Abstract
Objective
To compare the diagnostic accuracy of dilatation and curettage (D&C) versus endometrial aspiration biopsy in follow-up evaluation of patients treated with progestin for endometrial hyperplasia (EH)
Methods
A prospective multicenter study was conducted from 2015 to 2018. Patients with EH were treated with progestin, one of the following three treatment regimens: oral medroxyprogesterone acetate (MPA) 10 mg/day for 14 days per cycle, continuous MPA 10 mg/day or the levonorgestrel-releasing intrauterine system (LNG-IUS). At 3 or 6 months of treatment, endometrial tissues were obtained via 2 methods in each patient: aspiration biopsy, followed by D&C. The primary outcome was the consistency of the histologic results between the 2 methods. The secondary outcome was the regression rate at 6 months of treatment.
Results
The study population comprised 65 patients (55 with non-atypical hyperplasia, 10 with atypical hyperplasia). During the follow-up, a comparison of the pathologic results from aspiration biopsy and D&C was carried out for the 65 cases. Thirty-eight cases were diagnosed as EH by D&C. Among these, only 24 were diagnosed with EH from aspiration biopsy, for a diagnostic concordance of 63.2% (ĸ=0.59). Forty-four patients were followed up at 6 months, and the regression rate was 31.8% (14/44). Responses were obtained for 41.7% (5/12) of the cyclic MPA group, 58.3% (7/12) of the continuous MPA group and 10% (2/20) of the LNG-IUS group.
Conclusion
As a follow-up evaluation of patients treated with progestin for EH, aspiration biopsy is less accurate than D&C and might not be a reliable method.
Trial Registration
ClinicalTrials.gov Identifier: NCT02412072
Keywords: Endometrial Hyperplasia, Progesterone, Levonorgestrel, Intrauterine Device, Dilatation and Curettage, Biopsy
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries and the incidence is still rising [1]. Recently in Korea, the overall incidence of EC has increased by 6.9% per year [2]. Because endometrial hyperplasia (EH) is a precursor of EC, accurate diagnostics and adequate treatment of EH are clinically important to retard the rapid increase of EC.
The treatment modality for EH depends mostly on the histological diagnosis and the woman's desire to preserve fertility. Due to the risk of underlying malignancy or progression to cancer, hysterectomy is the recommended treatment of atypical EH [3,4]. Meanwhile, for patients with non-atypical EH or for young patients with atypical EH who wish to preserve their fertility, progestogen treatment has become the routine conservative therapy. Oral progestogen has long been the most commonly used method with various treatment regimens [5,6]. Moreover, the levonorgestrel-releasing intrauterine system (LNG-IUS), which achieves higher local concentrations of progestogens in the endometrium also has been successfully used to treat EH [7,8,9,10,11,12,13]. In the recent Royal College of Obstetricians and Gynaecologists/ British Society for Gynaecological Endoscopy guidelines on the management of EH, LNG-IUS is recommended as the first-line medical treatment because it has a higher disease regression rate with fewer adverse effects compared with oral progestogens [14].
However, there is no reliable data on the proper surveillance method to use during hormonal treatment of EH. As a follow-up evaluation method during hormonal treatment, inpatient endometrial sampling, dilatation and curettage (D&C) under anesthesia or outpatient endometrial biopsy using suction devices designed to aspirate endometrial tissue is generally used. A recent study comparing the histological results of pipelle aspiration biopsy and D&C reported almost equal EH-diagnostic success rates [15,16]. Meanwhile, these results had been obtained for cases where there were no progestin effects on the endometrium and moreover, where the LNG-IUS was not in the uterus. So far, there has been only limited data on the comparison of these methods' diagnostic accuracies during hormonal treatment of EH.
Therefore, we set out to conduct a multicenter prospective study comparing the diagnostic accuracy of D&C versus endometrial aspiration biopsy in follow-up evaluation of patients treated with progestin for EH. Additionally, we evaluated the treatment efficacy of three treatment regimens: cyclic oral medroxyprogesterone acetate (MPA), continuous oral MPA, and LNG-IUS.
