Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) account for a growing proportion of liver disease cases, and there is a need to better understand future disease burden. We used a modelling framework to forecast the burden of disease of NAFLD and NASH for Canada.
Methods:
We used a Markov model to forecast fibrosis progression from stage F0 (no fibrosis) to stage F4 (compensated cirrhosis) and subsequent progression to decompensated cirrhosis, hepatocellular carcinoma, liver transplantation and liver-related death among Canadians with NAFLD from 2019 to 2030. We used historical trends for obesity prevalence among adults to estimate longitudinal changes in the number of incident NAFLD cases.
Results:
The model projected that the number of NAFLD cases would increase by 20% between 2019 and 2030, from an estimated 7 757 000 cases to 9 305 000 cases. Increases in advanced fibrosis cases were relatively greater, as the number of model-estimated prevalent stage F3 cases would increase by 65%, to 357 000, and that of prevalent stage F4 cases would increase by 95%, to 195 000. Estimated incident cases of hepatocellular carcinoma and decompensated cirrhosis would increase by up to 95%, and the number of annual NAFLD-related deaths would double, to 5600.
Interpretation:
Increasing rates of obesity translate into increasing NAFLD-related cases of cirrhosis and hepatocellular carcinoma and related mortality. Prevention efforts should be aimed at reducing the incidence of NAFLD and slowing fibrosis progression among those already affected.
Nonalcoholic fatty liver disease (NAFLD), defined by the presence of excessive liver fat in the absence of another causative factor,1 is recognized to cause cirrhosis and hepatocellular carcinoma.2–4 Cases of NAFLD can be characterized as simple steatosis or nonalcoholic steatohepatitis (NASH), characterized by additional histologic features. Obesity rates are increasing in Canada, affecting over one-third of Canadians,5,6 and obesity is 1 component of metabolic syndrome, which is a risk factor for NAFLD.1,7 There is a growing disease burden associated with NAFLD, following the trajectory of increasing obesity in Canada and globally.8 Even if further increases in obesity in Canada are halted, NAFLD-related morbidity and mortality are projected to increase for decades.
Analyses of the disease and economic burden associated with NAFLD based on data in the existing literature have recently been reported.9–11 To our knowledge, there are currently no studies reporting estimates of NAFLD prevalence or disease burden in the general Canadian population, but estimates have been reported in selected populations12 in other areas.13,14 Given the substantial health and socioeconomic burden of NAFLD,7 there is an increasing need to forecast the burden of disease. A modelling framework can provide a range of outcomes that can help in effective resource use and the development of strategies to prevent further increases in disease burden. The current analysis describes the results of such modelling to forecast the burden of disease of NAFLD and NASH for Canada, from 2019 to 2030.
Methods
A Markov model (Appendix 1, Supplemental Figure S1, available at www.cmajopen.ca/content/8/2/E429/suppl/DC1) was built with the use of Microsoft Excel to estimate the number of NAFLD cases by disease stage in Canada beginning in 1950.15
Model
The model tracked the Canadian population by age group and gender over time, with adjustment for background mortality at every step. New NAFLD cases entered the model based on longitudinal adult obesity trends. We calculated progression of disease through fibrosis,16 decompensated cirrhosis, hepatocellular carcinoma and liver transplantation stages (Appendix 1, Supplemental Figure S1) after accounting for both all-cause mortality (with adjustment for excess cardiovascular and non–liver-cancer mortality) and liver mortality attributable to NAFLD. Fibrosis transition rates were varied by gender (males experience faster progression) and age (Appendix 1, Supplemental Tables S2 and S3).
We implemented a Delphi process in which expert consensus was used to develop key model inputs (Appendix 1, Supplemental Table S1). Experts were invited based on research expertise and represented multiple disease areas including hepatology and diabetology. In addition, the expert panel included representation from provinces representing a majority of the Canadian population.
