Abstract
A significant fraction of the total immune cells in the body are located in several hundred lymph nodes, in which lymphocyte accumulation, activation and proliferation are organized. Therefore, targeting lymph nodes provides the possibility to directly deliver drugs to lymphocytes and lymph node-resident cells and thus to modify the adaptive immune response. However, owing to the structure and anatomy of lymph nodes, as well as the distinct localization and migration of the different cell types within the lymph node, it is difficult to access specific cell populations by delivering free drugs. Materials can be used as instructive delivery vehicles to achieve accumulation of drugs in the lymph nodes and to target specific lymph node-resident cell subtypes. In this Review, we describe the compartmental architecture of lymph nodes and the cell and fluid transport mechanisms to and from lymph nodes. We discuss the different entry routes into lymph nodes and how they can be explored for drug delivery, including the lymphatics, blood capillaries, high endothelial venules, cell-mediated pathways, homing of circulating lymphocytes and direct lymph node injection. We examine different nanoscale and microscale materials for the targeting of specific immune cells and highlight their potential for the treatment of immune dysfunction and for cancer immunotherapy. Finally, we give an outlook to the field, exploring how lymph node targeting can be improved by the use of materials.
Lymph nodes are essential tissues of the immune system, providing a structure to gather immunogenic information from peripheral tissues1. Lymph nodes are one of the primary organs in which the adaptive immune response of the body occurs, and, therefore, their health is important for maintaining a functioning immune system2–4. The lymph nodes in the body are connected — immunologically speaking — by migrating lymphocytes, which enter the lymph node to find their cognate antigen and then re-enter the circulation to provide protective immunity in the periphery. Thus, delivering drugs directly to lymph nodes provides an opportunity to address a variety of local and systemic immunological challenges, as well as diseases that afflict cells of the immune system or are regulated by the adaptive immune system.
The efficacy of an administered drug is determined by the therapeutically relevant drug bioavailability and the duration of action at the target site. Deleterious off-target effects and toxicities reduce the maximum tolerable dose, requiring either alterations to the route of administration or advanced formulations to improve the specificity of tissue and cell delivery. Biomaterials- based delivery systems can be applied to address these challenges owing to the potential of materials to prolong circulation times of intravenously infused agents or their retention after administration in peripheral tissues, to leverage specific physiological structures and pathways to improve tissue targeting or clearance pathways and to target specific cells within tissues. Therefore, drug carriers, such as polymers, lipids and inorganic materials, can alter the pharmacokinetics and biodistribution of their associated small molecule drug. A variety of materials are being explored for lymph node drug delivery, including synthetic micelles5–10, dendrimers11,12, inorganic nanoparticles13,14 and liposomes15,16. Each of these materials has advantages for specific applications and/or targets; however, in general, drug carriers improve lymph node targeting by increasing the molecular weight of the drug, which favourably affects lymphatic uptake, by reducing vasculature permeability to improve lymphatic drainage, by targeting phagocytic cells in peripheral tissues to facilitate transport to the lymph nodes or through a combination of these effects.
Various physiochemical properties of materials can be tailored to target the lymph nodes for drug delivery17 and for lymph node imaging18. In this Review, we discuss materials that are designed to target specific cells within the lymph node. We examine lymph nodes and their specific cell subtypes as valuable immunotherapeutic and drug targets, investigate the mechanisms of endogenous molecular and cellular transport to and within the lymph nodes and highlight the use of bioinspired systems and materials for basic immunology studies and as drug delivery systems exploiting these pathways.
Targeting lymph nodes
One of the most obvious rationales for targeting lymph nodes is in the context of vaccination, which is generally used to generate adaptive immunity but also to induce immune tolerance. For vaccination, antigens are often delivered in conjunction with co-stimulatory agents that induce immunity or with immunosuppressive and/or tolerogenic agents that induce tolerance signals in antigen-presenting cells (APCs), which take up and process antigens for presentation to lymphocytes. APCs comprise a diverse collection of phagocytes with antigen presentation functions, including professional APCs — dendritic cells, macrophages, Langerhans cells and B cells — and non-classical APCs with important stromal functions within lymph nodes4. The quality and quantity of the immune response are fine-tuned by the activation state of these APCs and the microenvironment in which antigen presentation and recognition take place. If the adaptive immune system can be considered an orchestra, dendritic cells are the conductors. They can present both self-antigen and exogenous antigen and mediate a range of co-stimulatory signalling pathways, and thus have a key role in coordinating antigen uptake, processing and presentation and in the priming of lymphocytes. Therefore, a variety of material designs are being explored for the modulation of dendritic cell function by, for example, co-stimulation, antigen presentation and targeting19. Macrophages are also important cells within both peripheral tissues and lymph nodes, especially for the barrier and siphon functions of lymph nodes20 (FIG. 1). They further provide viral reservoirs during infection and can exert local immunomodulatory effects within lymph nodes. Therefore, materials are also being developed for targeted delivery, modulation and ablation of macrophages21.
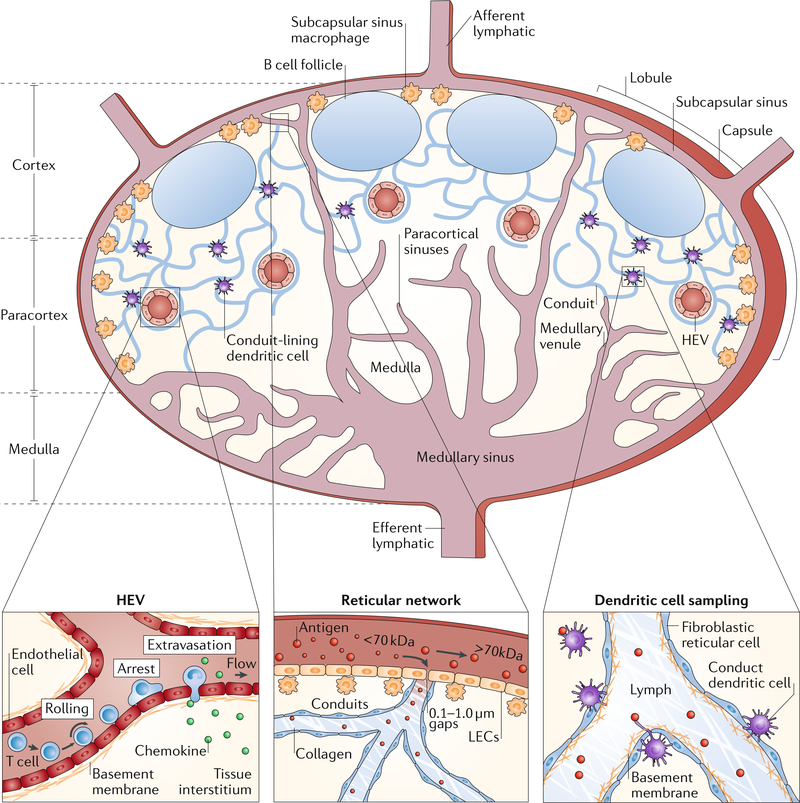
Fig. 1 |. Lymph node structure and physiology.
A cross section of a lymph node is shown. The architecture of the lymph node can be divided into distinct areas: fluid-filled lumen structures (lymphatics, high endothelial venules (HEVs), capillaries and sinuses), cellular locations (B cells in follicles, dendritic cells and T cells in the paracortex and macrophages in the subcapsular sinus and medulla) and structural units (cortex, paracortex and medulla). Lymphocyte extravasation occurs in the HEVs. The distribution of antigens within the reticular structure is regulated by haemodynamic size and molecular weight by the capsule and conduit. Circulating lymphocytes enter through the vasculature and exit through the efferent lymphatics. Dendritic cells sample the conduit and conduit structures. LEC, lymphatic endothelial cell. HEV and lymph node cross section adapted from REF40, Springer Nature Limited.
Aside from vaccination strategies aimed primarily at APCs, directly targeting lymphocytes is therapeutically desirable because immunomodulatory agents that directly act on T and B cells can regulate their differentiation, activation and function in response to antigen recognition. Lymph node drug delivery is also especially important for the elimination of lymph node-resident cancers and metastases, including lymphomas, which can reside within the lymph node. Moreover, latent viral reservoirs, such as HIV in T cells, are also localized within lymph nodes and difficult to treat22. Therefore, materials engineering can take advantage of the localization of these cell subtypes within the lymph nodes by targeting the specific endogenous structural features and transport mechanisms that access both the lymph nodes and sub-compartments within lymph nodes that house the cells.
Further materials design opportunities exist given that the tissue in pre-metastatic and metastatic lymph nodes undergoes extensive remodelling23–30, which affects tissue structure and makes them potentially more accessible to drugs than healthy lymph nodes. For example, abnormal lymphatic and blood vasculature (similar to the canonical enhanced permeability and retention effect in primary tumours), altered cell phenotypes (generally more suppressive) and aberrant chemokine and cytokine milieus provide potentially exploitable, microenvironment-specific features for lymph node-directed drug delivery.
Lymph node structure
The lymph node provides a specialized microenvironment to connect peripheral immunological information (antigens and other immune-modulatory molecules and cells) and circulating lymphocytes. Lymph nodes are composed of basic units called lymphoid lobules, each of which is drained by a single afferent lymphatic vessel sampling lymph from different drainage basins31 (FIG. 1). The base of the lobule consists of slender cords that are anchored by vascular roots and form part of the lymph node medulla, in which the arterioles, high endothelial venules and paracortical sinuses reside. The apex of the lobule is separated from the surrounding lymph node capsule by the subcapsular sinus31–33. The lobule is structurally supported by the reticular network, which is a fibrous sponge-like tissue composed of fibroblastic reticular cells and their reticular fibres. The reticular network provides a 3D scaffold for the interaction and migration of lymphocytes, APCs and macrophages34 (FIG. 1). Within this mesh, conduits of the reticular network are formed by extracellular matrix (ECM) proteins, with a central core composed of the interstitial matrix molecules collagen types I and III and a surrounding basement membrane-like structure ensheathed by a layer of fibroblastic reticular cells35.
