Abstract
The World Health Organization (WHO) classified the novel coronavirus (i.e., coronavirus disease 2019 [COVID-19]) as a global public health emergency. COVID-19 threatens to curtail patient access to evidence-based treatment. Medicine is changing, basically due to the limited available resources. In the field of gynecologic oncology, we have to re-design our treatments' paradigm. During COVID-19 pandemic outbreak, the highest priority is to achieve the maximum benefit from less demanding procedures. Extensive procedures should be avoided, in order to reduce hospitalization and postoperative events that might increase the in-hospital spread of the virus. There are ongoing concerns on the use of laparoscopic procedures, related to the possible contamination of the staff working in the operation room. Other minimally invasive techniques, including, vaginal surgery as well as robotic-assisted and isobaric procedures would be preferred over laparoscopy. A fair allocation of resources is paramount adequate treatments.
Keywords: COVID-19, SARS-CoV-2, Gynecologic Oncology, Surgery
INTRODUCTION
The coronavirus is a positive-sense single-stranded RNA virus (+ssRNA), infecting humans [1]. Coronavirus disease 2019 (i.e., coronavirus disease 2019 [COVID-19]) is an infectious disease caused by severe acute respiratory syndrome. It is the seventh known coronavirus able to infect people, after 229E, NL63, OC43, HKU1, Middle East respiratory syndrome coronavirus (MERS-CoV), and the original severe acute respiratory syndrome-related coronavirus (SARS-CoV) [1]. The disease was first identified in 2019 in China, and has spread globally, resulting in the 2020 coronavirus pandemic. In January 2020, the World Health Organization (WHO) formally declared a Public Health Emergency of International Concern (PHEIC) [2] and on 11 March 2020 the WHO declared it a pandemic. COVID-19 is highly infective, having COVID-19 infected more than 100,000 people in 100 countries. As of 27 April 2020 (05:50 UTC), there were more than 2,900,000 confirmed cases of infection [2]. The pandemic has changing our life dramatically, being the enemy of “dense urban life”. Individuals are working at home, mass transit is down, cities are doomed. Medicine is changing too. To reduce the spread of COVID-19, health system is hustling to switch to telemedicine. Many physicians and health care resources are dedicated to the COVID-19 emergency. In Italy, intensive care units will then be at maximum capacity; up to 4,000 hospital beds will be needed by mid-April 2020 [3]. All these features impact dramatically also on the health on patients without COVID-19 infections. In fact, the lack of resources reduces the possibility to take care of other diseases, including cancer. In light of possible in-hospital contamination by COVID-19 and the lack of resources treatment plan are carefully defined. Plans have to be tailored on the basis on patients' and disease characteristics as well as treatments-related morbidity.
In the field of gynecologic oncology, we are shifting our diagnostic and therapeutic paradigms, in order to maintain an adequate level of cancer related treatment for women affected by gynecological disease. The most common gynecological diseases that deserve to be treated included: ovarian cancer, endometrial cancer, uterine sarcoma, vulvar and vaginal cancer as well as pre-neoplastic lesions of the lower genital tract. Here, we evaluate possible surgical strategies for women affected by gynecological cancer and premalignant lesions harboring in the genital tract.
OVARIAN CANCER
Ovarian cancer is considered one of the most lethal malignancies in developed countries, due to its high death incidence ratio [4]. In the United States, more than 22,400 newly diagnosed cases and 14,000 cancer-related deaths are estimated, every year [4]. Generally, surgery performed in patients affected by ovarian cancer includes: i) primary surgical treatment, ii) surgery for recurrent disease, and iii) palliative surgery.
1. Primary surgical treatment of ovarian cancer
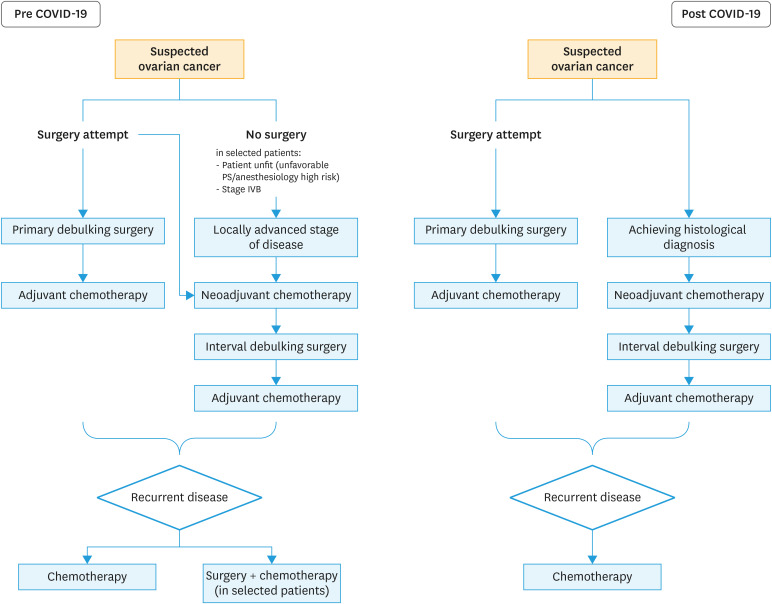
The mainstay of treatment for patients with ovarian cancer should include a combination of surgery and chemotherapy. The treatment modality of ovarian cancer is directly related to the stage of presentation and patients' characteristics. In the early stage of disease, surgery is mandatory even in the era of COVID-19 outbreak. Surgery (with or without chemotherapy) allow tumor removal, staging and guarantees a high possibility of long-term cure for those patients [5]. In patients with apparent early-stage ovarian cancer, the choice to have lymphadenectomy should be tailored on patients' and diseases' characteristics. When possible, it should be avoided. In particular, lymphadenectomy should be avoided in mucinous tumors while, in other histologic type (e.g., serous histology) at high risk of nodal spread lymphadenectomy could be executed [6]. In fact, no clear data support the beneficial effects of full lymphadenectomy in those patients, especially if adjuvant chemotherapy is planned [6].