Population and mortality
We organized the total Canadian population and background mortality rates by year, gender and 5-year age groups.17 Increased background mortality among NAFLD cases was based on reported hazard ratios for all-cause mortality by fibrosis stage,18 adjusted to remove the impact of increased liver-related mortality,19 as this was calculated as a separate progression in the model.2,20–22 After adjustment, there was no increased non–liver-related mortality in stages F0–F2; increased mortality ratios of 1.42 and 1.43 were applied to prevalent F3 and F4 cases, respectively (Appendix 1, Supplemental Table S2). For the oldest age groups (age ≥ 75 yr), no excess mortality was assumed given the already high mortality rates in this age group and reported decreases in the incremental impact of cardiovascular disease mortality among people aged 75 years or older.23
New cases of nonalcoholic fatty liver disease
We extrapolated the annual number of new NAFLD cases based on longitudinal changes in the prevalence of adults in different body mass index categories, with the assumption that relative increases in NAFLD prevalence would mirror changes in obesity prevalence24–26 (Appendix 1, Incidence [new cases] calculations). Owing to variations in cut-off levels for obesity that vary by race or ethnicity27 and differences in health risk by body mass index class,28 we calculated the prevalence of obesity as a weighted average using data for body mass index of 25 or greater for the population classified by 1996–2016 census data as South Asian, Chinese, Filipino, Southeast Asian, Korean or Japanese,29 and data for body mass index of 30 or greater for the remaining population. We estimated the total population with a body mass index of 25 or greater to be 7.3% of the total Canadian population in 2006, 14.2% in 2016 and, with linear trending, 19.0% in 2030.
Using the weighted average for the body mass index categories as described above, we extrapolated changes in obesity prevalence based on trending of data for the adult prevalence of body mass index of 25 or greater and of 30 or greater,24–26 where the adjusted obesity prevalence rate was estimated at 9.1% in 1975 and 32.7% in 2014 (Appendix 1, Supplemental Figure S2).
Prevalence of nonalcoholic fatty liver disease
Among people aged 20 years or older in 2018, there was an assumed NAFLD prevalence rate of 25% (Delphi range 22.5%–27.5%) based on expert consensus. The prevalence of NAFLD was assumed to decline among the youngest age groups (≤ 18 yr), who are often not included in studies based on the general population. 30,31 We based the age and gender distribution of prevalent NAFLD cases on data from general population studies, in which prevalence increases with increasing age.30,32,33 Based on a cohort of patients with NAFLD in Calgary, we assumed that prevalence would be approximately equal between men and women.34 Owing to elevated mortality, we assumed that prevalence would naturally decline among the oldest age groups (≥ 80 yr), with peak prevalence occurring in late middle age.
Liver transplantation
Annual liver transplantation procedures were reported by Canadian Blood Services.35 Based on expert input and review of diagnostic categories for transplant recipients,36 we estimated that about one-quarter of current liver transplantation procedures could be attributable to NAFLD or NASH.
Statistical analysis
We simulated disease progression by multiplying the total number of cases at a particular stage of the disease by a progression rate to the next stage, with adjustments for mortality at every step (Appendix 1, equation 1). We used Monte Carlo simulation to identify model inputs that accounted for the greatest variation in future disease burden and to produce 95% uncertainty intervals for selected model outputs (Appendix 1, Uncertainty and sensitivity analysis).
Ethics approval
Since this was a modelling study, no ethics approval was needed.