Within each lobule, B and T cells home to separate locations (FIG. 1). B cells reside in follicles, in which they primarily interact with follicular dendritic cells. Once activated, B cells proliferate and undergo clonal expansion within the follicle, which leads to the formation of germinal centres containing proliferating B cells and areas of displaced resting B cells, called the secondary follicles36,37. By contrast, T cells migrate to the deeper interfollicular cortex and paracortex of the lobule, where they interact with migratory dendritic cells from peripheral tissues or lymph node-resident dendritic cells to become activated and proliferate38,39. Therefore, the reticular network, the lobular blood vessels and the sinuses are key components of the lymph node providing the specific structure that enables the relatively small number of lymphocytes to efficiently circulate and monitor antigen in the lymph node network40.
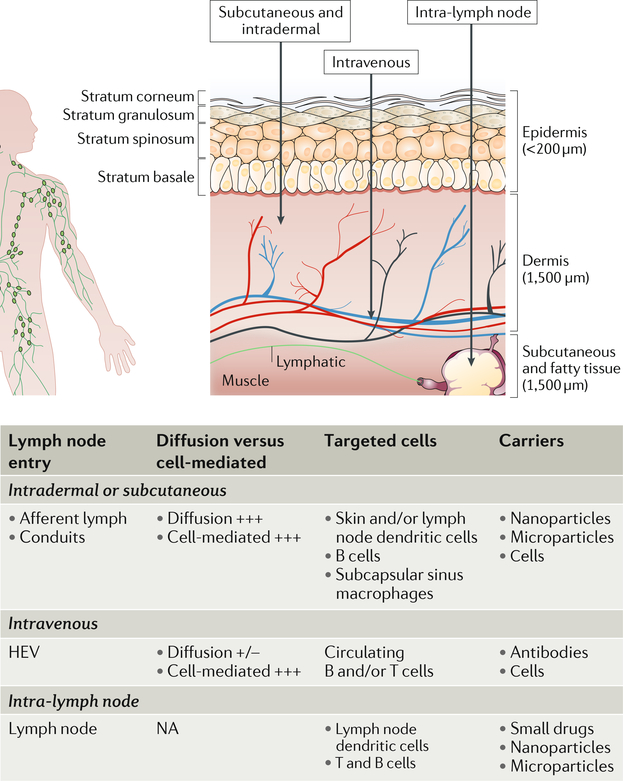
Solutes, biomolecules and cells can enter the lymph node by afferent lymphatics, lymph node blood capillaries or high endothelial venules41 (FIG. 1), resulting in a specific distribution of molecules and cells within the lymph node. The distribution depends on the interfaces of the entry pathways with the other structural components and resident cells of the lymph node. Therefore, the specific structure and location of the different lymph node components are important design factors for materials targeting specific lymph node-resident cell types. Thus, materials need to be designed to leverage the different entry pathways to lymph nodes to enable targeted lymph node drug delivery: diffusive or convective delivery through the afferent lymphatics or capillaries, active cell-mediated migration from the peripheral tissue interstitium, transport in the circulating vasculature and entry through the blood capillaries and high endothelial venules, or direct injection.
Accessing lymph nodes via lymphatics
Unlike the circulatory system, which contains a central pump, the lymphatics operate on a local level2. Fluid uptake and transport in the interstitium of a tissue are thought to be driven by expansion and compression of the initial lymphatics: expansion leads to percolation of interstitial fluid through the endothelial microvalves, which causes filling of the initial lymphatics. The lymphatics are then compressed by the surrounding tissue, triggering the transport of the fluid (now termed lymph) to the large collecting lymphatics31.
The initial lymphatics are blind-ended and composed of non-fenestrated overlapping endothelial cells with filaments anchoring them to the surrounding ECM, which provides mechanical support against the low pressure inside the initial lymphatic vessel lumen42. Owing to permeability differences between the non-fenestrated vascular capillaries and the lymphatics, only molecules with a certain size (10–100 nm in hydrodynamic radius) can efficiently convect into the lymphatics, which has important ramifications on drug formulation and delivery to the lymphatics43.
In the collecting lymphatic vessels, lymph is propelled by the synchronized movement of lymphatic vessel compartments called lymphangions, which contain one-way valves to propel the lymph in a unidirectional manner44. Once the lymph arrives at the draining lymph node through one of the afferent lymphatic vessels, it enters the subcapsular sinus45 (FIG. 1). The lymph then spreads into the subcapsular sinus and moves through the transverse sinuses, covering each lobule before finally exiting into the medullary sinuses, which merge from all lobules into a single efferent lymphatic vessel that may filter through subsequent lymph nodes in the same chain, before the lymph eventually returns back to the blood through the thoracic duct1 (FIG. 1).
Within each lymph node, the lymph flowing over the lobules through the subcapsular sinus is sampled by percolating through the conduits created by the reticular structure46. The reticular network restricts the access of lymph-borne material to the paracortex, which is important for preserving the naive state of the lymphocyte microenvironments and for controlling immunogenic molecules that adversely affect the immune response in the cortex, for example, exosomes from tumours or soluble products produced by microbial infections47–49 (FIG. 1). The efficiency of this barrier depends on the size of the lymph-borne molecules with high molecular weight (>70 kDa) molecules being virtually excluded from conduit and cortex access by the subcapsular sinus. Conversely, lower molecular weight species are gradually excluded, with molecules <70 kDa having some access to the conduits48,49. Permeation of low molecular weight molecules from the conduits to the lymphocytes within the paracortex is mostly restricted. For immune challenges with low antigen concentration, this restriction poses a significant barrier to the generation of a robust adaptive immune response. However, higher antigen concentrations could enable direct lymphocyte access on a physiologically relevant scale.
Lymphatic uptake
To be transported to lymph nodes in the afferent lymph, drug delivery systems must overcome barriers, such as vasculature clearance, penetration of the epithelium of the skin and traversing the mucosa and gut barriers. In the tissue interstitium, where afferent lymphatic access is maximized, transport is restricted by the gel-like ECM, which is composed of fluid, solutes, fibrillar proteins and proteoglycans, which inform the design parameters for size, shape and charge of the drug delivery system50.
Drug delivery formulations that lead to prolonged retention at the injection site can result in improved lymphatic uptake51. Similarly, drug delivery systems that prevent adsorption of the drug to the ECM interstitial biopolymer network52 show improved diffusivity through the interstitium and therefore better lymphatic uptake. Uptake from the interstitium by the lymphatics is sensitive to the size of the administered agent, and molecules with hydrodynamic diameters of 10–100 nm are most efficiently taken up11,12,51,53. The transport of larger molecules is limited by the pore size of the ECM50.
Comparing the biodistribution to the local draining lymph node with clearance to and accumulation in systemic tissues (liver, spleen, lungs and kidney) shows that transport through the afferent lymph results in an ~1,000-fold increase in accumulation within local draining lymph nodes54, which can substantially reduce the risk of off-target effects, owing to lower doses than would otherwise be required to achieve a therapeutic effect when administered systemically (for example, intravenously). Interestingly, the same level of locoregional enrichment of the afferent lymph occurs in diseased tissues, for example, in tumours54, demonstrating the relevance of afferent lymph transport for sentinel lymph node targeting. Therefore, a variety of materials have been explored for lymph node targeting through the afferent lymph, including den- drimers11,12, synthetic polymer nanoparticles55,56, lipid- based drug delivery vehicles57, inorganic particles58 and cell-derived exosomes59.
Targeting antigen-presenting cells
APCs, including some dendritic cell subtypes, are located in peripheral tissues and lymph nodes. Materials-based delivery strategies have been explored to target vaccines to dendritic cells55,60 (FIG. 2) because these cells are more sensitive to phagocytosing large particulate materials than small molecules. The shape61–63 and charge64 of materials affect dendritic cell targeting by modulating cell-particle interactions through membrane strain energy65 and membrane electrostatic interaction66. Accordingly, several approaches using inorganic13,14, polymer5,6,8–10 and lipid-based15,57 nanoparticles have been employed to improve lymphatic and dendritic cell uptake and thus lymph node targeting.
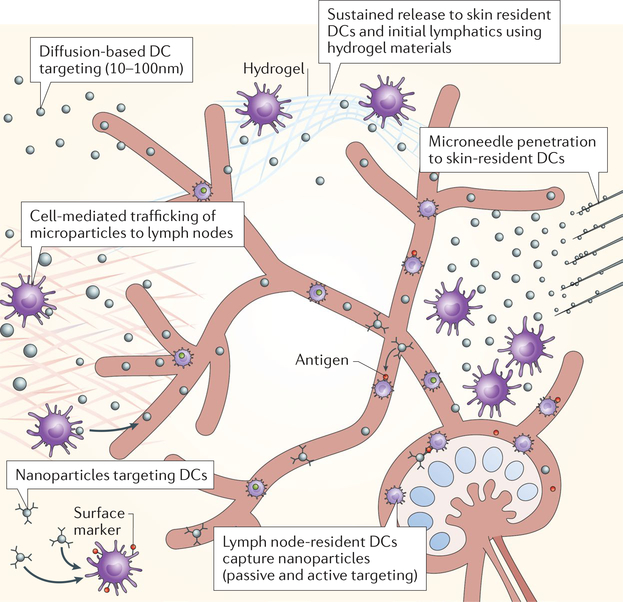
Fig. 2 |. Targeting dendritic cells.
