Abstract
Objective
To assess the outcomes and toxic effects of 5-day actinomycin D (Act-D) salvage therapy and to explore the predictors of Act-D resistance in patients with low-risk gestational trophoblastic neoplasia (GTN)who failed 5-day methotrexate (MTX) chemotherapy.
Methods
This retrospective study analyzed patients with low-risk GTN administered Act-D salvage therapy after failing MTX chemotherapy at Women's Hospital, School of Medicine Zhejiang University between January 2000 and December 2015. The clinical parameters of these patients were collected and analyzed.
Results
The final analysis included 89 cases. Of these, 73 cases (82.02%) responded to salvage Act-D. The remaining 16 resistant cases were switched to etoposide, MTX, Act-D/cyclophosphamide, and vincristine chemotherapy and achieved complete remission. Serum human chorionic gonadotrophin levels before Act-D salvage therapy (hCGAct-D)in the Act-D-resistant cases were significantly higher than those in the Act-D responders (median 605 vs. 103 IU/L, p=0.009). However, the range of hCGAct-D values in Act-D responders was wider than that in Act-D-resistant cases (5.76–16,664 IU/L vs. 11.43–6,732 IU/L). Thus, assigning a general cut-off value was difficult considering the individual setting. Except for 2 cases requiring other salvage regimens due to Act-D toxicity, 97.80% of cases (89/91) tolerated the toxicity. During at least 1-year follow-up, the survival rate was 100.00% and no case developed recurrence.
Conclusion
Based on the good therapeutic effect and tolerable toxicity, we recommend Act-D salvage therapy for all patients with low-risk GTN who fail primary MTX chemotherapy. The higher serum hCG levels before Act-D salvage therapy may be associated with resistance to this treatment.
Keywords: Gestational Trophoblastic Neoplasia, Methotrexate, Salvage Therapy, Actinomycin D
INTRODUCTION
Gestational trophoblastic neoplasia (GTN) is a rare malignancy that can be cured by cytotoxic chemotherapy. The International Federation of Gynecology and Obstetrics (FIGO) system recommends single-agent chemotherapy for low-risk GTN patients (FIGO prognostic score ≤6) and multi-agent chemotherapy for high-risk GTN patients (FIGO prognostic score ≥7). Methotrexate (MTX) as a first-line treatment remains the most commonly prescribed drug for low-risk GTN patients [1,2,3]. However, 4.00%–46.73% of patients develop resistance to MTX [1,3,4,5,6,7,8,9], while 1.41%–3.69% of patients experience intolerable toxicity to MTX [1,2,10,11], requiring salvage chemotherapy or even surgery [12].
Various salvage regimens for cases of primary MTX failure have been described in the literature, including single-agent pulsed actinomycin D (Act-D) [13,14]; 5-day Act-D [1,3,4,7,15,16]; etoposide and Act-D (EA) [17,18]; etoposide, MTX, Act-D/cyclophosphamide, and vincristine (EMA-CO) [1,3,15]; and bleomycin, etoposide and cisplatin (BEP) [19]. Among these regimens, the use of etoposide in multi-agent chemotherapy (e.g., EMA-CO, EA, and BEP) increases the risk of secondary tumors such as leukemia, melanoma, breast cancer, and colon cancer [20]. EMA-CO treatment also increases the risk of early menopause [21]. Thus, the Act-D regimen is currently preferred due to its simplicity, limited short- and long-term toxicity, and low risk of carcinogenicity [22,23,24].
However, 5.21%–25.00% of MTX-resistant patients develop Act-D resistance [3,7,14,15,16] and switch to multi-agent chemotherapy. Some researchers recommended that patients with high levels of serum human chorionic gonadotropin (hCG) at the time of MTX failure be treated directly with multi-agent chemotherapy to reduce drug resistance and shorten the chemotherapy cycle. However, the definition of high serum hCG level is unclear. In the current literature, high serum hCG levels range from 100–1,000 IU/L [3,14,15,16]. Identification of an accurate threshold is necessary to determine whether to administer a single-agent Act-D or multiple-agent regimen.
Our hospital is a tertiary medical institute and one of the GTN centers in China. We have previously reported on the treatment and management of GTN [4,25,26,27,28,29,30], including the reasons for treatment failure in low-risk GTN patients administered initial single-agent MTX chemotherapy [4,25,26]. The present study describes our 16-year experience in treating low-risk GTN patients who failed MTX chemotherapy. Our purpose was to assess the outcomes and side effects of the 5-day Act-D salvage therapy after MTX failure and to explore predictors of Act-D resistance.
MATERIALS AND METHODS
This retrospective cohort study included low-risk GTN patients who failed primary MTX chemotherapy at the Women's Hospital, School of Medicine Zhejiang University between January 2000 and December 2015. This study was approved by the Ethics Committee of Women's Hospital, School of Medicine Zhejiang University (09/27/2018, No.20180106). All patients included in this study were anonymized.