Results
Nonalcoholic fatty liver disease
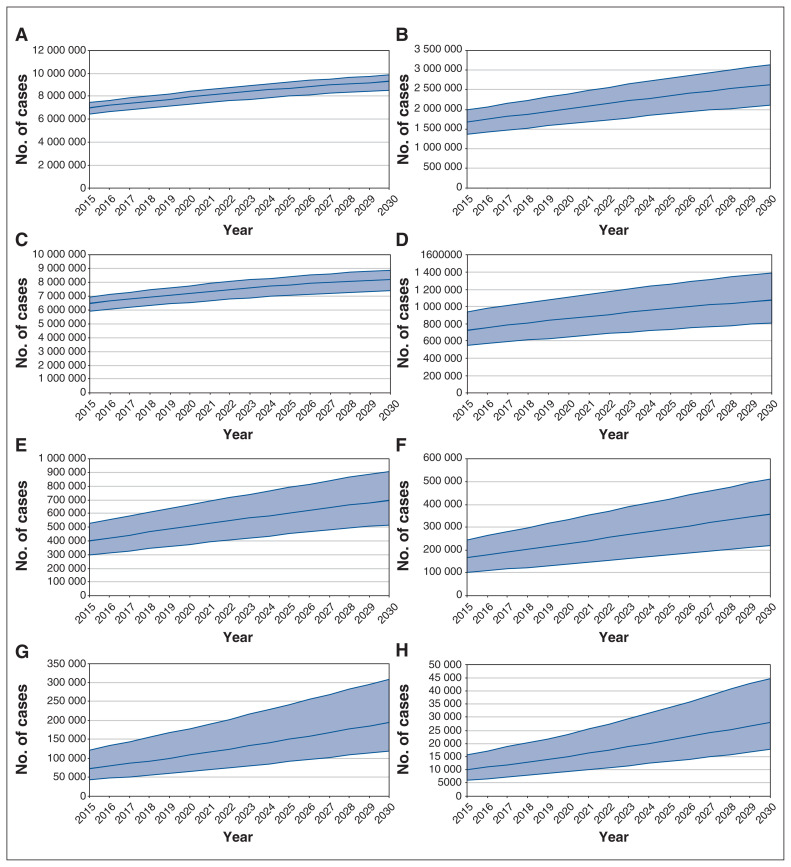
Between 2019 and 2030, the number of model-estimated NAFLD cases in Canada would increase by 20%, to 9 305 000 (uncertainty interval 8 550 000–9 875 000) (Figure 1). Accounting for population growth, the estimated prevalence would increase from 20.8% (19.1%–22.1%) to 22.9% (21.1%–24.3%), and the age-adjusted prevalence would increase from 20.8% (19.1%–22.1%) to 22.3% (20.5%–23.7%) (Table 1).
Figure 1:
Model-estimated prevalent cases of nonalcoholic fatty liver disease (NAFLD) (A), nonalcoholic steatohepatitis (B), stage F0 NAFLD (C), stage F1 NAFLD (D), stage F2 NAFLD (E), stage F3 NAFLD (F), compensated cirrhosis NAFLD (G) and decompensated cirrhosis, hepatocellular carcinoma and liver transplantation related to NAFLD (H) for Canada, 2015–2030. Shaded areas represent 95% uncertainty interval.
Table 1:
Model-estimated burden of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Canada, 2019–2030
| Variable | Year; no. (uncertainty interval)* | |||
|---|---|---|---|---|
| 2019 | 2020 | 2025 | 2030 | |
| Population of Canada | 37 280 000 | 37 603 000 | 39 173 000 | 40 618 000 |
| Model-estimated prevalent cases | ||||
| NAFLD | 7 757 000 | 7 930 000 | 8 712 000 | 9 305 000 |
| (7 138 000–8 232 000) | (7 298 000–8 414 000) | (8 012 000–9 244 000) | (8 550 000–9 875 000) | |
| Crude all-ages NAFLD prevalence rate, % | 20.8 (19.1–22.1) | 21.1 (19.4–22.4) | 22.2 (20.5–23.6) | 22.9 (21.1–24.3) |
| Adjusted all-ages NAFLD prevalence rate, %† | 20.8 (19.1–22.1) | 21.0 (19.4–22.3) | 22.0 (20.2–23.4) | 22.3 (20.5–23.7) |
| Stage F0 | 6 436 000 (5 784 000–6 956 000) | 6 553 000 (5 884 000–7 086 000) | 7 060 000 (6 300 000–7 655 000) | 7 400 000 (6 577 000–8 048 000) |
| Stage F1 | 630 000 (425 000–872 000) | 649 000 (437 000–899 000) | 736 000 (496 000–1 022 000) | 807 000 (542 000–1 123 000) |
| Stage F2 | 360 000 (235 000–508 000) | 376 000 (245 000–530 000) | 451 000 (298 000–636 000) | 518 000 (341 000–730 000) |
| Stage F3 | 216 000 (131 000–316 000) | 228 000 (139 000–334 000) | 293 000 (179 000–424 000) | 357 000 (219 000–511 000) |
| Compensated cirrhosis | 101 000 (60 400–167 000) | 108 000 (65 200–179 000) | 150 000 (91 300–242 000) | 195 000 (120 000–309 000) |
| Decompensated cirrhosis, hepatocellular carcinoma and liver transplantation | 14 000 (8600–21 700) | 15 100 (9300–23 500) | 21 300 (13 300–33 700 | 28 200 (17 700–44 700) |
| NASH | 1 953 000 (1 582 000–2 320 000) | 2 020 000 (1 635 000–2 401 000) | 2 345 000 (1 888 000–2 792 000) | 2 630 000 (2 107 000–3 136 000) |
| Crude all-ages NASH prevalence rate, % | 5.2 (4.2–6.2) | 5.4 (4.3–6.4) | 6.0 (4.8–7.1) | 6.5 (5.2–7.7) |
| Adjusted all-ages NASH prevalence rate, %† | 5.2 (4.2–6.2) | 5.3 (4.3–6.3) | 5.8 (4.7–6.9) | 6.1 (4.9–7.2) |
| Model-estimated incident cases | ||||