In advanced stage of disease (International Federation of Obstetrics and Gynecologists [FIGO] stage IIIB–IV) surgery followed adjuvant chemotherapy (generally 6 cycles) is considered the standard of care [5]. In women presenting with suspected advanced stage ovarian cancer, surgery allows to i) make a precise histological diagnosis, ii) evaluate the burden of disease, identifying the real diffusion of the tumor into the peritoneal cavity, and iii) more important to remove all macroscopic lesions. In fact, residual disease is one of the most important prognostic factors in patients with ovarian cancer. Although primary cytoreductive surgery should be the preferred treatment modality in women with advanced disease growing evidence support that the execution of neoadjuvant chemotherapy followed by interval debulking surgery is a valuable alternative to primary cytoreductive surgery. Neoadjuvant chemotherapy followed by interval debulking surgery seems reducing surgery-related morbidity (since the reduction of complexity of surgery) and to be related to similar long-term outcomes than primary cytoreductive surgery. Two randomized controlled trials (EORTC5597 and the CHORUS study) suggested the non-inferiority and reduced invasiveness of neoadjuvant chemotherapy followed by interval debulking surgery in comparison to open surgery in patients affected by advanced disease [7]. To date, the use of neoadjuvant chemotherapy followed by interval debulking surgery is not fully accepted [8]. The gynecologic oncology community have concerns related to the low number of patients having complete (no residual disease) and optimal (residual disease <1 cm) cytoreduction included in the primary debulking arms. The ongoing TRUST study will clarify the role of neoadjuvant chemotherapy in advanced stage ovarian cancer [9]. Until now, in light of the evidence provided, the use of primary cytoreductive surgery should be avoided in patients for whom extensive cytoreductive procedure are anticipated. Patients with a high burden of disease should be counseled to have neoadjuvant chemotherapy in order to reduce treatment related morbidity and to possibly allow the execution of surgery (generally after 3 months) after the COVID-19 pandemic outbreak. By this point of view patients diagnosed with a high burden of disease (those with FIGO stages IIIC and IVB) could be considered to have neoadjuvant chemotherapy after having acquired histological diagnosis by laparoscopic examination or radiological-guided biopsy. Histological assessment is mandatory before starting chemotherapy. The decision to have primary cytoreductive surgery in stage IIIC ovarian cancer with a high burden of disease patients should be carefully discussed and have to be reserved in a selected group of patients.
2. Surgical treatment of recurrent ovarian cancer
Another important issue regards the execution of secondary cytoreductive surgery in recurrent ovarian cancer. Generally, patients with oligomestatic disease and long-term progression free survival are ideal candidate for surgical removal of the recurrent disease. Accumulating data highlighted that secondary and tertiary cytoreductive surgery might improve outcomes of ovarian cancer patients, when complete cytoreduction is achieved [10,11,12]. The ongoing randomized phase III AGO DESKTOP OVAR III is evaluating the role of secondary cytoreduction followed by chemotherapy vs. chemotherapy alone will clarify the role of surgery in patients with recurrent ovarian cancer [13].
To date the only randomized trial (GOG 213) investigating the role of secondary surgery in recurrent ovarian cancer patients failed to demonstrate any positive effect of surgery in those patients. Median progression-free survival for surgery plus chemotherapy and chemotherapy alone was 18.9 months and 16.2 months, respectively. The hazard ratio for disease progression or death (surgery vs. no surgery) was 0.82 (95% confidence interval=0.66–1.01). The present investigation highlighted that about one out of ten patients is at risk of developing postoperative complications [14]. Additionally, patient-reported quality of life decreased significantly after surgery; albeit it did not differ significantly between the two groups after recovery [14]. Therefore, on the light of the available evidence supporting the use of chemotherapy only in recurrent ovarian cancer patients, the secondary surgery would be reserved only in super-selected patients. Considering the COVID-19 outbreak, extensive surgical procedures should be avoided in this group of patients. Fig. 1 shows a flow chart for the treatment of advanced / recurrent ovarian cancer in the pre and post COVID-19 outbreak.
Fig. 1. Flow chart for patients with advanced ovarian cancer in pre and post COVID-19 outbreak.
COVID-19, coronavirus disease 2019.
3. Palliative surgery for ovarian cancer
Palliative care is performed in case of poor clinical conditions and serious illness. It focuses on relieving the symptoms, and pain related to end-stage ovarian cancer. Palliative care is critical for patients' quality of life and should not be avoided if it is judged to be necessary. Bowel obstruction, unremitting pelvic pain, fistula formation, tumor necrosis, pelvic sepsis, and chronic hemorrhage are the main indications for palliative surgery. The primary reason of palliative surgery in patients with end-stage ovarian cancer is relief of bowel obstruction. This goal may be accomplished by ostomies. Bowel resection and bypass should be avoided. Of note, in case of bowel obstruction, it is estimated that about 10%–20% present with multiple concomitant small and large bowel obstructions [15]. A medical attempt should be carried out before surgery in almost every case. Palliative chemotherapy and/or stereotactic body radiotherapy should be taken in consideration in order to possible avoid surgeries during the COVID-19 outbreak.
BORDERLINE OVARIAN TUMORS
Around 15% of all ovarian tumor are classified as borderline ovarian tumors. They are also described as atypical proliferative tumors and used to be called tumors of low malignant potential. Borderline ovarian tumors usually affect young women aged less than 50 years of age. They are usually diagnosed at an early stage, when the abnormal cells are still within the ovary. Surgical removal of the cysts or the ovary is the treatment of choice for patients affected by borderline ovarian tumors, at primary diagnosis and recurrence [16]. However, in the era COVID-19 outbreak only primary diagnosis deserves a surgical treatment; while observation would be a safe and effective modality to follow patients with suspected recurrent borderline ovarian tumors. At the time of primary diagnosis surgery is mandatory to achieve a correct histopathological diagnosis. Histological diagnosis and pathology slides review by an expert pathologist is of paramount importance. In case of confirmed diagnosis surgical extirpation of recurrent disease could be safely delayed. Although in experienced hands ultrasonographic examination is able to discriminate patients having borderline and invasive ovarian tumors, all patients with a suspected malignant ovarian mass must have a histological diagnosis, even during COVID-19 outbreak. Ultrasonographic images/films would be evaluated by an expert ultrasonographer (possibly using telemedicine—see the last chapter) in order to reduce unnecessary procedures.
