Abstract
The PARADIGM-HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial reported that sacubitril/valsartan (S/V), an angiotensin receptor-neprilysin inhibitor, significantly reduced mortality and heart failure (HF) hospitalization in HF patients with a reduced ejection fraction (HFrEF). However, fewer than 1% of patients in the PARADIGM-HF study had New York Heart Association (NYHA) functional class IV symptoms. Accordingly, data that informed the use of S/V among patients with advanced HF were limited. The LIFE (LCZ696 in Hospitalized Advanced Heart Failure) study was a 24-week prospective, multicenter, double-blinded, double-dummy, active comparator trial that compared the safety, efficacy, and tolerability of S/V with those of valsartan in patients with advanced HFrEF. The trial planned to randomize 400 patients ≥18 years of age with advanced HF, defined as an EF ≤35%, New York Heart Association functional class IV symptoms, elevated natriuretic peptide concentration (B-type natriuretic peptide [BNP] ≥250 pg/ml or N-terminal pro–B-type natriuretic peptide [NT-proBNP] ≥800 pg/ml), and ≥1 objective finding of advanced HF. Following a 3- to 7-day open label run-in period with S/V (24 mg/26 mg twice daily), patients were randomized 1:1 to S/V titrated to 97 mg/103 mg twice daily versus 160 mg of V twice daily. The primary endpoint was the proportional change from baseline in the area under the curve for NT-proBNP levels measured through week 24. Secondary and tertiary endpoints included clinical outcomes and safety and tolerability. Because of the COVID-19 pandemic, enrollment in the LIFE trial was stopped prematurely to ensure patient safety and data integrity. The primary analysis consists of the first 335 randomized patients whose clinical follow-up examination results were not severely impacted by COVID-19. (Entresto [LCZ696] in Advanced Heart Failure [LIFE STUDY] [HFN-LIFE]; NCT02816736)
Key Words: heart failure, NYHA functional class IV, sacubitril/valsartan, valsartan
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; BNP, B-type natriuretic peptide; HFrEF, heart failure with a reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; S/V, sacubitril/valsartan; V, valsartan
Central Illustration
The use of evidence-based medical therapies has been shown to improve survival, reduce heart failure (HF) hospitalizations, and improve quality of life for patients with HF and reduced ejection fraction (HFrEF) who have mild to moderate symptoms (1,2). However, evidence for the use of medical therapy among patients with HFrEF and advanced symptoms is less comprehensive insofar as it is often difficult to achieve the dose(s) of neurohormonal antagonist recommended in clinical trials in those patients, because of dose-limiting symptomatic hypotension or worsening renal function, or both (3). Consequently, contemporary guidelines for patients with advanced HFrEF do not focus on medical therapy and instead recommend that these patients be considered for mechanical circulatory support, cardiac transplantation, or palliative care (1,4).
The global PARADIGM-HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) randomized trial compared sacubitril/valsartan (S/V) with enalapril in ambulatory patients with HFrEF. S/V therapy reduced the rates of cardiovascular (CV) mortality or hospitalization for patients with HF by a relative 20% and all-cause mortality by a relative 16% (5,6). Based on actuarial estimates of event rates and life expectancy, S/V was expected to prolong survival by approximately 1 to 2 years in ambulatory patients with HFrEF, across a wide range of age groups (7). The 5-year estimated number needed to treat was 14, when S/V was compared to enalapril, for the primary outcome of CV death or HF hospitalization (8). As a result of these findings, the U.S. Food and Drug Administration (FDA) approved S/V for treatment of HFrEF, and the American College of Cardiology/American Heart Association/Heart Failure Society of America updated their guidelines to recommend (Class I) the use of S/V to further reduce morbidity and mortality in patients with HFrEF (9,10).
Although S/V was approved by the FDA for patients with HFrEF with New York Heart Association (NYHA) functional class II to IV symptoms, <1% of patients in PARADIGM-HF had NYHA functional class IV symptoms at the time of enrollment. In order to be randomized into the PARADIGM-HF trial, patients had to be receiving and tolerating a stable dose of angiotensin II receptor blocker (ARB) and an angiotensin-converting enzyme (ACE) inhibitor that was equivalent to ≥10 mg of enalapril daily for 4 weeks, as well as have a screening systolic blood pressure ≥100 mm Hg. Moreover, nearly 20% of patients who were screened for PARADIGM-HF were unable to complete the 2 run-in periods, which required that patients tolerate a maximal dose of 10 mg twice daily of enalapril for 2 weeks, followed by a 4- to 6-week treatment with up to 97 mg/103 mg of S/V twice daily. Variables associated with noncompletion of the run-ins included lower blood pressure and lower glomerular filtration rate, both hallmarks of advanced HF (11,12). The recent PIONEER-HF (Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) trial, which tested S/V therapy in a patient population at higher risk for hospitalization for acute HFrEF, included only 9% of patients with NYHA functional class IV symptoms (13). In light of this evidence gap among patients with chronic HFrEF with severe symptoms, current guidelines do not provide guidance with regard to the use of S/V for HFrEF patients with advanced HF (9,10). This paper reports on the design of the LIFE (LCZ696 in Advanced Heart Failure) clinical trial, which tested the hypothesis that treatment with S/V would improve levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP) and clinical status compared to treatment with valsartan alone in HFrEF patients with NYHA functional class IV symptoms.