| Decompensated cirrhosis | 3400 (1900–5800) | 3700 (2100–6200) | 5100 (2900–8500) | 6700 (3800–10 900) |
| Hepatocellular carcinoma | 660 (440–990) | 710 (470–1100) | 940 (620–1400) | 1200 (770–1800) |
| Liver death | 2700 (1500–4500) | 2900 (1700–4800) | 4200 (2400–6800) | 5600 (3200–9000) |
Note: NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis.
Except where noted otherwise.
Adjusted to 2019 Canadian population.17
In 2019, the largest prevalent NAFLD age group would be 55–59 years, with 995 000 estimated NAFLD cases. By 2030, the largest prevalent age group would be 65–69 years, with 1 079 000 predicted cases (Appendix 1, Supplemental Figure S3).
Over the same period, the unadjusted number of stage F0 NAFLD cases would increase by 15%, to 7 400 000 (6 577 000–8 048 000). The number of prevalent stage F1 cases would increase by 30%, to 807 000 (542 000–1 123 000).
Even greater relative increases are projected in later disease stages: the estimated number of stage F2 cases would increase by 45%, the estimated number of stage F3 cases would increase by 65%, and the estimated number of compensated cirrhosis cases would increase by 95%.
The number of estimated prevalent cases classified as hepatocellular carcinoma, decompensated cirrhosis or liver transplantation related to NAFLD would increase the most, by 100%, to 28 200 (17 700–44 700).
Nonalcoholic steatohepatitis
The number of prevalent NASH cases would increase by 35% between 2019 and 2030, to 2 630 000 (2 107 000–3 136 000) (Figure 1). Among the total NAFLD population, 25.2% of cases were projected to have NASH in 2019, increasing to 28.3% in 2030. The crude all-ages prevalence of NASH would increase from 5.2% (4.2%–6.2%) to 6.5% (5.2%–7.7%), and the age-adjusted prevalence would increase from 5.2% (4.2%–6.2%) to 6.1% (4.9%–7.2%) (Table 1).
Of NASH cases in 2019, 349 000 would be estimated to be stage F3–F4 fibrosis, decompensated cirrhosis, hepatocellular carcinoma or liver transplantation, encompassing 17% of estimated NASH cases and 0.9% of the Canadian population (all ages). By 2030, this number would be expected to increase by 65%, to 578 000 cases, and would account for 22% of predicted NASH cases and 1.0% of the total population.
Decompensated cirrhosis and hepatocellular carcinoma related to nonalcoholic fatty liver disease
Estimated incident cases of decompensated cirrhosis would almost double between 2019 and 2030, from 3400 (1900–5800) in 2019 to 6700 (3800–10 900) in 2030 (Figure 2). The modelled cumulative incidence during the period would number 59 800 cases. The estimated number of incident hepatocellular carcinoma cases would increase by 80%, from 660 (440–990) to 1200 (770–1800), and the modelled cumulative incidence would number 11 000 cases. Estimated incident hepatocellular carcinoma was compared with reported estimates for 1992–2010, and model results were within reported ranges (Appendix 1, Supplemental Figure S4).