ENDOMETRIAL CANCER
Endometrial cancer is the most common gynecologic malignancy in developed countries, with more than 65,000 new cases estimated for the year 2020 in the United States [4]. Surgery is the mainstay of treatment of endometrial cancer in apparent early- and advanced- stage disease. In apparent early stage of disease (uterine confined endometrial cancer), hysterectomy with salpingo-oophorectomy allows to remove primary tumor and to identify patients at high-risk of developing recurrences. There is still no consensus on the execution of retroperitoneal staging. Two randomized clinical trials comparing hysterectomy plus lymphadenectomy vs. hysterectomy alone in the management of early stage endometrial cancer suggested that the execution of lymphadenectomy do not improve outcomes of endometrial cancer patients, but it is associated with an increased risk of treatment-related morbidity [17]. However, several international guidelines (including the American College of Obstetricians and Gynecologists [ACOG]) recommend lymphadenectomy, thus judging the execution of lymph node staging as an important part in endometrial cancer treatments [18].
Similarly, the European Society of Medical Oncology (ESMO)/European Society of Gynaecological Oncology (ESGO)/European Society for Radiotherapy & Oncology (ESTRO) guidelines confirmed the role and the indication to the lymph node status evaluation as part of the surgical staging in patients with apparent early-stage endometrial cancer [19]. Lymphadenectomy allows the identification of disease harboring in the lymph nodes thus allowing to plan postoperative treatment. Accumulating data support the adoption of sentinel node mapping instead of lymphadenectomy [20]. Recently, the National Comprehensive Cancer Network (NCCN) guidelines included sentinel lymph node mapping in the treatment algorithm of apparent early-stage endometrial cancer [21]. Retrospective data suggested that the execution of sentinel node mapping provide similar oncologic results in comparison to full lymphadenectomy [22,23]. Additionally, sentinel node mapping seems to improve the identification of patients with nodal involvement (FIGO stage IIIC disease) thanks to the detection of low volume disease (micrometastasis and isolated tumor cells detectable by ultrastaging). Data of centers implementing sentinel node mapping highlighted that sentinel node mapping is a simple and not time-consuming procedures, furthermore it does not increase significantly complication rate in comparison to hysterectomy alone.
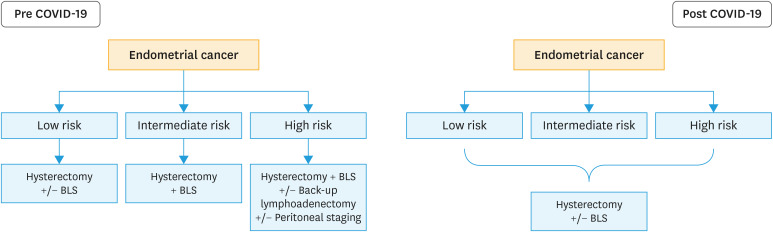
In the era of COVID-19 outbreak, in apparent early-stage endometrial cancer the adoption of sentinel node mapping would be preferred over the execution of lymphadenectomy, even in high-risk disease. The execution of back-up lymphadenectomy (lymphadenectomy performed after sentinel node mapping) should be omitted. Open surgery should be avoided when possible. Patients should be submitted to mini-laparotomic procedures (thorough Pfannenstiel incision), minimally invasive surgery (preferring isobaric procedures or robotic-assisted surgery) and vaginal procedures. In elderly patients with low- (FIGO stage I endometrioid endometrial carcinoma [EC], grades 1 and 2 with myometrial invasion <50%, and without lymphovascular space invasion [LVSI]) and intermediate low- risk disease (FIGO stage I endometrioid EC, grades 1 and 2 with myometrial invasion ≥50%, and LVSI negative) vaginal hysterectomy can be considered a valid treatment option. However, there are some patients for whom surgery might be safely delayed. For morbidly obese patients affected by FIGO grade 1 endometrioid endometrial cancer might be receive a hormonal treatment (e.g., progesterone releasing intrauterine device) in order to reduce possible postoperative pulmonary complications. It can be safely done, especially after ruling out myometrial involvement with magnetic resonance imaging (MRI) or ultrasonographic examination. Radiotherapy (external beam radiotherapy plus vaginal brachytherapy) can be considered a definitive treatment option for elderly patients who are not fit to tolerate surgery. Fig. 2 shows a flow chart for the treatment of apparent early stage endometrial cancer in the pre and post COVID-19 outbreak.
Fig. 2. Flow chart for patients with endometrial cancer in pre and post COVID-19 outbreak.
COVID-19, coronavirus disease 2019; BLS, bilateral salpingo-oophorectomy.
In case of advanced disease, cytoreduction have to be considered. Although no mature data still exist, patients with gross abdominal disease (especially those affected by serous endometrial cancer) might be submitted to neoadjuvant chemotherapy in order to reduce treatment related morbidity and to possibly allows the execution of surgery (generally after 3 months) after the COVID-19 outbreak.
UTERINE SARCOMA
Uterine sarcoma includes a group of rare uterine lesions with differing tumor biology, natural history and response to treatment [24]. The most common uterine sarcoma is leiomyosarcoma, followed by endometrial stromal sarcoma, adenosarcoma (with or without overgrowth) and undifferentiated uterine sarcoma. Surgery is used to diagnose, stage, and treat uterine sarcoma [24]. Generally, surgery for uterine confined sarcoma is not highly demanding. Treatment of uterine sarcoma include the execution of open abdominal hysterectomy with or without salpingo-oophorectomy. In case of advanced peritoneal dissemination, cytoreduction is recommended. But, selection of cases is mandatory in order to reduce the risk of developing morbidity and intensive care unit admission. The execution of nodal dissection is not recommended even in high-grade endometrial stromal sarcoma. Owing to the high aggressiveness of uterine sarcoma, surgery cannot be omitted.
