Fig. 4F). In contrast to HG, treatment with MRT alone did not affect CYP19A1 mRNA levels.
Knockdown of SIK2 enhances FSH actions
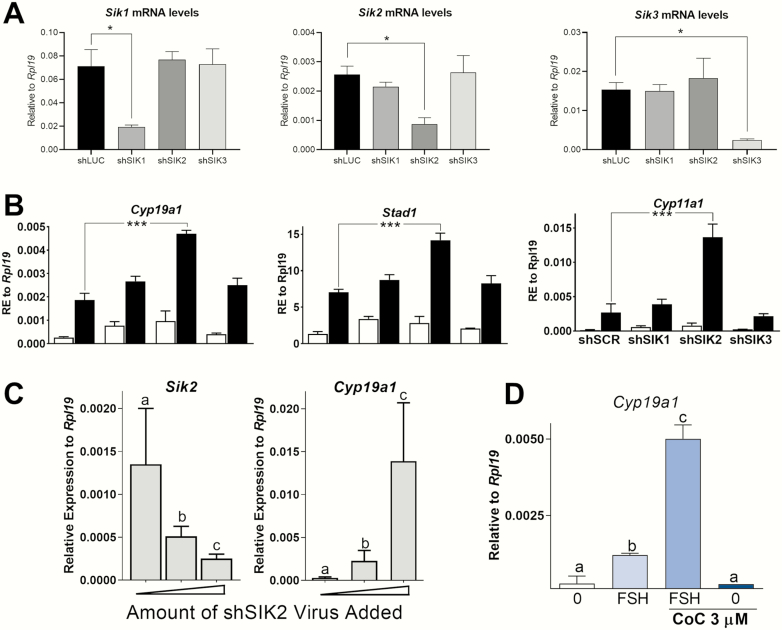
Next, we utilized small interference RNAs to selectively knockdown SIK1, SIK2, or SIK3 and gain insight into the role of individual SIK isoforms. Rat GCs were infected with lentivirus carrying small hairpin (sh)RNA specific for each isoform, as described previously (30). FSH or vehicle was added to the media 48 hours after virus infection. Then, cells were incubated for 48 hours before the determination of gene expression. Each Sik shRNA significantly knocked down its respective SIK isoform when compared with cells infected with a control shRNA (Fig. 5A). Furthermore, each Sik shRNA targeted specifically the intended isoform without affecting the others (Fig. 5A).
Figure 5.
SIK2 knockdown mimics the pharmacological inhibition of SIK activity. (A) SIK isoforms expression in rat GCs exposed to scrambled oligos (shSCR) or anti-SIK1 (shSIK1), SIK2 (shSIK2), or SIK3 (shSIK3) shRNAs. *P < .05, t-test, N = 3. (B) Cyp19a1, Stard1, and Cyp11a1 relative mRNA expression levels after exposure to shSCR, shSIK1, shSIK2, or shSIK3. Cells were treated with vehicle (white bars) or FSH (black bars). ***P < .001, t-test, N = 3. (C) Inverse correlation between sik2 knockdown with Cyp19a1 expression in rat GCs. Cells were exposed to increasing concentrations of shSIK2 for 48 hours and then treated with FSH for an additional 48 hours, N = 3. (D) Cells were treated with Compound C (CoC, a SIK2 inhibitor) for 1 hour before treatment with FSH. Cyp19a1 expression was determined 48 hours after the addition of FSH. Different letters represent significant differences, 1-way ANOVA followed by Tukey, P < .05 (N = 3).
The knockdown of Sik1 or Sik3 had no effect on Cyp19a1, Stard1, or Cyp11a1 mRNA expression in the presence or absence of FSH (Fig. 5B). In contrast, Sik2 knockdown potentiated the stimulatory effect of FSH on all 3 genes, suggesting a leading role for SIK2 in the regulation of the response of GCs to FSH.
To further confirm the role of SIK2 in Cyp19a1 expression, GCs were infected with increasing amounts of anti-Sik2 shRNA (shSIK2) and cultured in the presence of FSH for 48 hours. We observed an increase in Cyp19a1 mRNA levels proportional and concomitant with a decrease in Sik2 mRNA levels (Fig. 5C). Finally, we treated GCs with Compound C (CoC), which has been previously shown to prevent SIK2-mediated suppression of a cAMP reporter without suppressing SIK1 and SIK3 activity (44). Fig. 5D shows that CoC strongly potentiated the stimulatory effect of FSH on Cyp19a1. However, CoC alone failed to upregulate Cyp19a1 mRNA levels.
