Abstract
Objective
This study estimated nationally representative medical expenditures of gynecologic cancers, described treatment patterns and assessed key risk factors associated with the economic burden in the United States.
Methods
A retrospective repeated measures design was used to estimate the effect of gynecologic cancers on medical expenditures and utilization among women. Data were extracted from the Medical Expenditure Panel Survey (weighted sample of 609,787 US adults) from 2007 to 2014. Using the behavioral model of health services utilization, characteristics of cancer patients were examined and compared among uterine, cervical, and ovarian cancer patients. Multivariable linear regression models were conducted on medical expenditure with a prior logarithmic transformation.
Results
The estimated annual medical expenditure attributed to gynecologic cancers was $3.8 billion, with an average cost of $6,293 per patient. The highest annual cost per person was ovarian cancer ($13,566), followed by uterine cancer ($6,852), and cervical cancer ($2,312). The major components of medical costs were hospital inpatient stays (53%, $2.03 billion), followed by office-based visits (15%, $559 million), and outpatient visits (13%, $487 million). Two key prescription expenditures were antineoplastic hormones (10.3%) and analgesics (9.2%). High expenditures were significantly associated with being a married woman (p<0.001), having private health insurance (p<0.001), being from a low- and middle-income family (p<0.001), or living in the Midwest or the South (p<0.001).
Conclusion
The key risk factors and components were well described for the economic burden of gynecologic cancers. With a growing population of cancer patients, efforts to reduce the burden of gynecologic cancers are warranted.
Keywords: Health Expenditures, Drug Utilization, Uterine Cervical Neoplasms, Ovarian Neoplasms, Cost of Illness
INTRODUCTION
In 2015, there were approximately 1.3 million women living with a history of gynecologic cancers in the United States. Among them, an estimated 56%, 20%, and 17% were patients of uterine, cervical, and ovarian cancers, respectively [1,2,3,4,5]. It is estimated that in 2018 there were approximately 110,070 new diagnoses of gynecologic cancers, and 32,120 gynecologic cancer deaths in the United States [6]. While gynecologic cancers account for 12.5% of all estimated new female cancer diagnoses, they account for 11.2% of all estimated female deaths. This high mortality relative to prevalence indicates the severity of these diseases. Uterine cancer is the fourth most common cancer in female patients, accounting for 7% of new female cancer cases in 2018 [6]. Ovarian cancer is the fifth leading cause of cancer death among all female cancer deaths worldwide. It accounts for 2.5% of all female cancer cases, yet 5% of cancer deaths are due to ovarian cancer [7].
Gynecologic cancers place a considerable economic burden on society [8]. The national direct medical costs for cancer were estimated at $80.2 billion in the United States in 2015 [9]. Due to advances in diagnostic technology and targeted treatments, the costs of cancer care are expected to rise substantially [10]. Mariotto et al. [10] estimated that ovarian cancer had the highest cost ($6.03 billion) followed by uterine ($3.05 billion) and cervical ($1.54 billion) in 2020. There is no study of gynecologic cancer costs on a national level in the United States [4,5]. In the United States, treatment patterns were studied by using cancer registry data linked to Medicare claims for the elderly [11]. To fill the gap for the non-elderly, this study includes all adults with gynecologic cancers to examine their treatment patterns. Economic burden studies of gynecologic cancer were also conducted using claims data from one large health plan [12] and multiple datasets in a single state [13,14]. To our knowledge, no study quantifies direct medical spending and describes treatment patterns of gynecologic cancers by cancer site at the national level in the United States.
The purpose of this study was to estimate the economic burden, describe treatment patterns, and assess direct medical expenditures associated with key risk factors for patients with gynecologic cancers using nationally representative data. The economic burden has been estimated previously in studies using nationally representative data [15,16,17]. Understanding the costs and treatment patterns of these conditions can help determine potential resource allocation to reduce the economic burden on the patients' families, as well as on society in general.
MATERIALS AND METHODS
1. Data source and study population
A retrospective cross-sectional repeated measures study was conducted to analyze all patient-reported medical expenditures related to gynecologic cancers using Medical Expenditure Panel Survey (MEPS) data from 2007 to 2014. MEPS is the largest nationally representative survey of the United States civilian noninstitutionalized population. Each year, the MEPS sample is drawn from reporting units in the previous year's National Health Interview Survey. The MEPS has a complex design consisting of stratification, clustering, and multistage and disproportionate sampling with oversampling of minorities. Participants are interviewed every 6 months, and all surveys are recorded annually to provide nationally representative estimates of socio-demographics, medical conditions, characteristics, and healthcare expenditure and utilization. After data collection, Agency for Healthcare Research and Quality researchers allocated person-weights and variance estimation stratum to reflect survey nonresponse and national population [18]. We included MEPS data from 2007 to 2014. Eight years of data were pooled to ensure sufficient sample size and to increase the precision of estimates.