CANCER OF THE UTERINE CERVIX
Cervical cancer still represents a major health concern, being the third most common malignancy among women aged <39 years, and the second most common cause of death for cancer among females between 20 and 39 years in the United States [4].
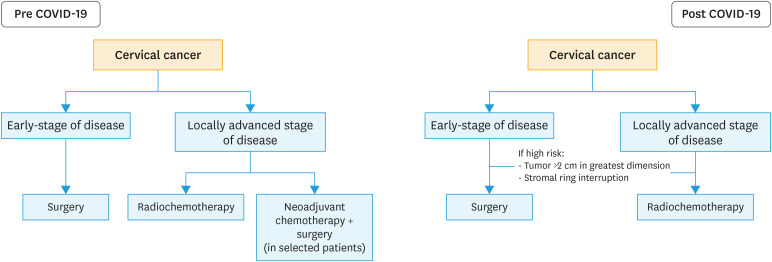
Patients affected by cervical cancer are classified in early-stage disease (FIGO stages I and IIA <4 cm), locally advanced stage of disease (FIGO stage IIA <4 cm, IIB, III, and IVA) and metastatic disease (FIGO stage IVB). Surgery and radiotherapy showed equivalent oncologic safety in patients affected by early-stage cervical cancer. However, radiotherapy correlates with long-term sequelea, and should be avoided in young patients. Therefore, surgery represent the mainstay of treatment for cervical cancer patients in the early-stage of disease, being associated with favorable oncologic outcomes [25]. Open abdominal radical hysterectomy represents the standard of care. Surgery should be performed via Pfannstiel or Kusner incisions in order to improve postoperative recovery, shorter hospitalization and a rapid workflow. In last decades, minimally-invasive surgery has replaced open surgery for the treatment of several malignant conditions, including cervical cancer [26]. Accumulating data from retrospective studies suggested that minimally invasive approach upholds oncologic results, improving short-term postoperative course in comparison to conventional open surgery [27,28]. Recently, the unexpected results of the Laparoscopic Approach to Cervical Cancer (LACC) trial have brought a strong debate into the gynecologic oncology community [29]. The LACC trial randomized patients to have minimally invasive and open abdominal radical hysterectomy, suggesting that patients undergoing minimally invasive surgery experience an increased risk of developing local recurrences and worse overall survival than patients undergoing open surgery [29]. Nodal evaluation is crucial in cervical cancer patients. As aforementioned for retroperitoneal staging of endometrial cancer, sentinel node mapping would replace the execution of full (pelvic) lymphadenectomy, thus minimizing possible complications and improving postoperative course [21]. Although surgery is the preferred treatments modality in early stage cervical cancer patients we have to point out that surgery could be avoided in 2 particular class of patients affected by early stage cervical cancer. First, in a setting lacking of adequate resources, we have to take in consideration the execution of definitive radiotherapy in elderly (aged 70 years of age or older) patients affected by early-stage cervical cancer. Second, patients affected by early stage cervical cancer but with tumor larger more than 2 cm and with sign of the interruption of stromal ring (detected at preoperative workup) should be considered to have definitive radiotherapy (with or without chemotherapy). In fact, this group of patients is characterized by a high need of adjuvant radiotherapy [30].
In patients with locally advanced stage of disease, definitive radio-chemotherapy should be considered the standard of care. Growing evidence support that neoadjuvant chemotherapy followed by radical surgery might be consider a suitable treatment modality for patients affected by locally advanced stage of disease, thus reducing long-term morbidity related to radiation therapy, especially in young women. However, the adoption of neoadjuvant chemotherapy followed by radical surgery is not still accepted. In fact, no level A evidence support the use of neoadjuvant chemotherapy instead of radiation [31]. Radio chemotherapy remains the standard of care for those patients. Therefore, the execution of neoadjuvant chemotherapy should be avoided. Chemotherapy (including cisplatinum/carboplatinum and paclitaxel with or without bevacizumab) still remains the standard of care in case of metastatic disease. The role of salvage surgery (i.e., pelvic exenteration) in case of metastatic and recurrent disease has to be taken into consideration only in a group of very selected patients. Additionally, chemotherapy should be considered a valuable option for patients with recurrent cervical cancer according to the results of the GOG 240 trial. The GOG 240 investigated various chemotherapeutic agents in recurrent/metastatic cervical cancer, suggesting that platinum, paclitaxel and bevacizumab would be the preferred chemotherapeutic regimen [32]. Fig. 3 shows a flow chart for the treatment of cervical cancer in the pre and post COVID-19 outbreak.
Fig. 3. Flow chart for patients with cervical cancer in pre and post COVID-19 outbreak.
COVID-19, coronavirus disease 2019.
VULVAR CANCER
Vulvar cancer represents an uncommon gynecological malignancy, accounting for less than 5% of all gynecological cancers. Squamous cell carcinoma is the most prevalent type of vulvar cancer. Traditionally, curative treatment of squamous cell vulvar cancer includes radical vulvectomy plus (superficial and deep) inguinal-femoral lymphadenectomy [33]. However, growing evidence suggested that less invasive procedures (i.e., simple vulvectomy, wide local excision) in combination with sentinel node mapping provide similar long-term results, minimizing morbidity related-treatment. Surgical treatment of early-stage vulvar cancer is generally well tolerated and rarely it is associated with severe surgical related morbidity. The procedure could be performed safely under spinal anesthesia, thus promoting a faster recovery for the patients. In case of extensive locally advanced disease involving the urethra and the anus, chemo-radiation would be the preferred treatment modality [33]. Those patients (<65 years) with locally advanced disease have to be counseled about the possible adoption of neoadjuvant platinum-based chemotherapy. Owing to the rarity of locally advanced vulvar cancer no phase III trial had investigated the role of neoadjuvant chemotherapy in vulvar cancer. However, few retrospective series support this practice in a selected group of women [33].