We searched the clinical records in the Medical Record Review System. Our recruitment criteria were: 1) FIGO prognostic score ≤6. 2) First-line regimen of MTX (0.4 mg/kg per day, maximum 25 mg) injected intramuscularly or intravenously injection for 5 days every other week. 3) Patients switched to 5-day Act-D salvage regimen (10 μg/kg per day, intravenous injection, for 5 days every 2 weeks) for MTX resistance or intolerable toxicity. 4) Patients completing the entire treatment in our hospital. FIGO prognostic score and stage were assessed according to the FIGO 2000 statement [26].
The following clinical information was recorded for each subject: 1) Clinical characteristics: age, antecedent pregnancy, interval months from antecedent pregnancy, stage, and FIGO prognostic score. 2) Imaging exams including pelvic ultrasound, chest X-ray, chest computed tomography (CT), abdominal and brain CT, or magnetic resonance imaging: detectable lesions, maximum lesion diameters, and number of metastases. 3) Therapeutic regimen: number of MTX cycles, the reasons switching to second-line Act-D or/and other salvage regimens, and treatment outcome. 4) Serum hCG monitoring before each chemotherapy cycle. 5) Chemotherapy-related toxicity: oral mucositis, alopecia, nausea, vomiting, complete blood cell count, platelet count, and renal and liver function tests (serum creatinine, serum alanine aminotransferase, aspartate aminotransferase, and bilirubin).
The drug resistance criteria were: serum hCG levels not showing a logarithmical decline, remaining at a plateau level, or increasing during 2 successive chemotherapy cycles or imaging files indicating that the tumor size did not decrease or increase or that new lesions appeared [31]. The trends in hCG levels were classified as elevation and declination. An elevation in hCG level was defined as an increase in serum hCG level during 2 successive MTX chemotherapy cycles, while the opposite situation was defined as a declination. Drug toxicity was assessed in each cycle according to World Health Organization criteria [32]. First-line MTX chemotherapy was stopped in the cases of drug resistance, severe side effects, or intolerable toxicity. Complete remission was defined as cases in which the hCG level remained normal within 3 months after treatment. To determine recurrence and survival, we performed a follow-up at least 1 year.
The normality of all continuous data was tested by 1-sample Kolmogorov–Smirnov tests. If the data were normally distributed, t-tests were used to compare means between the 2 groups. Otherwise, nonparametric Wilcoxon-Mann–Whitney tests were used. The heterogeneity of all distributions was assessed by χ2 or Fisher's exact tests. Receiver operating characteristic (ROC) curves were used to test the discriminating potential of important factors identified in the above-described comparative analysis. An area under the curve (AUC) with a lower 95% confidence interval (CI) of >0.5 was considered significantly discriminant. The p<0.05 (2-tailed) was considered statistically significant. All analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA) on Windows XP (Microsoft Corp., Redmond, WA, USA).
RESULTS
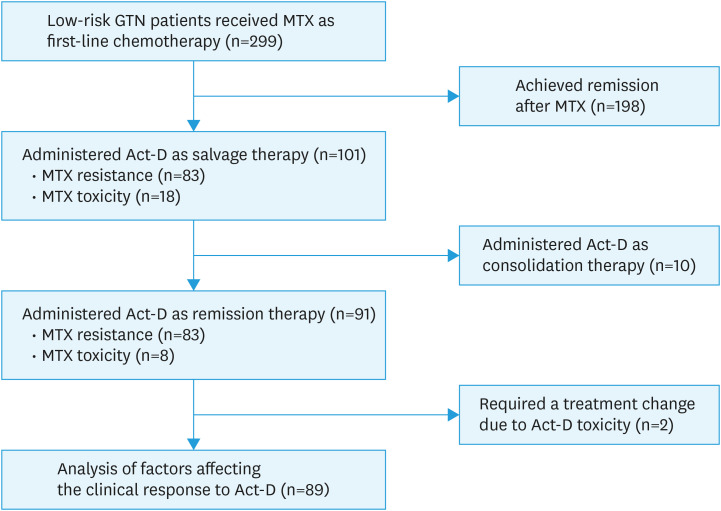
At our center, a total of 299 low-risk GTN patients received a 5-day MTX regimen as first-line chemotherapy between January 2000 and December 2015. Among these patients, 101 cases switched to Act-D as second-line chemotherapy, including 83 cases developing MTX resistance and 18 cases with intolerable toxicity. Except for 10 cases administered Act-D as consolidation therapy and 2 cases that stopped Act-D due to intolerable toxicity, 89 patients were included in the final analysis. Fig. 1 shows the flow chart of subject inclusion.
Fig. 1. Flowchart of the inclusion of patients with low-risk GTN administered Act-D salvage therapy after failing primary MTX chemotherapy.
Act-D, actinomycin D; GTN, gestational trophoblastic neoplasia; MTX, methotrexate.