Small nanoparticles (10–100 nm in diameter) are taken up by the lymphatics and diffuse to the lymph node to target lymph node-resident dendritic cells (DCs). Large nanoparticles (>100 nm in diameter) and microparticles are mostly entrapped in the interstitial matrix at the site of injection and require capture by peripheral DCs or Langerhans cells (skin) for cell-mediated delivery to lymph nodes. Peripheral and lymph node-resident DCs can be actively targeted using cell subtype-specific surface markers. Hydrogels can be used for the controlled release of molecules in peripheral tissues to enable sustained lymphatic uptake and prolonged DC interactions. Microneedles enable transdermal delivery of particle depots and delivery to DC subtypes that reside within discrete skin layers by adjusting the length of the needles. Lymph node-resident DCs take up passively drained nanoparticles and receive cell-delivered particles.
Drainage of the afferent lymphatics can be exploited to deliver nanoparticles to draining lymph node-resident dendritic cells67, for example, to transport immunotherapeutic adjuvant drugs to the tumour-draining lymph node. The tumour-draining lymph node is full of lymph- transported tumour antigen, and thus delivery of only adjuvant rather than synthetic or purified tumour antigen is sufficient to induce an immune response against the endogenous tumour antigen — a method called in situ vaccination. To deliver adjuvant to the tumour-draining lymph node, lymphatic-draining micellar Pluronic F127 nanoparticles can be used. Pluronic F127 is an amphiphilic block copolymer made from polyethylene glycol (PEG)–poly(propylene glycol)–PEG. The micellar poly(propylene sulfide) nanoparticles68 with a diameter of 30 nm can then be conjugated to or encapsulate Toll-like receptor 4 (TLR4) and TLR9 ligands as adjuvants. Following administration into the skin of C57Bl/6 mice ipsilateral to a B16F10 melanoma, the adjuvanted (TLR ligand-formulated) nanoparticles accumulate only in the tumour-draining lymph nodes, leading to a decrease in tumour growth, as compared with delivery to the non-tumour-draining lymph nodes or of free (non-encapsulated and/or non-conjugated) TLR ligand. The difference in efficacy can be attributed to the increase in the maturation and activation status of tumour-draining lymph node-resident dendritic and T cells, resulting in an increase in tumour antigen- specific T cells infiltrating (and presumably eliminating) the tumour.
Alternatively, draining lymph nodes can be targeted using endogenous albumin as a carrier, delivering a TLR9 CpG oligodeoxynucleotide adjuvant69. Albumin drains into lymphatics and thus is transported to the lymph nodes. CpG can be modified with engineered lipid chains that associate with albumin. Following a diacyl lipid modification and administration in mice, CpG- albumin accumulates in the lymph nodes at significantly higher levels than free CpG and associates with B cells, macrophages and dendritic cells.
APCs can also be transfected with a tumour- associated antigen to promote a cytotoxic T cell response using lipid nanoparticles that intracellularly deliver mRNA70. The lipid nanoparticle formulation can be optimized for lipid complexation with mRNA, cellular uptake, endosomal escape, particle stability and in vivo distribution by varying the lipids, for example, by using ionizable lipids, phospholipids, cholesterol, additives and PEGylated lipids. This system can then be used for the generation of antigen-specific T cells. The optimal lipid nanoparticle formulation has a diameter between 50 nm and 150 nm and a charge between −3 mV and −15 mV. These particles can be used to transfect dendritic cells, neutrophils, macrophages and B cells in draining lymph nodes following subcutaneous injection. Therefore, lymph node-resident APCs can be targeted with a variety of drug carriers through peripheral bolus injection.
Alternatively, hydrogels can be applied as sustained release platforms to target lymph node-resident APCs (FIG. 2). For example, a self-assembled filomicelle scaffold can be engineered that degrades into monodisperse micellar nanocarriers (~30 nm in diameter)71. Following subcutaneous injection, these scaffolds degrade over the course of a month through photooxidation or physiological oxidation and thus can be used for the sustained delivery of micellar nanocarriers to lymph node-resident phagocytic immune cells, including dendritic cells (MHCII+ and MHCII−) and macrophages. Similarly, nanoparticles can be encapsulated in a self-assembled pH-degradable hydrogel. The core polymer blocks of the nanoparticles can be ligated with the TLR7 and/or TLR8 agonist imiquimod (IMDQ)72, resulting in polymeric nanoparticles with a diameter of 50 nm. These ‘nanogels’ slowly break down over the course of a week, and the individual nanoparticles diffuse away from the injected gel. After subcutaneous administration in the mouse footpad, the IMDQ nanogels are retained in the footpad and drain to the lymph node for at least 24 hours. Passive diffusion of the IMDQ nanogels to the draining lymph node was confirmed in CC-chemokine receptor 7 (CCR7) knockout mice (which do not show dendritic cell homing to lymph nodes through the lymphatics). Furthermore, IMDQ ligation led to a 10-fold, 5-fold, 3-fold and 26-fold increase in the uptake of nanogels by B cells, dendritic cells, macrophages and monocytes, respectively, compared with control nanogels without IMDQ. Applying the IMDQ nanogels to initiate an adaptive immune response against the Mycobacterium tuberculosis antigen PPE44 demonstrated that they induced greater serum antibody titres and elicited increased interferon-γ-secreting CD4+ and CD8+ T cells compared with soluble IMDQ.
Targeting lymph node tumours
Lymph node-resident tumours can be targeted and treated by exploiting the afferent lymphatics. Primary and metastatic tumours disrupt the regular architecture of lymph nodes, which causes an increase in the diffusivity of fluids and molecules, enabling deeper lymph node penetration of drug carriers than that seen with healthy lymph nodes73. Nanoparticles accumulate in lymph node-resident tumours and therefore, in combination with photothermal therapy, can be applied for treatment through thermally triggered drug effects, which reduces adverse side effects74,75 (TABLE 1). For example, neutral PEGylated polymeric gold nanorods (~10 nm in diameter) can be delivered to lymph node-resident tumours through the lymphatics to enable local photothermal therapy75. The gold nanorods rapidly accumulate in the lymph nodes and are retained at the injection site adjacent to the axillary lymph node. In combination with photothermal therapy, the gold nanorods show robust efficacy against lymph node metastasis, providing an alternative strategy to systemic delivery approaches for the treatment of metastasis.
Table 1 |.
Lymph node cells and delivery methods
| Cell type | Immune response | Implicated diseases | Carriers and delivery methods | Refs |
|---|---|---|---|---|
| Peripheral tissue-resident dendritic cells | Adaptive T and B (humoral) cell immunity | Pathogenic infection; cancer (vaccination); and autoimmunity (tolerance induction) | Large particles (>500 nm diameter); microneedles; hydrogels; and topical application | 70,71,105 |
| Lymph node-resident dendritic cells | Adaptive T and B (humoral) cell immunity | Pathogenic infection; cancer (vaccination); and autoimmunity (tolerance induction) | Small delivery vehicles (10–100 nm diameter); liposomes; and cell-mediated transport | 55,67,69,70,121,156 |
| B cells | Humoral immunity | Pathogenic infection | Small antigens (<70 kDa); nanoparticles (<200 nm diameter); viruses; exosomes; and protein–immunoglobulin complexes | 47,77,79,80,84,85 |
| Effector CD8+ T cells | Antigen-specific cellular immunity | Viral infection and cancer | Blood-circulating T cells; ex vivo T cell labelling and adoptive cell transfer; HEV-targeting carriers; small molecules (<70 kDa) in conduits; and intra-lymph node microparticles | 95,138,141 |
| Regulatory CD4+ T cells | Tolerance against self-antigen | Autoimmunity; transplantation; and cancer (inhibition of immune suppression) | Blood-circulating T cells; ex vivo T cell labelling and adoptive cell transfer; HEV-targeting carriers; small molecules (<70 kDa) in conduits; and intra-lymph node microparticles | 93,142,150 |
| Lymph node-resident cancer cells | NA | Lymphoma and cancer metastasis | Small molecule chemotherapy and nanoparticles | 74,75,94,140 |
HEV, high endothelial venule; NA, not applicable.
Targeting B cells
B cells are crucial for the generation of humoral immunity and thus are of great interest for lymph node-directed drug delivery. However, access of B cells to antigen is tightly controlled by the subcapsular sinus, and therefore, delivering large antigens to B cells requires transit by an intermediate cell, such as CD169+ subcapsular sinus macrophages or fibroblastic reticular cells, which line the lymph node conduits. Owing to the location of B cells in the follicles adjacent the subcapsular sinus, they can be accessed by three different approaches: soluble antigen permeation through the conduits (antigens <70 kDa); large particulate antigen (for example, viral particles), immune complexes (antigen-antibody complex) or material coated in complement proteins shuttled by barrier capsule cells; and cellular delivery by tissue-resident fibroblastic reticular cells76 (FIG. 3). Although of great importance, strategies for material-mediated B cell targeting remain limited thus far; however, immunological studies characterizing antigen capture by B cells can provide instructive insights for the design of drug carriers (TABLE 1).
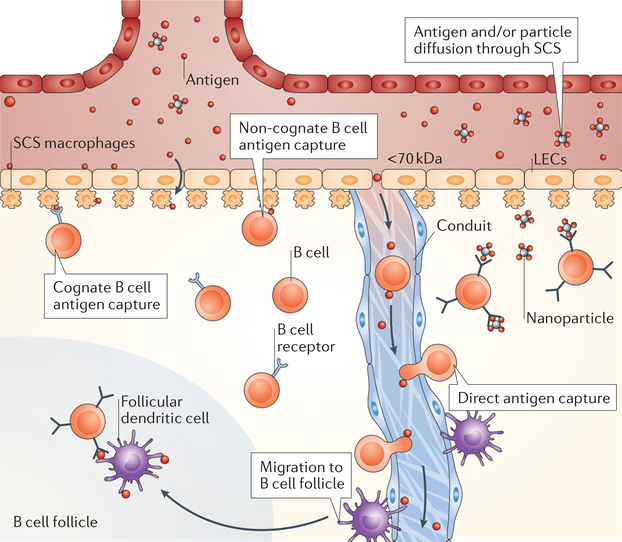
Fig. 3 |. Targeting B cells.