Effect of SIK inhibition in in vitro fertilization patients with different etiologies
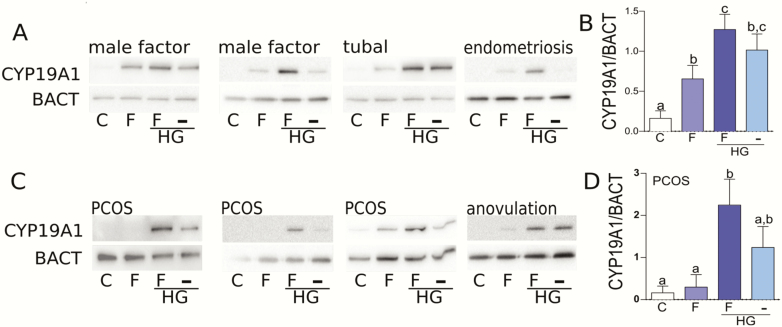
Next, we examined the effect of SIK inhibition using HG at 1 µM on CYP19A1 protein expression in the GCs of patients with normal (tubal, male factors, endometriosis) or abnormal (polycystic ovarian syndrome (PCOS), anovulation) ovarian function. In patients with normal ovarian function, FSH stimulated CYP19A1 protein levels, an effect that was potentiated by the inhibition of SIK activity (Fig. 6A). In the absence of FSH, SIK inhibition stimulated CYP19A1 protein expression in 3 of the 4 patients with normal ovarian function. Fig. 6B depicts the quantification of the data presented in Fig. 6A.
Figure 6.
SIK inhibition rescues FSH actions in human GCs from patients with different etiologies of infertility. (A) Primary GCs were obtained from patients with normal ovarian function or (C) patients diagnosed with PCOS or anovulation. Cells were treated with vehicle, FSH, FSH plus HG, or HG alone for 48 hours. CYP19A1 and β-actin (BACT) protein levels were measured 48 hours after the initiation of treatment by Western blot. The intensity of the CYP19a1 and BACT bands was quantified and the data expressed as a ratio between CYP19A1 and BACT. (B) Quantification of the data presented in Fig. 6A). (D) Quantification data from PCOS patients. Different letters represent significant differences, 1-way ANOVA followed by Tukey test, P < .05, N = 5.
In contrast, FSH was unable to stimulate CYP19A1 in 2 of the 3 patients with PCOS, while SIK inhibition with HG rescued FSH induction of CYP19A1 in these 2 patients (Fig. 6C). In the PCOS patient that responded to FSH alone, the presence of HG potentiated FSH actions. In the patient with anovulation, FSH stimulated CYP19A1 protein levels marginally, while HG alone or in the presence of FSH stimulated CYP19A1 strongly (Fig. 6C). Fig. 6D shows quantification data from PCOS patients only.
Effect of SIK inhibition in mouse GCs in vitro and in vivo
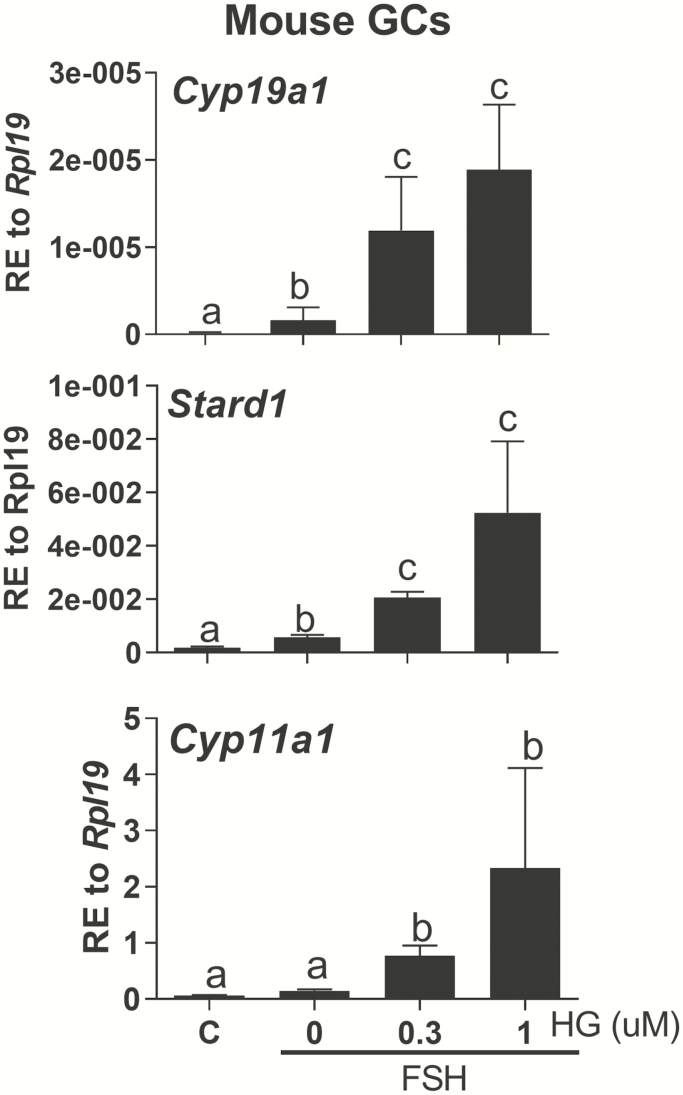
To study the role of SIK in ovarian function using genetic models, we first examined the effect of SIK inhibition on FSH actions in GCs isolated from wild-type mice in vitro. The results of these experiments mirrored those in rat and human GCs. Thus, as shown in Fig. 7, inhibition of SIK activity potentiated the stimulation of Cyp19a1, Stard1, and Cyp11a1 mRNA levels by FSH in a dose-dependent manner.