Table 1 shows the clinical characters of 89 patients administered Act-D salvage therapy after MTX failure. The median patient age was 28 years, ranging from 16 to 50 years. The response rate for Act-D was 82.02% (73/89). Sixteen cases with Act-D resistance were switched to multiple-agent EMA-CO chemotherapy. All patients continued to receive at least 2 cycles of consolidation chemotherapy after hCG levels decreased to normal (<5.3 IU/L) and were defined as achieving complete remission. Sixty-six cases were monitored for 1–16 years after complete remission. No case of recurrence was observed and the overall survival rate was 100%.
Table 1. Factors associated with resistance to Act-D salvage therapy for low-risk GTN.
| Characteristics | Response | Resistance | p-value | |
|---|---|---|---|---|
| No. of patients | 73 (82.02) | 16 (17.98) | ||
| Age (yr) | 0.114 | |||
| <40 | 60 (78.95) | 16 (21.05) | ||
| ≥40 | 13 (100) | 0 (0.00) | ||
| Antecedent pregnancy | 0.409 | |||
| Mole | 65 (83.33) | 13 (16.67) | ||
| Abortion or term | 8 (72.73) | 3 (27.27) | ||
| Interval time from the end of antecedent pregnancy to treatment (mo) | 0.186 | |||
| <4 | 65 (84.42) | 12 (15.58) | ||
| 4–6 | 5 (71.43) | 2 (28.57) | ||
| ≥7 | 3 (60.00) | 2 (40.00) | ||
| Size of the largesttumor (cm) | 0.363 | |||
| <3 | 53 (82.81) | 11 (17.19) | ||
| ≥3 and <5 | 17 (85.00) | 3 (15.00) | ||
| ≥5 | 3 (60.00) | 2 (40.00) | ||
| No. of metastases | 1.000 | |||
| 0 | 54 (81.82) | 12 (18.18) | ||
| 1–4 | 17 (80.95) | 4 (19.05) | ||
| ≥5 | 2 (100.00) | 0 (0.00) | ||
| FIGO stage | 1.000 | |||
| I | 17 (80.95) | 4 (19.05) | ||
| II–III | 56 (82.35) | 12 (17.65) | ||
| FIGO prognostic score | 0.401* | |||
| 0–2 | 40 (86.96) | 6 (13.04) | ||
| 3–4 | 24 (75.00) | 8 (25.00) | ||
| 5–6 | 9 (81.82) | 2 (18.18) | ||
| hCGMTX (IU/L) | 3,597 (50.42–183,505) | 13,360 (159.9–71,749) | 0.262 | |
| hCGAct-D (IU/L) | 103 (5.76–16,664) | 605 (11.43–6,732) | 0.009 | |
| Trend in hCG during MTX chemotherapy† | 1.000 | |||
| Elevation | 11 (84.62) | 2 (15.38) | ||
| Declination | 62 (81.58) | 14 (18.42) | ||
| No. of MTX chemotherapy courses | 3 (1-6) | 2.5 (2-6) | 0.154 | |
| Reason for switching to Act-D | 1.000 | |||
| MTX resistance | 67 (81.71) | 15 (18.29) | ||
| MTX toxicity | 6 (85.71) | 1 (14.29) | ||
Continuous data were expressed as medians (range) and categorical data as absolute values (%).
Act-D, actinomycin D; FIGO, International Federation of Gynecology and Obstetrics; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotrophin; hCGAct-D, serum hCG level before Act-D salvage therapy; hCGMTX, serum hCG level before primary MTX therapy; MTX, methotrexate.
*The p-value was calculated using χ2 test, the others were calculated using Fisher exact tests since more than 20% of cells had expected frequencies <5; †An elevation in hCG level referred to an increase in serum hCG level during 2 successive MTX chemotherapy cycles, while the opposite was defined as a declination in hCG level.
We compared the clinical features of Act-D-resistant cases to those of Act-D responders (Table 1). The results showed that the serum hCG levels before Act-D salvage therapy (hCGAct-D) were significantly higher in the Act-D-resistant cases than those in the Act-D responders (median 605 vs. 103 IU/L, p=0.009). However, other factors, such as age, FIGO prognostic score, and serum hCG level before primary MTX therapy, did not show significant differences.
The trends in serum hCG levels during primary MTX therapy did not differ significantly between the 2 groups, although the FIGO statement indicated that elevation in hCG level might be associated with Act-D resistance [33]. In our 16-year data, while 13/89 patients (14.61%) had elevated serum hCG levels during primary MTX chemotherapy, most (84.62%, 11/13) responded to Act-D. Even the case with the largest increase in serum hCG level (from 1,517 to 12,630 IU/L) in successive MTX cycles still responded to Act-D salvage therapy. Furthermore, the trend in serum hCG level during primary MTX therapy was not significantly associated with age, FIGO prognostic score, number of Act-D cycles, or other clinical factors (Table 2).
