Prime-only vaccination with ChAdOx1 MERS protects against HCoV-EMC/2012 in NHP and a variety of MERS-CoV strains in mice.
Abstract
Developing a vaccine to protect against the lethal effects of the many strains of coronavirus is critical given the current global pandemic. For Middle East respiratory syndrome coronavirus (MERS-CoV), we show that rhesus macaques seroconverted rapidly after a single intramuscular vaccination with ChAdOx1 MERS. The vaccine protected against respiratory injury and pneumonia and reduced viral load in lung tissue by several orders of magnitude. MERS-CoV replication in type I and II pneumocytes of ChAdOx1 MERS–vaccinated animals was absent. A prime-boost regimen of ChAdOx1 MERS boosted antibody titers, and viral replication was completely absent from the respiratory tract tissue of these rhesus macaques. We also found that antibodies elicited by ChAdOx1 MERS in rhesus macaques neutralized six different MERS-CoV strains. Transgenic human dipeptidyl peptidase 4 mice vaccinated with ChAdOx1 MERS were completely protected against disease and lethality for all different MERS-CoV strains. The data support further clinical development of ChAdOx1 MERS.
INTRODUCTION
Coronaviruses (CoV) pose a continuous emerging virus threat, as demonstrated by the emergence of three previously unidentified coronaviruses in the past 18 years. Severe acute respiratory syndrome coronavirus (SARS-CoV) was first detected in 2003 and went on to infect >8000 people, resulting in 774 fatalities (1). In 2012, Middle East respiratory syndrome coronavirus (MERS-CoV) was first detected. MERS-CoV continues to infect humans from the dromedary camel host and has thus far infected >2500 people, resulting in 866 fatalities (2). An outbreak of pneumonia with an unknown cause in Wuhan, China was first reported in December 2019. Although information is still limited, we now know that this outbreak is caused by a third emerging CoV. Currently, >200,000 cases are associated with this outbreak (3).
The clinical spectrum of MERS-CoV infection in humans varies from asymptomatic to severe respiratory disease and death. Patients present with influenza-like symptoms such as a fever and shortness of breath. Thereafter, they may develop pneumonia, which can require mechanical ventilation and support in an intensive care unit (2). Human-to-human transmission of MERS-CoV is relatively limited and occurs mainly in nosocomial settings but has been reported in local communities as well (1). In 2015, a traveler from the Middle East to South Korea caused an outbreak involving 186 people and 38 fatalities (4). The nosocomial outbreak lasted from May to July, and 16,752 people were isolated with MERS-CoV–like symptoms. At least three superspreaders were identified in the outbreak, who infected 28, 85, and 23 patients, respectively (5). The introduction and spread of MERS-CoV in South Korea underscore the potential of this virus to cause epidemics outside of the Arabian Peninsula. MERS-CoV has continued to cause disease in humans, and in 2019, 152 cases have been reported in the Kingdom of Saudi Arabia (KSA), of which 33% were fatal (2). The ongoing circulation of MERS-CoV and subsequent outbreaks in the human population highlight the need for an efficient MERS-CoV vaccine.
MERS-CoV is mainly prevalent in the Arabian Peninsula, with the majority of cases occurring in KSA (84%) (6). However, phylogenetically diverse MERS-CoV strains have been isolated from Africa and the Middle East (7–9), and antigenic differences have been reported between spike (S) proteins, the main antigen used in MERS-CoV vaccines, from the Middle East and Africa (8). Thus, it is important that a MERS-CoV vaccine is protective against a variety of diverse MERS-CoV strains.
Thus far, only four vaccines have been tested in rhesus macaques. These are a DNA vaccine (10); a vaccine based on the receptor-binding domain of the MERS-CoV S adjuvanted with alum (11); a combination of S plasmid DNA and S1 protein (12); and virus-like particles based on MERS-CoV S, matrix, and envelope proteins (13). Of the four vaccine studies, only three studies included challenge studies, all of which used MERS-CoV isolates from 2012. To date, there have been no reports of efficacy of a single-dose MERS-CoV vaccine in nonhuman primates (NHPs).
We recently demonstrated that vaccination of mice with a replication-deficient simian adenovirus vaccine vector (ChAdOx1) encoding full-length MERS-CoV S protein (ChAdOx1 MERS) elicited high-titer MERS-CoV–neutralizing antibodies and a robust CD8+ T cell response against the S protein (14). In addition, ChAdOx1 MERS vaccination resulted in full protection of human dipeptidyl peptidase 4 (hDPP4) transgenic mice against a lethal challenge with MERS-CoV (15). ChAdOx1 MERS vaccination of dromedary camels was immunogenic and reduced MERS-CoV shedding after challenge in a highly stringent natural transmission model with multiday exposure to infectious MERS-CoV (16). ChAd-vectored vaccines against malaria (17), HIV (18), influenza (19), hepatitis C (20), tuberculosis (21), Ebola (22), and others show an excellent immunogenicity and safety profile in humans. In the current manuscript, we show that a single dose of ChAdOx1 MERS vaccine protects rhesus macaque model against a mucosal challenge with HCoV-EMC/2012. Serum obtained from vaccinated rhesus macaques was able to neutralize six diverse MERS-CoV strains. Furthermore, a single dose of ChAdOx1 MERS vaccine protects hDPP4 transgenic mice against all evaluated MERS-CoV strains.
RESULTS
ChAdOx1 MERS elicits a productive immune response in rhesus macaques
Three groups of six animals each were vaccinated with ChAdOx1 MERS via a prime-boost regimen [−56 and −28 DPI (days post infection)] or prime-only regimen (−28 DPI) or with ChAdOx1 green fluorescent protein (GFP) (−56 and −28 DPI). Animals were then challenged with 7 × 106 TCID50 (median tissue culture infectious dose) of HCoV-EMC/2012 on 0 DPI via combined intratracheal, intranasal, oral, and ocular route (23). Blood samples were taken at −56, −42, −28, −14, and 0 DPI (Fig. 1A). The humoral immune response to vaccination was examined by enzyme-linked immunosorbent assay (ELISA) using S protein and by virus neutralization assay. S protein–specific immunoglobulin G (IgG) antibodies were detected as early as 14 days after vaccination in both ChAdOx1 MERS–vaccinated groups. All animals in these groups had S protein–specific antibodies at 0 DPI, whereas no antibodies against S protein were found before vaccination or at any time in the ChAdOx1 GFP–vaccinated group. A significant difference in ELISA titers was observed between prime-boost and prime-only animals at 0 DPI (Fig. 1B). Neutralizing antibodies were detected in 11 of 12 ChAdOx1 MERS–vaccinated animals at the time of challenge. One animal in the prime-only vaccination group did not have detectable neutralizing antibodies at this time and had the lowest antibody titer as measured by ELISA (1600). A significant difference in VN (virus-neutralizing) titers was observed between prime-boost and prime-only animals at 0 DPI (Fig. 1C). A second ChAdOx1 MERS vaccination at −28 DPI resulted in a statistically significant increase in S protein–specific ELISA titer (geometric mean titer −28 DPI = 1600; 0 DPI = 10,159; P < 0.0001) and neutralizing antibody titer (geometric mean titer −28 DPI = 24; 0 DPI = 148; P < 0.0001) as determined via two-tailed t test, although neutralizing antibodies against the ChAdOx1 vector could be detected at the time of the second vaccination (fig. S1A). This suggests that the presence of neutralizing antibodies against ChAdOx1 does not prevent the vaccine vector from boosting the immune response.