We identified 477 US adults with gynecologic cancers using an International Classification of Diseases, Ninth Revision code from the MEPS, Household Component. All data files within MEPS were merged using the unique personal identifier (DUPERSID) on a one-to-one match. MEPS collects detailed information on demographics, socioeconomic characteristics, healthcare use, health status, expenditures, sources of payment, the status of health insurance coverage, and prescription information. Healthcare use and expenditures were collected from both participants and their medical providers. This study was exempt from Institutional Review Board (IRB) review and approval by the University of Cincinnati IRB (IRB ID: 2019-0750).
2. Theoretical framework and covariates
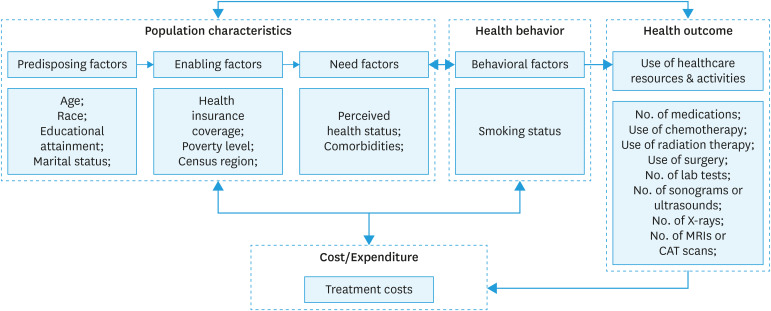
The Behavioral Model of Health Services Utilization was used as the framework [19] to identify the relationship between individual and population-level factors associated with health outcomes in gynecologic cancers and medical expenditures. The framework includes participants' characteristics, health behaviors, health outcomes, and medical costs (Fig. 1). Participants' characteristics were grouped by 3 categories: predisposing factors, enabling factors, and need factors.
Fig. 1. Andersen behavior model of treatment cost among gynecologic cancer patients.
CAT, computerized axial tomography; MRI, magnetic resonance imaging.
Covariates were age, race, educational attainment, census region, marital status, health insurance coverage, poverty level, perceived health status, smoking status, and comorbidities. Age was categorized into 18–49, 50–64, and ≥65 years. Sex was dichotomized as female vs. male, race as white and non-white. For health insurance coverage, we included any private health insurance and non-private health insurance. The educational attainment included “≤ high school graduate” or “≥ some college”. The marital status was defined as married or not married. All respondents who reported “widowed”, “divorced”, “separated”, or “never married” were grouped in the “not married” category. The census region included the Northeast, Midwest, South, and West (Appendix 1). Income level was defined as a percentage of poverty level and grouped into 3 categories: poor & near-poor (<125% federal poverty level), low & middle income (125% to less than 400% federal poverty level), and high income (≥400% poverty level) [20]. Perceived health status was defined as either “excellent/good” or “fair/poor”. The comorbidities included hypertension, stroke, emphysema, high cholesterol, diabetes, arthritis, and asthma.
3. Outcomes
The medical expenditures were the total direct healthcare costs for the calendar year for each individual, including emergency department, inpatient, ambulatory, home health care, prescribed medicines, and other services, including nursing home, rehabilitation, vision, medical supplies, and dental. The source of payment includes out-of-pocket, private health insurance, Medicare, Medicaid, Veterans Administration, and the Civilian Health and Medical Program of the Department of Veterans Affairs, etc. The total, mean, and median medical expenditures for gynecologic cancer patients were calculated. The cost over the 2007–2014 period was adjusted to 2014 United States dollars using the Consumer Price Index-Medical summary from the Bureau of Labor Statistics [21].
Treatment patterns were described using MEPS medical event files. All medications were identified using their brand name and generic name. We identified initiators of analgesics (Codeine, Oxycodone, Diclofenac, Endocet, Fentanyl, Hydrocortisone, Hydromorphone, Ibuprofen, Lortab, Methadone, Morphine, Motrin, Oxycontin, Percocet, Tylenol, Vicodin), antidepressants (Citalopram Hydrobromide, Duloxetine, Effexor Xr, Fluoxetine, Trazodone, Zoloft), antineoplastic hormones (Anastrozole, Arimidex, Femara, Letrozole, Lupron Depot, Medroxyprogesterone, Megestrol, Tamoxifen), sex hormones (Climara, Esterified Estrogens/Methyltestosterone, Estradiol, Necon, Premarin, Ortho), antiemetic or antivertigo agents (Emend, Reglan, Prochlorperazine, Ondansetron, Metoclopramide). All the records of chemotherapy, radiation therapy, prescription medications, psychotherapy/counseling, lab tests, procedures, and imaging, such as sonograms or ultrasounds, X-rays, computed tomography (CT) scans, magnetic resonance imaging (MRI) were also collected.