Study Design
Overview and eligibility criteria
The LIFE trial was a 24-week prospective, multicenter, double-blinded, double-dummy, active comparator trial to assess the safety, efficacy, and tolerability of S/V compared with treatment with valsartan in patients with advanced HFrEF. Key inclusion and exclusion criteria are shown in Table 1 . Briefly, approximately 400 patients between >18 and <85 years of age with advanced HF and the capacity to provide written informed consent were randomized. Advanced HF was defined as the following: a left ventricular ejection fraction (LVEF) <35%, NYHA functional class IV symptoms (i.e., chronic dyspnea or fatigue at rest or with minimal exertion at the time of screening or within the previous 3 months), and a minimum of 3 months of guideline-directed medical therapy for HF and/or intolerance to such therapy. Patients were enrolled during either an index HF hospitalization or in the outpatient setting. Key exclusion criteria included active use of S/V, a history of hypersensitivity or unmodifiable intolerance to S/V or ACE inhibitor/ARB therapy; currently had a left ventricular assist device (LVAD) or were scheduled for LVAD implantation within 30 days; were currently hospitalized and listed for cardiac transplantation as status 1A/1B prior to October, 2018, or status 1 to 4 after October 2018; had systolic blood pressure <90 mm Hg, an estimated glomerular filtration rate (eGFR) of <20 ml/min/1.73 m2, and a serum potassium concentration of >5.5 mmol/l.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|
| Exclusion Criteria |
|
6-MWT = 6-min walk test; ACE = angiotensin-converting enzyme; ACS = acute coronary syndrome; ARB = angiotensin receptor blocker; CABG = coronary artery bypass graft; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GDMT = guideline directed medical therapy; HFrEF = heart failure with a reduced Ejection Fraction; IV = intravenous; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; RER = respiratory exchange ratio; SBP = systolic blood pressure; VO2 = oxygen consumption.
Treatment protocol and follow-up
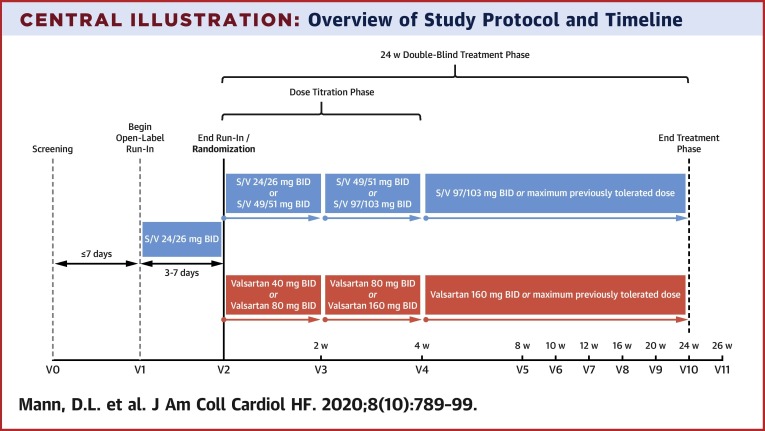
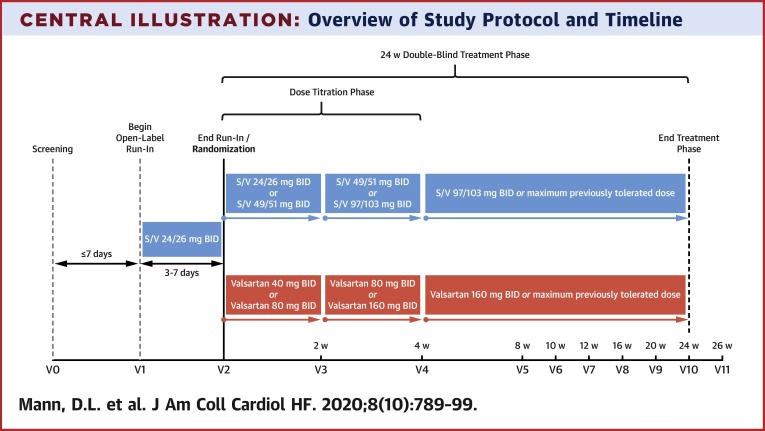
The study was composed of 3 phases: 1) a screening visit; 2) an open-label run-in period; and 3) a double-blind treatment phase (Table 2 , Central Illustration ). At the screening visit, complete history, physical examination, and laboratory evaluation were performed to assess eligibility. In subjects taking an ACE inhibitor at baseline, the ACE inhibitor was held for >36 h before beginning the run-in period with open-label S/V (2). Patients meeting all eligibility criteria were enrolled and began an unblinded run-in period of 3 to 7 days with oral S/V, 24 mg/26 mg twice daily. Of note, the screening visit, enrollment, and run-in period could begin on the same day for outpatients who were not taking an ACE inhibitor and for whom laboratory results had been reviewed and eligibility confirmed. The run-in period had to begin within 7 days of the screening visit.
Table 2.