Fig. 1. Vaccination of rhesus macaques with ChAdOx1 MERS elicits a humoral immune response.
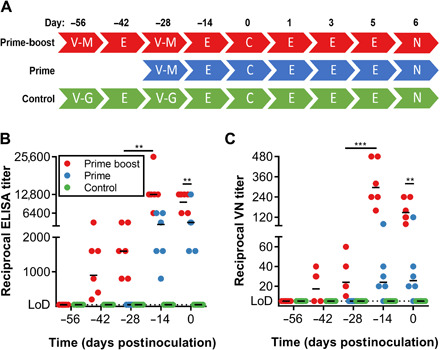
Serum samples were collected from NHPs at times of vaccination (−56 and −28 DPI), 14 days later, and at challenge. (A) Overview of experimental timeline. V-M, vaccination with ChAdOx1 MERS; V-G, vaccination with ChAdOx1 GFP; E, exam; C, challenge and exam; N, exam and necropsy. (B) Twofold serial diluted serum samples were tested for MERS-CoV S–specific antibodies using enzyme-linked immunosorbent assay (ELISA). (C) Twofold serial diluted serum samples were tested for neutralizing antibodies against MERS-CoV in VeroE6 cells. Line, geometric mean; dotted line, limit of detection (LoD). Statistical significance between −28 and −14 DPI in the prime-boost group was determined via one-tailed paired Student’s t test. Statistical significance between prime-boost and prime-only groups on 0 DPI was determined via two-tailed unpaired Student’s t test. **P < 0.01; ***P < 0.001.
Vaccination with ChAdOx1 MERS reduces disease severity
Animals vaccinated with ChAdOx1 GFP and challenged with MERS-CoV showed similar clinical signs as previously reported (23), such as a decreased appetite and increased respiratory rate. Clinical signs were scored using a standard NHP scoring sheet, focusing on areas such as general appearance, nasal discharge, and food intake. On average, ChAdOx1 MERS–vaccinated animals had a lower clinical score than ChAdOx1 GFP–vaccinated animals (Fig. 2A). All animals underwent exams on 0, 1, 3, 5, and 6 DPI. No significant changes in weight or body temperature were observed for the duration of the study. Peripheral capillary oxygen saturation (spO2) measurements supported a decrease in oxygen saturation from the baseline in ChAdOx1 GFP–vaccinated animals, but not in ChAdOx1 MERS–vaccinated animals (Fig. 2B).
Fig. 2. Clinical scoring and spO2 values are improved in ChAdOx1 MERS–vaccinated animals compared to ChAdOx1 GFP–vaccinated animals.
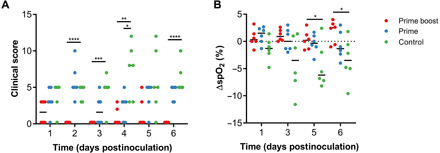
(A) Animals were evaluated daily, and clinical score was assessed using an established scoring sheet. (B) Changes in oxygen saturation from pre-inoculation values [Δ% peripheral capillary oxygen saturation (spO2)] were determined on exam days. Statistical significance between groups was determined via two-tailed unpaired Student’s t test. Line, median; dotted line, baseline value; *P < 0.025; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Ventrodorsal and lateral thoracic radiographs were collected on all exam days. All animals vaccinated with ChAdOx1 GFP showed moderate to severe pulmonary interstitial infiltrations, whereas animals vaccinated with ChAdOx1 MERS showed only mild signs of respiratory disease. A severe collapsed lung was observed on 3 DPI in two animals in the prime-boost group, likely caused by the bronchoalveolar lavage (BAL) performed on 1 DPI. Full details of all observed signs can be found in table S2 (Fig. 3A and table S2). All lung lobes (right cranial, right middle, right caudal, left cranial, left middle, and left caudal) of each individual animal were scored for severity of disease signs for each day radiographs were taken, and average scores were compared. Scores obtained from animals vaccinated with ChAdOx1 GFP were significantly higher from animals vaccinated with a prime-boost regimen of ChAdOx1 MERS on 3, 5, and 6 DPI (Fig. 3B).
Fig. 3. Single-dose vaccination with ChAdOx1 MERS protects rhesus macaques against bronchointerstitial pneumonia caused by MERS-CoV challenge.
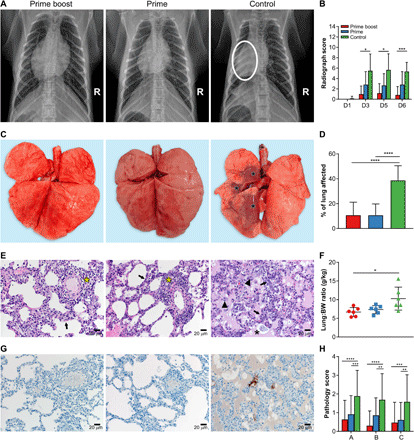
Rhesus macaques were vaccinated with a prime-boost or prime-only regimen of ChAdOx1 MERS, or with ChAdOx1 GFP and challenged with MERS-CoV. (A) Ventrodorsal thoracic radiographs collected on 6 DPI. A marker (R) indicates the right side of animal. No pathologic changes were observed in animals vaccinated with ChAdOx1 MERS via a prime-boost or prime-only regimen. Animal vaccinated with ChAdOx1 GFP shows focally extensive area of increased pulmonary opacity and deviation of the cardiac silhouette, highlighted in the circle located in the middle and caudal lung lobes. (B) Thoracic radiographs of each animal were scored per lung lobe, resulting in a maximum score of 18. Values were averaged per group per day (D), and mean with SD is shown (see table S2 for more details). (C) Gross pathology of lungs shows no pathologic changes in ChAdOx1 MERS–vaccinated animals and focally extensive areas of consolidation in left cranial, middle, and caudal lung lobes in control animals (asterisks). (D) Gross lung lesions were scored for each lung lobe, ventral and dorsal. Values were averaged per group, and mean with SD is shown. (E) Lung tissue sections were stained with hematoxylin and eosin. Moderate numbers of lymphocyte accumulation around pulmonary arterioles (asterisks) and mild thickening of alveolar septae by lymphocytes and macrophages (arrows) in lung tissue of animals vaccinated with ChAdOx1 MERS. Marked bronchointerstitial pneumonia with abundant pulmonary edema and fibrin (asterisks), type II pneumocyte hyperplasia (arrows), and increased numbers of alveolar macrophages (arrowheads) in lung tissue of control animals. Magnification, ×200. (F) Lung–to–body weight (BW) ratio was determined for all animals at necropsy. Mean with SD is shown. (G) Lung tissue sections were stained with antibody against MERS-CoV antigen, which is visible as a red-brown staining. No immunoreactivity was found in ChAdOx1 MERS–vaccinated animals, whereas multifocal immunoreactivity of type I and II pneumocytes could be found in lung tissue of ChAdOx1 GFP–vaccinated animals. (H) Lung tissue sections were scored on severity of lesions (0, no lesions; 1, 1 to 10%; 2, 11 to 25%; 3, 26 to 50%; 4, 51 to 75%; and 5, 76 to 100%) and averaged per group. Mean with SD is shown. A, bronchointerstitial pneumonia; B, type II pneumocyte hyperplasia; C, hemorrhages, edema, and fibrin deposits. Statistical significance between groups was determined via two-tailed unpaired Student’s t test. *P < 0.025; **P < 0.01; ***P < 0.001; ****P < 0.0001. Photo credit: Neeltje van Doremalen, NIAID/NIH.