Figure 7.
Effect of SIK inhibition in mouse GCs. Primary mouse GCs were treated with vehicle, FSH, or FSH plus increasing concentrations of HG. Cyp19a1, Stard1, and Cyp11a1 mRNA levels were quantified 48 hours later and expressed as relative expression (RE) to Rpl19. Different letters represent significant differences (mean ± SEM, N = 5, 1-way ANOVA followed by Tukey, P < .05).
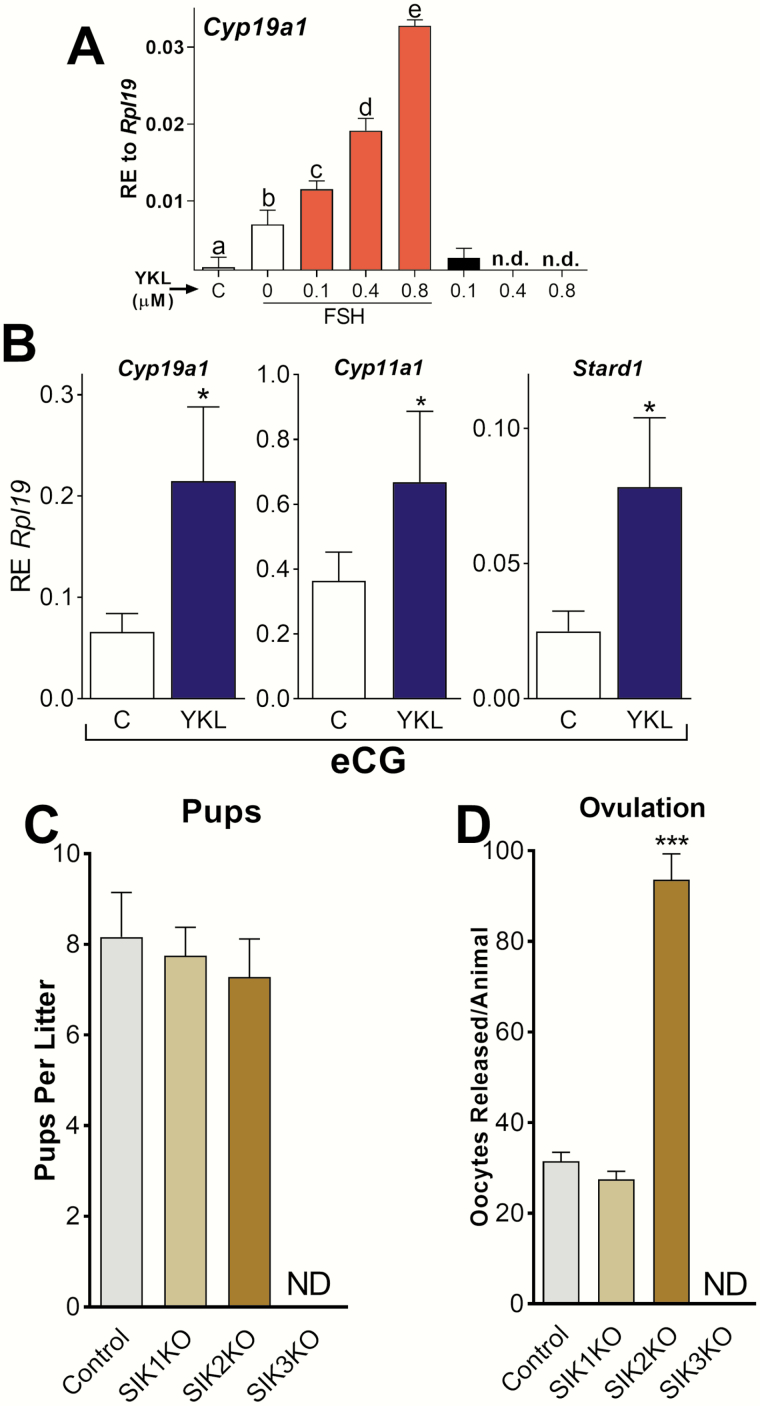
As mouse GCs in vitro responded similarly to rat and human cells, we examined the effect of SIK inhibition in vivo. The inhibitors used above have a short half-life in vivo, and therefore it was necessary to test SIK inhibitors suitable for in vivo studies. We examined the effect of YKL-05-099 (YKL) that achieves free median inhibitory(IC50) serum concentrations for SIK inhibition for more than 16 hours, reduces the phosphorylation of known SIK substrates in vivo, and is more tolerable and soluble than other SIK inhibitors (33). In vitro experiments demonstrated that YKL enhances the stimulatory effect of FSH on Cyp19a1 expression in a concentration-dependent manner (Fig. 8A).