Subcapsular sinus (SCS) macrophages transfer complementdecorated particles via the complement receptor, whereas they transfer immune complexes bound to particles or materials via Fc receptors to the basal side of the sinus to non-cognate and cognate B cells, respectively. Small antigen can be cleaved from microparticles by proteases and released in the sinus. Antigens then diffuse through the SCS directly to B cells. Materials can enter through gaps (0.1–1.0 μm) in the SCS, enabling diffusion of the materials to B cell follicles for direct B cell sampling. Small materials (<70 kDa) can enter the conduits, where they can be directly captured by B cells. LEC, lymphatic endothelial cell.
Owing to their phagocytic nature, the expression of the B cell receptor and their spatial location in the lymph node, B cells can directly sample and capture lymph- borne antigens (FIG. 3). One strategy applied by B cells is direct sampling of the lymph node conduits. The conduits bypass the subcapsular sinus barrier and pass through the follicles. Although they are less prevalent than in the paracortex because they are replaced by follicular dendritic cells during lymph node development, conduits are an important pathway for distributing molecules throughout the follicles of the lymph node, in particular, antigens. B cells use this structural feature to directly sample small conduit-accessible antigens to become activated47.
Using multiphoton intravital microscopy, it was demonstrated how follicular B cells have access to soluble antigen47. Following subcutaneous injection of a fluorescently labelled small antigen (~14 kDa) and adoptive transfer of fluorescently labelled B cells with a B cell receptor specific for this antigen, the antigen was transported to the draining lymph node within several minutes, and a majority of the B cells remained closely associated with antigen-filled conduits. Using electron microscopy, it was shown that the follicular conduits have gaps in their surrounding stromal cell layer through which the B cell pseudopods can come into direct contact with the collagen core, enabling them to sample antigen from the conduits. To investigate how B cells have access to large antigens, multivalent protein conjugates (~70 kDa) have been subcutaneously injected. B cells bind the large antigen in the follicles within minutes following subcutaneous injection, which would not occur if the antigen would be strictly confined to the conduits. Interestingly, antigen-specific B cells also mirror the position of the diffusing wave of antigen in relation to the subcapsular sinus, demonstrating that large antigens also have the capability to diffuse through the small 0.1–1.0 pm fenestrations in the subcapsular sinus and thus are directly accessed by follicular B cells.
To test how B cells react to large particulate antigens, fluorescent particles with a diameter of 1 μm, surface-decorated with a model antigen through covalent bond linkages, were intradermally injected in the ears of mice77. All antigen-specific B cells acquired the antigen, but only ~10% of these cells were positive for the microsphere carrier, which was the same as for the uptake of non-antigen-conjugated microspheres, indicating nonspecific uptake; moreover, this number did not change over time. These data suggest that most antigen-specific B cells acquire antigen conjugated to the microsphere without actually taking up the microsphere, which is confined to the subcapsular sinus owing to its size. The presence of lymph protease near the subcapsular sinus allows for the speculation that endogenous protease or administration of exogenous protease could induce cleavage of the antigen from the large carrier, resulting in direct B cell access to the small antigen. Therefore, these studies suggest that lymph-accessible, small antigens have direct access to B cells that are proximal to the subcapsular sinus owing to their small size.
Immune complex (antigen-antibody complex) trafficking to B cell follicles and B cell capture are also being explored for B cell-directed drug delivery. Immune complexes are generally more effective in generating antibody responses than free antigen78. Intralymph node immune complex capture and trafficking are tightly orchestrated and coordinated by several cell types, including subcapsular sinus macrophages, follicular dendritic cells and follicular B cells (FIG. 3). After subcutaneous administration, immune complexes are rapidly captured by poorly phagocytic subcapsular sinus macrophages and shuttled to follicular B cells, which relay the immune complexes to the germinal centre. In the germinal centre, the antigen is transferred to follicular dendritic cells or to cognate B cells, which has been demonstrated using phycoerythrin immune complexes79,80.
The process of immune complex capture is mediated in vivo by complement C3-coating and the Fc region of the antibody coating, which are recognized by the subcapsular sinus macrophage complement receptor 3 (CR3) and Fc receptor Ilb (FcRIIb), respectively81. Upon capture, immune complexes are shuttled to the basal side of the capsule, where follicular B cells retrieve the complex through the receptors CR1 and CR2 and subsequently migrate into the follicles80. In the follicles, follicular dendritic cells scavenge B cell-borne immune complexes owing to a higher level of CR1 and CR2 and retain antigen on their surface for up to 16 days, enabling constant immune complex cycling and potential interactions with cognate B cells82,83.
Antigen can also be transferred to B cell follicles in a B cell receptor-dependent manner (FIG. 3), which can be explored for material design strategies. For example, nanoparticles that can be transported to the lymph node can be used to investigate the presentation of large antigens (>70 kDa) to cognate B cells for the induction of antibody responses84. Fluorescent avidin-coated nanoparticles with a diameter of 0.2 μm can be decorated with biotinylated antigen85. B cells acquire the conjugated antigen in a B cell receptor-specific manner through direct transfer from subcapsular sinus macrophages, which translocate antigen-laden nanoparticles from the sinus to the follicle. Consequent activation of the B cells leads to an increase in MHCII and CD86 expression as well as IgM downregulation on their way to the T cell at the follicular border. Therefore, a variety of antigens could be conjugated to nanoparticles to be captured by subcapsular sinus macrophages and immediately recognized by cognate B cells, avoiding the need to be trafficked into B cell follicles.
Targeting subcapsular sinus macrophages
Subcapsular sinus macrophages play important roles in lymph node physiology by serving as a cellular barrier mediating the exposure of antigens and other lymph- borne species to lymphocytes residing in the follicles and paracortex45,86. Therefore, they can also be thought of as regulators of lymph node immune function86–88. Subcapsular sinus macrophages are non-degradative phagocytes, that is, they do not process particles, in contrast to conventional macrophages. Instead, subcapsular sinus macrophages present non-degraded antigen to B cells at the follicular side of the subcapsular sinus79. Liposomes are commonly used carriers for delivery to subcapsular sinus macrophages owing to their amphipathic composition, which promotes internalization by endocytosis rather than scavenging by phagocytosis16,89,90 (FIG. 3; TABLE 1). Once internalized, liposomes are processed by phospholipases, which disrupt their structure, causing the intracellular release of encapsulated cargo91. Thus, liposomes have been applied for the encapsulation of dichloromethylene-bisphosphonate (clodronate) for the selective depletion of subcapsular sinus macrophages. Presumably, any phagocytic cell takes up clodronate liposomes, but subcapsular sinus macrophages are the first cells encountering and interacting with material entering through the afferent lymphatics and eventually depleting the material. Therefore, liposomes can be used to deliver cargo intracellularly to subcapsular sinus macrophages and to deliver clodronate to explore the effect of macrophage depletion on the adaptive immune responses within lymph nodes85,86, which may be of interest for delivering cargo deeper into the lymph node.
Blood vasculature
The blood vasculature provides an alternative transport pathway to the lymph nodes. The infiltration of circulating lymphocytes into the lymph node is controlled by high endothelial venules, which are specialized tissues lined with high (full rounded shaped) cuboidal endothelial cells with receptors that facilitate intravascular lymphocyte transmigration through the endothelial layer into the reticular meshwork3 (FIG. 1). Therefore, owing to the fact that the blood capillaries perform filtration functions, materials can be designed to leverage the diffusive and convective transport through these vascular structures to target cells in the lymph node.
Targeting T cells
T cells primarily reside in the paracortex near the blood capillaries, and thus the blood vasculature is an attractive potential route to target lymph node-resident T cells (TABLE 1), for example, by mimicking homeostatic T cell trafficking from the blood to the lymph node through high endothelial venules (FIG. 4). The entry of lymphocytes through high endothelial venules is initiated by the homing receptor L-selectin (CD62L), which recognizes peripheral node addressin (PNAd), which is expressed on high endothelial venules in lymph nodes and upregulated at sites of chronic inflammation. This natural homing process can be explored for drug delivery by functionalizing microparticles with the 6-sulfo-sialyl Lewis X-targeting antibody MECA-79, which binds to PNAd. The functionalized particles accumulate in draining lymph nodes downstream from rejected transplants following intravenous injection92,93, as draining lymph nodes have higher expression levels of PNAd than non-draining lymph nodes owing to chronic inflammation, enabling selective targeting. Administration of free MECA-79 before microparticle injection leads to blocking of PNAd and therefore to a decrease in particle accumulation in draining lymph nodes, indicating MECA-79-mediated accumulation. Therefore, drugs can be selectively delivered to draining lymph nodes, where they can then be delivered to T cell populations, for example, to decrease effector CD4+ helper T cell levels in murine cardiac allograft recipients, leading to prolonged survival compared with free drug or drug-loaded microparticles without targeting ligands93.