Data Collection and Schedule of Assessments
| Visit Number |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
Unscheduled Visit for Dose Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of Visit | Enrollment | Run-In∗ | First Dose | Dose Titration (2 weeks) | Dose Titration (4 weeks) | 8 Weeks |
10 Weeks |
12 Weeks |
16 Weeks |
20 Weeks |
24 Weeks s |
26 Weeks | |
| Inclusion/exclusion criteria | x | x | |||||||||||
| Information and informed consent | x | ||||||||||||
| Physical examination | x | x | x | x | x | x | x | x | |||||
| KCCQ questionnaire | x | x | x | x | |||||||||
| Dispense study medication | x1 | x | x | x | x | x | x | x | |||||
| Laboratory test (routine)† | x‡ | x | x | x | x | x | x | x | x | ||||
| Laboratory test (core) | x2 | x3 | x2 | x2 | x2 | x2 | |||||||
| Adverse events | x | x4 | x | x | x | x | x | x | x | x | x | ||
| Telephone follow-up | x | x | x | ||||||||||
| Telephone safety assessment | x | ||||||||||||
1 = Open-label sacubitril/valsartan (24 min/26 mg orally twice per day) during the run-in phase. 2 = BNP, NT-proBNP, and cystatin C. 3 = BNP and NT-proBNP only. 4 = Patients in whom run-in failed were to be contacted approximately 2 weeks after their last dose of study drug.
BNP = B-type natriuretic peptide; BUN = blood urine nitrogen; KCCQ = Kansas City Cardiomyopathy Questionnaire; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
The screening visit and visit 1 were combined at the investigator’s discretion for subjects who were stable and for whom laboratory results were reviewed as long as the investigator ensured the first dose of sacubitril/valsartan ≥36 h after the last ACE inhibitor dose (if applicable). If data were not combined, visit 1 would take place within 7 days of the screening visit.
Local laboratory tests included sodium, potassium, chloride, Co2/bicarbonate, total calcium, magnesium, BUN, and creatinine concentrations.
For screening and study visit 0 only, standard of care laboratory tests were acceptable, using results within 24 h prior for hospitalized patients and within 7 days prior for outpatients.
Central Illustration.
Overview of Study Protocol and Timeline
The screening visit (visit 0) and visit 1 may was allowed to be combined at the investigator’s discretion for subjects who were stable and for whom laboratory results were reviewed as long as the investigator ensured the first dose of sacubitril/valsartan was given >36 h after the last dose of ACE inhibitor (if applicable). If visit data were not combined, visit 1 timeline should have taken place within 7 days of the screening visit. BID = twice daily; S/V = sacubitril/valsartan; w = week.
Patients who tolerated the run-in period with low-dose, open-label S/V were randomized in a double-blind fashion 1:1 to receive S/V plus placebo orally twice per day or valsartan plus placebo orally twice per day. The dose of the study drug was titrated to the target dose of S/V 97 mg/103 mg plus placebo orally twice per day or valsartan, 160 mg plus placebo orally twice per day (Table 3 ). The initial dose was selected based on FDA labeling and the package insert for S/V, as follows:
-
•
For patients who were ACE inhibitor/ARB naive, previously taking a low-dose of an ACE inhibitor/ARB (e.g., ≤10 mg lisinopril daily or other dose equivalent) or who had eGFR <30 ml/min/1.73 m2, the starting dose of S/V was 24 min/26 mg plus placebo orally twice per day, and the starting dose of valsartan was 40 mg plus placebo orally twice per day.
-
•
For patients taking an ACE inhibitor/ARB at greater than the low dose, the starting dose of S/V was 49 mg/51 mg plus placebo orally twice per day, and the starting dose of valsartan was 80 mg plus placebo orally twice per day.
Table 3.
Treatment Dose Levels
| Dose Level | Sacubitril/Valsartan | Valsartan |
|---|---|---|
| 1 | 24/26 mg BID | 40 mg BID |
| 2 | 49/51 mg BID | 80 mg BID |
| 3 | 97/103 mg BID | 160 mg BID |
BID = twice daily.
Study participants returned for follow-up visits at 2, 4, 8, 12, and 24 weeks, with a final phone visit at 26 weeks to assess clinical stability and any applicable adverse events. Each in-person study visit included an interim history, review of medications, focused physical examination, routine laboratory testing, core laboratory testing, quality of life assessments, and/or adverse event monitoring. Telephone encounters were performed at weeks 10, 16, and 20 to assess dosage compliance, record the occurrence of applicable adverse events and events of interest, and remind the subject of the date and time of their next in-person visit.
Dose adjustments of the study drug were performed every 2 weeks by doubling the dose of study medication up to the target or maximally tolerated subtarget dose. Criteria for doubling the dose were based on a systolic blood pressure ≥90 mm Hg, the absence of symptomatic hypotension, and the absence of worsening renal function or a serum creatinine concentration >2 mg/dl. For patients who did not tolerate the dose of study medication administered, the dose was reduced to the dose previously tolerated. At the conclusion of the 24-week double-blind treatment phase, the patient was transitioned to open-label S/V or valsartan at the discretion of the treating physician. The study participants and study team remain blinded until after the completion of the trial and the database had been locked and made available.