Upon necropsy on 6 DPI, gross lung lesions were more prevalent in animals vaccinated with ChAdOx1 GFP than in animals vaccinated with ChAdOx1 MERS (Fig. 3, C and D). Animals vaccinated with ChAdOx1 GFP showed focally extensive areas of consolidation in all lung lobes and lungs generally failed to collapse. Mediastinal lymph nodes were often edematous and enlarged. Animals that received a vaccination with ChAdOx1 MERS, either via a prime-boost or prime-only regimen, had either no lesions or limited small multifocal areas of consolidation and congestion.
An increased lung:body weight ratio is an indicator of pulmonary edema. Animals in the control group had significantly higher lung:body weight ratios compared to ChAdOx1 MERS–vaccinated animals, and there was minimal difference between prime-boost and prime-only ChAdOx1 MERS–vaccinated animals (Fig. 3F).
Lung tissue sections were stained with hematoxylin and eosin or with MERS-CoV–specific antibodies. All slides were evaluated by a board-certified veterinary pathologist blinded to study group allocations. In animals that received a vaccination with ChAdOx1 MERS, either prime-boost or prime-only, a minimal to mild bronchointerstitial pneumonia was present, characterized by mild thickening of the alveolar septae by lymphocytes and macrophages. Pulmonary vessels were bound by moderate numbers of lymphocytes. In stark contrast, in lung tissue obtained from animals vaccinated with ChAdOx1 GFP, moderate to marked bronchointerstitial pneumonia was present throughout the lung lobes characterized by thickening of the alveolar septae by lymphocytes and macrophages and edema and fibrin. Alveoli contained abundant edema and fibrin and moderate to abundant numbers of alveolar macrophages, neutrophils, and necrotic debris. Inflammation often surrounded bronchioles and pulmonary vasculature, and type II pneumocyte hyperplasia was prominent. In addition, the presence of MERS-CoV antigen by immunohistochemistry was found only in lungs of animals vaccinated with ChAdOx1 GFP within type I and II pneumocytes and was not found in lung tissue of ChAdOx1 MERS–vaccinated animals. Severity of bronchointerstitial pneumonia, type II pneumocyte hyperplasia and hemorrhages, edema, and fibrin deposits was scored. Statistically significant differences between animals vaccinated with ChAdOx1 MERS or ChAdOx1 GFP were found for all three categories (Fig. 3, E, F, and H). Thus, vaccination with ChAdOx1 MERS, either via a prime-boost or prime-only regimen, significantly decreased the severity of pulmonary pathology and protected rhesus macaques against bronchointerstitial pneumonia.
Vaccination with ChAdOx1 MERS limits virus replication in the respiratory tract
BAL was performed on all animals on 1, 3, 5, and 6 DPI, and the amount of viral RNA, mRNA, and infectious virus was determined. In the prime-boost group, infectious virus was only detected at 1 DPI (n = 3) after inoculation, and in the prime-only group at 1 DPI (n = 6) and 3 DPI (n = 3) but not thereafter. In contrast, infectious virus was detected on all days in BAL fluid from the control group (Fig. 4A). Viral mRNA in BAL fluid of animals in the prime-boost group was only detected at 1 DPI (n = 5) and 3 DPI (n = 1). In contrast, viral mRNA in BAL fluid from animals that received a single vaccination with ChAdOx1 MERS mRNA was detected on all days, but the amount was reduced compared to animals vaccinated with ChAdOx1 GFP (fig. S1B). Viral RNA as measured by UpE quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay could be detected in all groups up to 6 DPI. However, the number of genome copies per milliliter detected was lower in animals vaccinated with ChAdOx1 MERS compared to animals vaccinated with ChAdOx1 GFP (Fig. 4A). A significant association was found between higher ELISA titer or VN titer and lower levels of viral RNA, mRNA, or infectious virus in BAL fluid for all days, except 6 DPI for infectious virus (Spearman’s rank correlation coefficient; table S2).
Fig. 4. Vaccination with ChAdOx1 MERS results in reduced virus replication in the respiratory tract.
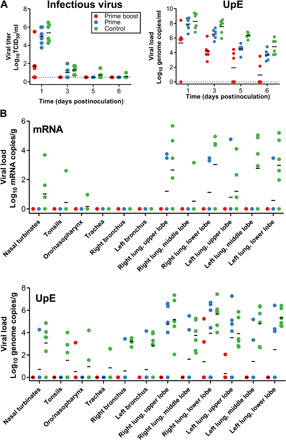
(A) Infectious virus titers and viral load were determined in BAL fluid. Individual values are depicted. (B) UpE and mRNA copies were determined in respiratory tract tissues collected at 6 DPI. Individual values are depicted. Line, geometric mean; dotted line, limit of detection.
All animals were euthanized on 6 DPI, and tissues were analyzed for the presence of viral RNA, mRNA, or infectious virus. Infectious virus titers were only found in nasal turbinate tissue (n = 1) and lung lobe tissue (n = 4) from control animals. No viral mRNA was found in all tissues obtained from animals that received a prime-boost regimen. In tissue from animals that received a prime-only regimen, limited viral mRNA could be found in upper and lower lung lobe tissues (n = 4). In contrast, mRNA could be found in respiratory tract tissues of all control animals and in conjunctiva (Fig. 4B). Viral RNA was detected in tissues from all groups but was mainly found in lung lobes and bronchi. Viral load was higher for lower respiratory tract tissue obtained from animals vaccinated with ChAdOx1 GFP (n = 6) than from animals receiving a prime-only (n = 6) or a prime-boost regimen of ChAdOx1 MERS (n = 2) (Fig. 4B).