4. Statistical analysis
Patient characteristics were examined and compared among uterine, cervical, and ovarian cancer patients using Pearson's χ2 test. Univariate regression models were used for calculating medical expenditure estimates with a prior logarithmic transformation among cancer patients by factoring differences in age, race, educational attainment, census region, marital status, health insurance coverage, comorbidities, poverty level, perceived health status, and current smoking status. Only predictors with a p-value less than 0.05 were kept in the multivariable linear regression models. Multivariable linear regression models were used to identify the factors associated with the medical expenditures of all gynecologic cancer patients. Statistical comparisons were 2-tailed, and statistical significance was defined at a p-value of less than 0.05. All the analyses, sampling weight, variance estimation stratum, and primary sampling unit/clustering were used to adjust for the complex survey designs of the MEPS data set and required to correct for sources of statistical bias (e.g., nonresponse) to provide nationally representative estimates. All analyses were performed in Statistical Analysis System software, version 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
We identified 477 patients with gynecologic cancers representing 609,787 patients nationally from 2007 to 2014. The characteristics of patients with gynecologic cancers and subtypes in the United States are shown in Table 1. There were 31.9%, 36.9%, 12.4%, and 18.9% living with uterine, cervical, ovarian, and other female genital organ cancers, respectively. Cervical cancer patients tended to be younger (165,180 were younger than 50 [78.1%]), had lower education attainment (70.1%), and were more likely to have smoked or to live in households whose income was below 125% of the federal poverty line, compared to patients with uterine, ovarian, or other gynecologic cancers. Uterine cancer patients were more likely to report that they have comorbid conditions, such as hypertension (61.0%), arthritis (52.4%), diabetes (27.9%), and stroke (13.5%), than individuals with other gynecologic cancers (p<0.05).
Table 1. Sample demographics among adults with gynecologic cancers, 2007–2014.
| Patient-level characteristics | Overall (n=609,787) | Uterine cancer (n=221,848) | Cervical cancer (n=212,101) | Ovarian cancer (n=65,937) | p-value† | |
|---|---|---|---|---|---|---|
| Age | <0.001 | |||||
| 18–34 | 94,314 (16.1) | 10,122 (4.7) | 70,466 (33.3) | 2,149 (3.8) | ||
| 35–49 | 153,010 (26.2) | 26,744 (12.5) | 94,714 (44.8) | 13,118 (23.1) | ||
| 50–64 | 188,647 (32.3) | 85,425 (40.1) | 29,301 (13.9) | 28,649 (50.6) | ||
| 65–79 | 111,490 (19.1) | 73,862 (34.7) | 14,649 (6.9) | 10,599 (18.7) | ||
| ≥80 | 36,750 (6.3) | 16,968 (8) | 2,338 (1.1) | 2,157 (3.8) | ||
| Race | 0.982 | |||||
| White | 534,332 (87.6) | 193,648 (87.3) | 186,757 (88.1) | 56,624 (85.9) | ||
| Non-white | 75,455 (12.4) | 28,200 (12.7) | 25,345 (11.9) | 9,313 (14.1) | ||
| Marital status | 0.065 | |||||
| Married | 306,318 (50.2) | 128,581 (58) | 84,846 (40) | 34,382 (52.1) | ||
| Not married* | 303,469 (49.8) | 93,267 (42) | 127,256 (60) | 31,555 (47.9) | ||
| Educational attainment | <0.001 | |||||
| ≤High school | 329,884 (63.2) | 134,921 (68.3) | 126,905 (70.1) | 38,632 (63.8) | ||
| ≥Some college | 192,383 (36.8) | 62,604 (31.7) | 54,220 (29.9) | 21,965 (36.2) | ||
| Family income Poverty level | 0.017 | |||||
| Poor & near poor | 151,346 (24.8) | 39,846 (18) | 68,621 (32.4) | 13,766 (20.9) | ||
| Low & middle income | 253,701 (41.6) | 93,801 (42.3) | 93,772 (44.2) | 32,514 (49.3) | ||
| High income | 204,741 (33.6) | 88,200 (39.8) | 49,708 (23.4) | 19,657 (29.8) | ||
| Health insurance Coverage | 0.306 | |||||
| Private | 371,689 (61) | 144,528 (65.1) | 115,822 (54.6) | 42,912 (65.1) | ||
| Non-private | 238,098 (39) | 77,320 (34.9) | 96,279 (45.4) | 23,025 (34.9) | ||
| Census region | 0.396 | |||||
| Northeast | 143,976 (24.6) | 62,364 (29.3) | 39,262 (18.6) | 9,947 (17.6) | ||
| Midwest | 124,520 (21.3) | 43,300 (20.3) | 44,412 (21) | 13,196 (23.3) | ||
| South | 171,861 (29.4) | 65,736 (30.8) | 66,491 (31.4) | 15,305 (27) | ||
| West | 143,854 (24.6) | 41,722 (19.6) | 61,302 (29) | 18,223 (32.2) | ||
| Comorbidities | ||||||
| Hypertension | 284,807 (47) | 134,501 (61) | 66,310 (31.3) | 32,838 (49.8) | <0.001 | |
| Stroke | 55,937 (9.2) | 29,743 (13.5) | 8,033 (3.8) | 5,171 (7.8) | 0.040 | |
| Emphysema | 24,334 (4) | 8,674 (3.9) | 8,210 (3.9) | 809 (1.2) | 0.384 | |
| High cholesterol | 232,911 (38.4) | 102,140 (46.4) | 64,765 (30.5) | 28,380 (43) | 0.056 | |
| Diabetes | 121,979 (20.1) | 61,561 (27.9) | 21,704 (10.2) | 20,855 (31.6) | 0.001 | |
| Arthritis | 261,621 (43.2) | 115,386 (52.4) | 67,779 (32) | 31,786 (48.2) | 0.019 | |
| Asthma | 79,678 (13.1) | 32,397 (14.7) | 26,874 (12.7) | 6,044 (9.2) | 0.770 | |
| Currently smoke | 0.001 | |||||
| Yes | 99,027 (18.4) | 24,489 (12.8) | 61,072 (30.5) | 3,596 (7.3) | ||
| No | 439,144 (81.6) | 166,955 (87.2) | 138,913 (69.5) | 45,989 (92.7) | ||
| Perceived health status | 0.210 | |||||
| Excellent/good | 390,206 (65.7) | 150,663 (68.7) | 144,099 (68.1) | 31,770 (52.5) | ||
| Fair/poor | 203,942 (34.3) | 68,610 (31.3) | 67,368 (31.9) | 28,687 (47.5) | ||
Values are presented as number (%). Number is indicated total number of patients in the US population (calculated by using Medical Expenditure Panel Survey weights) and % is indicated weighted percentage. Bold text indicates a statistically significant difference with a p-value less than 0.05.