Figure 2:
Model-estimated incident cases of hepatocellular carcinoma (A), decompensated cirrhosis (B) and incident liver-related death (C) related to nonalcoholic fatty liver disease for Canada, 2015–2030. Shaded areas represent 95% uncertainty interval.
Mortality
Over the study period, the estimated number of incident liver-related deaths in the total NAFLD population would double, from 2700 (1500–4500) to 5600 (3200–9000) (Figure 2), and the cumulative number of liver-related deaths would number 48 700. The total estimated number of deaths in the NAFLD population would increase by 75%, from 66 100 to 115 000. Estimated liver-related mortality would account for 4.0% of deaths in 2019 and 4.8% of deaths in 2030. Among the NASH population, the number of annual deaths was estimated to be 24 400 in 2019, doubling to 49 100 in 2030. Estimated liver-related death in the NASH population was estimated to account for 10.9% of total deaths in 2019 and 11.3% in 2030.
Uncertainty and sensitivity analysis
The leading drivers of uncertainty for the number of estimated NASH cases in 2030 included ranges around the rate of transition from stage F0 to stage F1, the starting prevalence among adults aged 20 years or more in 2018, and the standard mortality ratios applied to background mortality rates (Appendix 1, Supplemental Figure S5).
Interpretation
The current analysis shows that NAFLD may represent a growing burden on the Canadian health care system over the next decade. The population of Canada is aging17 and will be prone to increased disease progression to advanced fibrosis in the coming years.
Model estimates from this analysis are similar to projections for other countries where the same modelling was applied.13,15 Differences between countries in changes in disease burden are a result of different length of model study period, changes in the background population (population change and aging), and the timing and magnitude of the obesity epidemic in each country.
Other investigators have applied a modelling framework to consider the economic impact of NAFLD, including quality-adjusted life-years.8,14 A large portion of estimated costs were among patients with simple steatosis, who were the largest estimated group in the current analysis. However, it is unknown what proportion of patients with simple steatosis are diagnosed, under care and incurring costs, given the burden of occult liver disease, including compensated cirrhosis.37
Estimation of quality-adjusted life-years is an important measure for estimating disease burden, but challenges exist for understanding NAFLD in this context. Given the common comorbidities among patients with NAFLD,38 decreased quality of life may be negligible, as utility measures are not additive. 39 Future research should focus on the burden of NAFLD in terms of quality-adjusted life-years, with attention to advanced cases and adjustment for comorbidities.
Challenges exist for modelling based on health records. Chronic liver disease, even cirrhosis, may go unrecognized in primary care,37 but algorithms exist for identifying advanced cases.40 Recorded hospital admissions for NAFLD increased sharply during 2007–2014 in the United States;41 however, varied diagnostic modalities with different sensitivity levels, along with increased awareness, likely contributed to an unknown degree. Mortality data are subject to unusable or insufficient coding of cause of death,42 and cause of death in NAFLD cases may be coded as complications of cirrhosis or other complications of metabolic syndrome.43 Rates of mortality related to cardiovascular disease and nonliver cancers44–48 are elevated in NAFLD cases, and the relation between these comorbid conditions and elevated mortality risk has not been fully quantified.
The time frame of our analysis aligns with 2030 targets to reduce mortality from noncommunicable disease by one-third.49 Interventions for obesity will reduce NAFLD disease burden. They should follow a multipronged approach, including “1) health services and clinical interventions that target individuals, 2) community-level intervention to influence behaviours, and 3) public policies that target broad social or environmental determinants.”50 Lifestyle modification interventions that result in weight loss of more than 10% can result in resolution of NASH and regression of fibrosis.51,52
There are opportunities for intervention among Canadians aged 6–17 years, as one-third were classified as overweight or obese based on the Canada Health Measures Survey, and this rate remained stable between 2004 and 2014.53 Without intervention, these people will contribute to an ever-growing disease burden in future decades and may experience progression to advanced disease at an earlier age.54
Limitations
Challenges to understanding NAFLD disease burden include the lack of consistent definitions for measuring prevalence in general populations55 and the potential failure to identify a substantial portion of the NAFLD population owing to the lack of simple and widely available diagnostic techniques with high positive predictive value.56 Estimates of disease burden are usually based on historical data, which may not represent the current situation in an epidemic of increasing obesity, especially among younger people.57 A lack of consistent diagnostic measures means that reported NAFLD prevalence rates vary between studies,11 with NAFLD diagnosis often considered an incidental diagnosis when steatosis is found on ultrasonography.58 Longitudinal studies of disease using noninvasive measures in general populations59–63 should inform modelling, as in such studies more data are reported for longer periods and noninvasive measures are refined.