0 tissue of ChAdOx1 GFP–vaccinated animals compared to ChAdOx1 MERS–vaccinated animals
The presence of 23 cytokines was evaluated in lung tissue. Several cytokines were up-regulated in animals vaccinated with ChAdOx1 GFP compared to animals vaccinated with ChAdOx1 MERS, including interleukin-2 (IL-2), IL-6, IL-8, IL-18, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1a (MIP-1α), and transforming growth factor–α (TGF-α), although only IL-2 and MCP-1 were significantly different in tissue obtained from control animals compared to vaccinated animals. A significant difference was observed in IL-1ra levels in lung tissue obtained from the prime-only and prime-boost groups. It is not clear what the clinical significance of this difference is (fig. S2). Overall, these results show local increased immune activity in animals vaccinated with ChAdOx1 GFP, but not in animals vaccinated with ChAdOx1 MERS, 6 days after challenge with MERS-CoV. The likely explanation for this is that vaccinated animals have controlled the infection rapidly, whereas in the control animals, more viral replication has taken place followed by the development of a local immune response and increased cytokine levels in the lungs.
Antibodies elicited by ChAdOx1 MERS vaccination neutralize different MERS-CoV strains
ChAdOx1 MERS is based on the S protein from Camel/Qatar/2/2014, and we currently do not have access to an isolate. We thus already show cross-protection of ChAdOx1 MERS against HCoV-EMC/2012 (99.78% S protein amino acid identity). Here, we extend that analysis to five other strains. We selected six different strains of MERS-CoV (fig. S2). S protein identity for all strains to the vaccine S protein was >99.3%. Amino acid identity was lowest for Camel/Burkina Faso/CIRAD-HKU785/2015 (99.33%) and highest for Hu/Korea/Seoul/SNU1-035/2015 (99.85%). We tested neutralizing capability of serum obtained at 0 DPI. Strains were selected on the basis of geographical location (KSA, South Korea, and Burkina Faso), host (dromedary camel or human), and time of isolation (2012 to 2018). All six strains were neutralized by antibodies elicited by ChAdOx1 MERS vaccination. Although we were not able to detect neutralizing ability of serum obtained from animal 12 against HCoV-EMC/2012, antibodies in the serum were able to neutralize four of six tested MERS-CoV strains (Table 1).
Table 1. Neutralizing titer of serum obtained from animals vaccinated with a prime-boost regimen of ChAdOx1 MERS against different MERS-CoV strains.
U, unassigned.
| Full virus name | Abbreviation |
GenBank accession no. |
Lineage (9, 38) |
Animal number | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| Hu/HCoV-EMC/2012 | EMC/12 | JX869059 | A | 120 | 480 | 120 | 240 | 240 | 120 | 120 | 40 | 40 | 40 | 80 | <20 |
| Hu/KSA/Rs924/2015 | KSA/15 | KY688119 | B4 | 240 | 160 | 80 | 160 | 120 | 60 | 120 | 60 | 60 | 40 | 60 | 20 |
| Hu/Korea/Seoul/ SNU1-035/2015 |
SK/15 | KU308549 | B5 | 160 | 120 | 60 | 160 | 60 | 60 | 80 | 40 | 30 | 30 | 30 | 20 |
| Riyadh/ KSA-18013832/2018 |
KSA/18 | MN723544 | U | 320 | 320 | 160 | 480 | 120 | 80 | 40 | 30 | 30 | 30 | 20 | <20 |
| Camel/KSA/ KFU-HKU1/2013 |
C/KSA/13 | KJ650297 | B2 | 160 | 240 | 80 | 120 | 120 | 80 | 240 | 60 | 60 | 40 | 30 | 20 |
| Camel/Burkina Faso/ CIRAD-HKU785/2015 |
C/BF/15 | MG923471 | C1 | 240 | 240 | 160 | 160 | 240 | 80 | 80 | 60 | 120 | 80 | 60 | 30 |
ChAdOx1 MERS protects mice against different strains of MERS-CoV
To investigate whether vaccination with ChAdOx1 MERS vaccination provides protection against a variety of different MERS-CoV strains, we vaccinated BALB/c mice transgenic for hDPP4 with ChAdOx1 MERS or ChAdOx1 GFP 28 days before challenge with 104 TCID50 of one of six diverse MERS-CoV strains (fig. S3) via the intranasal route. All mice vaccinated with ChAdOx1 MERS survived challenge with MERS-CoV, independent of the challenge virus used, whereas most control mice were euthanized because of >20% weight loss or poor body condition (Fig. 5A). Four animals per group were euthanized at 3 DPI, and infectious MERS-CoV titers in lung tissue were evaluated. Whereas infectious virus could be found in lung tissue of control animals, we were unable to find infectious virus in lung tissue of ChadOx1 MERS–vaccinated animals (Fig. 5B). Thus, ChAdOx1 MERS protects against a variety of different MERS-CoV strains in hDPP4 transgenic mice.
Fig. 5. ChAdOx1 MERS provides cross-protection against different MERS-CoV strains in the mouse model.
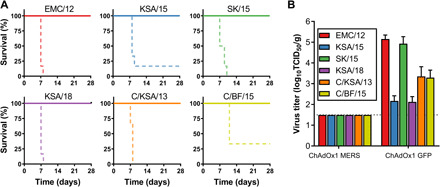
(A) Survival curves of ChAdOx1 MERS–vaccinated (solid line) and ChAdOx1 GFP–vaccinated (dashed line) hDPP4 mice challenged with MERS-CoV. (B) Infectious virus titers in lung tissue collected at 3 DPI from hDPP4 mice challenged with MERS-CoV. Mean titer with SD is shown.
DISCUSSION
MERS-CoV is circulating in the dromedary camel population and continuously reintroduced into the human population (24). MERS is associated with a high case-fatality rate (34.5%), and human-to-human transmission is a major contributor to patient infections (1). Currently, no MERS-CoV vaccine is available. Ideally, such a vaccine would only require a single administration and would protect against a wide variety of different MERS-CoV strains.
Several studies have evaluated different types of MERS-CoV vaccines in animal models, but few have taken these vaccines into NHPs. In the current study, we show the efficacy of ChAdOx1 MERS in rhesus macaques. Unlike other NHP vaccine studies (10–13), we investigate vaccine efficacy after a single dose. Animals that received a single dose of ChAdOx1 MERS showed an induction of a neutralizing antibody response associated with mostly normal clinical parameters, showing no breathing irregularities or reduced lung function by spO2 values, limited evidence of infiltration by radiograph analysis after challenge, and no signs of gross pathological lesions. Vaccination reduced viral RNA load in tissues collected at 6 DPI compared to ChAdOx1 GFP–vaccinated animals by several logs. In contrast to the abundant presence of viral mRNA and infectious virus in control animals, we found no evidence of infectious virus, and only a limited presence of viral mRNA, in the lungs of prime-only vaccinated macaques. Viral mRNA and infectious virus was completely absent from animals vaccinated with a prime-boost regimen of ChAdOx1 MERS. Although both vaccine regimens protected rhesus macaques from the clinical symptoms of MERS-CoV inoculation, we detected lower virus replication in animals that received a prime-boost regimen compared to animals receiving a prime-only regimen, suggesting that the prime-boost regimen is a superior therapeutic approach. It should be noted that in the current model, animals are inoculated with a high dose of virus (7 × 106 TCID50 per animal). This is likely a higher inoculum than most humans are exposed to. It is thus difficult to extrapolate this observation to humans vaccinated with a single dose of ChAdOx1 MERS.