Figure 8.
SIK inhibition enhances FSH actions in vivo. (A) Primary rat GCs were treated with vehicle, FSH, FSH plus YKL, or YKL alone. Cyp19a1 mRNA levels were quantified 48 hours later and expressed as RE to Rpl19. Different letters represent significant differences (mean ± SEM, N = 5, 1-way ANOVA followed by Tukey, P < .05). n.d., non-detectable. (B) Immature 23-day-old female mice were injected intraperitoneally with YKL or vehicle (PBS). Two hours later, animals were treated with eCG or eCG plus YKL. The expression of Cyp19a1, Cyp11a1, and Stard1 was quantified 48 hours later. *P < .05, t-test, N = 7. (C) SIK1, SIK2, or SIK3 knockout mice were placed in cages with males of proven fertility and the number of pups per litter registered, N ≥ 7. (D) Wild-type (control) or SIK1, SIK2, and SIK3 knockout mice were treated with eCG (7.5 IU SC [subcutaneously]) and 48 hours later treated with hCG (7.5 IU SC). Ovulated oocytes were counted 17 hours after the injection of hCG. ***P < .001 vs control, t-test, N ≥ 6. ND, none detected.
Next, we injected immature 23-day-old female mice with vehicle or YKL before the administration of eCG at 4 IU, a hormone with FSH activity. The mRNA levels for Cyp19a1, Cyp11a1, and Stard1 were high in the GCs of animals treated with eCG. Treatment with YKL enhanced the stimulatory effect of eCG on Cyp19a1, Cyp11a1, and Stard1 significantly (Fig. 8B).
Effect of the global knockdown of SIKs on the fertility of female mice
Next, we examined the fertility of SIK1, SIK2, or SIK3 knockout mice. SIK1 knockout females were fertile, producing normal litter sizes and releasing a number of oocytes similar to control females (Fig. 8C). Mice lacking SIK2 were also fertile and produced average litter sizes (Fig. 8C). However, SIK2 knockout mice released 3 times more oocytes than control mice in response to a superovulation protocol (Fig. 8D). Noteworthy, some SIK2 knockout females produced up to 120 oocytes in response to gonadotropins. These findings reveal that SIK2 deficiency strongly enhances ovarian stimulation by exogenous gonadotropins. In contrast, SIK3 knockout females were infertile and failed to release oocytes when stimulated with a superovulation protocol (Fig. 8C and 8D).
SIK3 is required to maintain ovarian structures and function in mice
Since SIK3 knockout mice were insensitive to exogenous gonadotropins, we examined their ovarian histology. The ovaries of 4-week-old SIK3 knockout mice contain follicle structures that appear to be smaller and with less-defined theca layers than those in control ovaries (Fig. 9A). Unexpectedly, the ovaries of adult 16-week-old SIK3 knockout mice lacked preovulatory follicles and corpora lutea and contained numerous hemorrhagic follicles (Fig. 9B). Most noticeable, the ovaries of SIK3 knockout mice presented an abnormally developed stroma containing depositions of a homogeneous, acellular, eosinophilic material. Blood vessels in the ovaries of animals lacking SIK3 showed strong angiectasis characterized by an expanded lumen and deformations (Fig. 9B). The acellular depositions advanced rapidly with age, taking over the entire ovary in mutant animals (Fig. 9C). Thus, only a few abnormal follicles were present in the ovaries of 1-year-old SIK3 knockout mice. Corpora lutea, which are representative of successful ovulation, were present in the ovaries of 1-year-old wild-type animals but not in the ovaries of SIK3 knockout females of the same age (Fig. 9C). In addition, the uteri of SIK3 knockout mice were noticeably smaller than the uteri of wild-type females (Fig. 9D).
Figure 9.
SIK3 is required to maintain normal ovarian structures. (A) Representative ovarian histology of 4-week-old wild-type and SIK3 knockout mice (N = 3). (B) Representative ovarian histology of 16-week-old SIK3 knockout mice showing acellular and eosinophilic depositions (#) and illustrating the absence of preovulatory follicles and corpora lutea. As shown on the right, large hemorrhagic follicles (*) were observed in SIK3 knockout mice. The arrowheads point to deformations and extreme dilatation of blood vessels characteristic of angiectasis, N = 5. (C) Left: Representative ovarian histology of 1-year-old wild-type females showing follicles at various stages of follicle maturation and several corpora lutea (CL). Right: Representative ovarian histology of one-year-old SIK3 knockout mice illustrating the complete involution of all ovarian structures, N = 4. (D) Anatomy of the uterus of 6-month-old wild-type and SIK3 knockout mice, N = 3.