*All respondents reported “widowed,” “divorced,” “separated,” or “never married” were grouped in the “not married” category; †All statistical tests were 2-sided, and all p-values were calculated using Pearson's χ2 test. Statistically significant (p<0.05).
1. Economic burden
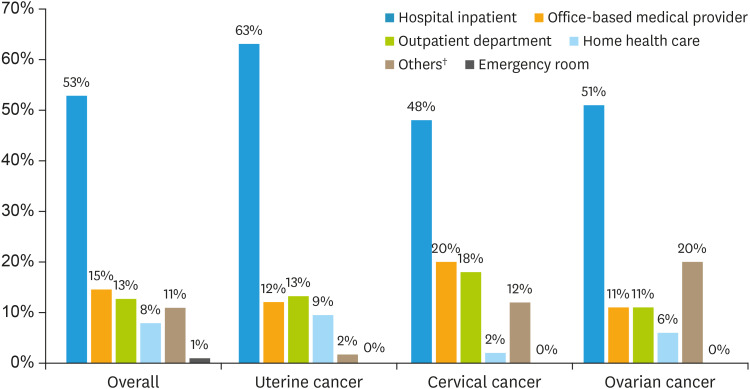
The estimated annual total medical expenditure attributed to gynecologic cancers was $3.8 billion, with an average annual cost of $6,293 per patient. The 8-year median cost for patients with gynecologic cancers were $492.6. During 2007–2014, the most considerable contributions of medical costs were hospital inpatient stays (53%, $2.03 billion), followed by office-based visits (15%, $559 million), and hospital outpatient visits (13%, $487 million) as shown in Fig. 2 and Table 2. The average annual medical expenditure for a patient with ovarian cancer was $13,566 (±$1,123), which was 2 to 6 times higher than for uterine ($6,852±$615) and cervical cancers ($2,312±$418). The median medical cost for a patient with ovarian cancer was $1,653.0 (±$489.9), which was nearly 4 times higher than that of uterine ($426.6±$42.6) and cervical cancers ($422.1±$35.6). During the same period, the largest portion of total costs on prescribed medication was apportioned to the following 3 categories: antineoplastic hormones ($217,591), analgesics ($194,118), and sex hormones ($68,841). The costs of chemotherapy were included in the expenditures of hospital inpatient stay and outpatient visit because chemotherapy requires the patients to go to the hospital to receive the treatment with help from health professionals.
Fig. 2. Sum of medical expenditures of gynecologic cancer patients by type of service and cancer type*, 2007–2014.
*All monetary values were converted to 2014 dollars using the price indices recommended by Agency for Healthcare Research and Quality; †Others medical expenditures include visual aids, medical equipment, supplies, and other medical items.
Table 2. Sum of medical expenditures of gynecologic cancer patients by type of service and cancer type, 2007–2014*.
| Costs by type of service | Overall (n=609,787) | Uterine cancer (n=221,848) | Cervical cancer (n=212,101) | Ovarian cancer (n=65,937) | ||||
|---|---|---|---|---|---|---|---|---|
| Hospital inpatient stay | $2,028,247,359 | 53% | $959,270,979 | 63% | $235,549,768 | 48% | $454,728,050 | 51% |
| Office-based medical provider | $559,358,549 | 15% | $183,164,214 | 12% | $98,811,698 | 20% | $100,637,439 | 11% |
| Outpatient department | $487,649,416 | 13% | $201,434,489 | 13% | $88,188,227 | 18% | $98,832,487 | 11% |
| Home health care | $302,984,914 | 8% | $144,385,891 | 9% | $9,132,439 | 2% | $56,619,941 | 6% |
| Emergency room | $36,702,336 | 1% | $5,340,525 | 0% | $612,547 | 0% | $1,907,735 | 0% |
| Prescription medication | $2,107,417 | 0% | $589,257 | 0% | $133,866 | 0% | $1,206,905 | 0% |
| Others† | $420,361,715 | 11% | $25,885,286 | 2% | $57,928,166 | 12% | $180,596,142 | 20% |
| Total | $3,837,411,706 | $1,520,070,641 | $490,356,711 | $894,528,699 | ||||
Number is indicated total number of patients in the US population (calculated by using Medical Expenditure Panel Survey weights) and % is indicated weighted percentage.