MATERIALS AND METHODS
1. Study design
The prospective multicenter study was conducted from May 2015 to May 2018. Five institutions belonging to the Korean Gynecologic-Oncology Group were registered. Eligible subjects were women with histologically confirmed EH who desired to avoid hysterectomy. The initial histologic diagnosis was made by D&C or hysteroscopic biopsy with the patient anesthetized. All the patients were fully informed of the study purposes and procedures, and their voluntary informed consent to participate (as approved by the institutional review board of each clinical trial institution) was obtained. This trial was registered at ClinicalTrials.gov (NCT02412072) and published in October 2015 [17].
2. Treatment and follow-up
Patients were treated with progestin, one of the following 3 treatment regimens according to the clinician's judgment: oral MPA 10 mg/day for 14 days per cycle, continuous oral MPA 10 mg/day, or LNG-IUS. The follow-up with clinical review, transvaginal ultrasonography and endometrial histological surveillance was undertaken at 3 or 6 months of treatment. Endometrial tissues were obtained via 2 methods: endometrial aspiration biopsy using a pipelle, followed by D&C (in the case of LNG-IUS, aspiration biopsy with the LNG-IUS remaining in the uterus, followed by D&C after removal of the LNG-IUS). The histologic diagnoses of the specimens were made by central pathologic review.
3. Outcome measures
The primary outcome was the consistency of the histologic results between the aspiration biopsy and the D&C. To evaluate the diagnostic accuracy of the aspiration biopsy as compared with the D&C, the histological results of the two methods were compared.
The secondary outcome was the regression rate at 6 months of treatment. This was determined by comparing the diagnosis of the follow-up D&C at 6 months after hormonal treatment with the initial histologic diagnosis. For assessment of the histological response, complete regression was defined as regular proliferative, secretory endometrium, progesterone effect with atrophic glands or pseudo-decidualization without evidence of hyperplasia. During the follow-up period, if there was histological evidence of EC or progression of non-atypical EH to atypical EH, hormonal treatment was suspended and an alternative specific treatment option, namely hysterectomy, was suggested.
4. Sample size calculation and statistical considerations
Kappa statistics were used to assess the agreement of the 2 methods: endometrial aspiration biopsy and D&C. κ values <0 indicated no agreement, 0 to 0.20 slight, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 substantial, and 0.81 to 1 almost perfect agreement. The sample size was estimated as 75 with an expected kappa value of 0.7, with a margin of error of 11%, assuming a 10% dropout or withdrawal rate.
RESULTS
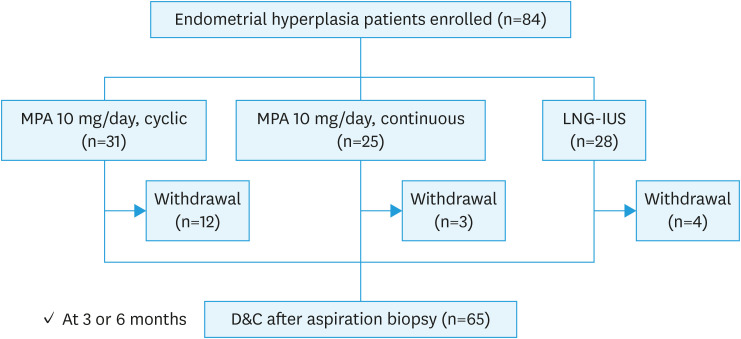
A total of 84 patients meeting the inclusion criteria were enrolled. All the patients were assigned to three treatment groups, 31 to the cyclic MPA group, 25 to the continuous MPA group, and 28 to the LNG-IUS group. Nineteen patients voluntarily withdrew from the total group, and 65 patients completed the protocol treatment. Twelve withdrawals were reported for the cyclic MPA group, 3 for the continuous MPA group, and 4 for the LNG-IUS group (Fig. 1).
Fig. 1. Study design and flow diagram.
D&C, dilation and curettage; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate.
The study population included 27 patients with simple hyperplasia (SH) without atypia; 1 with atypical SH; 28 with complex hyperplasia (CH) without atypia and 9 with atypical CH. The patients' baseline characteristics are provided in Table 1. The mean age of the patients was 38.8±7.3 years (range, 18–58 years) and the mean body-mass index (BMI) was 25.4±5.3 kg/m2 (range, 18.6–40.9 kg/m2).