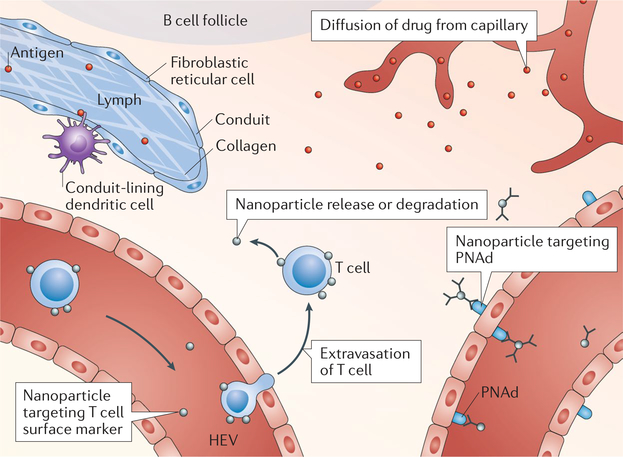
Fig. 4 |. Targeting T cells.
Conduit-lining dendritic cells sample antigen for subsequent presentation to proximal T cells. Circulating T cells can be targeted for T cell-mediated nanoparticle trafficking into the lymph node T cell zone. Lymph node blood capillaries that are leaky as a result of disease allow for diffusion-mediated transport to lymph node T cells. Microparticles and nanoparticles can be actively targeted to high endothelial venules (HEVs) using anti-peripheral node addressin (PNAd) antibodies, such as MECA-79, followed by diffusion of the delivered agent into the lymph node.
Targeting lymph node tumours
The lymph node vasculature also enables access to metastatic lymph nodes through exploiting enhanced permeability (TABLE 1). For example, systemic intravenous administration of polymeric micelles with a diameter of 30 nm (REF94) loaded with chemotherapeutic drugs leads to their selective accumulation in lymph node-resident tumours, presumably facilitated by the permeable blood vasculature around the tumour. Accumulation caused by enhanced permeability was quantified by intravenous administration of gadolinium-conjugated albumin. Interestingly, micelles with a diameter of 70 nm also accumulate in the lymph nodes at similar levels but do not have the same antitumour efficacy as micelles with a diameter of 30 nm, which can be explained by the lower accumulation of 70 nm micelles in the metastatic foci than of the smaller 30 nm micelles. These results suggest that sub-100 nm carriers passively accumulate in metastatic lymph nodes via the blood vasculature; however, smaller carriers (30 nm) accumulate at higher levels in the metastatic region owing to enhanced diffusivity.
Similarly, liposomes with diameters of ~190 nm can be conjugated with an antibody specific for the T cell surface antigen Thyl.1 using an antibody binding (Fab) fragment or an antibody fragment for the interleukin-2 (IL-2) protein and intravenously injected to target adoptively transferred cells that express the target ligand95. Evaluation of lymph nodes 24 hours after lymphodepletion showed that, if liposomes are administered immediately after adoptive T cell transfer, ~80% of adoptively transferred T cells are labelled with the targeted liposomes in the lymph nodes. Interestingly, if liposomes are administered 3 days after T cell transfer, less binding is observed, with only ~20% of adoptively transferred T cells being labelled with liposomes in lymph nodes. These results suggest that targeted nanoparticles and/or liposomes can be used to target adoptively transferred T cells in vivo resulting in efficient lymph node delivery; however, repeated dosing with targeted systems may not lead to sustained lymph node accumulation presumbly owing to decreased expression of target ligands and/or incomplete recirculation of T cells from lymph nodes to the blood.
Cell-mediated lymph node entry
Antigen-presenting cells in peripheral tissue
APCs that reside in the periphery are primed for phagocytosis and actively consume particulates to scavenge antigen for degradation and processing into peptides, which are then loaded onto MHCII96,97. Therefore, most of the antigen sampled by dendritic cells is self-antigen, which does not activate the dendritic cell98. By contrast, during an infection, the foreign antigen is often located in proximity to pathogen-associated molecular patterns (PAMPs), which are highly conserved molecules with structures that are not found in the human body. For example, coatings of pathogenic organisms, such as viral coatings and bacterial carbohydrates, are types of PAMPs99. PAMP molecules are taken up by dendritic cells together with the antigen and bind to endosomal receptors (of note, some PAMP molecules also bind to external cell membrane receptors), causing the dendritic cell to become activated100. The dendritic cell then matures and loses the ability to phagocytose and process antigen101. In the mature dendritic cell, expression of receptors for inflammatory chemokines is downregulated and expression of the lymphoid chemokines CCR7, CXC-chemokine receptor 4 (CXCR4) and CCR4 is upregulated allowing the cell to become motile and enter the lymph vessels102.
In the migrating dendritic cell, the co-stimulatory ligands CD80 and CD86 are also upregulated. These ligands are involved in the activation of T cells through binding to CD28. The dendritic cells further produce high levels of peptide-MHC, which interacts with the cognate T cell receptor in the lymph node, as well as chemokines that attract naive T cells to the lymph node through the high endothelial venules. The dendritic cells then migrate to the lymph node through the afferent lymphatic vessels, and once in the subcapsular sinus, they settle onto the sinus floor and migrate through the sinus-lobule membrane to the paracortex, where they present their antigen to lymph node-resident T cells103.
APCs reside in all peripheral tissues. Skin-resident dendritic cells and MHCII+ Langerhans cells reside in different skin tissue layers104 and exhibit distinct time frames of lymph node homing105. Following migration through the skin, they localize in discrete draining lymph node locations105 and exert specific immunomodulatory functions104,106,107. Alveolar macrophages are the main phagocytic population in the lung; however, they are more involved in clearance of foreign material than in initiating adaptive immune responses108. The adaptive immune response in the lung is generated by lung-resident dendritic cells, in particular, CD11b+ and CD103+ cells109, which recognize, internalize and present antigen on their MHCII and subsequently migrate to lymph nodes for T cell activation109,110. Of these cells, CD103+ dendritic cells are thought to be the main migratory population109. In the intestine, the mucosal surface is protected by specialized innate and adaptive sites called gut-associated lymphoid tissues, which contain B cells, T cells and other APCs capable of generating specific immune responses111. The lumen of the intestinal mucosa is further covered by epithelial cells and microfold cells, which are phagocytic and take up antigen from the intestinal lumen and transfer it to the basal side, where APCs can process the antigen for lymphocyte activation. Upon activation, dendritic cells leave the initial site of infection and transit through the lymph to draining lymph nodes, where they activate T cells or differentiate into memory or effector cells112–115. Therefore, targeting immune cells by oral delivery is different than targeting cells in the lung or skin.
Targeting lymphatic cutaneous antigen-presenting cells
Many materials for targeting skin dendritic cells have been explored, including hydrogels93,116–118 and large particulates105,119,120, with the common aim of localizing the materials to the site of administration to increase the likelihood of APC uptake and migration (FIG. 2; TABLE 1) through increasing retention half-life50,51. For example, methyl vinyl ether/maleic anhydride microneedles with a length of 600 pm can be applied to intradermally deliver antigen encapsulated within poly(lactic-co-glycolic acid) (PLGA) nanoparticles105 (FIG. 2). The microneedles locally deposit large PLGA nanoparticles, which retain and protect the vaccine antigen until uptake by skin- resident dendritic cells. In vitro, the nanoparticles are efficiently taken up by bone marrow-derived dendritic cells, which subsequently become activated and induce antigen-specific T cell proliferation. Optical coherence tomography was further used to evaluate microneedle- mediated delivery in vivo. The microneedles reach 70 μm in penetration depth and dissolve within 15 minutes after application, which leads to local deposition of the nanoparticles within the dermal layer, causing a local inflammatory response. Owing to the local effect, only dendritic cells originally migrating from the skin are positive for the uptake of nanoparticle-delivered antigen in the draining lymph node. Furthermore, owing to sustained degradation of the PLGA nanoparticles, skin-resident dendritic cells can trigger proliferation of antigen-specific T cells up to 7 days later, indicating localized and stable vaccination. The microneedle system was tested in a parainfluenza virus murine model, demonstrating that it can confer antigen-specific protective immunity against viral challenge, highlighting the importance of skin-resident dendritic cells in initiating vaccine responses105.
Cutaneous dendritic cells can also be targeted using Fc receptors, scavenger receptors and antibodies (FIG. 2; TABLE 1). For example, a model antigen and TLR ligands can be encapsulated in PLGA nanoparticles, which can be functionalized to target distinct dendritic cell surface molecules using conjugated antibodies, for example, anti-CD40, an antibody against a tumour necrosis factor family receptor, which is a marker of maturation; anti- DEC-205, an antibody against a C-type lectin receptor; and anti-CD11c, an antibody against an integrin receptor121. In vivo, subcutaneous injection of anti-CD40 functionalized nanoparticles in the mouse tail leads to the highest activation and expansion of ex vivo lymph node T cells as compared with the other antibodies, demonstrating the benefits of active targeting of dendritic cells in draining lymph nodes to elicit an immune response.
Pulmonary delivery to target lung antigen-presenting cells
Pulmonary delivery of nanoparticles to target APCs in the lung has been explored for various immunological applications122–125, including cationic gold nanoparticles for CD4+ T cell expansion126 and small interfering RNA (siRNA) polymeric vectors for asthma therapy127. The size and charge of administered nanoparticles have an effect on APC capture and lymph node accumulation. For example, comparing the effect of nanoparticles with 20, 50, 100, 200 and 1,000 nm diameters 2 and 24 hours after administration123 shows that the majority of nanoparticles are taken up by alveolar macrophages in the respiratory tract regardless of their size; however, nanoparticles with 20 and 50 nm diameters show the highest dendritic cell uptake 24 hours after administration compared with the other nanoparticles. In draining lymph nodes, the dendritic cells that have taken up 20, 50 and 100 nm nanoparticles are significantly more migratory than lymph node-resident dendritic cells, indicating an active transport process of the cells to the draining lymph nodes. However, nanoparticles with diameters <34 nm were also shown to be transported from the lung to mediastinal lymph nodes within minutes after administration, suggesting that small nanoparticles can passively diffuse to draining lymph nodes124.