*All monetary values were converted to 2014 dollars using the price indices recommended by Agency for Healthcare Research and Quality; †Others medical expenditures include visual aids, medical equipment, supplies, and other medical items.
2. Treatment patterns
Types of treatments and services were analyzed from emergency department visits and ambulatory visits. An estimated 26.3% (95% confidence interval [CI]=22.1–30.5) of patients received chemotherapy, 23.4% (95% CI=17.8–29.0) of patients received radiation therapy, and 0.4% (95% CI=0.3–0.4) of patients received psychotherapy or counseling therapy. Additionally, 32.4% (95% CI=29.4–35.4), 2.5% (95% CI=1.5–3.5), 7.1% (95% CI=6.0–8.2), and 1.9% (95% CI=0.9–3.0) of patients received lab tests, sonograms or ultrasounds, MRIs or CT scans, and X-rays, respectively. Compared with other gynecologic cancer patients, ovarian cancer patients were more likely to be treated using chemotherapy (33.7% vs. 26.3%).
The results of the most frequently prescribed medications for gynecologic cancers and their expenditures are summarized in Table 3. Of 9,670 prescriptions, the most frequently prescribed medications were analgesics (39%, 95% CI=28.4–49.6), followed by sex hormones (17.1%, 95% CI=11.6–22.6), antineoplastic hormones (9.9%, 95% CI=7.2–12.6), and antiemetic/antivertigo agents (3.2%, 95% CI=1.9–4.5).
Table 3. Top 5 prescription medications frequency and expenditure of gynecologic cancer patients by drug category, 2007–2014*.
| Cancer type | Category | Rx No.† (%) | Mean (95% CI) | Sum |
|---|---|---|---|---|
| Overall | Analgesics‡ | 3,772 (39) | 51.5 (15.1, 87.9) | $194,118 |
| Sex hormones§ | 1,649 (17.1) | 41.7 (31.4, 52.1) | $68,841 | |
| Antineoplastic hormones‖ | 958 (9.9) | 227.2 (148.9,305.5) | $217,591 | |
| Antiemetic/antivertigo agents¶ | 313 (3.2) | 135.8 (90.3, 181.4) | $42,488 | |
| Antidepressants** | 276 (2.9) | 64.7 (34.5, 94.8) | $17,832 | |
| Uterine cancer | Analgesics | 1,046 (25.8) | 29.6 (21.4, 37.9) | $31,011 |
| Sex hormones | 1,005 (24.8) | 38.7 (5.48, 82.9) | $38,923 | |
| Antineoplastic hormones | 484 (11.9) | 293.2 (68.8, 517.5) | $141,786 | |
| Antidepressants | 235 (5.8) | 57.6 (57.6, 57.6) | $13,516 | |
| Iron products | 231 (5.7) | 9.1 (9.1, 9.1) | $2,107 | |
| Cervical cancer | Analgesics | 960 (51.2) | 43.7 (41.4, 45.9) | $41,894 |
| Sex hormones | 479 (25.6) | 56.9 (21.4, 92.5) | $27,276 | |
| Penicillins | 78 (4.2) | 4.4 (4.4, 4.4) | $345 | |
| Laxatives | 68 (3.6) | 35.8 (35.8, 35.8) | $2,449 | |
| Antineoplastic hormones | 60 (3.2) | 749.2 (749.2, 749.2) | $45,056 | |
| Ovarian cancer | Sex hormones | 165 (17.8) | 16.0 (16.0, 16.0) | $2,642 |
| Antiemetic/antivertigo agents | 97 (10.4) | 91.8 (−125.42, 309.0) | $8,884 | |
| Analgesics | 95 (10.2) | 52.6 (43.3, 61.8) | $4,980 | |
| Miscellaneous antineoplastics | 74 (8) | 8,201.6 (8,201.6, 8,201.6) | $607,715 | |
| Colony stimulating factors | 74 (8) | 7,540.2 (7,540.2, 7,540.2) | $558,708 |
CI, confidence interval.