Study endpoints
The primary endpoint was the proportional change in the area under the curve for NT-proBNP levels measured at baseline and at weeks 2, 4, 8, 12, and 24. The secondary efficacy endpoint assessed over a 24-week period included the number of days when the patient was alive, out of hospital, and free from any of the following events: listing for cardiac transplantation (status 1 to 4), cardiac transplantation, LVAD implantation or placed on continuous inotropic therapy for ≥7 days, hospitalization for HF on >2 occasions (other than the index admission). Secondary tolerability endpoints included analysis through 24 weeks of the number of subjects who: 1) achieved a target dose of 25%, 50%, or 100% of valsartan or S/V; 2) developed hypotension (systolic blood pressure ≤85 mm Hg) with symptoms; 3) developed worsening renal function (eGFR <20 ml/min/1.73 m2); or 4) developed moderate (>5.5 mmol/l) or severe (≥6 mmol/l) hyperkalemia. Tertiary endpoints included a time-to-event analysis through 24 weeks for death, first hospitalization for HF, first hospitalization for HF, or death. Additional tertiary endpoints comparing S/V with valsartan therapy over 24 weeks included the total number of HF hospitalizations, as well as the number of patients who required chronic inotropic support >7 days after discharge from the index hospitalization; were listed for heart transplantation (i.e., status 1 to 4); underwent heart transplantation or LVAD implantation; had a change in baseline levels of eGFR and cystatin C measured at 4, 8, 12, and 24 weeks; had unanticipated use of intravenous diuretic agents (outpatient, emergency room, or inpatient); and had a change in the area under the curve (AUC) in patient-reported quality of life as measured by the Kansas City Cardiomyopathy Questionnaire. In addition, there was a prespecified exploratory hierarchical endpoint with 4 levels including: 1) death; 2) LVAD or heart transplantation (including listing status of 1A, 1B or 1 to 4); 3) multiple HF hospital admissions; and 4) single HF admission. There was also an exploratory endpoint of CV death or HF hospitalization.
Statistical considerations
The final analytic cohort included all randomized patients. Descriptive statistics were provided for demographics and baseline clinical characteristics. All continuous data were reported as mean ± SD or median (interquartile range [IQR]) (25th, 75th). Categorical data were reported as frequencies and percentages. Baseline differences between treatment groups were assessed using chi-square or Fisher exact test for categorical variables and the Wilcoxon 2-sample test for continuous variables.
Analyses of all study endpoints were based on intention-to-treat. The primary hypothesis was that the AUC for NT-proBNP levels measured at weeks 2, 4, 8, 12, and 24 would be smaller among patients randomized to S/V than those taking valsartan. A general linear model was used to estimate and compare the log-transformed AUCs in NT-proBNP values between the 2 treatment groups. Analysis of the NT-proBNP values used for the primary analysis were determined by Biomarker Core Laboratory (Burlington, Vermont). Each patient had 1 response based on the log of the proportional change from baseline in the AUC from the 2-, 4-, 8-, 12-, and 24-week measurements. The treatment effect was summarized using a point estimate and 95% confidence interval. Based on a 2-sample Student's t-test with type I error of 0.05 2-sided, the total sample size of 400 randomized subjects provided 80% and 90% power to detect differences of 19% and 21%, respectively for S/V compared to valsartan therapy. If there were missing NT-proBNP values at week 24, the last observation from the previous post-baseline time point with available NT-proBNP data were carried forward with available NT-proBNP data. For the primary analysis, no adjustment was made for missing baseline results.
The secondary and tertiary endpoints were analyzed using models that contained an indicator variable for the treatment assignment and atrial fibrillation status at enrollment. Continuous endpoints were modeled using general linear regression, whereas categorical endpoints were modeled using logistic regression. Cox regression modeling was used for the time-to-event analyses to estimate hazard ratios between the treatments. Kaplan-Meier curves were generated to graphically display the event rates as a function of time from randomization in each treatment arm. Mixed models were used for the analysis of longitudinal data. The unmatched Win Ratio estimator was used to analyze the exploratory 4-level hierarchical endpoint (14).
Funding and study organization
The LIFE trial was funded primarily by the National Heart, Lung, and Blood Institute (NHLBI) as part of the Heart Failure Clinical Research Network. Novartis supplied study drug and supplemental funding for coordinating center operations to support trial completion. The Duke Clinical Research Institute (Durham, North Carolina) was the coordinating center. Overall responsibility for the oversight and management of the trial lay with the LIFE Steering Committee, consisting of academic investigators and representatives from the NHLBI. The data and safety monitoring board included HF specialists and independent statistician and was responsible for active surveillance of safety data, including all adverse events. The LIFE trial protocol was approved by the Institutional Review Boards at all of the participating recruiting centers. Novartis Pharmaceuticals Corporation is providing the study drug and partial funding through the Investigator Initiated Trial (IIT) program (CLCZ696BUS04T).