The charge of nanoparticles also plays a crucial role for their translocation from the lungs to lymph nodes. Both anionic and cationic nanoparticles are internalized by alveolar macrophages; however, lung-resident dendritic cells preferentially associate with cationic nanoparticles128. Cationic and anionic nanoparticles are also found at similar levels in draining lymph nodes following administration, indicating that their charge does not influence lymph node accumulation. Overall, smaller (<50 nm diameter), slightly cationic nanoparticles achieve higher levels of lymph node accumulation following pulmonary administration — a process that is primarily achieved through active cell-mediated transport.
Oral delivery to target mucosal antigen-presenting cells
Oral delivery to target intestinal APCs for vaccination has been of interest for many decades129 owing to patient compliance and the potential generation of a systemic immune response130. Following microfold cell or enterocyte capture, the antigen is either transferred to APCs on the basal side of the epithelial layer or packaged for mesenteric lymphatic entry. Once APCs capture macromolecules, they become activated, migrate through the mesenteric lymphatics and accumulate in mesenteric lymph nodes112–114. The physical properties of materials, including size, charge and surface ligands, impact the targeting of phagocytic microfold cells131,132. Particles with a diameter below <1 μm are taken up by microfold cells, whereas larger particles with diameters >3 μm are taken up by Peyer’s patches and are retained there131. Moreover, non-ionic particles are better taken up by microfold cells than charged particles. Surface ligands further promote uptake by these cells; however, the particles remain bound to the cells rather than being translocated to the mesenteric lymphatics131. It has also been shown that lymphatic uptake of orally delivered nanoparticles is minimal owing to a variety of factors, including material properties and variation in methodologies and techniques used in the field to assess lymphatic absorption131,132. Therefore, the exact underlying mechanisms of nanoparticle-mucosal APC interactions and subsequent immune responses remain elusive thus far.
Circulating lymphocytes
Antigen-specific T and B cells are rare, and the vast majority of naive lymphocytes are circulating between lymph nodes and the lymphatics, spending less than half an hour in circulation before homing to a lymphoid organ, where they take a few hours or days to find their cognate antigen4. Lymphocytes primarily migrate into lymph nodes along the entire length of HEVs, and exit through efferent lymphatics, with T and B cell trafficking being substantially increased during lymph node inflammation133. Following a tightly orchestrated adhesion cascade134, adhesive ligands and chemokines direct lymphocyte diapedesis through the inter-endothelial junctions of the high endothelial venules. Once inside the lymph node, T and B cells home to their respective areas in the paracortex and to the follicles, guided by chemokine cues135–137.
Drug delivery via lymphocyte homing
Cell homing to the lymph node can be exploited to target T cells in the lymph node by using cells for ‘backpacking’, that is, drug-loaded nanoparticles or carriers are covalently or non-covalently bound to T cells and thus shuttled to lymph nodes (FIG. 4; TABLE 1) following adoptive transfer. For example, this method can be used to prolong autocrine stimulation of transferred T cells, triggered by conjugated nanoparticles that are tethered with anti-CD45 antibodies and release IL-15 superagonist (IL-15Sa). This approach can be applied to support the antitumour activity of therapeutic T cells and increase their lymph node accumulation138.
Active targeting by cell homing can also be used for the treatment of lymphomas in lymph nodes. For example, T cells can be functionalized ex vivo with nanocapsules loaded with a chemotherapeutic, which is then delivered to the lymphoma139,140. By engineering the T cells to be resistant to the chemotherapy, high-payload delivery to lymph nodes can be achieved, which ultimately leads to a decrease in tumour growth rate compared with traditional systemic dosing139. T cells migrating to the lymph nodes can also be targeted in the blood using antibody-nanoparticle conjugates, such as anti-programmed cell death 1 (PD-1), anti-CD8 and anti-CD4 conjugates141,142 (FIG. 4).
Direct lymph node injection
Administration of drugs in peripheral tissues or intravenously achieves low yet sustained levels of lymph node delivery, mediated by convection and active cell-mediated trafficking. Alternatively, drugs can be directly injected into the lymph node — a method that has been used for over half a century to treat lymph node metastasis143 (FIG. 5). Direct lymph node injection is invasive and often used only if delivery via the lymph or blood is not sufficient to achieve the required drug levels in the lymph nodes (TABLE 1). Usually, the draining lymph node is identified by administration of lymph-draining chromogenic colloid in peripheral tissues.
Fig. 5 |. Route of administration into lymph nodes.
Different regions of skin-draining lymph nodes can be targeted by injections and administration. + and −, scale; HEV, high endothelial venule; NA, not applicable.
Intra-lymph node injection has also been explored to improve vaccine potency144–147. The potency of antigen–adjuvant formulations of ~300–900 nm in diameter comprising synthetic or biopolymers and liposomes is substantially improved by intra-lymph node injection148, which is not surprising owing to the low lymph node accumulation of cargos at this scale54. For example, intra-lymph node injection of PLGA microparticles leads to increased accumulation of the TLR3 ligand polyinosinic-polycytidylic acid (poly(I:C)) within lymph node-resident APCs as compared with soluble poly(I:C)149. Injection of microparticles with both soluble poly(I:C) and conjugated poly(I:C) shows sustained lymph node retention and uptake by lymph node-resident APCs, including by dendritic cells, macrophages and B cells. Similarly, microparticles can be directly injected into lymph nodes to deliver a self-antigen and rapamycin (a regulatory signal)150. In a mouse multiple sclerosis model, the delivery of both molecules rather than of single agents induces systemic antigen-specific tolerance. Moreover, intra-lymph node injection of the microparticles leads to increased amounts of immune-suppressive regulatory T cells in treated and non-treated lymph nodes and thus to improved therapeutic effects compared with delivery of rapamycin and a protein control using the microparticle platform.
Perspectives and conclusions
The physiology and the cellular and fluid transport mechanisms in lymph nodes offer a blueprint for the rational design of materials to target specific cell types in the lymph nodes. The afferent lymphatics provide an entry point for nanomaterials to deliver cargo to lymph node-resident and lymph-sampling cells, such as dendritic cells, macrophages and B cells. However, it remains challenging to supersede the scavenger functions of these cells and at the same time exploit their afferent lymphatic delivery route. Affinity-based targeting could be used to better discriminate between the different lymph-sampling APCs and thus to optimize delivery to specific cell types. For example, subcapsular sinus macrophages, which constitute a lymph node barrier, could be disrupted using clodronate liposomes; subsequent delivery of dendritic cell-targeting drug vehicles would then allow targeting of dendritic cells and lymphocytes that reside deeper within the lymph node structure and are not readily accessible through the lymph. Alternatively, APC scavenging can be overcome by shedding the delivery carrier, which promotes lymphatic uptake and pinocytosis, and by consequent release of the smaller active agent directly into the lymph node, which can then act on the target cell without being taken up by APCs. For example, nanoparticles functionalized with anti-CD169 antibodies could be used to label subcapsular sinus macrophages. The conjugated nanoparticles would then remain on the sinus side of the subcapsular sinus macrophage barrier and would not translocate to B cells. Using degradable nanomaterials or externally triggered systems could then enable the release of small agents, which could penetrate into the lymph node or provide sustained antigen release to the conduits.
Materials that are used for the probing of B cell interactions with antigen could also be exploited for drug delivery. Antigens with molecular weights of <70 kDa entering through the conduits can directly target B cells; however, small antigens suffer from inefficient lymphatic uptake, which decreases the abundance of administered agent and therefore the concentration available within the conduits. By using carriers that are smaller than traditional 30–200 nm particle delivery vehicles, a balance between lymphatic uptake and conduit and follicle access could be achieved. For example, an antigen with a molecular weight of 14 kDa is directly taken up by B cells from the conduits47. To improve direct uptake in the conduits, the size of the conjugate could be increased by linking adjuvant and antigen for combination B cell vaccination or by using polymer or macromolecular conjugates, such as cyclodextrins or PEG, for B cell delivery of small molecule drugs. Alternatively, small antigen can be linked to large particles that are cleaved by proteases to release the antigen and target B cells77. Similarly, large particles, for example, avidin-coated microparticles with a diameter of 0.2 μm (REF.85), could be used to transport small biotinylated molecules and antigens to subcapsular sinus macrophages and then to B cells. Finally, subcapsular sinus macrophage capture and presentation to B cells could be improved by functionalization with complement or Fc fragments, which could potentially also be applied for therapeutic drugs or diagnostic agents.
The high endothelial venules provide an entry point to target the paracortex in the lymph nodes and thus T lymphocytes. However, specific targeting of high endothelial venules remains challenging because most antibodies that target cells of the high endothelial venules also recognize the 6-sulfo-sialyl Lewis X epitope (for example, MECA- 79). Alternatively, antibodies that recognize O-glycan and N-glycan epitopes, for example, CL40 (REF.151) and S2 (REF152), bind stronger to high endothelial venules than MECA-79, enabling more specific targeting. Effective penetration of the lymph node after extravasation is also a challenge for drug delivery approaches via the vasculature. Therefore, nanoparticles rather than microparticles93 could provide a possibility to increase diffusion into the lymph node to enable interaction with more T cells. Additionally, T cell targeting and uptake could further be improved by providing a controlled release platform rather than by attempting to target individual cells with particles. This platform could be used to first deliver drugs to high endothelial venules and T cell zones and then release the drugs through particle degradation.
Peripheral APC targeting could be further improved by using active targeting or microneedle patches to overcome interstitial efflux and to control delivery to specific cell subtypes, such as plasmacytoid dendritic cells (during inflammation), Langerhans cells and dermal dendritic cells, which reside within different skin layers104. Taking advantage of the antigen transfer capabilities of migratory dendritic cells153, drug-loaded nanoparticles, which are taken up by dendritic cells, can be transported to draining lymph nodes. The same approach could also be used to deliver high concentrations of drugs deep into the lymph node. Various methods including modifying size, charge and surface lipophilicity of particles have been developed to improve the uptake of molecules by pulmonary and intestinal APCs64,154,155; however, such approaches have not yet been extensively explored for lymph node-directed drug delivery.