The study was not designed to determine correlates of protection, which must be determined separately for each vaccine candidate, but it is of interest that here, one animal was protected despite not having detectable neutralizing antibodies against HCoV-EMC/2012, and in general, the neutralizing antibody titer was not high. In clinical trials, ChAdOx1-vectored vaccines prime strong T cell responses against the vaccine antigen (17–22). In a clinical study of patients that recovered from MERS infection, some had strong CD8+ T cell responses without detectable antibodies (25). Further studies addressing correlates of protection for ChAdOx1 MERS should assess CD8+ T cell responses.
Following the successful induction of protective immunity after vaccination as demonstrated here, the duration of immunity and ability to induce the development of memory B and T cells should be assessed. The phenotype of memory cells induced by vaccination may not necessarily mimic that induced by infection with the pathogen. In the first clinical trial of ChAdOx1 MERS (ClinicalTrials.gov identifier: NCT03399578) both humoral- and T cell–mediated responses were assessed at multiple times and persisted up to 1 year after vaccination. Further work will be required to assess memory B and T cell phenotypes. We are currently planning studies to look at long-term protection by ChAdOx1 MERS vaccination in our hDPP4 mouse model.
A variety of different MERS-CoV strains have been isolated from dromedary camels and humans over the past 8 years of MERS-CoV emergence (24). Dromedary camels are distributed throughout Africa, the Middle East, Asia, and Australia (26). Although MERS-CoV has not been detected in dromedary camels in Australia (27), strains have been isolated from Africa (9) and the Middle East (7), and seropositive dromedary camels have been found in Asia (28, 29). Phylogenetic analyses show a clustering of MERS-CoV by geographical location (8, 9), and analysis of 219 complete MERS-CoV genomes, which only included one African strain, showed the presence of two clades, with human isolates in both clades (30). Notably, antigenic differences have been reported between S proteins from the Middle East and Africa (8), potentially affecting the efficacy of a vaccine based on S protein. It is important that a MERS-CoV vaccine not only provides homologous protection but also protects against other MERS-CoV strains. We previously showed full protection of ChAdOx1 MERS (based on the Camel/Qatar/2/2014 strain)–vaccinated hDPP4 transgenic mice after heterologous challenge with HCoV-EMC/2012 (15). Here, we repeated this challenge and extended the experiment to include five other MERS-CoV strains. We used strains from KSA, South Korea, and Burkina Faso, obtained from dromedary camels or humans, and isolated between 2012 and 2018 and showed full protection against all strains when mice were vaccinated with ChAdOx1 MERS in a prime-only regimen. Moreover, sera obtained at the day of challenge from rhesus macaques vaccinated with ChadOx1 MERS efficiently neutralized all six MERS-CoV strain, indicating that vaccination with ChAdOx1 MERS can protect against a variety of different MERS-CoV strains. On the basis of these results, future clinical development studies are planned with this ChAdOx1 MERS vaccine, supported by the Coalition for Epidemic Preparedness Innovations.
In conclusion, we show that a single vaccination with ChAdOx1 MERS results in protection against disease progression and virus replication associated with MERS-CoV challenge in the rhesus macaque, and a prime-boost regimen reduced viral replication further. Furthermore, ChAdOx1 MERS vaccination protected against a diverse panel of contemporary MERS-CoV strains in hDPP4 mice. This is the first time that broad protection after a single vaccination has been shown for any MERS-CoV vaccine. Last, ChAdOx1 vaccines can be produced rapidly, have been shown to be safe in human patients, and are protective against MERS-CoV in rhesus macaques and hDPP4 mice. We conclude that the ChAdOx1 platform is ideal for the development of vaccines against novel emerging coronaviruses, such as HCoV-19/SARS-CoV-2.
MATERIALS AND METHODS
Ethics statement
Animal experiment approval was provided by the Institutional Animal Care and Use Committee at Rocky Mountain Laboratories. All animal experiments were executed in an Association for Assessment and Accreditation of Laboratory Animal Care–approved facility by certified staff, following the guidelines and basic principles in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, the U.S. Department of Agriculture, and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Rhesus macaques were housed in individual primate cages allowing social interactions, in a climate-controlled room with a fixed light-dark cycle (12/12 hours). Rhesus macaques were monitored a minimum of twice daily throughout the experiment. Commercial monkey chow, treats, and fruit were provided twice daily by a trained personnel. Water was available ad libitum. Environmental enrichment consisted of a variety of human interaction, commercial toys, videos, and music. The Institutional Biosafety Committee (IBC) approved work with infectious MERS-CoV virus strains under BSL3 conditions. All sample inactivation was performed according to IBC-approved standard operating procedures for the removal of specimens from high containment.
Vaccine generation and production
The S protein gene from MERS-CoV strain Camel/Qatar/2/2014 (GenBank accession no. KJ650098.1) was codon optimized for humans and synthesized by GeneArt (Thermo Fisher Scientific). The synthesized S gene was cloned into a transgene expression plasmid comprising a modified human cytomegalovirus immediate early promoter (CMV promoter) with tetracycline operator sites and the polyadenylation signal from bovine growth hormone. The resulting expression cassette was inserted into the E1 locus of a genomic clone of ChAdOx1 using site-specific recombination (31). The virus was rescued and propagated in T-REx-293 cells (Invitrogen). Purification was by CsCl gradient ultracentrifugation, and the virus was titered, as previously described (32). Doses for vaccination were based on infectious units (IUs) (33).
NHP study
Eighteen adult rhesus macaques (17 males and 1 female) were purchased from Morgan Island and randomly divided into three groups of six animals each. The group 1 was vaccinated with ChAdOx1 MERS at −56 and −28 DPI, the group 2 was vaccinated with ChAdOx1 MERS at −28 DPI, and the group 3 was vaccinated with ChAdOx1 GFP at −56 and −28 DPI. All vaccinations were done with 3.9 × 108 IU per animal per vaccination. Blood samples were obtained before vaccination and 14 days thereafter. Animals were challenged with MERS-CoV strain HCoV-EMC/2012 on 0 DPI with administrations of 4 ml intratracheally, 1 ml intranasally, 1 ml orally, and 1 ml ocularly of 107 TCID50/ml virus solution. Clinical exams were performed on −56, −42, −28, −14, 0, 1, 3, and 5 and 6 DPI; animals were euthanized at 6 DPI. All exams existed of the following: weight and temperature measurements, radiographs, spO2 measurements using pulse oximetry, and blood sampling. BAL was performed on 1, 3, 5, and 6 DPI by insertion of an endotracheal tube and bronchoscope into the trachea, then past the third bifurcation, and subsequent installation of 10 ml of sterile saline. Manual suction was applied to retrieve the BAL sample. Necropsy was performed on 6 DPI. Radiographs were evaluated and scored by a board-certified veterinarian who was blinded to the group assignment of the animals according to the following criteria: 0, normal examination; 1, mild interstitial pulmonary infiltrates; 2, moderate interstitial infiltrates, perhaps with partial cardiac border effacement and small areas of pulmonary consolidation (alveolar patterns and air bronchograms); and 3, pulmonary consolidation as the primary lung pathology, seen as a progression from grade 2 lung pathology (34).