Discussion
These findings reveal novel roles for SIKs in the regulation of folliculogenesis, ovulation, and fertility in rodents and the control of GC differentiation in humans and rodents. Strikingly, the 2 most highly expressed SIK isoforms in the ovary, SIK2 and SIK3, have distinct and unique roles. SIK2 blunts GC differentiation and restricts the number of follicles reaching ovulation. Conversely, SIK3 is required for the maintenance of ovarian structures, including preovulatory follicles, stroma, and corpora lutea.
The use of pharmacological inhibitors and genetic knockdowns established that SIK2 is an important regulator of steroidogenesis in rodents and humans. SIK1 has been shown previously to control the expression of StAR (23, 26, 45) and Cyp11a1 (24). Thus, the literature suggests that increased levels of SIK1 expression inhibit adrenal steroidogenesis by attenuating cAMP response element-binding protein (CREB) transcriptional activity (24). However, SIK1 does not appear to be a critical regulator of ovarian steroidogenesis. In contrast, SIK2 is the only isoform involved in the regulation of GC steroidogenesis targeting not only StAR and CYP11A1 but, more importantly, aromatase, which plays a central role in the regulation of folliculogenesis. Our results demonstrate that SIK2 inhibits aromatase expression in GCs, and, therefore, this kinase appears to play an essential role in the regulation of estradiol production, a central hormone in female fertility.
The mechanism by which SIK2 inhibition increases aromatase expression and estradiol production remains to be fully elucidated. Luciferase reporter studies indicate that SIK inhibition increases the transcriptional activity of the proximal promoter of the aromatase gene. Consistent with this observation, SIK1 inhibition of adrenal steroidogenesis has been shown to be mediated by the repression of CREB transcriptional activity (24). This effect of SIK1 appears to be mediated by the phosphorylation and inactivation of CREB regulated transcription coactivator 2, also known as CRTC2, which is a coactivator of CREB (46). We hypothesize that SIK2 controls Cyp19a1 gene expression by reducing CREB activity indirectly through the inhibition of CRTC2. In support of this hypothesis, it has been shown that FSH increases CRTC2 translocation to the nucleus in GCs (47, 48). However, it remains to be determined if this effect is controlled by SIK2 and whether CRTC2 plays any role in the regulation of aromatase expression in ovarian GCs. Thus, the exact mechanism by which SIK2 regulates steroidogenesis remains to be elucidated and warrants further research.
The inhibitory effect of SIK2 on estradiol production and ovulation suggests a crucial role for this kinase among the checkpoints controlling folliculogenesis. In humans, SIK2 activity might limit the number of early antral follicles recruited at the beginning of the follicular phase of the menstrual cycle when follicles are exposed to the highest levels of circulating gonadotropins. Thus, it is possible to postulate that by limiting follicle recruitment, SIK2 may play a role in preventing premature ovarian insufficiency.
Interestingly, although SIK2 knockout mice have an extraordinary response to exogenous gonadotropins, they are fertile with normal litter sizes. We propose that SIK2 is 1 of several mechanisms involved in the control of ovulation. Thus, the lack of SIK2 may not be enough to augment follicle recruitment by endogenous gonadotropins since other pathways maintain ovulation within physiological parameters. However, in the presence of exogenous pharmacological levels of gonadotropins, the absence of the antagonistic effects of SIK2 leads to the maximal stimulation of follicle recruitment and ovulation. Further research will be required to test these possibilities. Noteworthy, oocytes obtained from SIK2 knockout mice are viable and develop normally after fertilization, which also makes it possible to preserve fertilized oocytes and to produce SIK2 knockout mice (Dr. Takemori, personal communication).
In contrast to the negative regulatory effects of SIK2 in ovarian function, SIK3 appears to be required to maintain the structure and function of the ovary. The ovaries of adult SIK3 knockout mice were observed to degenerate rapidly, are dysfunctional, and administration of exogenous gonadotropins did not rescue ovulation, strongly suggesting an ovarian defect. Our findings demonstrate that secondary follicles of SIK3 knockout mice fail to progress to the preovulatory stage and often became hemorrhagic cysts. A second striking abnormality found in the ovaries of SIK3 knockout mice is the progressive accumulation of homogeneous, acellular, eosinophilic material that resembles amyloid lesions. It appears that the buildup of this material with age hinders follicle growth and survival. Finally, another prominent anomaly is the expanded lumen and deformations of the ovarian blood vessels. Thus, SIK3 is required for maintaining stromal, and blood vessel structures, which agrees with the higher levels of SIK3 found in the ovarian parenchyma. It is known that ovarian amyloidosis and angiectasis increase with age in mice (49, 50); therefore, SIK3 knockout mice could be used as a model of accelerated ovarian senescence.