Finally, most material-based approaches leveraging homing of circulating immune cells for lymph node- directed drug delivery have focused on T cell targeting. However, using T cells as carriers could lead to systemic effects in the lymph nodes because lymphocytes constantly traffic between the lymph nodes in the body; whether such a systemic effect is advantageous depends on the application. Cell-mediated targeting techniques have yet to be extended to circulating B cells, which would allow modulation of the humoral immunity, or to myeloid progenitor cells, which have the potential to enable delivery to distinct lymph node locations.
Lymph nodes are key tissues for initiating immune responses because they physically coordinate the interactions of peripheral immune information with circulating lymphocytes. The physiology, local structural motifs and transport mechanisms into and within the lymph node should inform the design criteria for drug delivery systems, and a holistic consideration of lymph node cell types and areas, cell-cell interactions and mechanisms of action of drugs will open up new opportunities for targeting specific cells and regions in the lymph node.
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grant R01CA207619, a CCR15330478 grant from Susan G. Komen and US Department of Defense grant CA150523.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sainte-Marie G The lymph node revisited: development, morphology, functioning, and role in triggering primary immune responses. Anat. Rec. 293, 320–337 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Kaldjian EP, Gretz JE, Anderson AO, Shi Y & Shaw S Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 13, 1243–1253 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Chaplin DD Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy K, Travers P, Walport M & Janeway C Janeway’s Immunobiology 8th edn (Garland Science, 2012). [Google Scholar]
- 5.Jeanbart L et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2, 436–447 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Kourtis IC et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLOS ONE 8, e61646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schudel A, Sestito LF & Thomas SN Winner of the society for biomaterials young investigator award for the annual meeting of the society for biomaterials, April 11–14, 2018, Atlanta, GA: S-nitrosated poly(propylene sulfide) nanoparticles for enhanced nitric oxide delivery to lymphatic tissues. J. Biomed. Mater. Res. A 106, 1463–1475 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller S et al. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses. J. Control. Release 191, 24–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster S, Duvall CL, Crownover EF, Hoffman AS & Stayton PS Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T cell production and prophylactic vaccine efficacy. Bioconjug. Chem. 21, 2205–2212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leleux JA, Pradhan P & Roy K Biophysical attributes of CpG presentation control TLR9 signaling to differentially polarize systemic immune responses. Cell Rep. 18, 700–710 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Kaminskas LM et al. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control. Release 140, 108–116 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Ryan GM et al. PEGylated polylysine dendrimers increase lymphatic exposure to doxorubicin when compared to PEGylated liposomal and solution formulations of doxorubicin. J. Control. Release 172, 128–136 (2013). [DOI] [PubMed] [Google Scholar]
- 13.An M, Li M, Xi J & Liu H Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl. Mater. Interfaces 9, 23466–23475 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Kang S et al. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 256, 56–67 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Wang C et al. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine 32, 5475–5483 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Delemarre FG, Kors N, Kraal G & van Rooijen N Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J. Leukoc. Biol. 47, 251–257 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Singh I, Swami R, Khan W & Sistla R Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin. Drug Deliv. 11, 211–229 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Nune SK, Gunda P, Majeti BK, Thallapally PK & Forrest ML Advances in lymphatic imaging and drug delivery. Adv. Drug Delivery Rev. 63, 876–885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacken PJ, de Vries IJ, Torensma R & Figdor CG Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 7, 790–802 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Gray EE & Cyster JG Lymph node macrophages. J. Innate Immun. 4, 424–436 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahsan F, Rivas IP, Khan MA & Torres Suarez AI Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Release 79, 29–40 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Lederman MM & Margolis L The lymph node in HIV pathogenesis. Semin. Immunol. 20, 187–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas SN, Rohner NA & Edwards EE Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu. Rev. Biomed. Engineer. 18, 207–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohner NA et al. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 29, 4512–4522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira ER, Jones D, Jung K & Padera TP The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Biol. 38, 98–105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyaji K et al. The stiffness of lymph nodes containing lung carcinoma metastases: a new diagnostic parameter measured by a tactile sensor. Cancer 80, 1920–1925 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Chung MK et al. Lymphatic vessels and high endothelial venules are increased in the sentinel lymph nodes of patients with oral squamous cell carcinoma before the arrival of tumor cells. Ann. Surg. Oncol. 19, 1595–1601 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Nathanson SD & Mahan M Sentinel lymph node pressure in breast cancer. Ann. Surg. Oncol. 18, 3791–3796 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Nathanson SD, Shah R, Chitale DA & Mahan M Intraoperative clinical assessment and pressure measurements of sentinel lymph nodes in breast cancer. Ann. Surg. Oncol. 21, 81–85 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Qian CN et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 66, 10365–10376 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Willard-Mack CL Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 34, 409–424 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Kelly RH Functional anatomy of lymph nodes. I. The paracortical cords. Int. Arch. Allergy Appl. Immunol. 48, 836–849 (1975). [DOI] [PubMed] [Google Scholar]
- 33.Forkert PG, Thliveris JA & Bertalanffy FD Structure of sinuses in the human lymph node. Cell Tissue Res. 183, 115–130 (1977). [DOI] [PubMed] [Google Scholar]
- 34.Gretz JE, Kaldjian EP, Anderson AO & Shaw S Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J. Immunol. 157, 495–499 (1996). [PubMed] [Google Scholar]
- 35.Gretz JE, Anderson AO & Shaw S Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997). [DOI] [PubMed] [Google Scholar]
- 36.MacLennan IC Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Kroese FG, Timens W & Nieuwenhuis P Germinal center reaction and B lymphocytes: morphology and function. Curr. Top. Pathol. 84, 103–148 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Romani N et al. Migration of dendritic cells into lymphatics-the Langerhans cell example: routes, regulation, and relevance. Int. Rev. Cytol. 207, 237–270 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Belisle C & Sainte-Marie G Tridimensional study of the deep cortex of the rat lymph node. III. Morphology of the deep cortex units. Anat. Rec. 199, 213–226 (1981). [DOI] [PubMed] [Google Scholar]
- 40.Girard JP, Moussion C & Forster R HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Randolph GJ, Ivanov S, Zinselmeyer BH & Scallan JP The lymphatic system: integral roles in immunity. Annu. Rev. Immunol. 35, 31–52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baluk P et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swartz MA The physiology of the lymphatic system. Adv. DrugDeliv. Rev. 50, 3–20 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Schmid-Schonbein GW Mechanisms causing initial lymphatics to expand and compress to promote lymph flow. Arch. Histol Cytol. 53 (Suppl), 107–114 (1990). [DOI] [PubMed] [Google Scholar]
- 45.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T & Germain RN A spatially- organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150, 1235–1248 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rantakari P et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 16, 386–396 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Roozendaal R et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE & Shaw S Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sixt M et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Thomas SN & Schudel A Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 7, 65–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohner NA & Thomas SN Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater. Sci. Eng. 3, 153–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stroh M et al. Multiphoton microscopy guides neurotrophin modification with poly(ethylene glycol) to enhance interstitial diffusion. Nat. Mater. 3, 489–494 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Reddy ST, Berk DA, Jain RK & Swartz MA A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J. Appl. Physiol. 101, 1162–1169 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Rohner NA & Thomas SN Melanoma growth effects on molecular clearance from tumors and biodistribution into systemic tissues versus draining lymph nodes. J. Control. Release 223, 99–108 (2016).This study details quantification of the relative efficiency of nanocarrier versus microcarrier accumulation within lymph nodes versus systemic tissues after administration in the periphery and investigation of the effects of disease on the extent of specific targeting.
- 55.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA & Swartz MA In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Rao DA, Forrest ML, Alani AW, Kwon GS & Robinson JR Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J. Pharm. Sci. 99, 2018–2031 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A & Moon JJ Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peinado H et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan S, Vannberg FO & Dixon JB Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 6, 24436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy ST, Swartz MA & Hubbell JA Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27, 573–579 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Sunshine JC, Perica K, Schneck JP & Green JJ Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 35, 269–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wibroe PP et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 12, 589–594 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Anselmo AC, Banerjee A, Zakrewsky M & Mitragotri S Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 220, 141–148 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Ryan GM et al. A comparison of the pharmacokinetics and pulmonary lymphatic exposure of a generation 4 PEGylated dendrimer following intravenous and aerosol administration to rats and sheep. Pharm. Res. 33, 510–525 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Agarwal R et al. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl Acad. Sci. USA 110, 17247–17252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foged C, Brodin B, Frokjaer S & Sundblad A Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 298, 315–322 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Thomas SN, Vokali E, Lund AW, Hubbell JA & Swartz MA Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 (2014).This study provides a demonstration of the principle and therapeutic benefit of lymph node targeting using lymphatic-draining nanoparticles in mediating delivery of immune-stimulatory small molecule adjuvants to tumour-draining lymph nodes to potentiate adaptive immune responses against endogenously produced tumour antigen co-draining to targeted lymph nodes.
- 68.Rehor A, Hubbell JA & Tirelli N Oxidationsensitive polymeric nanoparticles. Langmuir 21, 411–417 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Liu H et al. Structure-based programming of lymph- node targeting in molecular vaccines. Nature 507, 519–522 (2014).This study details the engineering of a modified subunit vaccine, specifically a lipid-modified peptide and adjuvant oligonucleotide CpG that leverage endogenous albumin transport of hydrophobic lipids to the lymph node to improve vaccine effects.