Table 2. Factors associated with trends in serum hCG levels during primary MTX chemotherapy.
| Characteristics | Elevation | Declination | p-value | |
|---|---|---|---|---|
| No. of patients | 13 (14.61) | 76 (85.39) | - | |
| Age (yr) | 0.395 | |||
| <40 | 10 (13.16) | 66 (86.84) | ||
| ≥40 | 3 (23.08) | 10 (76.92) | ||
| Antecedent pregnancy | 0.199 | |||
| Mole | 10 (12.82) | 68 (87.18) | ||
| Abortion or term | 3 (27.27) | 8 (72.73) | ||
| Interval time from the end of antecedent pregnancy to treatment (mo) | 0.078 | |||
| <4 | 9 (11.69) | 68 (88.31) | ||
| 4–6 | 2 (28.57) | 5 (71.43) | ||
| ≥7 | 2 (40.00) | 3 (60.00) | ||
| Size of the largest tumor (cm) | 0.663 | |||
| <3 | 11 (17.19) | 53 (82.81) | ||
| ≥3 and <5 | 2 (10.00) | 18 (90.00) | ||
| ≥5 | 0 (0.00) | 5 (100.00) | ||
| No. of metastases | 1.000 | |||
| 0 | 10 (15.15) | 56 (84.85) | ||
| 1–4 | 3 (14.29) | 18 (85.71) | ||
| ≥5 | 0 (0.00) | 2 (100.00) | ||
| FIGO stage | 0.496 | |||
| I | 4 (19.05) | 17 (80.95) | ||
| II–III | 9 (13.24) | 59 (86.76) | ||
| FIGO prognostic score | 0.485 | |||
| 0–2 | 6 (13.04) | 40 (86.96) | ||
| 3–4 | 4 (12.50) | 28 (87.50) | ||
| 5–6 | 3 (27.27) | 8(72.73) | ||
| hCGMTX (IU/L) | 2,005 (280–183,505) | 5,827 (50.42–84,783) | 0.457 | |
| No. of MTX chemotherapy courses | 3 (2–6) | 3 (1–6) | 0.479 | |
| hCGAct-D (IU/L) | 156 (6.4–12,630) | 149 (5.76–16,664) | 0.794 | |
| No. of Act-D chemotherapy courses | 5 (3–8) | 4 (1–9) | 0.243 | |
| Reason for switching to Act-D | 0.588 | |||
| MTX resistance | 13 (15.85) | 69 (84.15) | ||
| MTX toxicity | 0 (0.00) | 7 (100.00) | ||
| Treatment outcome | 1.000 | |||
| Act-D response | 11 (15.07) | 62 (84.93) | ||
| Act-D resistance | 2 (12.50) | 14 (87.50) | ||
Continuous data were expressed as medians (range) and categorical data as absolute values (%). P-values were calculated using Fisher exact tests since more than 20% of cells had expected frequencies of <5 in each factor.
Act-D, actinomycin D; FIGO, International Federation of Gynecology and Obstetrics; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotrophin; hCGAct-D, serum hCG level before Act-D salvage therapy; hCGMTX, serum hCG level before primary MTX therapy; MTX, methotrexate.
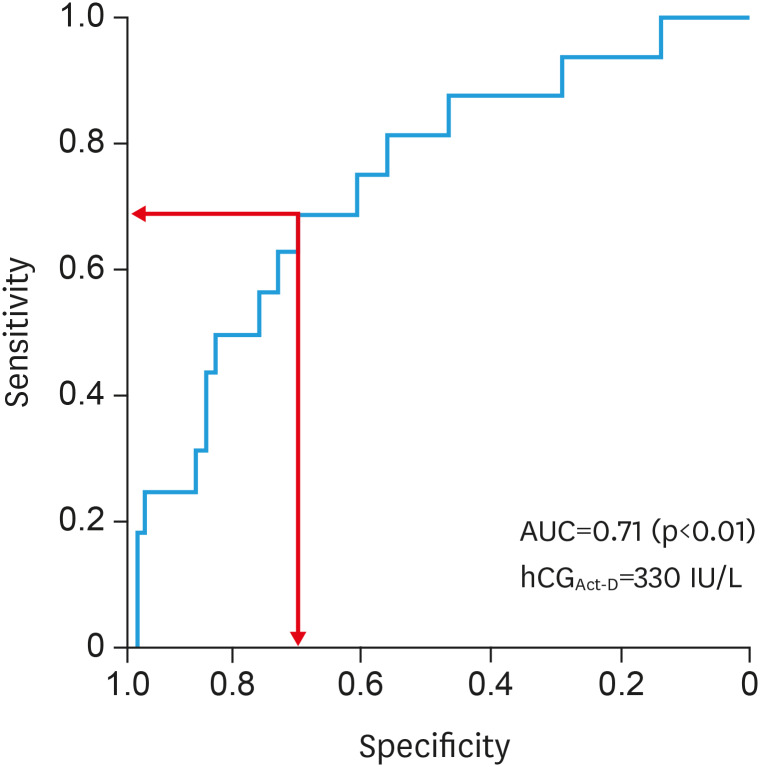
Thus, hCGAct-D was the only factor associated with Act-D resistance in our study. ROC curve analysis showed an AUC of hCGAct-D of 0.71 (95% CI, 0.58–0.84), indicating its discriminatory potential (Fig. 2). The optimal hCGAct-D cut-off value for Act-D resistance was 330 IU/L, with 68.80% sensitivity and 69.90% specificity. Patients with hCGAct-D ≥330 IU/L had a 5-fold higher risk of Act-D resistance compared to that in cases with hCGAct-D <330 IU/L (risk ratio [RR]=5.10; 95% CI=1.58–16.42; p=0.004).