Discussion
The guideline-directed medical therapies recommended for all patients with chronic NYHA functional class IV HF include β-blockers, renin-angiotensin system inhibitors (ACE inhibitor/ARB) and mineralocorticoid receptor antagonists; whereas isosorbide dinitrate/hydralazine was also indicated as an adjunct to standard therapy in self-identified black patients with NYHA functional classes III to IV symptoms. Although S/V is FDA approved for use in NYHA functional classes II to IV patients with HFrEF, current guidelines do not provide guidance regarding the use of S/V in class IV HFrEF patients because of the limited evidence base to inform use in this group of patients (9). The LIFE trial was designed to fill the knowledge gap with respect to the safety, efficacy, and tolerability of S/V in HFrEF patients with chronic NYHA functional class IV symptoms and would provide important information regarding the use of S/V in the management of patients with advanced HF. Apart from the specific characteristics of the target population in the LIFE trial, the following features of the trial warrant discussion.
In comparison with PARADIGM-HF, in which there were 2 sequential run-in phases that required all patients to tolerate high-dose enalapril (10 mg twice daily) for 2 weeks followed by 4 to 6 weeks of maximal dose S/V (97 mg/103 mg twice daily), the run-in period in the LIFE trial was only 3 to 7 days and required that the patient tolerate only low-dose S/V (24 mg/26 mg twice daily) (6).
The comparator arm of the LIFE study was valsartan, as opposed to enalapril, which was used in the PARADIGM-HF study. Although valsartan was used as the active comparator in trials of patients with HFpEF (15,16), the LIFE trial was the first trial to provide a direct comparison of the incremental effect of the neprilysin inhibitor sacubitril in patients with HFrEF.
The LIFE trial did not exclude patients taking intravenous inotropic agents and, therefore, provided information on the use of S/V in this challenging patient population. In addition, patients with low systolic blood pressure (90 to 100 mm Hg) and eGFR (20 to 30 ml/min/1.73) were enrolled.
Although HFrEF trials have usually been separated into studies of either hospitalized patients or outpatients, the LIFE trial was 1 of the first multicenter HF trials that included patients in both categories (17,18). Patients meeting objective criteria consistent with advanced HF and other selection criteria were eligible regardless of inpatient or outpatient status. With increasing recognition that decision making regarding hospital admission and discharge is subject to a variety of nonclinical factors independent of HF severity (e.g., local health care infrastructure, geographic region or country, medicolegal liability, and reimbursement factors), results of the LIFE may apply broadly to patients with advanced HF who are cared for in a wide variety of health systems (17,18).
Study limitations
Several limitations of the LIFE trial should be noted. First, the classification and eligibility criteria for advanced HF (e.g., EF, cardiopulmonary exercise testing, and 6-min walk testing) were determined on the basis of local testing and local clinical judgment and may therefore have been subject to some variability across study sites. Likewise, institutional and clinician practice patterns for use of inotropic agents may also vary across sites. Nonetheless, these differences were consistent with the realities of routine care delivery in the United States, which may enhance the application of trial results to real-world clinical care. Finally, although mortality and hospitalization data were collected during the study follow-up, the LIFE trial was underpowered to detect statistically significant differences among these endpoints, and findings for these study outcomes should be viewed as exploratory.
Conclusions
Data regarding effectiveness of medical therapy for patients with advanced HFrEF is limited (Table 4 ), and survival without heart transplantation or LVAD therapy remains exceedingly poor (3). Although the use of S/V has been shown to have clinical benefits among HFrEF patients with mild to moderate symptoms, the evidence with respect to the safety, efficacy, and tolerability for use of S/V in patients with advanced HF is limited, and it is unclear whether the clinical benefits of S/V will be of similar or different magnitude in patients with more advanced HFrEF. The LIFE trial was designed to address a critical knowledge gap regarding the use of S/V in severely symptomatic patients with HFrEF and will provide important new information that will inform the use of S/V as a treatment option for patients with advanced HF.
Table 4.