*All monetary values were converted to 2014 dollars using the price indices recommended by Agency for Healthcare Research and Quality; †Total number of prescriptions; ‡Analgesics include Codeine, Oxycodone, Diclofenac, Endocet, Fentanyl, Hydrocortisone, Hydromorphone, Ibuprofen, Lortab, Methadone, Morphine, Motrin, Oxycontin, Percocet, Tylenol, and Vicodin; §Sex hormones include Climara, Esterified Estrogens/Methyltestosterone, Estradiol, Necon, Premarin, and Ortho; ‖Antineoplastic hormones include Anastrozole, Arimidex, Femara, Letrozole, Lupron Depot, Medroxyprogesterone, Megestrol, and Tamoxifen; ¶Antiemetic or antivertigo agents include Emend, Reglan, Prochlorperazine, Ondansetron, and Metoclopramide; **Antidepressants include Citalopram Hydrobromide, Duloxetine, Effexor Xr, Fluoxetine, Trazodone, and Zoloft.
High medical costs were significantly associated with being a married woman, having private health insurance, being from a low- and middle-income family, or living in the Midwest or South regions. For gynecologic cancer patients, married women paid 63% more of medical expenditures compared to unmarried women (p<0.001). Compared to patients from high-income families, patients from low- and middle-income families paid 24% more (p<0.001), as well as patients from poor or near-poor families, paid 19% less (p=0.003). Patients from the Northeast, the Midwest, and the South paid 27%, 136%, and 83% more, respectively, compared to patients living in the West (p<0.05). Moreover, patients with private health insurance paid 98% more compared to patients without private health insurance (p<0.001, Table 4).
Table 4. Survey summary of multiple log linear regression for medical expenditures for patients with gynecologic cancers (n=609,787)*.
| Predictor variable | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Age (yr) | |||||
| 18–49 | 0.85 | 0.76–0.95 | 0.006 | ||
| 50–64 | 1.27 | 1.09–1.49 | 0.003 | ||
| ≥65 | Reference | ||||
| Marital status | |||||
| Married | 1.63 | 1.44–1.86 | <0.001 | ||
| Not married | Reference | ||||
| Family income poverty level | |||||
| Poor & near poor | 0.81 | 0.70–0.93 | 0.003 | ||
| Low & middle income | 1.24 | 1.12–1.37 | <0.001 | ||
| High income | Reference | ||||
| Health insurance coverage | |||||
| Private | 1.98 | 1.68–2.33 | <0.001 | ||
| Non-private | Reference | ||||
| Census region | |||||
| Northeast | 1.27 | 1.05–1.54 | 0.015 | ||
| Midwest | 2.36 | 2.14–2.61 | <0.001 | ||
| South | 1.83 | 1.49–2.24 | <0.001 | ||
| West | Reference | ||||
| Perceived health status | |||||
| Excellent/good | 0.43 | 0.39–0.47 | <0.001 | ||
| Fair/poor | Reference | ||||
| Comorbidities | |||||
| High cholesterol | |||||
| Yes | 0.86 | 0.75–0.98 | 0.025 | ||
| No | Reference | ||||
| Diabetes | |||||
| Yes | 2.36 | 1.90–2.94 | <0.001 | ||
| No | Reference | ||||
CI, confidence interval; OR, odds ratio.
*All statistical tests were 2-sided, and all p-values were derived from regressions. A p-value of less than 0.05 means that cancer patients bear statistically significant higher economic burden. Statistically significant (p<0.05).
DISCUSSION
To our knowledge, this is the first study to estimate medical expenditures and describe treatment patterns of gynecologic cancers using recent nationally representative databases in the United States. The study results suggest that gynecologic cancers place a considerable economic burden with substantial healthcare costs. The annual medical spending attributed to gynecologic cancers was approximately $3.8 billion. Within this, uterine cancer was the most costly for the healthcare system, followed by ovarian cancer, and cervical cancer. However, the annual medical expenditure per patient with ovarian cancer was the largest, at 2 to 6 times higher than that of uterine and cervical cancers. More than half of the annual medical spending of gynecologic cancers was attributable to inpatient hospital stays. In the United States, prices of hospitalizations vary slightly determined by the type of hospital, but one inpatient day typically costs around $2,000 [22]. Thus, chemotherapy treatment has shifted from inpatient to outpatient settings such as patient homes or outpatient hospital departments. This shift occurred in the early 1990s driven by the United States government finial restriction [23]. Outpatient therapy was associated with significant savings and improved patient satisfaction. Additionally, patients with high medical costs were more likely to live in the Northeast, Midwest, or South. Prior work suggests that healthcare utilization and expenditure vary widely across the United States [24]. Individuals in high-spending regions received approximately 60% more in healthcare services than those who live in low-spending areas [25]. The Western region of the United States had lower population rate and fewer hospitals, especially specialty hospitals, than other parts of the country [26]. Patients receiving services from specialty hospitals faced far greater medical spending compared to the same care provided at non-specialty hospitals [27]. So, this may the reason that patient from Northeast, Midwest, and South had greater expenditure. This study suggests that patients with high medical costs were more likely to have private health insurance and/or were from low- and middle-income families. Compared to higher-income population, low-income women face greater barriers to receive human papillomavirus vaccination (HPV) vaccination, screening, and new drugs [28]. They are less likely to have access to primary and specialty care . In addition, high medical expenditure in lower-income groups can be mainly attributed to presentation at more advanced stages of cancer and poor treatment compliance [29,30]. Women ages 50–64 have significant higher expenditures compared to those over 65 mainly due to prevalence of cancer.