VAGINAL CANCER
Vaginal cancer represents an uncommon gynecological malignancy, accounting for less than 1% of all gynecological cancers. More than 90% of vaginal cancer cases are squamous cell carcinomas; while approximately 5% are adenocarcinomas [33]. Squamous cell vaginal carcinomas initially spread superficially, but few women could be diagnosed with metastatic disease. Frequent sites of distant metastases are no regional lymph nodes, liver and lungs. The risk of having lymph node involvement is directly related to stage at presentation. Basically, patients with disease confined within the vaginal mucosa (FIGO stage I) have a risk of nodal involvement of about 6%–16%; while in patients with tumor involving the sub-vaginal tissue but has not extend to the pelvic wall (FIGO stage II) this risk ranged between 30%–35% [33]. Patients affected by FIGO stage I vaginal cancer might receive either surgery or radiotherapy (external beam radiotherapy associated with vaginal brachytherapy). Surgical procedures should guarantee the presence of free surgical margins. The procedures needed might include the execution of simple wide vaginal excision to the execution of radical colpectomy and pelvic exenteration. The mainstay of treatment for FIGO stage II and III vaginal cancer includes the use of radiotherapy (external beam radiotherapy associated with vaginal brachytherapy) with or without chemotherapy. In the era of COVID-19 outbreak, surgical treatments should reserve in selected patients (young women with FIGO stage I disease is strongly recommended in vaginal cancer patients. Staging lymphadenectomy is not recommended during the COVID-19 outbreak.
CONSERVATIVE SURGERY
Although the majority of gynecological malignancies occur in post-menopausal women, it is estimated that approximately 3%–14% of invasive ovarian cancer, 3%–5% of endometrial cancer and 40% of cervical cancer are diagnosed in women aged less than 40 years old [34]. These patients are in some cases identified at early stage of disease and could potentially receive a conservative surgical treatment, with the preservation of the uterus and the adnexa (contra-lateral ovary in case of ovarian cancer). Fertility and hormonal preservations are considered as one of the most important quality of life (QoL) indicators among pre-menopausal women affected by cancer. The adoption of fertility sparing treatment is strongly recommended, even during the COVID-19 outbreak. The two main reasons included: i) quality of life preservation, and ii) the execution of less demanding procedures (in whom the uterus and the ovaries are preserved). All patients were evaluated at baseline by pelvic examination, transvaginal ultrasound and computed tomography or magnetic resonance imaging. All examination should be performed by expert physician specifically trained in gynecologic oncology. Patients should be counseled about the risk of having preservation of then genital organs. A careful evaluation by an expert in fertility is needed before adopting a conservative approach. In fact, women with low ovarian reserve should be counseled of the low possibility to conceive spontaneously after the treatment [34].
Fertility sparing treatments for women affected by ovarian cancer include the execution of salpingo-oophorectomy (one side) plus peritoneal (omentectomy and peritoneal biopsies) with or without retroperitoneal (pelvic and para-aortic nodal dissection) staging. Adjunctive procedures including appendectomy and endometrial sampling should be tailored on the basis of patients' and disease characteristics. Fertility sparing treatments for women affected by endometrial cancer include the execution of progestin treatment (oral or progesterone-releasing intra-uterine system), hysteroscopic examination with endometrial sampling and diagnostic laparoscopy (in order to exclude the presence of a synchronous ovarian cancer). No mature data support the execution of sentinel node mapping in this phase of treatment. Fertility sparing treatments for women affected by cervical cancer include the execution of cervical conization plus sentinel node mapping. The execution of cervical conization should preferred to trachelectomy since it is simplest and due to the low-risk of developing postoperative events. Neoadjuvant chemotherapy could be administered in patients with tumor >2 cm, in order to making possible cervical conization in young patients wishing to preserve their fertility [34]. Table 1 reports indications and recommendation for fertility-sparing treatment in young women affected by gynecological cancer.
Table 1. Fertility-sparing treatment in gynecological cancers during COVID-19 pandemic outbreak.
| Gynecological cancer | Taget population | Reccomandation |
|---|---|---|
| Ovarian cancer | Stage IA–IB, G1–2 | Salpingo-oophorectomy and peritoneal staging (with or without lymphadenectomy, endometrial sampling, and appendectomy) |
| Endometrial cancer | Endometrioid histology, FIGO stage IA, G1 | Progestin therapy |
| Hysteroscopy ± diagnostic laparoscopy | ||
| Cervical cancer | FIGO stage IA, IB1 | Cervical conization ± sentinel node mapping |
COVID-19, coronavirus disease 2019; FIGO, International Federadation of Gynecologist and Obstetrics.
RISK REDUCING SURGERY
Patients harboring mutations of the BRCA1 and BRCA2 genes as well as patients affected by Lynch syndrome are ideal candidate to risk reduction surgery. This prophylactic surgery includes the use of simple procedures (generally performed via minimally invasive surgery) in order to reduce the risk of developing invasive cancer arising from the genital system. Additionally, it is important to underline that about 5% of women having prophylactic surgery are diagnosed with occult cancer, detected by pathological examination only [35]. Although these procedures have a minimal impact on patients' status due to the low risk of developing morbidity and the rapid workflow of patients, the routine use of risk reduction surgery is not recommended during COVID-19 pandemic outbreak. Patients harboring BRCA1 and BRCA2 mutations have to be counseled about their risk. If possible medical treatments (low-dose estroprogestinic therapy) could be administered. During COVID-19 outbreak, risk reduction surgery should be performed only in presence of a grade of suspicious (i.e., high CA125 levels, presence of an adnexal mass or presence of abnormal levels of free-fluid).