Placebo-Controlled Studies in Ambulatory Severe Heart Failure
| Class Trial (Ref. #) | n | Agent | Entry Criteria | Average Follow-Up Period |
Primary Endpoint | Findings |
|---|---|---|---|---|---|---|
| ACE Inhibitors | ||||||
| CONSENSUS (19) | 253 | Enalapril vs. placebo | NYHA functional class IV | 6 months | All-cause mortality | Placebo 44% Enalapril 26% (HR: 0.60; p = 0.002) |
| Beta-Blockers | ||||||
| COPERNICUS (20) | 2,289 | Carvedilol vs. placebo | LVEF <25% NYHA functional class IIIB–IV |
10 months | All-cause mortality | Placebo 17% Carvedilol 11% (HR: 0.65; p = 0.0014) |
| U.S. Carvedilol HF Study Group (severe) (21) | 131 | Carvedilol vs. placebo | LVEF ≤35% NYHA functional class III–IV 6-MWD ≤150 m |
6 months | Quality of life (MLWHQ) | Placebo ↑8.8 points Carvedilol ↑11.6 points (p = 0.60) |
| Mineralocorticoid Receptor Antagonists | ||||||
| RALES (22) | 1,663 | Spironolactone vs. placebo | LVEF ≤35% NYHA functional class III–IV |
24 months | All-cause mortality | Placebo 46% Spironolactone 35% (HR: 0.70; p < 0.001) |
| Fixed Dose Hydralazine Isosorbide (H-I) | ||||||
| A-HeFT (23) | 1,050 | H-I vs placebo | LVEF ≤35% NYHA functional class III–IV | 10 months | Composite score of death, first hospitalization for heart failure and QOL (MLWHQ) | H-I −0.1 ± 1.9 Placebo −0.5 ± 2.0 (p = 0.01) (HR: death 0.57; p = 0.01) |
| Calcium Channel Blockers | ||||||
| PRAISE (24) | 1,153 | Amlodipine vs. placebo | LVEF <30% NYHA functional class III–IV |
14 months | All-cause mortality or CV hospitalization | Placebo 42% Amlodipine 39% (HR: 0.91; p = 0.31) |
| PRAISE-2 (25) | 1,654 | Amlodipine vs. placebo | NICM LVEF <30% NYHA functional class III–IV |
33 months | All-cause mortality | Placebo 31.7% Amlodipine 33.6% (HR: 1.09; p = 0.33) |
| Guanylate Cyclase Stimulators | ||||||
| VICTORIA (26) | 5,050 | Vericiguat vs. placebo | LVEF <45% NYHA functional class II–IV Worsening HF∗ |
11 months | CV death or first HF hospitalization | Placebo 38.5% Vericiguat 35.5% (HR: 0.90; p = 0.02) |
| Oral Inotropes | ||||||
| PROMISE (27) | 1,088 | Milrinone vs. placebo | LVEF ≤35% NYHA functional class III–IV |
6 months | All-cause mortality | Placebo 24% Milrinone 30% (HR: 1.28; p = 0.038) |
| ESSENTIAL (28) | 1,854 | Enoximone vs. placebo | LVEF ≤30% NYHA functional class III-IV Worsening HF |
17 months | All-cause mortality or CV hospitalization | Placebo 50.1% Enoximone 49.5% (HR: 0.98; p = 0.71) |
| EMOTE (29) | 201 | Enoximone vs. placebo | LVEF ≤25% Need for IV inotrope |
30 days | Alive and free of inotrope | Placebo 51% Enoximone 61% (p = 0.17) |
| PERSIST (30) | 307 | Levosimendan vs. placebo | LVEF ≤30% NYHA functional class IIIB–IV Worsening HF |
60 days | Patient journey† | Placebo 0.44–0.53 No difference in patient journey scores (p = 0.57) |
| PROFILE (31) | 2,354 | Flosequinan vs. placebo | LVEF ≤35% NYHA functional class III–IV |
10 months | All-cause mortality | Placebo 16.3% Flosequinan 21.8% (HR: 1.39; p = 0.0006) |
| PRIME II (32) | 1,906 | Ibopamine vs. placebo | LVEF <35% NYHA functional class III–IV |
12 months | All-cause mortality | Placebo 20% Ibopamine 24% (HR: 1.26; p = 0.017) |
| Xamoterol in severe HF (33) | 516 | Xamoterol vs. placebo | LVEF <35% NYHA functional class III–IV |
12 weeks | Exercise duration All-cause mortality |
Placebo 381 s Xamoterol 384 s (p = NS) Placebo 3.7% Xamoterol 9.2% (HR: 2.54; p = 0.02) |
| Amrinone in severe HF (34) | 99 | Amrinone vs. placebo | LVEF <40% NYHA functional class III–IV |
8 weeks | Exercise improvement Withdrawal due to AE |
Placebo 35% Amrinone 37% (p = NS) Placebo 2% Amrinone 34% (p = 0.01) |
6-MWD = 6-min walk distance; AE = adverse event; A-HeFT = Combination of Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure; CONSENSUS = Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode; COPERNICUS = Effect of Carvedilol on the Morbidity of Patients With Severe Chronic Heart Failure; CV = cardiovascular; EMOTE = Oral Enoximone in Intravenous Inotrope-Dependent Subjects; ESSENTIAL = The Studies of Oral Enoximone Therapy in Advanced Heart Failure; HF = heart failure; IV = intravenous; LVEF = left ventricular ejection fraction; MLWHQ = Minnesota Living with Heart Failure Questionnaire; NICM = nonischemic cardiomyopathy; NYHA = New York Heart Association; PERSIST = Oral levosimendan in patients with severe chronic heart failure—The PERSIST study; PRAISE-2 = Prospective Randomized Amlodipine Survival Evaluation 2; PRIME II = Randomised Study of Effect of Ibopamine on Survival in Patients With Advanced Severe Heart Failure. Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy; PROFILE = Prospective Randomized Flosequinan Longevity Evaluation; PROMISE = Prospective Randomized Milrinone Survival Evaluation; RALES = Randomized Aldactone Evaluation Study; VICTORIA = Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction.
Enrolled patients had NYHA functional class II–IV heart failure with worsening HF defined as recent HF hospitalization or need for IV diuretic therapy.
This exploratory primary endpoint was measured by repeated symptom assessments, worsening heart failure events and mortality during 60 days after randomization to one of two doses of levosimendan or placebo.