Fig. 2. ROC curve of hCGAct-D as a predictor for resistance to Act-D salvage therapy. The hCGAct-D has an AUC of 0.71 (95% confidence interval, 0.58–0.84). A cut-off of 329.3 IU/L has a 68.80% sensitivity and 69.90% specificity for the highest Youden's index (indicated by arrows). Thus, we selected 330 IU/L as the optimal cut-off.
Act-D, actinomycin D; AUC, area under the ROC curve; hCGAct-D, serum hCG level before Act-D salvage therapy; ROC, receiver operating characteristic.
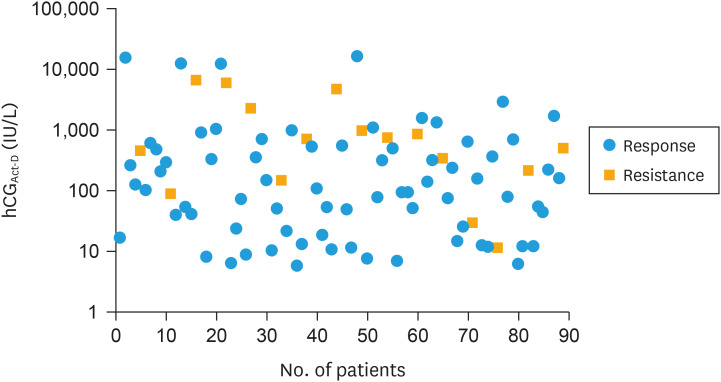
However, the scatter diagram showed that the range of hCGAct-D values in Act-D responders was wider than that in the Act-D-resistant cases (5.76–16,664 IU/L vs. 11.43–6,732 IU/L, Fig. 3). Most (71.43%, 10/14) cases with high hCGAct-D level (>1,000 IU/L) responded to Act-D. Therefore, hCGAct-D values did not provide overwhelming evidence to predict whether individual cases would develop Act-D resistance.
Fig. 3. Scatter plot of hCGAct-D in Act-D responders and Act-D-resistant cases. The y-axis shows the log-hCG values due to their wide range of values (5.76–16,664 IU/L) and 84.27% of cases with values under 1,000 IU/L. The scatter spot shows the hCGAct-D for all patients and the overlap of hCGAct-D between the Act-D-resistant cases and responders.
Act-D, actinomycin D; hCGAct-D, serum hCG level before Act-D salvage therapy.
Except for 2 cases requiring other salvage regimens due to Act-D toxicity (Fig. 1), nearly all patients (97.80%, 89/91) tolerated the toxicity. Assessment of Act-D side effects for each cycle revealed that 59.55% of patients (53/89) experienced mild (grade I or II) chemotherapy-related toxicity. Neutropenia occurred at the highest frequency, developing at grade IV and III in 6.74% (6/89) and 23.60% (21/89) of patients. All patients were administered granulocyte colony-stimulating factor instead of stopping Act-D salvage therapy (Table 3). Patients with mild to moderate stomatitis were successfully treated with various “stomatitis cocktails.” No grade III hepatotoxicity related to Act-D salvage therapy was observed. Grade III nausea/vomiting was not a common side effect.
Table 3. Chemotherapy-related toxicity* of salvage therapy with Act-D (n=89).
| Toxicity | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Neutropenia | 27 (30.34) | 21 (23.60) | 21 (23.60) | 6 (6.74) |
| Hepatotoxicity | 42 (47.19) | 10 (11.24) | 1 (1.12) | NA† |
| Stomatitis | 17 (19.10) | 18 (20.22) | 9 (10.11) | NA† |
| Alopecia | 37 (41.57) | 14 (15.73) | 13 (14.61) | NA† |
| Nausea/vomiting | 29 (32.58) | 45 (50.56) | 7 (7.87) | NA† |
Categorical data are expressed as absolute values (%).
Act-D, actinomycin D; NA, not available.
*The number of patients was defined as those experiencing the maximum observed grade of toxicity; †No grade IV toxicity was observed.
DISCUSSION
This study reported our 16-year experience in managing low-risk GTN patients who failed primary MTX chemotherapy. These patients were administered an Act-D salvage regimen and 82.02% responded to 5-day Act-D. The response rate was consistent with that in our previous paper, which showed 81.25% response rate in 16 patients from 1999 to 2004 [25]. Both datasets stated the appropriate therapeutic effect of the 5-day Act-D salvage therapy on the low-risk GTN patients with MTX failure. To our knowledge, the number of subjects in our study was larger than that in many existing studies on 5-day Act-D salvage therapy in low-risk GTN [3,6,7,15,16,34].