Table 1. Patients' characteristics (n=65).
| Characteristics | Values | |
|---|---|---|
| Age (yr) | 38.8±7.3 (18–58) | |
| BMI (kg/m2) | 26.3±7.0 (16.3–49.1) | |
| Parity | ||
| 0 | 41 (63.1) | |
| 1 | 11 (16.9) | |
| 2 | 11 (16.9) | |
| 3 | 2 (3.1) | |
| Histologic type | ||
| SH without atypia | 27 (41.5) | |
| CH without atypia | 28 (43.1) | |
| SH with atypia | 1 (1.5) | |
| CH with atypia | 9 (13.9) | |
| Treatment | ||
| MPA 10 mg/day, cyclic | 19 (29.2) | |
| MPA 10 mg/day, continuous | 22 (33.9) | |
| LNG-IUS | 24 (36.9) | |
Data are expressed as the means ± standard deviation (range) or number (%).
BMI, body mass index; CH, complex hyperplasia; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; SH, simple hyperplasia.
A comparison of the pathologic results from the aspiration biopsy and D&C was carried out for 65cases. The histologic results by D&C were 27 (41.5%) with normal endometrium and38 (58.5%) with EH. Overall, 41 of 65 the cases (63.1%) showed diagnostic concordance between D&C and aspiration biopsy: 17 cases with normal endometrium and 24 cases with EH. Among the 38 cases of EH on D&C, only 24 were diagnosed with EH from aspiration biopsy, for a diagnostic concordance of 63.2% (ĸ=0.59). There were no correlations between the diagnostic concordance and clinical characteristics including age, BMI, histology and treatment method.
In the histologic results by aspiration biopsy, 26 patients were diagnosed as normal endometrium. Among these 26 cases of normal endometrium by aspiration biopsy, 9 were diagnosed as EH by D&C. In addition, there were 15 of 65 cases (23.1%) of insufficient tissue for pathologic evaluation in the histologic results by aspiration biopsy. Among these 15 cases with insufficient tissue, 5 were diagnosed as EH by D&C (Table 2).
Table 2. Comparison of pathologic results from endometrial aspiration biopsy and D&C.
| D&C | No. (%) | Aspiration biopsy | No. | Concordance to D&C (%) |
|---|---|---|---|---|
| Normal | 27 (41.5) | Normal | 17 | 63.0 |
| Material insufficiency | 10 | |||
| EH | 38 (58.5) | EH | 24 | 63.2 |
| Normal | 9 | |||
| Material insufficiency | 5 | |||
| Total | 65 (100) | 63.1 |
Kappa value, 0.59 (moderate).
D&C, dilatation and curettage; EH, endometrial hyperplasia.
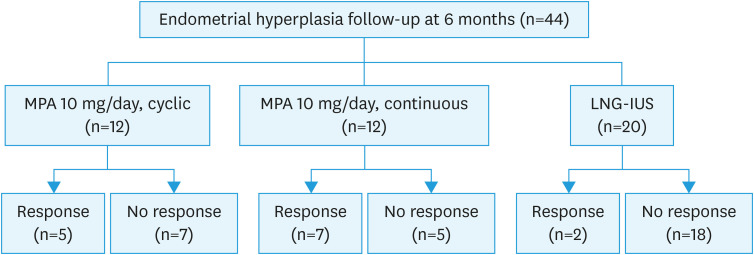
Forty-four patients (44/65, 67.7%) were followed up at 6 months of therapy, 12 in the cyclic MPA group, 12 in the continuous MPA group and 20 in the LNG-IUS group.
The regression rate was 31.8% (14/44) for the total group. Responses were obtained for 41.7% (5/12) in the cyclic oral MPA group, 58.3% (7/12) in the continuous MPA group and 10% (2/20) in the LNG-IUS group (Fig. 2). Treatment generally was well tolerated, and there were no cases of treatment-related complication. There was no significant difference among the three groups with respect to age or BMI (Table 3).