PREMALIGNANT LESIONS OF THE LOWER GENITAL TRACT AND THE UTERUS
Secondary prevention is aimed to reduce the burden of cervical cancer and other lesions related to human papillomavirus (HPV). Identification of pre-malignant lesions including vulvar (VIN), vaginal (VaIN) and cervical (CIN) intraepithelial neoplasia is paramount in order to minimize the risk of developing cancer arising in the lower genital tract. In case of diagnosis of severe VIN and VaIN, outpatient surgical treatment could be safely performed. In case of large lesions deserving wide excision, medical treatments including the use of 5-FU or other local antiblastic agents would be a safe option [36]. In case of diagnosis of severe cervical dysplasia, cervical conization is recommended. The treatment of these lesions is simple and not require hospitalization. They can be safely performed in an out-patient setting, thus minimizing possible in hospital diffusion of COVID-19. Additionally, we have to point out that in absence of macroscopic lesions, conization could be safely delayed (<3 months) [36]. Histological examination should be achieved also in case of ablative procedures in order to identify possible malignant conditions.
Similarly, patients having suspected endometrial lesions (i.e., patients having uterine bleeding) deserve to be evaluated in order to identify possible malignant and pre-neoplastic lesions. Endometrial sampling should be achieved in out-patients setting using hysteroscopic examination or other simplest endometrial sample techniques. Histological examinations should be reserve to patients presenting with symptoms; while in absence of uterine bleeding histological examination is not mandatory, regardless the endometrial thickness [37].
INTRODUCING TELEMEDICINE FOR FOLLOW-UP EVALUATION AND 2ND OPINION
Telemedicine refers to the practice of taking care patients remotely when the provider and patient are not physically present with each other. Modern technology has enabled doctors to consult patients by using personal computers and various types of software packages. According to the new dispositions issued by the Government we are facing with an increase of telemedicine use during the COVID-19 outbreak. Patients can consult a physician at the comfort of their home, potentially reducing COVID-19 spread. Follow-up evaluations should be performed using telemedicine. There are not data supporting routine follow-up in asymptomatic patients; therefore, we suggest to postponing follow up visits in asymptomatic women. Additionally, telemedicine would be useful in case of patients requiring second opinion in specialized cancer centers. Today, there are telemedicine solutions that allow patients to seek a 2nd opinion from their home. Sending another physician copy of their medical images and more can easily be done by uploading the content to their secure website. This is very convenient for those who need a specialist but they have to travel, potentially spreading the virus.
THE ROLE OF MINIMALLY INVASIVE SURGERY AND SELF-PROTECTION OF THE SURGICAL STAFF
Preoperative triage is an essential part in identify patients without COVID-19 that may be admitted in COVID-19-free hubs. To date there is not consensus regarding the best way of triage methods of those patients. Microbiological test plus computed tomography (CT) scan of the thorax in the last 24 hours would be the preferred methods for triaging patients. However, these methods do not guarantee a detection rate of COVID-19 infection in all patients. Interestingly, about 80% of patients infected by COVID-19 are asymptomatic or mild symptomatic. There are concerns on the adoption of laparoscopy in potentially infected patients. The Royal College of Surgeons warns against the use of laparoscopic surgery during COVID-19 pandemic outbreak [38]. Since surgical smoke and intra-operative aerosol (normally occurring during minimally invasive procedures) might promote the diffusion of COVID-19 in the operative room (OR), thus infecting surgeons and operative staff. Personal protective equipment (PPE) are necessary to all staff working into the operative theater. The minimum necessary number of individuals should allow to enter in the theater. Levels of pneumoperitoneum pressure and the power settings of electrocautery should be as low as possible in order to reduce possible aerosol formation [39]. In patients having ongoing COVID-19 surgery should avoided (when possible); while in a COVID-19-free setting the use of minimally invasive surgery would be the preferred surgical approach. Minimally invasive surgery guarantees a rapid patients' workflow, reducing hospitalization and the occurrence of postoperative complications in comparison to traditional open surgery. However, laparoscopic approach should be performed only if the protection of the staff working in the OR is guaranteed [39]. To date, the risk of contamination during laparoscopy is only theoretical and it has not been proven yet. However, until new evidence would be available, surgeons and the other health care providers working in the OR should use PPE, including respiratory masks (e.g., N95, FPP2, FFP3) and medical eye protection. The use of trocar port filters (ultra-filtration systems) is highly recommended, but no evidence is still support they clinical utility [40]. Surgeons have to choose the best surgical approach that guarantee safety for patients and for the OR staff.
Theoretically, robotic-assisted surgery would be preferred to laparoscopy since it allows to perform minimally invasive procedures at low pneumoperitoneum pressure. The surgeon controls the arms while seated at a computer console located in a safe area. During minimally invasive surgery, the use of filters is highly recommended. The use of vaginal surgery for patients affected by endometrial cancer as well as robotic-assisted and isobaric minimally invasive techniques should be promoted. During COVID-19 outbreak, any surgical procedure (including open surgery) should be considered at high risk for patient and health care providers. Surgeons, anesthesiologists and all OR staff must be protected using adequate PPE. Centralization of oncologic patients is paramount in order to improve safety of patients and health care providers.
DISCUSSION
This paper highlighted the need of fair allocation of scarce medical resources in the time of COVID-19 and stresses the importance of avoid unnecessary procedures. Allocations of resources during COVID-19 pandemic outbreak is of paramount importance. Hospitals should be classified into two categories: dedicated hubs for highly specialized treatments (including oncological practice) and hubs for patients deserving treatment of COVID-19 (these latter hubs should have a limited surgical staff and operative rooms for COVID-19 patients needing immediate surgery.