We calculated the number of new NAFLD cases over time using changes in obesity, classified on the basis of body mass index. Although waist circumference has been shown to be a better measure of visceral fat,64 which is central to the development of NAFLD, measures at the national level are available for limited time points,65 which makes comparisons problematic. We ultimately selected body mass index because of the availability of nationally representative longitudinal data over several decades. However, it is important to note that trends using body mass index may not capture changes in the burden of disease in people with normal body mass index who are metabolically unhealthy, among whom the prevalence of NAFLD has been estimated to be 7% in the US66 and 19% in Asia.67
We compared liver cancer incidence to model outputs. A limitation of this comparison is that overall liver cancer incidence may be underreported,68,69 and levels of underreporting are continuously changing, so that application of historical estimates may result in overestimation when applied to the latest data. There is further uncertainty and changes over time with regard to the cause of liver cancer, with viral hepatitis expected to contribute relatively fewer cases in the future.70
There are several uncertainties when considering future liver transplantation, including the overall demand for and availability of donor organs, and changes in disease burden for competing indications, such as viral hepatitis. We assumed that the total annual number of transplantation procedures would remain constant in the future, but there is evidence that the proportion of NAFLD-related transplantation procedures is growing in Western countries.71 Nonalcoholic steatohepatitis is also a growing cause of transplantation among patients with hepatocellular carcinoma.72 Furthermore, the fact that there are overlapping indications for transplantation means that some transplantation procedures for other primary indications may also be related to NAFLD but not identified as such.
Our model excluded data on comorbidities that are confounded with NAFLD, including diabetes and dyslipidemia,38 to adjust model inputs. However, we applied excess background mortality to adjust for competing mortality risks among NAFLD cases. Finally, we did not adjust base prevalence and disease progression for ethnicity, although this factor may be important for understanding disease burden.38
Conclusion
Nonalcoholic fatty liver disease may represent a growing burden on the Canadian health care system over the next decade. Increasing rates of obesity, in combination with an aging population, translate into increasing NAFLD-related cases of cirrhosis, hepatocellular carcinoma and related mortality. Prevention efforts should be aimed at reducing the incidence of NAFLD and slowing fibrosis progression among those already affected.
Supplementary Material
Footnotes
Competing interests: Funding for this project was provided by Gilead Sciences. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Homie Razavi and Chris Estes conceived and designed the study. All of the authors contributed data, analyzed and interpreted the data, drafted the manuscript and revised it critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: Funding for this project was provided by Gilead Sciences. Giada Sebastiani is supported by Junior 1 and 2 Salary Awards (27127 and 267806) from the Fonds de recherche du Québec – Santé and research salary from the Department of Medicine of McGill University.
Data sharing: Model results presented in the current analysis are available by contacting info@cdafound.org.