These findings are consistent with prior study [4]. Kamijo and Ichikawa [4] has examined the burden of gynecologic cancers particularly, and found the chemotherapy costs and other medical care expenditures for patients with cervical and uterine cancers. This study showed the total medical cost for each course of treatment, including supportive care and treatment for chemotoxic symptoms, ranged from $278 to $7,377. In agreement with Kamijo and Ichikawa [4] findings, our multiple regression analyses shows that the key factors related to the total medical expenditure for cervical cancer were complications and age. However, the estimated medical expenditures of gynecologic cancer patients who were newly diagnosed or at the end of their lives are lower than those reported in previous studies [31,32]. This is because health care costs are much higher at the end of life or right after cancer diagnoses than intermediate phases of care [33,34]. In our study, the cancer patient population from the MEPS database is a nationally representative sample of all the cancer patients in the United States of which more than sixty percent are estimated to live longer than 5 years after diagnosis [35]. As a result, the majority of cancer patients in the database were relatively with low-cost, intermediate phase of their cancer treatment.
Our study shows that the average medical expenditure for ovarian cancer patients was the highest compared to other gynecologic cancers. According to studies in multiple developed countries, 60%–74% of ovarian cancer patients were diagnosed with cancer in advanced stages [36]. Thus, they needed additional treatment. In addition, early detection of cancer can save people and reduce healthcare expenditures. HPV and Papanicolaou tests (pap smear) lead to a significant decline in cervical cancer and reduce healthcare costs accordingly [12].
Patients with gynecologic cancers not only experience an excessive burden as a result of their disease, but substantial medical expenditures and significant impairment to their quality of life. Additionally, the healthcare expenditure for gynecologic cancers is substantial for society and patients' families. In the near future, as the number of cancer patients grows, and more advanced treatments are used, such as immunotherapy and targeted therapies [37], the cancer medical expenditures may increase at a highest rate than overall medical expenditures. Understanding how the medical expenditures vary by gynecologic cancer type, demographic characteristics, health insurance coverage, comorbidities, and census region is important to shape healthcare policies to target areas where cancer patients are most vulnerable.
There are a few limitations to this study. First, some important clinical variables were not available in the MEPS database, including cancer stage at diagnosis and survival period [38]. The information from this database is not enough to be stratified by time since diagnosis. Second, this study relied on self-reported data from cancer patients, which may be subject to reporting bias. However, previous studies showed that there was good agreement between medical records and self-reported cancer history in MEPS [39]. Third, the use of population-based survey data may lead to an underestimation of cancers with short survival. Finally, the proportion receiving chemotherapy, lab tests, and screening tests was underestimated. Inpatient hospital services and costs were based on diagnosis-related groups, thus the database cannot provide specific treatment and medication information. Because chemotherapy treatments have shifted from inpatient to outpatient settings, there are not a lot of patients who are received chemotherapy in inpatient care. Due to this, the current study can establish general patterns of use and expense in the United States.
In conclusion, this recent national real-world data of gynecologic cancers yields substantial medical expenditures in the United States, which are associated with certain socioeconomic factors. Although the annual costs of gynecologic cancer patients are not as high as those who were newly diagnosed, the economic burden of those patients is long-lasting and considerable for many years after diagnosis. With gynecologic cancer patients estimated to increase to more than 1.5 million by 2026 in the United States [40], it is likely for the economic burden to increase. These finding may be helpful to develop gynecologic cancer prevention programs to reduces the cost of gynecologic cancers in the United States.
Appendix 1
Geographic variables
Geographic variables indicate the region. The values and states for each region included the following:
• Northeast: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont.
• Midwest: Indiana, Illinois, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin.
• South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
• West: Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming [20].
Footnotes
Presentation: Part of this information was presented as a poster at the 2018 Annual Conference of the International Society for Pharmacoeconomics and Outcomes Research in Baltimore, MD, USA.
Conflict of Interest: Dr. Hincapie, Dr. Pruemer, Dr. Almalki, and Ms. Yue have no conflicts of interest to report. Dr. Guo has received research grant or unrestricted grant funding from the following: The Ohio Department of Jobs and Family Services (Medicaid Agency), Ortho-McNeil Janssen Scientific Affairs LLC, Eli-Lilly Company, Novartis Company, and Roche-Genentech Company, none of which involved gynecologic cancers.
- Conceptualization: Y.X., H.A.L., G.J.J.
- Data curation: Y.X., A.Z.S.
- Methodology: Y.X., A.Z.S.
- Project administration: Y.X., G.J.J.
- Supervision: P.J.M., H.A.L., G.J.J.
- Validation:
- Visualization:
- Writing - original draft: Y.X.
- Writing - review & editing: Y.X., P.J.M., H.A.L., A.Z.S., G.J.J.