- 70.Oberli MA et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 17, 1326–1335 (2017).This paper details an investigation of a variety of liposomal formulations to achieve high mRNA encapsulation and cytosolic delivery to APCs for mRNA vaccines, achieving high transfection rates at the site of injection and in the draining lymph node.
- 71.Karabin NB et al. Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat. Commun. 9, 624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nuhn L et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl Acad. Sci. USA 113, 8098–8103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang F, Zhu L, Huang X, Niu G & Chen X Differentiation of reactive and tumor metastatic lymph nodes with diffusion-weighted and SPIO-enhanced MRI. Mol. Imaging Biol. 15, 40–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang C et al. Tumor metastasis inhibition by imaging- guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 26, 5646–5652 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Oladipo AO et al. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci. Rep. 7, 45459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez SF, Pitcher LA, Mempel T, Schuerpf F & Carroll MC B cell acquisition of antigen in vivo. Curr. Opin. Immunol. 21, 251–257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catron DM, Pape KA, Fife BT, van Rooijen N & Jenkins MK A protease-dependent mechanism for initiating T-dependent B cell responses to large particulate antigens. J. Immunol. 184, 3609–3617 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heyman B Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 18, 709–737 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Phan TG, Grigorova I, Okada T & Cyster JG Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8, 992–1000 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Phan TG, Green JA, Gray EE, Xu Y & Cyster JG Immune complex relay by subcapsular sinus macrophages and non-cognate B cells drives antibody affinity maturation. Nat. Immunol. 10, 786–793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez SF et al. Complement-dependent transport of antigen into B cell follicles. J. Immunol. 185, 2659–2664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heesters BA et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity 38, 1164–1175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roozendaal R & Carroll MC Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 219, 157–166 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Junt T et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Carrasco YR & Batista FD B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Pucci F et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352, 242–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saunderson SC, Dunn AC, Crocker PR & McLellan AD CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123, 208–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernhard CA, Ried C, Kochanek S & Brocker T CD169+macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells. Proc. Natl Acad. Sci. USA 112, 5461–5466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Rooijen N & Hendrikx E Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605, 189–203 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Hettiaratchi MH et al. A rapid method for determining protein diffusion through hydrogels for regenerative medicine applications. APLBioeng. 2, 026110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Rooijen N & van Kesteren-Hendrikx E Clodronate liposomes: perspectives in research and therapeutics. J. Liposome Res. 12, 81–94 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Bahmani B et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J. Clin. Invest. 128, 4770–4786 (2018).This study details the engineering of a modified subunit vaccine, specifically a lipid- modified anti- MECA-79 monoclonal antibody to prolong cardiac allograft survival compared with unmodified, drug- laden nanoparticles or free drug.
- 93.Azzi J et al. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 15, 1202–1213 (2016).This paper exploits the same targeting methods used in the work by Bahmani et al. (directed delivery to adhesive ligand that T cells use to home to the lymph node) using microparticles encapsulating immunomodulators to improve allograft survival.
- 94.Cabral H et al. Systemic targeting of lymph node metastasis through the blood vascular system by using size-controlled nanocarriers. ACS Nano 9, 4957–4967 (2015).This paper describes the investigation of the effects of the size of sub-100 nm nanoparticles on lymph node accumulation versus delivery to lymph node- localized metastatic tumour foci to improve antitumour therapeutic effects.
- 95.Zheng Y et al. In vivo targeting of adoptively transferred T cells with antibody- and cytokine-conjugated liposomes. J. Control. Release 172, 426–435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Kissenpfennig A et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Lutz MB & Schuler G Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 23, 445–449 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Joffre O, Nolte MA, Sporri R & Reis e Sousa C Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Gallo PM & Gallucci S The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front. Immunol. 4, 138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rescigno M, Granucci F, Citterio S, Foti M & Ricciardi-Castagnoli P Coordinated events during bacteria-induced DC maturation. Immunol. Today 20, 200–203 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Cyster JG Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Braun P, Foldi M, Kisfaludy S & Szabo G Free amino-acid content of the lymph. Nature 177, 1133–1134 (1956). [DOI] [PubMed] [Google Scholar]
- 104.Kaplan DH In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 31,446–451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zaric M et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 7, 2042–2055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seneschal J, Jiang X & Kupper TS Langerin+dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scarification with vaccinia virus. J. Invest. Dermatol. 134, 686–694 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagao K et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl Acad. Sci. USA 106, 3312–3317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guilliams M, Lambrecht BN & Hammad H Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 6, 464–473 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Kopf M, Schneider C & Nobs S P The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 16, 36–44 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Kim TH & Lee HK Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 14, 128–137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang S, Liu H, Zhang X & Qian F Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell 6, 480–503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu LM & MacPherson GG Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 177, 1299–1307 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang FP et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang FP, Farquhar CF, Mabbott NA, Bruce ME & MacPherson GG Migrating intestinal dendritic cells transport PrP(Sc) from the gut. J. Gen. Virol. 83, 267–271 (2002). [DOI] [PubMed] [Google Scholar]
- 115.Macpherson AJ & Smith K Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 203, 497–500 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ali OA, Huebsch N, Cao L, Dranoff G & Mooney DJ Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y et al. In situ modulation of dendritic cells by injectable thermosensitive hydrogels for cancer vaccines in mice. Biomacromolecules 15, 3836–3845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh A, Suri S & Roy K In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials 30, 5187–5200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahe B et al. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J. Invest. Dermatol. 129, 1156–1164 (2009). [DOI] [PubMed] [Google Scholar]
- 120.Baleeiro RB et al. Topical vaccination with functionalized particles targeting dendritic cells. J. Invest. Dermatol. 133, 1933–1941 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Cruz LJ et al. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J. Control. Release 192, 209–218 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Fromen CA et al. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine 12, 677–687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blank F et al. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am. J. Respir. Cell. Mol. Biol. 49, 67–77 (2013).This paper investigates the effects of inhaled particle size on association with APCs in regional lymph nodes draining the lung airway.
- 124.Choi HS et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 28, 1300–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hardy CL et al. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J. Immunol. 191, 5278–5290 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Seydoux E et al. Pulmonary delivery of cationic gold nanoparticles boost antigen-specific CD4(+) T cell proliferation. Nanomedicine 12, 1815–1826 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Xie Y & Merkel OM Pulmonary delivery of siRNA via polymeric vectors as therapies of asthma. Arch. Pharm. 348, 681–688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fromen CA et al. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc. Natl Acad. Sci. USA 112, 488–493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sabin AB Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J. Infect. Dis. 151, 420–436 (1985). [DOI] [PubMed] [Google Scholar]
- 130.Lavelle EC & O’Hagan DT Delivery systems and adjuvants for oral vaccines. Expert Opin. Drug Deliv. 3, 747–762 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Florence AT Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov. Today Technol. 2, 75–81 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Trevaskis NL, Kaminskas LM & Porter CJ From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 14, 781–803 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Ager A High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front. Immunol. 8, 45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.von Andrian UH Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996). [DOI] [PubMed] [Google Scholar]
- 135.Anderson AO & Shaw S T cell adhesion to endothelium: the FRC conduit system and other anatomic and molecular features which facilitate the adhesion cascade in lymph node. Semin. Immunol. 5, 271–282 (1993). [DOI] [PubMed] [Google Scholar]
- 136.Takeda A, Sasaki N & Miyasaka M The molecular cues regulating immune cell trafficking. Proc. Jpn Acad. Ser. B 93, 183–195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyasaka M & Tanaka T Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4, 360–370 (2004). [DOI] [PubMed] [Google Scholar]
- 138.Stephan MT, Moon JJ, Um SH, Bershteyn A & Irvine DJ Therapeutic cell engineering with surface- conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang B et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. TranslMed. 7, 291ra94 (2015).In this paper, chemotherapy-loaded nanocapsules with sustained release over 3 days are backpacked onto T cell surfaces capable of lymph node homing, achieving improved drug delivery to the lymph node-resident tumour.
- 140.Wu AA, Drake V, Huang H-S, Chiu S & Zheng L Reprogramming the tumor microenvironment: tumor- induced immunosuppressive factors paralyze T cells. Oncoimmunology 4, e1016700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmid D et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 8, 1747 (2017).This paper highlights the concept that cells that are the target of a drug can also be its carrier to enable targeted drug delivery.
- 142.McHugh MD et al. Paracrine co-delivery of TGF-beta and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials 59, 172–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanis PJ, Nieweg OE, Valdes Olmos RA, Th Rutgers EJ & Kroon BB History of sentinel node and validation of the technique. Breast Cancer Res. 3, 109–112 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maloy KJ et al. Intralymphatic immunization enhances DNA vaccination. Proc. Natl Acad. Sci. USA 98, 3299–3303 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johansen P et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur. J. Immunol. 35, 568–574 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Smith KA et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine 27, 2603–2615 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Ribas A et al. Intra-lymph node prime-boost vaccination against Melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin. Cancer Res. 17, 2987–2996 (2011). [DOI] [PubMed] [Google Scholar]
- 148.Mohanan D et al. Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J. Control. Release 147, 342–349 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Jewell CM, Bustamante López SC & Irvine DJ In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tostanoski LH et al. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 16, 2940–2952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arata-Kawai H et al. Functional contributions of N− and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am. J. Pathol. 178, 423–433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirakawa J et al. Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J. Biol. Chem. 285, 40864–40878 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Allan RS et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Hu L et al. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance oral bioavailability. Angew. Chem. 55, 13700–13705 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Han S et al. Constitutive triglyceride turnover into the mesenteric lymph is unable to support efficient lymphatic transport of a biomimetic triglyceride prodrug. J. Pharm. Sci. 105, 786–796 (2016). [DOI] [PubMed] [Google Scholar]
- 156.de Titta A et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl Acad. Sci. USA 110, 19902–19907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]