Mouse study
Groups of 10 mice were vaccinated with ChAdOx1 MERS or ChAdOx1 GFP (1 × 108 IU per mouse) intramuscularly 28 days before intranasal inoculation with 104 TCID50 of MERS-CoV. Mice were challenged with one of six MERS-CoV strains: HCoV-EMC/12 (EMC/12, JX869059), Aseer/KSA-Rs924/62015 (KSA/15, KY688119), Korea/Seoul/SNU1-035/2015 (SK/15, KU308549), Riyadh/KSA-18013832/2018 (KSA/18, MN723544), Camel/KSA/KFU-HKU1/2013 (C/KSA/13, KJ650297), or Camel/Burkina Faso/CIRAD-HKU785/2015 (C/BF/15, MG923471). Animals were monitored daily for signs of disease. Four animals were euthanized at 3 DPI, and lung tissue was harvested. The remaining animals were monitored for survival. Animals were euthanized upon reaching >20% of body weight loss or poor body condition.
Cells and virus
EMC/12 was provided by the Erasmus Medical Center, Rotterdam, The Netherlands; KSA/15 and KSA/18 were provided by the CDC (U.S. Centers for Disease Control and Prevention), Atlanta, USA; SK/15 was provided by the Seoul National University, Seoul, South Korea; and C/KSA/13 and C/BF/15 were provided by the Hong Kong University, Pok Fu Lam, Hong Kong. Virus propagation was performed in VeroE6 cells in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% fetal bovine serum (FBS), 1 mM l-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml). VeroE6 cells were maintained in DMEM supplemented with 10% fetal calf serum, 1 mM l-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml).
Virus titration assay
Virus titrations were performed by endpoint titration in VeroE6 cells, which were inoculated with 10-fold serial dilutions of virus. After 1-hour incubation at 37°C and 5% CO2, tissue homogenate dilutions were removed, and cells were washed twice with phosphate-buffered saline (PBS) and incubated in 100 μl of 2% DMEM. Cytopathic effect was scored at 5 DPI, and the TCID50 was calculated from four replicates by the Spearman-Karber method (33, 35).
Virus neutralization assay MERS-CoV
Sera were heat inactivated (30 min, 56°C), and twofold serial dilutions were prepared in 2% DMEM. Hereafter, 100 TCID50 of MERS-CoV was added. After 60-min incubation at 37°C, virus:serum mixture was added to VeroE6 cells and incubated at 37°C and 5% CO2. At 5 DPI, the cytopathic effect was scored. The virus neutralization titer was expressed as the reciprocal value of the highest dilution of the serum, which still inhibited virus replication (33).
Virus neutralization assay ChAdOx1
Chimpanzee adenovirus ChAdOx1-specific neutralizing antibody titers were assessed using a secreted placental alkaline phosphatase (SEAP) quantitation assay. Briefly, GripTite MSR 293 cells (Invitrogen, catalog no. R795-07) were cultured as per manufacturer’s instructions and were seeded at 3 × 104 cells per well in a 96-well plate the day before starting the assay (24 ± 2 hours). Cells were infected with the test sera dilutions (fourfold dilution series) at 1:18, 1:72, 1:288, 1:1152, and 1:4608 in phenol red–free 0% FBS DMEM (Life Technologies, catalog no. 31053028) and the ChAdOx1-SEAP reporter virus in a 1:1 mixture (pre-incubated for 1 hour to allow any neutralization to occur) for 1 hour before replacing with phenol red–free 10% FBS DMEM for a further 24 hours (±2 hours). Sample dilutions were tested in duplicate lanes. For each sample, SEAP concentration was assessed in 50 μl aliquots of culture supernatant, with CPSD as an indicator substrate (Tropix Phospha-Light Chemiluminescent Assay Kit, Life Technologies, catalog no. T1017), using a minor variant of the manufacturer’s instructions; luminescence intensity was measured using a Varioskan Flash luminometer (Thermo Fisher Scientific). Serum dilution neutralization titers were measured by linear interpolation of adjacent values (to 50% inhibition) to determine the serum dilution required to reduce SEAP concentration by 50% compared to wells with virus alone.
RNA extraction and qRT-PCR
Tissues (30 mg) were homogenized in RLT buffer, and RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA was extracted from BAL fluid using the QIAamp Viral RNA kit (Qiagen) on the QIAxtractor. The UpE MERS-CoV (36) or mRNA (37) detection assay was used for the detection of MERS-CoV viral RNA. Five microliters of RNA was tested with the Rotor-Gene probe kit (Qiagen) according to the instructions of the manufacturer. Dilutions of MERS-CoV virus stock with known genome copies were run in parallel. Genome copies were determined using Droplet Digital PCR (Bio-Rad) and the corresponding qRT-PCR.
Enzyme-linked immunosorbent assay
A soluble, trimeric recombinant S protein of MERS-CoV (isolate Ca/Jeddah/D42/2014) incorporating amino acids 1 to 1273 and a C-terminal trimerization domain was produced in Chinese hamster ovary cells (expiCHO; Thermo Fisher Scientific) and purified by immunoaffinity chromatography. MaxiSorp plates (Nunc) were coated overnight at room temperature with 5 μg of S protein per plate in PBS. Plates were blocked with 100 μl of casein (Thermo Fisher Scientific) for 90 min at room temperature. Serum (2× serial dilution in casein starting at 100× dilution) was incubated at room temperature for 2 hours. Antibodies were detected using affinity-purified polyclonal antibody peroxidase–labeled goat anti-monkey IgG (SeraCare, 074-11-021) in casein and 3,3′,5,5′-Tetramethylbenzidine (TMB) two-component peroxidase substrate (SeraCare) and read at 450 nm. All wells were washed three times with PBST (PBS-Tween 0.05%) in between steps. Threshold for positivity was set at 3× optical density (OD) value of negative control (serum obtained from NHPs before the start of the experiment).
Cytokine and chemokine profiles
Samples for analysis of cytokine/chemokine levels were inactivated with γ-radiation (2 mrad) according to the standard operating procedures. Concentrations of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF, interferon-γ, IL-1β, IL-1 receptor antagonist, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17, IL-18 MCP-1, and MIP-1α, MIP-1β, soluble CD40 ligand (sCD40L), TGF-α, tumor necrosis factor–α, and vascular endothelial growth factor were measured on a Bio-Plex 200 instrument (Bio-Rad) using the Non-Human Primate Cytokine MILLIPLEX map 23-plex kit (Millipore) according to the manufacturer’s instructions.