The main goal is to maximizing the benefit produced in a setting lacking of adequate resources. During COVID-19 outbreak we have to maximize the overall cure rate, reducing patients' hospitalization and postoperative events. Generally, less invasive procedures should be preferred in order to improve post-surgical recovery. Extensive surgical procedures for which the admission of intensive care unit would be necessary should be taken in consideration only in selected cases, while unfit and elderly patients should receive the possible less invasive procedures. Maximizing benefit requires consideration of patients' and disease characteristics as well as prognosis. Patients' characteristics that have to be considered should include mere chronological age as well as functional age, performance status, and a careful evaluation of comorbidity. Diseases' characteristics that have to be considered should include natural history of the disease, the burden of disease, and the possible risk of growing/spread. Additionally, we have to take in consideration the impact of hospitalization and treatment related morbidity on the outcomes of possibly compromised patients. We can speculate that surgery has an impact on asymptomatic patients harboring indolent COVID-19 infection. Abdominal surgical procedures (especially if they are performed via open approach) per se, are associated with a high risk of postoperative pulmonary complications [26,27]. Data from China highlighted that 80% of those infected either are asymptomatic or have mild symptoms. Anecdotally, in our surgical practice we are assisting an increasing number of asymptomatic patients, without sign of infection at preoperative CT of the thorax who develop postoperative interstitial pneumonia after surgery. We can speculate that advanced abdominal procedures, impacting on the efficacy of the immune systems, increase the risk of developing severe acute respiratory syndrome and interstitial pneumonia in patients harboring an asymptomatic COVID-19. On the light of the risk of developing severe acute respiratory syndrome and interstitial pneumonia, the need of surgery and possible alternatives have to be explored. A multidisciplinary evaluation of oncologic patients is mandatory in order to explore possible therapeutic options. Surgery has to avoided in patients in elderly and obese patients with poor general conditions. Minimally invasive surgery (especially using isobaric technique) has to be carried out instead of the performing open approach, in order to reduce morbidity and length of stay. Step Trendelenburg position should be avoided [38]. Ultra-filtration systems have to be applied in order to reduce possible spread of the virus in the operative theatre [38]. Patients who are scheduled to have extensive procedures should be tested per COVID-19 suing microbiological tests and chest imaging before having surgery. Self-protection is mandatory. PPE should be used by all health care providers, especially in the operative room. In conclusion during the COVID-19 outbreak we have to change our paradigm of treatments. Extensive surgical procedures should be performed only in ultra-selected cases; while, less demanding procedures producing the maximum benefit should have the highest priority. During the COVID-19 outbreak “less is more”. However, it is important to highlight that all these recommendations are based on common sense, an few empirical data. Owing to the lack of proper methodology, and the low level of evidence of these recommendations would be adopted with caution. These recommendations have to evaluated carefully and would be tailored on the basis of available resources. Possible, COVID-19 outbreak would push decision makers in improving quality of the health care system and prevent the paucity of medical resources, worldwide.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.G., B.C., G.R., L.S., S.M., D.A., R.F.
- Data curation: B.C., G.R.
- Methodology: B.G., B.C., G.R., L.S., S.M., D.A., R.F.
- Project administration: R.F.
- Supervision: R.F.
- Writing - original draft: B.G., B.C., G.R., L.S., S.M., D.A., R.F.
- Writing - review & editing: B.G., B.C., G.R., L.S., S.M., D.A., R.F.
References
- 1.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Indini A, Aschele C, Cavanna L, Clerico M, Daniele B, Fiorentini G, et al. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: a nationwide Italian survey. Eur J Cancer. 2020;132:17–23. doi: 10.1016/j.ejca.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 3.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Peres LC, Sinha S, Townsend MK, Fridley BL, Karlan BY, Lutgendorf SK, et al. Predictors of survival trajectories among women with epithelial ovarian cancer. Gynecol Oncol. 2020;156:459–466. doi: 10.1016/j.ygyno.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogani G, Tagliabue E, Ditto A, Signorelli M, Martinelli F, Casarin J, et al. Assessing the risk of pelvic and para-aortic nodal involvement in apparent early-stage ovarian cancer: a predictors- and nomogram-based analyses. Gynecol Oncol. 2017;147:61–65. doi: 10.1016/j.ygyno.2017.07.139. [DOI] [PubMed] [Google Scholar]
- 7.Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MK, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19:1680–1687. doi: 10.1016/S1470-2045(18)30566-7. [DOI] [PubMed] [Google Scholar]
- 8.Bogani G, Leone Roberti Maggiore U, Paolini B, Diito A, Martinelli F, Lorusso D, et al. The detrimental effect of adopting interval debulking surgery in advanced stage low-grade serous ovarian cancer. J Gynecol Oncol. 2019;30:e4. doi: 10.3802/jgo.2019.30.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuss A, du Bois A, Harter P, Fotopoulou C, Sehouli J, Aletti G, et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7) Int J Gynecol Cancer. 2019;29:1327–1331. doi: 10.1136/ijgc-2019-000682. [DOI] [PubMed] [Google Scholar]
- 10.Bogani G, Rossetti D, Ditto A, Martinelli F, Chiappa V, Mosca L, et al. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J Gynecol Oncol. 2018;29:e66. doi: 10.3802/jgo.2018.29.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harter P, Hahmann M, Lueck HJ, Poelcher M, Wimberger P, Ortmann O, et al. Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:1324–1330. doi: 10.1245/s10434-009-0357-0. [DOI] [PubMed] [Google Scholar]
- 12.Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21:289–295. doi: 10.1097/IGC.0b013e31820aaafd. [DOI] [PubMed] [Google Scholar]