Fig. 2. Flowchart of treatment outcomes.
LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate.
Table 3. Treatment outcomes of progestin therapy (n=44).
| Variables | MPA cyclic (n=12) | MPA continuous (n=12) | LNG-IUS (n=20) | p-value |
|---|---|---|---|---|
| Age (yr) | 41.1±4.8 (35–48) | 38.0±6.8 (24–48) | 38.2±5.5 (28–45) | 0.085 |
| BMI | 25.6±6.4 (19.6–40.9) | 25.6±3.7 (21.1–32.1) | 26.5±6.24 (18.6–37.9) | 0.870 |
| Response | 7 (58.3) | 5 (41.7) | 2 (10) | 0.012 |
Data are presented as means±standard deviation (range) or number (%).
BMI, body mass index; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate.
DISCUSSION
To date, there have been no reliable data on the response of the endometrium in follow-up for progestin treatment of EH. There was a study to evaluate the diagnostic accuracy of endometrial aspiration biopsy for EH patients treated with LNG-IUS, by comparing the pathologic result of aspiration biopsy with that of D&C at the 12th month of follow-up [13]. This study, however, failed to find satisfactory results in its analysis of the diagnostic concordance between the 2 methods, due to a small sample size (15 patients) and a high regression rate. In almost all patients (14/15), the pathologic result according to D&C was normal endometrium. Only 1 case was diagnosed by D&C as residual EH, but the histologic result by aspiration biopsy was normal endometrium.
Therefore, we designed a multicenter prospective study to compare the diagnostic accuracy of aspiration biopsy with that of D&C in EH patients undergoing progestin treatment. Our results showed that the diagnostic concordance was relatively low (63.2%) and that the kappa value was moderate (ĸ=0.59). Furthermore, 23.1% (15/65) of the endometrial tissue specimens obtained by aspiration biopsy were insufficient for pathologic evaluation, and among these 15 cases, 5 were diagnosed as EH by D&C. These findings, significantly, are consistent with that of an earlier study on the diagnostic accuracy of a proper surveillance method during hormonal treatment of early-stage EC. In a recent prospective study on the comparison of two methods' accuracies, during the combined oral MPA/LNG-IUS treatment for early stage EC, the diagnostic accuracy of aspiration biopsy with the LNG-IUS in the uterus versus D&C after removal of the LNG-IUS was compared. Among the 15 cases of EC on D&C, only 8 were diagnosed with EC from aspiration biopsy, for a diagnostic concordance of 53.3% (ĸ=0.55) [18].
These results can be considered to indicate that aspiration biopsy failed to obtain adequate amounts of tissue for diagnosis, due to endometrial atrophy induced by oral progestin or LNG-IUS and mechanical interference from the LNG-IUS. The clinical significance of this study is that it is the first prospective investigation to show that D&C is more accurate than aspiration biopsy in the evaluation of treatment response for progestin treatment of EH.
Another outcome that we wanted to investigate in this study was the regression rate of three different types of progestin treatment: cyclic oral MPA 10 mg/day, continuous oral MPA 10 mg/day, and LNG-IUS. According to the literature, both continuous oral progestin and LNG-IUS are highly effective in achieving regression of EH (89%–96%); several randomized controlled trials (RCTs) comparing use of the LNG-IUS and oral progestin found that the LNG-IUS achieved a higher regression rate [14]. Notwithstanding, in our results, the regression rate for the total group was 31.8% (14/44). Responses were obtained for 41.7% (5/12) in the cyclic oral MPA group, 58.3% (7/12) in the continuous MPA group and 10% (2/20) in the LNG-IUS group. These results were relatively lower regression rate compared with those of previous studies.
The relatively short treatment and follow-up periods might be the culprit. Moreover, the small sample size due to the low follow-up percentage (44/65, 67.7%) and the possibility of selection bias in the choice of three treatment regimens according to the clinician's judgment could be another limitation. Above all, there was a possibility of underestimation of residual EH in the evaluation of treatment response, when aspiration biopsy was used as follow-up evaluation method in other studies. Actually, we reviewed 7 RCTs comparing the LNG-IUS and oral progestin for treatment of EH, and found that 6 of the 7 studies used aspiration biopsy for follow-up evaluation (Table 4). Further studies are needed to clarify this point.