Histology and immunohistochemistry
Necropsies and tissue sampling were performed according to IBC-approved protocols. Lungs were perfused with 10% formalin and processed for histologic review. Harvested tissues were fixed for a minimum of 7 days in 10% neutral-buffered formalin and then embedded in paraffin. Tissues were processed using a VIP-6 Tissue-Tek (Sakura Finetek, USA) tissue processor and embedded in Ultraffin paraffin polymer (Cancer Diagnostics, Durham, NC). Samples were sectioned at 5 μm, and the resulting slides were stained with hematoxylin and eosin. Specific anti-CoV immunoreactivity was detected using MERS-CoV nucleocapsid protein rabbit antibody (Sino Biological Inc.) at a 1:4000. The tissues were processed for immunohistochemistry using the Discovery ULTRA automated IHC/ISH staining instrument (Ventana Medical Systems) with a Discovery Red (Ventana Medical Systems) kit. All tissue slides were evaluated by a board-certified veterinary anatomic pathologist blinded to study group allocations.
Statistical analyses
Tukey’s multiple comparison test or a two-tailed unpaired Student’s t test was conducted to compare differences between vaccine groups and the control group. A Bonferroni correction was used to control the type I error rate for the two comparisons (group 1 versus control and group 2 versus control), and thus, statistical significance was reached at P < 0.025. Spearman’s rank correlation coefficient test was used to interfere correlation.
Supplementary Material
Acknowledgments
We would like to thank K. Chappell, University of Queensland, Australia, for the clamped S protein used in ELISAs, the animal care takers, A. Mora and A. Athman, for assistance with figures, B. Carrasco for preparation of animal studies, and D. Scott, J. Lovaglio, A. Griffin, and K. Cordova for assistance during the animal studies. Funding: This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH (1ZIAAI001179-01) and the Department of Health and Social Care using UK Aid funding managed by the NIHR. S.C.G. is a Jenner investigator. The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health and Social Care. Author contributions: Conceptualization: N.v.D., T.L., S.C.G., and V.J.M.; methodology: N.v.D., E.H., F.F., K.M.-W., T.B., R.J.F., A.O., P.W.H., G.S., N.J.E., M.H.A.C., and T.L.; formal analysis: N.v.D. and G.S.; writing—original draft preparation: N.v.D.; writing—review and editing: T.L., S.C.G., and V.J.M. Competing interests: S.C.G. is a board member of Vaccitech and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines. The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. ChAdOx1 MERS can be provided by the Jenner Institute, University of Oxford pending scientific review and a completed material transfer agreement. Requests for the ChAdOx1 MERS should be submitted to S.C.G. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/24/eaba8399/DC1
REFERENCES AND NOTES
- 1.de Wit E., van Doremalen N., Falzarano D., Munster V. J., SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, Middle East Respiratory Syndrome (2019); www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html.
- 3.WHO, (2020); https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200515-covid-19-sitrep-116.pdf?sfvrsn=8dd60956_2.
- 4.Bin S. Y., Heo J. Y., Song M.-S., Lee J., Kim E.-H., Park S.-J., Kwon H.-i., Kim S. M., Kim Y.-i., Si Y.-J., Lee I.-W., Baek Y. H., Choi W.-S., Min J., Jeong H. W., Choi Y. K., Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in south korea. Clin. Infect. Dis. 62, 755–760 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K. H., Tandi T. E., Choi J. W., Moon J. M., Kim M. S., Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: Epidemiology, characteristics and public health implications. J. Hosp. Infect. 95, 207–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO, Epidemic and Pandemic-prone Disease, (2019); www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-may-2019.html.
- 7.Azhar E. I., El-Kafrawy S. A., Farraj S. A., Hassan A. M., Al-Saeed M. S., Hashem A. M., Madani T. A., Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 370, 2499–2505 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Shirato K., Melaku S. K., Kawachi K., Nao N., Iwata-Yoshikawa N., Kawase M., Kamitani W., Matsuyama S., Tessema T. S., Sentsui H., Middle East respiratory syndrome coronavirus in dromedaries in ethiopia is antigenically different from the Middle East isolate EMC. Front. Microbiol. 10, 1326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu D. K. W., Hui K. P. Y., Perera R., Miguel E., Niemeyer D., Zhao J., Channappanavar R., Dudas G., Oladipo J. O., Traoré A., Fassi-Fihri O., Ali A., Demissié G. F., Muth D., Chan M. C. W., Nicholls J. M., Meyerholz D. K., Kuranga S. A., Mamo G., Zhou Z., So R. T. Y., Hemida M. G., Webby R. J., Roger F., Rambaut A., Poon L. L. M., Perlman S., Drosten C., Chevalier V., Peiris M., MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl. Acad. Sci. U.S.A. 115, 3144–3149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthumani K., Falzarano D., Reuschel E. L., Tingey C., Flingai S., Villarreal D. O., Wise M., Patel A., Izmirly A., Aljuaid A., Seliga A. M., Soule G., Morrow M., Kraynyak K. A., Khan A. S., Scott D. P., Feldmann F., LaCasse R., Meade-White K., Okumura A., Ugen K. E., Sardesai N. Y., Kim J. J., Kobinger G., Feldmann H., Weiner D. B., A synthetic consensus anti–spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 7, 301ra132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W., Bao L., Deng W., Wei Q., Gao G. F., Qin C., Tan W., Recombinant receptor binding domain protein induces partial protective immunity in rhesus macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine 2, 1438–1446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Shi W., Joyce M. G., Modjarrad K., Zhang Y., Leung K., Lees C. R., Zhou T., Yassine H. M., Kanekiyo M., Yang Z.-y., Chen X., Becker M. M., Freeman M., Vogel L., Johnson J. C., Olinger G., Todd J. P., Bagci U., Solomon J., Mollura D. J., Hensley L., Jahrling P., Denison M. R., Rao S. S., Subbarao K., Kwong P. D., Mascola J. R., Kong W.-P., Graham B. S., Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 6, 7712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C., Zheng X., Gai W., Zhao Y., Wang H., Wang H., Feng N., Chi H., Qiu B., Li N., Wang T., Gao Y., Yang S., Xia X., MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget 8, 12686–12694 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alharbi N. K., Padron-Regalado E., Thompson C. P., Kupke A., Wells D., Sloan M. A., Grehan K., Temperton N., Lambe T., Warimwe G., Becker S., Hill A. V. S., Gilbert S. C., ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 35, 3780–3788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munster V. J., Wells D., Lambe T., Wright D., Fischer R. J., Bushmaker T., Saturday G., van Doremalen N., Gilbert S. C., de Wit E., Warimwe G. M., Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2, 28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alharbi N. K., Qasim I., Almasoud A., Aljami H. A., Alenazi M. W., Alhafufi A., Aldibasi O. S., Hashem A. M., Kasem S., Albrahim R., Aldubaib M., Almansour A., Temperton N. J., Kupke A., Becker S., Abu-Obaidah A., Alkarar A., Yoon I.