The question that emerges from these findings is the identity of the genes and proteins targeted by SIK3 in the ovary. In addition, the nature of the material that accumulates in the ovary of SIK3 knockout mice and why it accumulates are points that call for additional research. Our knockdown experiments indicate that steroidogenic genes are not targeted by SIK3, at least in the GCs. These findings, along with the striking ovarian defects observed in SIK3 knockout mice suggest that SIK3 targets stromal/theca genes. However, the strong expression of SIK3 in the GCs suggests that this kinase may regulate nonsteroidogenic genes in these cells. Thus, the phenotype observed in SIK3 knockout mice could be due to granulosa, theca, or stromal cell alterations. Furthermore, it is essential to determine how the ovarian phenotype described here relates to the bone and metabolic deficiencies observed in SIK3 knockout mice (41, 51). Current efforts are aimed at the identification of specific targets for SIK2 and SIK3 in GCs and to examine the fertility of mice with a conditional knockdown of SIK2 and SIK3 in the GCs.
SIKs have emerged as regulators of pathways that maintain glucose and lipid homeostasis, and since they respond to metabolic hormones, it is also clear that these kinases participate in the hormonal control of metabolism. Therefore, the fertility phenotype observed in these global knockout mice may be due to systemic changes in reproductive and metabolic hormones. For instance, SIKs have emerged as an important mediator of glucagon effectors in the regulation of hepatic gluconeogenesis (15, 16). Also, SIK2 is involved in the regulation of GLUT4 levels and glucose uptake in adipocytes by regulating insulin signaling (16, 52). SIK3 knockout mice have a malnourished, hypoglycemic, and hyperinsulin-sensitive phenotype that could be due to reduced energy storage (41). These effects on the metabolism of glucose and lipids along with alterations in insulin and glucagon signaling could lead to the depletion of energy storage that, in turn, may affect the circulating levels of leptin, which is known to be essential to maintain normal ovarian function. Leptin maintains gonadotropin secretion in the pituitary (53) and leptin participation in reproduction is illustrated by the hypogonadism and infertility of leptin-deficient mice (54). Moreover, the leptin receptor is expressed in theca and GCs of rodents and humans (55, 56). Thus, metabolic changes caused by the lack of SIKs and the consequent changes in insulin and leptin may also affect ovarian function in SIK2 and SIK3 global knockout mice.
Therapeutically, SIKs are drug targets (33, 57); therefore, it is possible to postulate that targeting specifically SIK2 activity could be used to improve fertility. For instance, acute inhibition of SIK2 could minimize the number of oocyte donors in mouse resource facilities, adhering to the 3Rs principle for good laboratory animal practice. SIK2 inhibitors could also provide agricultural benefits by setting the groundwork for novel strategies to enhance livestock productivity. Finally, maximizing the number of oocytes produced during assisted reproductive technology procedures may be helpful for the preservation of endangered mammals.
Our findings are clinically significant in at least two possible ways. First, the specific inhibition of SIK2 activity could improve the response of poor responders or older patients to controlled ovarian stimulation. Although SIK inhibition would not compensate for the scarcity of follicles found in these patients, they may benefit from the exploitation of pathways aimed to maximize the growth and response of the follicles that remain viable in their ovaries. In support of this idea, our results show that SIK inhibition rescues FSH sensitivity in primary human GCs extracted from PCOS and anovulatory patients. Furthermore, as mice injected with YKL displayed no signs of distress, short-term inhibition of SIK activity could be used to improve oocyte retrieval, and the likelihood of pregnancy in patients undergoing controlled ovarian stimulation. Second, pharmacological inhibition of SIK activity is being explored as a novel therapy in several types of cancers, including breast cancer (58). Therefore, the use of SIK inhibitors must be monitored closely in patients with estrogen receptor-positive tumors as the treatment also increases estradiol production.
These findings show, for the first time, the involvement of SIKs in the regulation of GC differentiation in humans and rodents. We describe the expression of all SIK isoforms in the ovary and GCs and show that SIK2 and SIK3 are the most relevant isoforms. In addition, functional studies demonstrate that SIK2 functions as a negative regulator of FSH actions in vitro and in vivo. Conversely, SIK3 appears to be required for normal ovarian structure and function. These findings establish SIKs as critical regulators of female fertility.
Acknowledgements
Financial Support: National Institutes of Health grant R01HD097202 to C.S.