Meanwhile, around 20% of cases in the present study developed Act-D resistance after MTX resistance. Repeated drug resistance prolongs the treatment period, delays pregnancy, and increases psychological stress. Several studies have explored predictors of resistance to Act-D salvage therapy. Kang et al. [35] analyzed 38 cases, reporting response rates of 89.29% (25/28) for cases with hCGAct-D <1,000 IU/L, 42.86% (3/7) for 1,000–10,000 IU/L, and 100% (0/3) for ≥10,000 IU/L. In our study, hCGAct-D levels in the Act-D-resistant cases were significantly higher than those in Act-D responders (p=0.009). Thus, the possibility of resistance to Act-D salvage therapy increased with increasing serum hCGAct-D level. Gynecological oncologists agree with prescribing Act-D as salvage therapy for patients with low hCGAct-D and EMA-CO for those with high hCGAct-D [3,14,15,16].
However, the definition of high hCGAct-D remains controversial, varying from 100 to 1,000 IU/L in the literature [3,14,15,16]. In our study, the optimal cut-off value for Act-D resistance was 330 IU/L with 68.80% sensitivity, 69.90% specificity, and RR of 5.1. However, the range of hCGAct-D values in Act-D responders was wider than that in the Act-D-resistant cases. Only 33.33% of patients (11/33) with hCGAct-D levels over the cut-off value showed Act-D resistance, while 28.57% of patients (4/14) showed Act-D resistance with hCGAct-D ≥1,000 IU/L, the threshold proposed by Prouvot et al. [16]. Although the risk of drug resistance increased with increasing hCGAct-D level, resistance developed in a small proportion of the population. Thus, it is reckless to use multiple agent regimens for the small number of potentially drug-resistant cases, which might sacrifice more patients to tolerate the side effects of multiple drugs. Therefore, the predictive power of hCGAct-D to identify individuals with Act-D resistance was limited.
The 2018 FIGO cancer report recommended the initiation of multiple agents in cases with significant elevation in hCG level during the first single-agent treatment [33]. However, this report did not give a precise numerical definition of “significant”. Furthermore, other literature also failed to define this term in this context [33,36]. In the 16 years of data of our institute, 14.61% of cases (13/89) administered Act-D salvage treatment experienced elevated hCG levels during MTX chemotherapy. While we have identified an association between elevation in hCG level during MTX chemotherapy and Act-D resistance, additional studies with more patients are required to reach clear conclusions regarding this relationship.
In terms of drug side effects, most of the patients in this study experienced mild chemotherapy-related toxicity. In comparison, half of the ultra-high-risk GTN patients (50.00%, 12/24) administered multiple-agent EMA-CO chemotherapy in our center experienced severe neutropenia (grade III or IV) during the same period [29]. Among these patients, 37.50% (9/24) stopped cyclophosphamide and vincristine (CO) chemotherapy for severe adverse side effects [29]. These results indicate that the toxicity of Act-D was much lower than that of EMA-CO [16,21]. No cases of relapse were observed in follow-ups performed at least 1 year. The overall survival rate was 100.00%. Because only a few cases developed resistance to Act-D salvage therapy, unless strongly indicated, we do not suggest the initial use of EMA-CO protocols due to the risk of serious side effects.
One of the limitations of this study was that we did not compare cases directly treated by EMA-CO to those subsequently treated by Act-D and EMA-CO after MTX failure. This comparison is difficult to perform in clinical studies. Moreover, data on long-term response were unavailable for the 23 patients (23/89, 25.84%) lost to follow-up. Finally, we retrieved data retrospectively, resulting in a potential bias that may have underestimated some of the toxicities.
In conclusion, the predictive value of hCGAct-D for Act-D salvage therapy could not be determined, although the hCGAct-D level was higher in resistant cases than that in responders. Based on the good therapeutic effect and tolerable toxicity, we recommended 5-day Act-D salvage therapy for all patients who fail primary MTX chemotherapy. Additional studies are required to determine the proper criteria for regimen selection.
Footnotes
Funding: This work was supported by Young Scientists Fund of the National Natural Science Foundation of China (grant number: 8160515, grant recipient: Jiale Qin), Zhejiang Province Medical and Health Technology Projects (grant number: 2016KYB164; 2019KY092; 2016KYA123, grant recipient: Jiale Qin; Jiale Qin; Lili Chen), Center for Uterine Cancer Diagnosis & Therapy Research of Zhejiang Province (grant number: JBZX-201803, grant recipient: Weiguo Lu), Commonwealth Technology Research and Social Development Project for Science Technology Development of Zhejiang Province (grant number: 2013C33151, grant recipient: Lili Chen), and Education Department of Zhejiang Province (grant number: Y201838996, grant recipient: Xiaodong Wu).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: W.X., L.W.
- Data curation: W.X., S.T.