Table 4. Characteristics of randomized controlled trials comparing the LNG-IUS and oral progestin for treatment of EH.
| Author | Population | Intervention | Comparison | Diagnostic method | Follow-up evaluation | Treatment outcome (regression rate) |
|---|---|---|---|---|---|---|
| Ismail et al., 2013 [19] | 90 women with non-atypical SEH | LNG-IUS (n=30) for 3 mo | MPA 10 mg/day for 10 day/cycle (n=30), or NET 15 mg/day for 10 day/cycle (n=30) | D&C | Aspiration biopsy | LNG-IUS group: 66.67%; MPA group: 36.66%; NET group: 40% |
| Abu Hashim et al., 2013 [11] | 120 women with non-atypical EH | LNG-IUS (n=59) for 3-6 mo | NET (n=61) 15 mg/day for 3 wk/cycle | Hysteroscopy with D&C | Aspiration biopsy | LNG-IUS group vs. NET group at the 3rd, 6th and 12th mo: 67.8% vs. 47.5%, RR, 1.42; 79.7% vs. 60.7%, RR, 1.31; and 88.1% vs. 55.7%, RR, 1.58, respectively |
| Dolapcioglu et al., 2013 [20] | 104 women with non-atypical EH | LNG-IUS (3, 6-month treatment subgroups; n=26 for each one) | MPA 10 mg/day for 10 d/cycle (3, 6-month treatment subgroups; n=26 for each one) | D&C; Aspiration biopsy | D&C; Aspiration biopsy | LNG-IUS group vs. MPA group: at the 3rd, 6th mo: 84% vs. 50%, 100% vs. 64%, respectively |
| Behnamfar et al., 2014 [21] | 60 women with non-atypical EH | LNG-IUS (n=30) for 3 mo | MPA (n=30), 10 mg/day for 12 day/cycle | - | Aspiration biopsy | LNG-IUS group: 89.3%; MPA group: 70.4% |
| El Behery et al., 2015 [22] | 100 women with non-atypical EH | LNG-IUS (n=50) for 6 mo | Dydrogesterone (n=50) 20 mg for 16 day/cycle | D&C | D&C | LNG-IUS group: 96%; Dydrogesterone group: 80% |
| Abdelaziz and Abosrie, 2013 [23] | 84 women with non-atypical SEH | LNG-IUS (n=42) for 3 mo | NET (n=42); 15 mg/day continuously | Hysteroscopy | Aspiration biopsy | LNG-IUS group: 73.8%; NET group: 57.1% |
| Ørbo et al., 2014 [24] | 153 women with non-atypical EH | LNG-IUS (n=53) for 6 mo | Cyclic MPA 10 mg/day for 10 day/cycle (n=52), or continuous MPA 10 mg daily (n=48) | Aspiration biopsy | Aspiration biopsy | LNG-IUS group: 100%; continuous MPA group: 96%; cyclic MPA group: 69% |
EH, endometrial hyperplasia; D&C, dilatation and curettage; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; NET, norethisterone acetate; RR, relative risk; SEH, simple endometrial hyperplasia.
In conclusion, as a follow-up evaluation of patients treated with progestin for EH, endometrial aspiration biopsy is less accurate than D&C. For accurate diagnosis and response assessment of hormonal treatment of EH, D&C might be a more reliable method than aspiration biopsy.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.S.J.
- Data curation: K.M.K., S.S.J., P.D.C., H.J.H., R.J.W., K.S.B.
- Investigation: P.D.C., R.J.W., K.S.B.
- Writing - original draft: K.M.K.
- Writing - review & editing: K.M.K., S.S.J., P.D.C., H.J.H., R.J.W., K.S.B.