Glossary
Abbreviations
- ANOVA
analysis of variance
- cAMP
3′,5′-cyclic adenosine 5′-monophosphate
- CoC
Compound C
- cDNA
complementary deoxyribonucleic acid
- eCG
equine chorionic gonadotropin
- FSH
follicle-stimulating hormone
- GOI
gene of interest
- GC
granulosa cell
- hCG
human chorionic gonadotropin
- IC50
median inhibitory concentration
- IGF2
insulin-like growth factor 2
- m
messenger
- PBS
phosphate-buffered saline
- PCOS
polycystic ovarian syndrome
- RNA
ribonucleic acid
- SEM
standard error of the mean
- sh
small hairpin
- SIK
salt-inducible kinase
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the national survey of family growth. Natl Health Stat Report. 2013(67):1-18. [PubMed] [Google Scholar]
- 2. Vollenhoven B, Hunt S. Ovarian ageing and the impact on female fertility. F1000Res. 2018;1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li RH, Ng EH. Management of anovulatory infertility. Best Pract Res Clin Obstet Gynaecol. 2012;26(6):757-768. [DOI] [PubMed] [Google Scholar]
- 4. Wyndham N, Marin Figueira PG, Patrizio P. A persistent misperception: assisted reproductive technology can reverse the “aged biological clock”. Fertil Steril. 2012;97(5):1044-1047. [DOI] [PubMed] [Google Scholar]
- 5. Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73-91. [DOI] [PubMed] [Google Scholar]
- 6. Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73(5):473-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerasimova T, Thanasoula MN, Zattas D, Seli E, Sakkas D, Lalioti MD. Identification and in vitro characterization of follicle stimulating hormone (FSH) receptor variants associated with abnormal ovarian response to FSH. J Clin Endocrinol Metab. 2010;95(2):529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on poor ovarian response definition ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624. [DOI] [PubMed] [Google Scholar]
- 9. Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L. Management of poor responders in IVF: is there anything new? Biomed Res Int. 2014;2014:352098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loutradis D, Vomvolaki E, Drakakis P. Poor responder protocols for in-vitro fertilization: options and results. Curr Opin Obstet Gynecol. 2008;20(4):374-378. [DOI] [PubMed] [Google Scholar]
- 11. Berkkanoglu M, Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders? Fertil Steril. 2010;94(2):662-665. [DOI] [PubMed] [Google Scholar]
- 12. George JW, Dille EA, Heckert LL. Current concepts of follicle-stimulating hormone receptor gene regulation. Biol Reprod. 2011;84(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landomiel F, Gallay N, Jégot G, et al. . Biased signalling in follicle stimulating hormone action. Mol Cell Endocrinol. 2014;382(1):452-459. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Takemori H, Halder SK, Nonaka Y, Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Lett. 1999;453(1-2):135-139. [DOI] [PubMed] [Google Scholar]
- 15. Wein MN, Foretz M, Fisher DE, Xavier RJ, Kronenberg HM. Salt-inducible kinases: physiology, regulation by cAMP, and therapeutic potential. Trends Endocrinol Metab. 2018;29(10):723-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakamoto K, Bultot L, Göransson O. The salt-inducible kinases: emerging metabolic regulators. Trends Endocrinol Metab. 2018;29(12):827-840. [DOI] [PubMed] [Google Scholar]
- 17. Clark K, MacKenzie KF, Petkevicius K, et al. . Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc Natl Acad Sci U S A. 2012;109(42):16986-16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wein MN, Liang Y, Goransson O, et al. . SIKs control osteocyte responses to parathyroid hormone. Nat Commun. 2016;7:13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang B, Moya N, Niessen S, et al. . A hormone-dependent module regulating energy balance. Cell. 2011;145(4):596-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horike N, Kumagai A, Shimono Y, et al. . Downregulation of SIK2 expression promotes the melanogenic program in mice. Pigment Cell Melanoma Res. 2010;23(6):809-819. [DOI] [PubMed] [Google Scholar]
- 21. Jefcoate CR, Lee J, Cherradi N, Takemori H, Duan H. cAMP stimulation of StAR expression and cholesterol metabolism is modulated by co-expression of labile suppressors of transcription and mRNA turnover. Mol Cell Endocrinol. 2011;336(1-2):53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takemori H, Kanematsu M, Kajimura J, et al. . Dephosphorylation of TORC initiates expression of the StAR gene. Mol Cell Endocrinol. 2007;265-266:196-204. [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Tong T, Takemori H, Jefcoate C. Stimulation of StAR expression by cAMP is controlled by inhibition of highly inducible SIK1 via CRTC2, a co-activator of CREB. Mol Cell Endocrinol. 2015;408:80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu Z, Hu J, Shen WJ, Kraemer FB, Azhar S. A novel role of salt-inducible kinase 1 (SIK1) in the post-translational regulation of scavenger receptor class B type 1 activity. Biochemistry. 2015;54(46):6917-6930. [DOI] [PubMed] [Google Scholar]