- Formal analysis: W.X., Q.J.
- Funding acquisition: W.X., Q.J.
- Methodology: S.T., F.W., C.L.
- Project administration: F.W., C.L., X.X.
- Resources: S.T., F.W.
- Software: S.T., F.W., C.L.
- Supervision: Q.J., X.X., L.W.
- Validation: Q.J., L.W.
- Writing - original draft: W.X., Q.J.
- Writing - review & editing: X.X., L.W.
References
- 1.Chalouhi GE, Golfier F, Soignon P, Massardier J, Guastalla JP, Trillet-Lenoir V, et al. Methotrexate for 2000 FIGO low-risk gestational trophoblastic neoplasia patients: efficacy and toxicity. Am J Obstet Gynecol. 2009;200:643.e1–643.e6. doi: 10.1016/j.ajog.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kang WD, Choi HS, Kim SM. Weekly methotrexate (50mg/m2) without dose escalation as a primary regimen for low-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2010;117:477–480. doi: 10.1016/j.ygyno.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Sita-Lumsden A, Short D, Lindsay I, Sebire NJ, Adjogatse D, Seckl MJ, et al. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000–2009. Br J Cancer. 2012;107:1810–1814. doi: 10.1038/bjc.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu XD, Chen LL, Shen T, Chen SS, Chen QQ, Qin JL, et al. Curative effects and influenced factors of primary chemotherapy with single-agent methotrexate on low-risk gestational trophoblastic neoplasia. Zhonghua Yi Xue Za Zhi. 2017;97:1769–1772. doi: 10.3760/cma.j.issn.0376-2491.2017.23.003. [DOI] [PubMed] [Google Scholar]
- 5.Matsui H, Iitsuka Y, Seki K, Sekiya S. Comparison of chemotherapies with methotrexate, VP-16 and actinomycin-D in low-risk gestational trophoblastic disease. Remission rates and drug toxicities. Gynecol Obstet Invest. 1998;46:5–8. doi: 10.1159/000009987. [DOI] [PubMed] [Google Scholar]
- 6.Hoekstra AV, Lurain JR, Rademaker AW, Schink JC. Gestational trophoblastic neoplasia: treatment outcomes. Obstet Gynecol. 2008;112:251–258. doi: 10.1097/AOG.0b013e31817f58ae. [DOI] [PubMed] [Google Scholar]
- 7.Chapman-Davis E, Hoekstra AV, Rademaker AW, Schink JC, Lurain JR. Treatment of nonmetastatic and metastatic low-risk gestational trophoblastic neoplasia: factors associated with resistance to single-agent methotrexate chemotherapy. Gynecol Oncol. 2012;125:572–575. doi: 10.1016/j.ygyno.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a gynecologic oncology group study. J Clin Oncol. 2011;29:825–831. doi: 10.1200/JCO.2010.30.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulia S, Bajpai J, Gupta S, Maheshwari A, Deodhar K, Kerkar RA, et al. Outcome of gestational trophoblastic neoplasia: experience from a tertiary cancer centre in India. Clin Oncol (R Coll Radiol) 2014;26:39–44. doi: 10.1016/j.clon.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Abrão RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecol Oncol. 2008;108:149–153. doi: 10.1016/j.ygyno.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JP, Lurain JR. Treatment of low-risk metastatic gestational trophoblastic tumors with single-agent chemotherapy. Am J Obstet Gynecol. 1996;174:1917–1923. doi: 10.1016/s0002-9378(96)70229-6. [DOI] [PubMed] [Google Scholar]
- 12.Alazzam M, Tidy J, Osborne R, Coleman R, Hancock BW, Lawrie TA. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016:CD008891. doi: 10.1002/14651858.CD008891.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covens A, Filiaci VL, Burger RA, Osborne R, Chen MD Gynecologic Oncology Group. Phase II trial of pulse dactinomycin as salvage therapy for failed low-risk gestational trophoblastic neoplasia: a Gynecologic Oncology Group study. Cancer. 2006;107:1280–1286. doi: 10.1002/cncr.22118. [DOI] [PubMed] [Google Scholar]
- 14.Winter MC, Tidy JA, Hills A, Ireson J, Gillett S, Singh K, et al. Risk adapted single-agent dactinomycin or carboplatin for second-line treatment of methotrexate resistant low-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2016;143:565–570. doi: 10.1016/j.ygyno.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 15.McNeish IA, Strickland S, Holden L, Rustin GJ, Foskett M, Seckl MJ, et al. Low-risk persistent gestational trophoblastic disease: outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol. 2002;20:1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 16.Prouvot C, Golfier F, Massardier J, You B, Lotz JP, Patrier S, et al. Efficacy and safety of second-line 5-day dactinomycin in case of methotrexate failure for gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2018;28:1038–1044. doi: 10.1097/IGC.