-K., Azhar E., Lambe T., Bayoumi F., Aldowerij A., Ibrahim O. H., Gilbert S. C., Balkhy H. H., Humoral immunogenicity and efficacy of a single dose of ChAdOx1 MERS vaccine candidate in dromedary camels. Sci. Rep. 9, 16292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehy S. H., Duncan C. J., Elias S. C., Collins K. A., Ewer K. J., Spencer A. J., Williams A. R., Halstead F. D., Moretz S. E., Miura K., Epp C., Dicks M. D., Poulton I. D., Lawrie A. M., Berrie E., Moyle S., Long C. A., Colloca S., Cortese R., Gilbert S. C., Nicosia A., Hill A. V., Draper S. J., Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol. Ther. 19, 2269–2276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayton E.-J., Rose A., Ibrahimsa U., Del Sorbo M., Capone S., Crook A., Black A. P., Dorrell L., Hanke T., Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLOS ONE 9, e101591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlan L., Sridhar S., Payne R., Edmans M., Milicic A., Venkatraman N., Lugonja B., Clifton L., Qi C., Folegatti P. M., Lawrie A. M., Roberts R., de Graaf H., Sukhtankar P., Faust S. N., Lewis D. J. M., Lambe T., Hill A., Gilbert S. C., Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine 29, 146–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly C., Swadling L., Capone S., Brown A., Richardson R., Halliday J., von Delft A., Oo Y., Mutimer D., Kurioka A., Hartnell F., Collier J., Ammendola V., Del Sorbo M., Grazioli F., Esposito M. L., Di Marco S., Siani L., Traboni C., Hill A. V., Colloca S., Nicosia A., Cortese R., Folgori A., Klenerman P., Barnes E., Chronic hepatitis C viral infection subverts vaccine-induced T-cell immunity in humans. Hepatology 63, 1455–1470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkie M., Satti I., Minhinnick A., Harris S., Riste M., Ramon R. L., Sheehan S., Thomas Z. M., Wright D., Stockdale L., Hamidi A., O'Shea M. K., Dwivedi K., Behrens H. M., Davenne T., Morton J., Vermaak S., Lawrie A., Moss P., McShane H., A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime – MVA85A boost in healthy UK adults. Vaccine 38, 779–789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatraman N., Ndiaye B. P., Bowyer G., Wade D., Sridhar S., Wright D., Powlson J., Ndiaye I., Dièye S., Thompson C., Bakhoum M., Morter R., Capone S., Del Sorbo M., Jamieson S., Rampling T., Datoo M., Roberts R., Poulton I., Griffiths O., Ballou W. R., Roman F., Lewis D. J. M., Lawrie A., Imoukhuede E., Gilbert S. C., Dieye T. N., Ewer K. J., Mboup S., Hill A. V. S., Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the united kingdom and senegal. J. Infect. Dis. 219, 1187–1197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wit E., Rasmussen A. L., Falzarano D., Bushmaker T., Feldmann F., Brining D. L., Fischer E. R., Martellaro C., Okumura A., Chang J., Scott D., Benecke A. G., Katze M. G., Feldmann H., Munster V. J., Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 110, 16598–16603 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudas G., Carvalho L. M., Rambaut A., Bedford T., MERS-CoV spillover at the camel-human interface. eLife 7, e31257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J., Alshukairi A. N., Baharoon S. A., Ahmed W. A., Bokhari A. A., Nehdi A. M., Layqah L. A., Alghamdi M. G., Al Gethamy M. M., Dada A. M., Khalid I., Boujelal M., Al Johani S. M., Vogel L., Subbarao K., Mangalam A., Wu C., Ten Eyck P., Perlman S., Zhao J., Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci. Immunol. 2, eaan5393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adney D. R., Letko M., Ragan I. K., Scott D., van Doremalen N., Bowen R. A., Munster V. J., Bactrian camels shed large quantities of Middle East respiratory syndrome coronavirus (MERS-CoV) after experimental infection. Emerg. Microbes Infect. 8, 717–723 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crameri G., Durr P. A., Barr J., Yu M., Graham K., Williams O. J., Kayali G., Smith D., Peiris M., Mackenzie J. S., Wang L. F., Absence of MERS-CoV antibodies in feral camels in Australia: Implications for the pathogen’s origin and spread. One Health 1, 76–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islam A., Epstein J. H., Rostal M. K., Islam S., Rahman M. Z., Hossain M. E., Uzzaman M. S., Munster V. J., Peiris M., Flora M. S., Rahman M., Daszak P., Middle East respiratory syndrome coronavirus antibodies in Dromedary Camels, Bangladesh, 2015. Emerg. Infect. Dis. 24, 926–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saqib M., Sieberg A., Hussain M. H., Mansoor M. K., Zohaib A., Lattwein E., Muller M. A., Drosten C., Corman V. M., Serologic evidence for MERS-CoV infection in Dromedary Camels, Punjab, Pakistan, 2012–2015. Emerg. Infect. Dis. 23, 550–551 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau S. K. P., Wong A. C. P., Lau T. C. K., Woo P. C. Y., Molecular evolution of MERS coronavirus: Dromedaries as a recent intermediate host or long-time animal reservoir? Int. J. Mol. Sci. 18, E2138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dicks M. D., Spencer A. J., Edwards N. J., Wadell G., Bojang K., Gilbert S. C., Hill A. V., Cottingham M. G., A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLOS ONE 7, e40385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottingham M. G., Carroll F., Morris S. J., Turner A. V., Vaughan A. M., Kapulu M. C., Colloca S., Siani L., Gilbert S. C., Hill A. V., Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors. Biotechnol. Bioeng. 109, 719–728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Doremalen N., Lambe T., Sebastian S., Bushmaker T., Fischer R., Feldmann F., Haddock E., Letko M., Avanzato V. A., Rissanen I., LaCasse R., Scott D., Bowden T. A., Gilbert S., Munster V., A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLOS Negl. Trop. Dis. 13, e0007462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brining D. L., Mattoon J. S., Kercher L., LaCasse R. A., Safronetz D., Feldmann H., Parnell M. J., Thoracic radiography as a refinement methodology for the study of H1N1 influenza in cynomologus macaques (Macaca fascicularis). Comp. Med. 60, 389–395 (2010). [PMC free article] [PubMed] [Google Scholar]
- 35.Karber G., Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn. Schmiedebergs. Arch. Exp. Pathol. Pharmakol. 162, 480–483 (1931). [Google Scholar]
- 36.Corman V. M., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M., van Boheemen S., Gopal R., Ballhause M., Bestebroer T. M., Muth D., Müller M. A., Drexler J. F., Zambon M., Osterhaus A. D., Fouchier R. M., Drosten C., Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 17, 20285 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Coleman C. M., Frieman M. B., Growth and quantification of MERS-CoV infection. Curr. Protoc. Microbiol. 37, 15E.2.1–15E.2.9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusof M. F., Queen K., Eltahir Y. M., Paden C. R., Al Hammadi Z., Tao Y., Li Y., Khalafalla A. I., Shi M., Zhang J., Mohamed M., Abd Elaal Ahmed M. H., Azeez I. A., Bensalah O. K., Eldahab Z. S., Al Hosani F. I., Gerber S. I., Hall A. J., Tong S., Al Muhairi S. S., Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg. Microbes Infect. 6, e101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/24/eaba8399/DC1