Addendum
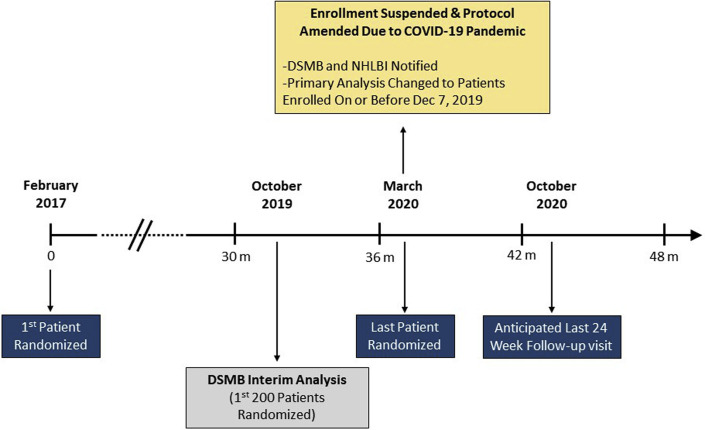
Because of the high risk for adverse outcomes related to COVID-19 infection in heart failure patients, the Executive Committee of the Heart Failure Network suspended enrollment into the LIFE trial on March 23, 2020. Subsequent to that suspension, the Executive Committee considered that patients enrolled in the LIFE trial would have great difficulty attending study visits on their planned schedule secondary to restrictions placed on outpatient visits at many institutions, which meant that patients would be unable to participate in study-related procedures such as biomarker sample collection that comprised the primary endpoint for this trial. Additionally, due to restrictions on clinic access and potential concerns with patients going to the emergency department where exposure risk to COVID-19 was increased, the Executive Committee anticipated that the reporting of clinical endpoints such as emergency department visits and even hospital admissions would be impacted significantly by the pandemic. Therefore, due to the unexpected disruption brought on by the COVID-19 pandemic, the LIFE protocol was amended to restrict the reported analyses to those patients who had their week 12 visit prior to March 1, 2020, when the COVID-19 pandemic became more active in the continental United States. The data analysis plan for the LIFE trial was changed so that the primary analyses includes only those patients who were randomized on December 7, 2019, or earlier (n = 335). Additionally, any study visits performed after March 1, 2020, were excluded from the primary analyses if the patients and were randomized on or prior to December 7, 2019. Patients enrolled after December 7, 2019 (n = 30) contributed to secondary analyses of the trial, so that all randomized patients contributed to the results of the LIFE trial. Based on a 2-sample Student's t-test with type I error of 0.05 2-sided, a revised conditional power analysis indicated that a total sample size of 335 randomized subjects would provide 72% and 84% power to detect differences of 19% and 21%, respectively, for S/V compared to valsartan therapy, respectively. The NHLBI concurred with the decision to suspend enrollment, and the above changes to the LIFE trial were communicated to the NHLBI and to the data and safety monitoring board on April 13, 2020. A timeline of the LIFE trial that included the original planned timeline for the trial, as well as how COVID-19 changed the timeline, is summarized in Figure 1 .
Figure 1.
Timeline for LIFE Trial
LIFE trial timelines are shown from the date on which the first patient was enrolled and from the first data and safety monitoring board interim analysis. Enrollment in the trial was suspended March 23, 2020, because of the high risk for adverse outcomes related to COVID-19. The plan for data analysis was adjusted to restrict the primary analysis to patients who had their week-12 visit prior to March 1, 2020. The last anticipated 24-week follow-up visit for patients randomized before March 23 was anticipated to be October 24, 2020. COVID-19 = coronavirus-2019; LIFE = LCZ696 in Hospitalized Advanced Heart Failure.
Footnotes
Dr. Mann has served as a consultant for Novartis. Dr. Greene has received research support from Amgen, AztraZeneca, Bristol-Myers Squibb, Merck, and Novartis; and is a consultant for Amgen and Merck; is a member of the advisory boards of Amgen and Cytokinetics. Dr. Starling has served as a consultant for Novartis; and is on the steering committee for the PARAGLIDE trial. Dr. Ambrosy has received research support from Novartis; and has received personal fees for the PIONEER-HF trial. Dr. Shah is an employee of Inova Heart and Vascular Institute; has received grant support from Merck, Abbott, Bayer, Medtronic, and Pulse CV; and is a consultant for NuPulse, Ortho Clinical Diagnostics, and Procyrion. Dr. Mahr is a consultant for Abbott, Medtronic, and Abiomed. Dr. Lewis has been a consultant for and received research support from Cytokinetics and Applied Therapeutics; and received research support from Amgen and AstraZeneca. Dr. Mohammed is a member of the advisory board for Pfizer; and has received research support from Cardiocell, Abbott, Actelion, Corvia, and Medtronic. Dr. Gilotra is a consultant for scPharmaceuticals. Dr. DeVore has received research support through his institution from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, American Regent, National Heart, Lung, Blood Institute, Novartis, and Patient-Centered Outcomes Research Institute; and is a consultant for Novartis. Dr. Desvigne-Nickens is an employee of the National Heart, Lung, and Blood Institute; and is a consultant for Novartis. Dr. Gorodeski has received research support and speaker and consultation compensation from Abbott. Dr. Hernandez has received research grants and consulting for AstraZeneca, Amgen, Bayer, Merck and Novartis. Dr. Braunwald has received research support through his institution from AstraZeneca, Daiichi-Sankyo, Merck, and Novartis; and is a consultant for Amgen, Cardurion, MyoKardia, and NovoNordisk. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institute of Arthritis and Infectious Diseases, the National Institutes of Health, or the U.S. Department of Health and Human Services.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Heart Failureauthor instructions page.