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 17.Dobson LS, Lorigan PC, Coleman RE, Hancock BW. Persistent gestational trophoblastic disease: results of MEA (methotrexate, etoposide and dactinomycin) as first-line chemotherapy in high risk disease and EA (etoposide and dactinomycin) as second-line therapy for low risk disease. Br J Cancer. 2000;82:1547–1552. doi: 10.1054/bjoc.2000.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevado-Gammad MS, Soriano-Estrella AL. Etoposide-actinomycin as salvage regimen for the treatment of nonmetastatic and low-risk metastatic gestational trophoblastic neoplasia: experience at the Philippine General Hospital. Int J Gynecol Cancer. 2016;26:977–983. doi: 10.1097/IGC.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 19.Al-Husaini H, Soudy H, Darwish A, Ahmed M, Eltigani A, Edesa W, et al. Gestational trophoblastic neoplasia: treatment outcomes from a single institutional experience. Clin Transl Oncol. 2015;17:409–415. doi: 10.1007/s12094-014-1251-1. [DOI] [PubMed] [Google Scholar]
- 20.Rustin GJ, Newlands ES, Lutz JM, Holden L, Bagshawe KD, Hiscox JG, et al. Combination but not single-agent methotrexate chemotherapy for gestational trophoblastic tumors increases the incidence of second tumors. J Clin Oncol. 1996;14:2769–2773. doi: 10.1200/JCO.1996.14.10.2769. [DOI] [PubMed] [Google Scholar]
- 21.Savage P, Cooke R, O'Nions J, Krell J, Kwan A, Camarata M, et al. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol. 2015;33:472–478. doi: 10.1200/JCO.2014.57.5332. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DP, Berkowitz RS, Horowitz NS. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2015;15:1293–1304. doi: 10.1586/14737140.2015.1088786. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematol Oncol Clin North Am. 2012;26:111–131. doi: 10.1016/j.hoc.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Aghajanian C. Treatment of low-risk gestational trophoblastic neoplasia. J Clin Oncol. 2011;29:786–788. doi: 10.1200/JCO.2010.31.0151. [DOI] [PubMed] [Google Scholar]
- 25.Chen YX, Shen YM, Qian JH, Xie X. Effects of primary chemotherapy with single methotrexate on low-risk gestational trophoblastic neoplasia and influencing factors thereof. Zhonghua Yi Xue Za Zhi. 2005;85:2109–2112. [PubMed] [Google Scholar]
- 26.Lu WG, Ding ZM, Xie X, Ye DF, Chen HZ, Feng SW. Single methotrexate chemotherapy for low-risk gestational trophoblastic tumor. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:414–417. [PubMed] [Google Scholar]
- 27.Lu WG, Ye F, Shen YM, Fu YF, Chen HZ, Wan XY, et al. EMA-CO chemotherapy for high-risk gestational trophoblastic neoplasia: a clinical analysis of 54 patients. Int J Gynecol Cancer. 2008;18:357–362. doi: 10.1111/j.1525-1438.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Wan X, Lv W, Xie X. Relapsed or refractory gestational trophoblastic neoplasia treated with the etoposide and cisplatin/etoposide, methotrexate, and actinomycin D (EP-EMA) regimen. Int J Gynaecol Obstet. 2007;98:44–47. doi: 10.1016/j.ijgo.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Shen T, Chen LL, Qin JL, Wang XY, Cheng XD, Xie X, et al. EMA/CO regimen for chemotherapy 24 patients with ultra high-risk gestational trophoblastic neoplasia. Zhonghua Fu Chan Ke Za Zhi. 2018;53:371–376. doi: 10.3760/cma.j.issn.0529-567x.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Zhao W, Zhang YQ, Huang XF. Curative effects and influenced factors of EMA-CO as an initial regimen for the treatment of high-risk gestational trophoblastic neoplasia. Zhonghua Yi Xue Za Zhi. 2018;98:3896–3899. doi: 10.3760/cma.j.issn.0376-2491.2018.47.017. [DOI] [PubMed] [Google Scholar]
- 31.Song HZ, Yang XY, Xiang Y. Trophoblastic tumor diagnosis & treatment. 2nd ed. Beijing: People's Medical Publishing House; 2004. [Google Scholar]
- 32.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143 Suppl 2:79–85. doi: 10.1002/ijgo.12615. [DOI] [PubMed] [Google Scholar]
- 34.Lurain JR, Elfstrand EP. Single-agent methotrexate chemotherapy for the treatment of nonmetastatic gestational trophoblastic tumors. Am J Obstet Gynecol. 1995;172:574–579. doi: 10.1016/0002-9378(95)90575-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang WD, Kim CH, Cho MK, Kim JW, Cho HY, Kim YH, et al. Serum hCG level and rising world health organization score at second-line chemotherapy (pulse dactinomycin): poor prognostic factors for methotrexate-failed low-risk gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2010;20:1424–1428. doi: 10.1111/IGC.0b013e3181f5873e. [DOI] [PubMed] [Google Scholar]
- 36.Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11–18. doi: 10.1016/j.ajog.2010.06.072. [DOI] [PubMed] [Google Scholar]