- 13.Bogani G, Tagliabue E, Signorelli M, Ditto A, Martinelli F, Chiappa V, et al. A score system for complete cytoreduction in selected recurrent ovarian cancer patients undergoing secondary cytoreductive surgery: predictors- and nomogram-based analyses. J Gynecol Oncol. 2018;29:e40. doi: 10.3802/jgo.2018.29.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929–1939. doi: 10.1056/NEJMoa1902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope JM, Pothuri B. The role of palliative surgery in gynecologic cancer cases. Oncologist. 2013;18:73–79. doi: 10.1634/theoncologist.2012-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plett H, Harter P, Ataseven B, Heitz F, Prader S, Schneider S, et al. Fertility-sparing surgery and reproductive-outcomes in patients with borderline ovarian tumors. Gynecol Oncol. 2020;157:411–417. doi: 10.1016/j.ygyno.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 17.May K, Bryant A, Dickinson HO, Kehoe S, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2010:CD007585. doi: 10.1002/14651858.CD007585.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez E American College of Obstericians and Gynecologists. ACOG Practice Bulletin number 65: management of endometrial cancer. Obstet Gynecol. 2006;107:952–953. doi: 10.1097/01.AOG.0000209463.53764.e7. [DOI] [PubMed] [Google Scholar]
- 19.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitao MM, Jr, Zhou QC, Gomez-Hidalgo NR, Iasonos A, Baser R, Mezzancello M, et al. Patient-reported outcomes after surgery for endometrial carcinoma: prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol Oncol. 2020;156:147–153. doi: 10.1016/j.ygyno.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogani G, Casarin J, Leone Roberti Maggiore U, Ditto A, Pinelli C, Dell'acqua A, et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: long-term results of a propensity-matched analysis. Gynecol Oncol. 2020 doi: 10.1016/j.ygyno.2020.04.691. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 22.Schlappe BA, Weaver AL, McGree ME, Ducie J, Zahl Eriksson AG, Dowdy SC, et al. Multicenter study comparing oncologic outcomes after lymph node assessment via a sentinel lymph node algorithm versus comprehensive pelvic and paraaortic lymphadenectomy in patients with serous and clear cell endometrial carcinoma. Gynecol Oncol. 2020;156:62–69. doi: 10.1016/j.ygyno.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multinu F, Ducie JA, Eriksson AG, Schlappe BA, Cliby WA, Glaser GE, et al. Role of lymphadenectomy in endometrial cancer with nonbulky lymph node metastasis: comparison of comprehensive surgical staging and sentinel lymph node algorithm. Gynecol Oncol. 2019;155:177–185. doi: 10.1016/j.ygyno.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cybulska P, Sioulas V, Orfanelli T, Zivanovic O, Mueller JJ, Broach VA, et al. Secondary surgical resection for patients with recurrent uterine leiomyosarcoma. Gynecol Oncol. 2019;154:333–337. doi: 10.1016/j.ygyno.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez PT, Pareja R, Eriksson AG, Frumovitz M. International Gynecologic Cancer Society 2019 meeting summary. Int J Gynecol Cancer. 2020;30:167–173. doi: 10.1136/ijgc-2019-001146. [DOI] [PubMed] [Google Scholar]
- 26.Bogani G, Rossetti D, Ditto A, Martinelli F, Chiappa V, Leone C, et al. Minimally invasive surgery improves short-term outcomes of nerve-sparing radical hysterectomy in patients with cervical cancer: a propensity-matched analysis with open abdominal surgery. J Gynecol Oncol. 2019;30:e27. doi: 10.3802/jgo.2019.30.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang C, Liu P, Cui Z, Liang Z, Bin X, Lang J, et al. Effect of laparoscopic versus abdominal radical hysterectomy on major surgical complications in women with stage IA–IIB cervical cancer in China, 2004–2015. Gynecol Oncol. 2020;156:115–123. doi: 10.1016/j.ygyno.2019.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Kim SI, Lee M, Lee S, Suh DH, Kim HS, Kim K, et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: a matching study of two institutional hospitals in Korea. Gynecol Oncol. 2019;155:75–82. doi: 10.1016/j.ygyno.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 30.Papadia A, Bellati F, Bogani G, Ditto A, Martinelli F, Lorusso D, et al. When does neoadjuvant chemotherapy really avoid radiotherapy? Clinical predictors of adjuvant radiotherapy in cervical cancer. Ann Surg Oncol. 2015;22 Suppl 3:S944–S951. doi: 10.1245/s10434-015-4799-2. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 32.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu S, Lairson DR, Chan W, Wu CF, Ramondetta L. Mean medical costs associated with vaginal and vulvar cancers for commercially insured patients in the United States and Texas. Gynecol Oncol. 2018;148:342–348. doi: 10.1016/j.ygyno.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Lenck C, Chopin N, Gouy S, Bonsang-Kitzis H, Martinez-Gomez C, Radosevic-Robin N, et al. The French national network dedicated to rare gynecological cancers diagnosis and management could improve the quality of surgery in daily practice of granulosa cell tumors. A TMRG and GINECO group Study. Gynecol Oncol. 2020;157:78–84. doi: 10.1016/j.ygyno.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Piedimonte S, Frank C, Laprise C, Quaiattini A, Gotlieb WH. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498–508. doi: 10.1097/AOG.0000000000003702. [DOI] [PubMed] [Google Scholar]
- 36.Bogani G, Ditto A, Martinelli F, Mosca L, Chiappa V, Rossetti D, et al. LASER treatment for women with high-grade vaginal intraepithelial neoplasia: a propensity-matched analysis on the efficacy of ablative versus excisional procedures. Lasers Surg Med. 2018;50:933–939. doi: 10.1002/lsm.22941. [DOI] [PubMed] [Google Scholar]
- 37.Gemer O, Segev Y, Helpman L, Hag-Yahia N, Eitan R, Raban O, et al. Is there a survival advantage in diagnosing endometrial cancer in asymptomatic postmenopausal patients? An Israeli Gynecology Oncology Group study. Am J Obstet Gynecol. 2018;219:181.e1–181.e6. doi: 10.1016/j.ajog.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 38.The Royal College of Surgeons of England. Coronovirus (COVID-19) London: The Royal College of Surgeons of England; 2020. [cited 2020 Mar 28]. Available from: https://www.rcseng.ac.uk/coronavirus/ [Google Scholar]
- 39.Bogani G, Raspagliesi F. Minimally invasive surgery at the time of COVID-19: the OR staff needs protection. J Minim Invasive Gynecol. 2020 doi: 10.1016/j.jmig.2020.04.010. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) SAGES AND EAES recommendations regarding surgical response to COVID-19 crisis [Internet] Los Angeles (CA): Society of American Gastrointestinal and Endoscopic Surgeons; 2020. [cited 2020 Apr 30]. Available from: https://www.sages.org/recommendations-surgical-response-covid-19/ [Google Scholar]