Disclaimer: The funders had no role in the study design, data collection, analysis or interpretation of data, or preparation of the manuscript.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/2/E429/suppl/DC1.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 5.Vogel L. Overweight or overfat? Many Canadians are both. CMAJ. 2017;189:E1202–3. doi: 10.1503/cmaj.109-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatty liver disease. Toronto: Canadian Liver Foundation; 2017. [accessed 2020 Mar. 26]. Available: www.liver.ca/patients-caregivers/liver-diseases/fatty-liver-disease/ [Google Scholar]
- 7.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33:1245–53. doi: 10.1007/s40273-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Zheng L, Stepanova M, et al. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49:222–7. doi: 10.1097/MCG.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 12.Wells MM, Li Z, Addeman B, et al. Computed tomography measurement of hepatic steatosis: prevalence of hepatic steatosis in a Canadian population. Can J Gastroenterol Hepatol. 2016;2016 doi: 10.1155/2016/4930987. 4930987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–86. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 15.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.World population prospects: the 2017 revision. New York: Department of Economic Social Affairs, United Nations; 2017. [Google Scholar]
- 18.Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–73. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–9. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 21.Rahman RN, Ibdah JA. Nonalcoholic fatty liver disease without cirrhosis is an emergent and independent risk factor of hepatocellular carcinoma: a population based study. Hepatology. 2012;56:241A. [Google Scholar]
- 22.Ries LAG, Young JL, Keel GE, et al., editors. SEER survival monograph: cancer survival among adults — U.S. SEER program, 1988 2001 patient and tumor characteristics. Bethesda (MD): National Cancer Institute; 2007. (SEER Program, NIH publ no 07-6215). [Google Scholar]
- 23.Nakanishi R, Li D, Blaha MJ, et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur Heart J Cardiovasc Imaging. 2016;17:1305–14. doi: 10.1093/ehjci/jev328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in bodymass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Country profile: Canada — body mass index. NCD Risk Factor Collaboration (NCD-RisC) [accessed 2018 Mar 18]. Available: www.ncdrisc.org/downloads/country-pdf/country-profile-Canada.pdf.
- 27.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 28.Chiu M, Austin PC, Manuel DG, et al. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34:1741–8. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Census profile, 2016 census. Ottawa: Statistics Canada; 2019. Aug 9, [accessed 2019 Jan 15]. Available: www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=01&Geo2=PR&Code2=01&Data=Count&SearchText=canada&SearchType=Begins&SearchPR=01&B1=All&TABID=1. [Google Scholar]
- 30.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YJ, Lee DH, Han KD, et al. Is nonalcoholic fatty liver disease associated with the development of prostate cancer? A nationwide study with 10,516,985 Korean men. PLoS One. 2018;13:e0201308. doi: 10.1371/journal.pone.0201308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caballería L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22:24–32. doi: 10.1097/MEG.0b013e32832fcdf0. [DOI] [PubMed] [Google Scholar]
- 33.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204–10. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Shaheen AA, Riazi K, Medellin A, et al. Implementation of a primary care shear-wave elastography-based pathway to identify non-alcoholic fatty liver disease patients with advanced fibrosis in a large North American urban population. J Hepatol. 2019;70(Suppl):E783. [Google Scholar]
- 35.Organ donation and transplantation in Canada: system progress report 2006–2015. Donation and Transplantation, Canadian Blood Services; 2016. [accessed 2019 Oct 16]. Available: https://blood.ca/sites/default/files/ODT_Report.pdf. [Google Scholar]
- 36.Treatment of end-stage organ failure in Canada, Canadian Organ Replacement Register, 2006 to 2015: data tables, liver transplants. Ottawa: Canadian Institute for Health Information; [accessed 2019 Oct 16]. Available: www.cihi.ca/sites/default/files/document/liver_transplant_section_v0.1_en_2017.xlsx. [Google Scholar]
- 37.Martini A, Ceranto E, Gatta A, et al. Occult liver disease burden: analysis from a large general practitioners’ database. United European Gastroenterol J. 2017;5:982–6. doi: 10.1177/2050640617696402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken) 2018;11:81. doi: 10.1002/cld.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu AZ, Kattan MW. Utilities should not be multiplied: evidence from the preference-based scores in the United States. Med Care. 2008;46:984–90. doi: 10.1097/MLR.0b013e3181791a9c. [DOI] [PubMed] [Google Scholar]