References
- 1.Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, Frigerio L, et al. Management of endometrial cancer: issues and controversies. Eur J Gynaecol Oncol. 2016;37:6–12. [PubMed] [Google Scholar]
- 2.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, 2nd, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812–819. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 5.Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006;125:259–264. doi: 10.1016/j.ejogrb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Reed SD, Voigt LF, Newton KM, Garcia RH, Allison HK, Epplein M, et al. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113:655–662. doi: 10.1097/AOG.0b013e318198a10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:547.e1–547.e10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Kim MK, Park H, Yoon BS, Seong SJ, Kang JH, et al. The effectiveness of levonorgestrel releasing intrauterine system in the treatment of endometrial hyperplasia in Korean women. J Gynecol Oncol. 2010;21:102–105. doi: 10.3802/jgo.2010.21.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, et al. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia--a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2008;139:169–175. doi: 10.1016/j.ejogrb.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Ørbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: a follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111:68–73. doi: 10.1016/j.ygyno.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Abu Hashim H, Zayed A, Ghayaty E, El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial. J Gynecol Oncol. 2013;24:128–134. doi: 10.3802/jgo.2013.24.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG. 2014;121:477–486. doi: 10.1111/1471-0528.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MK, Seong SJ, Kim JW, Jeon S, Choi HS, Lee IH, et al. Management of endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: a Korean gynecologic oncology group study. Int J Gynecol Cancer. 2016;26:711–715. doi: 10.1097/IGC.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 14.Royal College of Obstetricians and Gynaecologists (RCOG) with the British Society for Gynecological Endoscopy (BSGE) Management of endometrial hyperplasia. Green-top guideline No. 67. London: Royal College of Obstetricians and Gynaecologists; 2016. [Google Scholar]
- 15.Demirkiran F, Yavuz E, Erenel H, Bese T, Arvas M, Sanioglu C. Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C) Arch Gynecol Obstet. 2012;286:1277–1282. doi: 10.1007/s00404-012-2438-8. [DOI] [PubMed] [Google Scholar]
- 16.Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial hyperplasia. Acta Obstet Gynecol Scand. 2001;80:784–793. doi: 10.1034/j.1600-0412.2001.080009784.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim MK, Seong SJ, Lee TS, Ki KD, Lim MC, Kim YH, et al. Comparison of diagnostic accuracy between endometrial curettage and pipelle aspiration biopsy in patients treated with progestin for endometrial hyperplasia: a Korean Gynecologic Oncology Group Study (KGOG 2019) Jpn J Clin Oncol. 2015;45:980–982. doi: 10.1093/jjco/hyv106. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Seong SJ, Kang SB, Bae DS, Kim JW, Nam JH, et al. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: a Korean Gynecologic-Oncology Group Study. J Gynecol Oncol. 2019;30:e47. doi: 10.3802/jgo.2019.30.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail MT, Fahmy DM, Elshmaa NS. Efficacy of levonorgestrel-releasing intrauterine system versus oral progestins in treatment of simple endometrial hyperplasia without atypia. Reprod Sci. 2013;20:45–50. doi: 10.1177/1933719112459243. [DOI] [PubMed] [Google Scholar]
- 20.Dolapcioglu K, Boz A, Baloglu A. The efficacy of intrauterine versus oral progestin for the treatment of endometrial hyperplasia. A prospective randomized comparative study. Clin Exp Obstet Gynecol. 2013;40:122–126. [PubMed] [Google Scholar]
- 21.Behnamfar F, Ghahiri A, Tavakoli M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J Res Med Sci. 2014;19:686–690. [PMC free article] [PubMed] [Google Scholar]
- 22.El Behery MM, Saleh HS, Ibrahiem MA, Kamal EM, Kassem GA, Mohamed MS. Levonorgestrel-releasing intrauterine device versus dydrogesterone for management of endometrial hyperplasia without atypia. Reprod Sci. 2015;22:329–334. doi: 10.1177/1933719114542014. [DOI] [PubMed] [Google Scholar]
- 23.Abdelaziz AM, Abosrie M. Levonorgestrel-releasing intrauterine system is an efficient therapeutic modality for simple endometrial hyperplasia. J Am Sci. 2013;9:417–424. [Google Scholar]
- 24.Ørbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG. 2014;121:477–486. doi: 10.1111/1471-0528.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]