References
- 1.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Jessup M., Bozkurt B., et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Ambardekar A.V., Kittleson M.M., Palardy M., et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) registry. J Heart Lung Transplant. 2019;38:408–417. doi: 10.1016/j.healun.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J.C., Ewald G.A., Allen L.A., et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. doi: 10.1016/j.cardfail.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 5.McMurray J.J., Packer M., Desai A.S., et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 7.Claggett B., Packer M., McMurray J.J., et al. Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373:2289–2290. doi: 10.1056/NEJMc1509753. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava P.K., Claggett B.L., Solomon S.D., et al. Estimated 5-year number needed to treat to prevent cardiovascular death or heart failure hospitalization with angiotensin receptor-neprilysin inhibition vs standard therapy for patients with heart failure with reduced ejection fraction: an analysis of data from the PARADIGM-HF trial. JAMA Cardiol. 2018;3:1226–1231. doi: 10.1001/jamacardio.2018.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37 doi: 10.1093/eurheartj/ehw128. 2129–200. [DOI] [PubMed] [Google Scholar]
- 11.Desai A.S., Solomon S., Claggett B., et al. Factors associated with noncompletion during the run-in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM-HF trial. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002735. e002735. [DOI] [PubMed] [Google Scholar]
- 12.McMurray J.J., Packer M., Desai A.S., et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2014;16:817–825. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velazquez E.J., Morrow D.A., DeVore A.D., et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 14.Pocock S.J., Ariti C.A., Collier T.J., Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 15.Solomon S.D., Zile M., Pieske B., et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 16.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 17.Greene S.J., Mentz R.J., Felker G.M. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3:252–259. doi: 10.1001/jamacardio.2017.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene S.J., Felker G.M., Butler J. Outpatient versus inpatient worsening heart failure: distinguishing biology and risk from location of care. Eur J Heart Fail. 2019;21:121–124. doi: 10.1002/ejhf.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group C.T.S. Effects of enalapril on mortality in severe congestive heart failure: results of the cooperative north scandanavian enalapril survival study. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 20.Packer M., Coats A.J., Fowler M.B., et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 21.Cohn J.N., Fowler M.B., Bristow M.R., et al. Safety and efficacy of carvedilol in severe heart failure. The U.S. Carvedilol Heart Failure Study Group. J Card Fail. 1997;3:173–179. doi: 10.1016/s1071-9164(97)90013-0. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B., Zannad F., Remme W.J., et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 23.Taylor A.L., Ziesche S., Yancy C., et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 24.Packer M., O'Connor C.M., Ghali J.K., et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107–1114. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 25.Packer M., Carson P., Elkayam U., et al. Effect of amlodipine on the survival of patients with severe chronic heart failure due to a nonischemic cardiomyopathy: results of the PRAISE-2 study (prospective randomized amlodipine survival evaluation 2) J Am Coll Cardiol HF. 2013;1:308–314. doi: 10.1016/j.jchf.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong P.W., Pieske B., Anstrom K.J., et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 27.Packer M., Carver J.R., Rodeheffer R.J., et al. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 28.Metra M., Eichhorn E., Abraham W.T., et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30:3015–3026. doi: 10.1093/eurheartj/ehp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman A.M., Oren R.M., Abraham W.T., et al. Low-dose oral enoximone enhances the ability to wean patients with ultra-advanced heart failure from intravenous inotropic support: results of the oral enoximone in intravenous inotrope-dependent subjects trial. Am Heart J. 2007;154:861–869. doi: 10.1016/j.ahj.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Nieminen M.S., Cleland J.G., Eha J., et al. Oral levosimendan in patients with severe chronic heart failure---the PERSIST study. Eur J Heart Fail. 2008;10:1246–1254. doi: 10.1016/j.ejheart.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Packer M., Pitt B., Rouleau J.L., Swedberg K., DeMets D.L., Fisher L. Long-term effects of flosequinan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the PROFILE trial After 24 years. J Am Coll Cardiol HF. 2017;5:399–407. doi: 10.1016/j.jchf.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Hampton J.R., van Veldhuisen D.J., Kleber F.X., et al. Randomised study of effect of ibopamine on survival in patients with advanced severe heart failure. Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy (PRIME II) Investigators [see comments] Lancet. 1997;349:971–977. doi: 10.1016/s0140-6736(96)10488-8. [DOI] [PubMed] [Google Scholar]
- 33.Xamoterol in severe heart failure. The xamoterol in Severe Heart Failure Study group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- 34.Massie B., Bourassa M., DiBianco R., et al. Long-term oral administration of amrinone for congestive heart failure: lack of efficacy in a multicenter controlled trial. Circulation. 1985;71:963–971. doi: 10.1161/01.cir.71.5.963. [DOI] [PubMed] [Google Scholar]