- 40.Lapointe-Shaw L, Georgie F, Carlone D, et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13:e0201120. doi: 10.1371/journal.pone.0201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adejumo AC, Samuel GO, Adegbala OM, et al. Prevalence, trends, outcomes, and disparities in hospitalizations for nonalcoholic fatty liver disease in the United States. Ann Gastroenterol. 2019;32:504–13. doi: 10.20524/aog.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikkelsen L, Iburg KM, Adair T, et al. Assessing the quality of cause of death data in six high-income countries: Australia, Canada, Denmark, Germany, Japan and Switzerland. Int J Public Health. 2020;65:17–28. doi: 10.1007/s00038-019-01325-x. [DOI] [PubMed] [Google Scholar]
- 43.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepanova M, Rafiq N, Makhlouf H, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2013;58:3017–23. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 45.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Targher G, Byrne CD, Lonardo A, et al. Nonalcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis of observational studies. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Nseir W, Abu-Rahmeh Z, Tsipis A, et al. Relationship between non-alcoholic fatty liver disease and breast cancer. Isr Med Assoc J. 2017;19:242–5. [PubMed] [Google Scholar]
- 48.Harding JL, Shaw JE, Anstey KJ, et al. Comparison of anthropometric measures as predictors of cancer incidence: a pooled collaborative analysis of 11 Australian cohorts. Int J Cancer. 2015;137:1699–708. doi: 10.1002/ijc.29529. [DOI] [PubMed] [Google Scholar]
- 49.Global action plan for the prevention and control of noncommunicable diseases: 2013–2020. Geneva: World Health Organization; 2013. [Google Scholar]
- 50.Obesity in Canada — opportunities for intervention. Ottawa: Public Health Agency of Canada; 2011. Jun 23, [accessed 2020 Mar. 20]. Available: www.canada.ca/en/public-health/services/health-promotion/healthy-living/obesity-canada/opportunities-intervention.html. [Google Scholar]
- 51.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–46. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78.e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 53.Rao DP, Kropac E, Do MT, et al. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can. 2016;36:194–8. doi: 10.24095/hpcdp.36.9.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratziu V, Marchesini G. When the journey from obesity to cirrhosis takes an early start. J Hepatol. 2016;65:249–51. doi: 10.1016/j.jhep.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 55.European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 56.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–40. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 57.Alberti G, Zimmet P, Shaw J, et al. Consensus Workshop Group. Type 2 diabetes in the young: the evolving epidemic: the International Diabetes Federation consensus workshop. Diabetes Care. 2004;27:1798–811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 58.Wilkins T, Tadkod A, Hepburn I, et al. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician. 2013;88:35–42. [PubMed] [Google Scholar]
- 59.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of National Health and Nutrition Examination Survey data. Am J Gastroenterol. 2017;112:581–7. doi: 10.1038/ajg.2017.5. [DOI] [PubMed] [Google Scholar]
- 60.Kim D, Kim W, Adejumo AC, et al. Race/ethnicity-based temporal changes in prevalence of NAFLD-related advanced fibrosis in the United States, 2005–2016. Hepatol Int. 2019;13:205–13. doi: 10.1007/s12072-018-09926-z. [DOI] [PubMed] [Google Scholar]
- 61.Petta S, Di Marco V, Pipitone RM, et al. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: genetic and metabolic risk factors in a general population. Liver Int. 2018;38:2060–8. doi: 10.1111/liv.13743. [DOI] [PubMed] [Google Scholar]
- 62.Caballería L, Pera G, Arteaga I, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16:1138–45.e5. doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 63.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 64.Balakrishnan M, El-Serag HB, Nguyen T, et al. Obesity and risk of nonalcoholic fatty liver disease: a comparison of bioelectrical impedance analysis and conventionally-derived anthropometric measures. Clin Gastroenterol Hepatol. 2017;15:1965–7. doi: 10.1016/j.cgh.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shields M, Tremblay MS, Connor Gorber S, et al. Measures of abdominal obesity within body mass index categories, 1981 and 2007–2009. Health Rep. 2012;23:33–8. [PubMed] [Google Scholar]
- 66.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 67.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–73. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Törner A, Stokkeland K, Svensson A, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2017;65:885–92. doi: 10.1002/hep.28775. [DOI] [PubMed] [Google Scholar]
- 69.Hong TP, Gow P, Fink M, et al. Novel population-based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology. 2016;63:1205–12. doi: 10.1002/hep.28267. [DOI] [PubMed] [Google Scholar]
- 70.Report on hepatitis B and C in Canada: 2017. Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada; 2019. [accessed 2020 May 26]. Cat no HP37-22E-PDF. Available: https://www.canada.ca/en/services/health/publications/diseases-conditions/report-hepatitis-b-c-canada-2017.html#a4.1. [Google Scholar]
- 71.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 72.Younossi Z, Stepanova M, Ong JP, et al. Global Nonalcoholic Steatohepatitis Council. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–55.e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.