- 25. Doi J, Takemori H, Lin XZ, Horike N, Katoh Y, Okamoto M. Salt-inducible kinase represses cAMP-dependent protein kinase-mediated activation of human cholesterol side chain cleavage cytochrome P450 promoter through the CREB basic leucine zipper domain. J Biol Chem. 2002;277(18):15629-15637. [DOI] [PubMed] [Google Scholar]
- 26. Takemori H, Katoh Y, Horike N, Doi J, Okamoto M. ACTH-induced nucleocytoplasmic translocation of salt-inducible kinase. Implication in the protein kinase A-activated gene transcription in mouse adrenocortical tumor cells. J Biol Chem. 2002;277(44):42334-42343. [DOI] [PubMed] [Google Scholar]
- 27. Hobeika E, Armouti M, Kala H, et al. . Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. J Clin Endocrinol Metab. 2019;104(5):1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumgarten SC, Convissar SM, Zamah AM, et al. . FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab. 2015;100(8):E1046-E1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99(8):2995-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, Baumgarten SC, Zhou P, Stocco C. Testosterone-dependent interaction between androgen receptor and aryl hydrocarbon receptor induces liver receptor homolog 1 expression in rat granulosa cells. Mol Cell Biol. 2013;33(15):2817-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett J, Baumgarten SC, Stocco C. GATA4 and GATA6 silencing in ovarian granulosa cells affects levels of mRNAs involved in steroidogenesis, extracellular structure organization, IGF-I activity, and apoptosis. Endocrinology. 2013;154(12):4845-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett J, Wu YG, Gossen J, Zhou P, Stocco C. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology. 2012;153(5):2474-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundberg TB, Liang Y, Wu H, et al. . Development of chemical probes for investigation of salt-inducible kinase function in vivo. ACS Chem Biol. 2016;11(8):2105-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou P, Baumgarten SC, Wu Y, et al. . IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27(3):511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RRID:AB_10703956.
- 36. RRID:AB_306683.
- 37. RRID:AB_2756386.
- 38. RRID:AB_2301724.
- 39. RRID:AB_11140583.
- 40. RRID:AB_2042747.
- 41. Uebi T, Itoh Y, Hatano O, et al. . Involvement of SIK3 in glucose and lipid homeostasis in mice. PLoS One. 2012;7(5):e37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel K, Foretz M, Marion A, et al. . The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat Commun. 2014;5:4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77(1-2):27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sasaki T, Takemori H, Yagita Y, et al. . SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron. 2011;69(1):106-119. [DOI] [PubMed] [Google Scholar]
- 45. Lee J, Tong T, Duan H, et al. . Regulation of StAR by the N-terminal domain and coinduction of SIK1 and TIS11b/Znf36l1 in single cells. Front Endocrinol (Lausanne). 2016;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang WL, Lee MT, Wu LS, et al. . CREB coactivator CRTC2/TORC2 and its regulator calcineurin crucially mediate follicle-stimulating hormone and transforming growth factor β1 upregulation of steroidogenesis. J Cell Physiol. 2012;227(6):2430-2440. [DOI] [PubMed] [Google Scholar]
- 48. Lai WA, Yeh YT, Fang WL, et al. . Calcineurin and CRTC2 mediate FSH and TGFβ1 upregulation of Cyp19a1 and Nr5a in ovary granulosa cells. J Mol Endocrinol. 2014;53(2):259-270. [DOI] [PubMed] [Google Scholar]
- 49. Frith CH, Chandra M. Incidence, distribution, and morphology of amyloidosis in Charles Rivers CD-1 mice. Toxicol Pathol. 1991;19(2):123-127. [DOI] [PubMed] [Google Scholar]
- 50. Montgomery CA, Alison RH. Nonneoplastic lesions of the ovary in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 1987;73:53-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sasagawa S, Takemori H, Uebi T, et al. . SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development. 2012;139(6):1153-1163. [DOI] [PubMed] [Google Scholar]
- 52. Henriksson E, Säll J, Gormand A, et al. . SIK2 regulates CRTCs, HDAC4 and glucose uptake in adipocytes. J Cell Sci. 2015;128(3):472-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chehab FF, Qiu J, Mounzih K, Ewart-Toland A, Ogus S. Leptin and reproduction. Nutrition Reviews. 2002;60(10 Pt 2):S39-46; discussion S68-84, 85-37. [DOI] [PubMed] [Google Scholar]
- 54. Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190-1193. [DOI] [PubMed] [Google Scholar]
- 55. Karlsson C, Lindell K, Svensson E, et al. . Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab. 1997;82(12):4144-4148. [DOI] [PubMed] [Google Scholar]
- 56. Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65(3):223-228. [DOI] [PubMed] [Google Scholar]
- 57. Sundberg TB, Choi HG, Song JH, et al. . Small-molecule screening identifies inhibition of salt-inducible kinases as a therapeutic strategy to enhance immunoregulatory functions of dendritic cells. Proc Natl Acad Sci U S A. 2014;111(34): 12468-12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maxfield KE, Macion J, Vankayalapati H, Whitehurst AW. SIK2 restricts autophagic flux to support triple-negative breast cancer survival. Mol Cell Biol. 2016;36(24):3048-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]