References
- 1.National Cancer Institute. Cancer stat facts: cervical cancer [Internet] Bethesda, MD: National Cancer Institute; 2018. [cited 2018 Dec 4]. Available from: https://seer.cancer.gov/statfacts/html/cervix.html. [Google Scholar]
- 2.National Cancer Institute. Cancer stat facts: ovarian cancer [Internet] Bethesda, MD: National Cancer Institute; 2018. [cited 2018 Dec 4]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html. [Google Scholar]
- 3.National Cancer Institute. Cancer stat facts: uterine cancer [Internet] Bethesda, MD: National Cancer Institute; 2018. [cited 2018 Dec 4]. Available from: https://seer.cancer.gov/statfacts/html/corp.html. [Google Scholar]
- 4.Kamijo Y, Ichikawa M. Cost information of chemotherapy for cervical and endometrial cancer in Japan. Jpn J Nurs Sci. 2014;11:190–199. doi: 10.1111/jjns.12020. [DOI] [PubMed] [Google Scholar]
- 5.Bosanquet N, Sikora K. The economics of cancer care in the UK. Lancet Oncol. 2004;5:568–574. doi: 10.1016/S1470-2045(04)01569-4. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer facts & figures 2018 [Internet] New York, NY: American Cancer Society; 2018. [cited 2019 Dec 26]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. [Google Scholar]
- 7.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angioli R, Capriglione S, Aloisi A, Miranda A, de Cicco Nardone C, Terranova C, et al. Economic impact among family caregivers of patients with advanced ovarian cancer. Int J Gynecol Cancer. 2015;25:1541–1546. doi: 10.1097/IGC.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Economic impact of cancer [Internet] New York, NY: American Cancer Society; 2018. [cited 2018 Dec 4]. Available from: https://www.cancer.org/cancer/cancer-basics/economic-impact-of-cancer.html. [Google Scholar]
- 10.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JJ, Egorova N, Franco R, Prasad-Hayes M, Bickell NA. Ovarian cancer treatment and survival trends among women older than 65 years of age in the United States, 1995–2008. Obstet Gynecol. 2016;127:81–89. doi: 10.1097/AOG.0000000000001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjalma WA, Kim E, Vandeweyer K. The impact on women's health and the cervical cancer screening budget of primary HPV screening with dual-stain cytology triage in Belgium. Eur J Obstet Gynecol Reprod Biol. 2017;212:171–181. doi: 10.1016/j.ejogrb.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Max W, Rice DP, Sung HY, Michel M, Breuer W, Zhang X. The economic burden of gynecologic cancers in California, 1998. Gynecol Oncol. 2003;88:96–103. doi: 10.1016/s0090-8258(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 14.Insinga RP, Ye X, Singhal PK, Carides GW. Healthcare resource use and costs associated with cervical, vaginal and vulvar cancers in a large U.S. health plan. Gynecol Oncol. 2008;111:188–196. doi: 10.1016/j.ygyno.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 16.Guy GP, Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Yabroff KR, Guy GP, Jr, Han X, Li C, Banegas MP, et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst. 2015;108:djv382. doi: 10.1093/jnci/djv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: survey background [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2019. [cited 2019 Sep 25]. Available from: https://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp. [Google Scholar]
- 19.Phillips KA, Morrison KR, Andersen R, Aday LA. Understanding the context of healthcare utilization: assessing environmental and provider-related variables in the behavioral model of utilization. Health Serv Res. 1998;33:571–596. [PMC free article] [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality. MEPS HC-171 2014 full year consolidated data file [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2016. [cited 2019 Sep 24]. Available from: https://meps.ahrq.gov/data_stats/download_data/pufs/h171/h171doc.pdf. [Google Scholar]
- 21.U.S. Bureau of Labor Statistics. CPI inflation calculator [Internet] Washington, D.C.: U.S. Bureau of Labor Statistics; 1913. [cited 2019 Dec 26]. Available from: http://data.bls.gov/cgi-bin/cpicalc.pl. [Google Scholar]
- 22.Rappleye E. Average cost per inpatient day across 50 states [Internet] Chicago, IL: Becker's Hospital Review; 2015. [cited 2019 Dec 25]. Available from: https://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states.html. [Google Scholar]
- 23.Barbor M. Transitioning from inpatient to outpatient chemotherapy saves money, increases patient satisfaction [Internet] Cranbury, NJ: The Oncology Pharmacist; 2017. [cited 2019 Dec 26]. Available from: http://theoncologypharmacist.com/top-issues/2017-issues/february-2017-vol-10-no-1/17001-transitioning-from-inpatient-to-outpatient-chemotherapy-saves-money-increases-patient-satisfaction. [Google Scholar]
- 24.Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310:1227–1228. doi: 10.1001/jama.2013.278139. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 26.Freeman WJ, Weiss AJ, Heslin KC. Overview of U.S. hospital stays in 2016: variation by geographic region [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2018. [cited 2019 Dec 25]. Available from: www.hcup-us.ahrq.gov/reports/statbriefs/sb246-Geographic-Variation-Hospital-Stays.pdf. [PubMed] [Google Scholar]
- 27.America's Health Insurance Plans (AHIP) Care significantly more expensive at specialty hospitals vs. non-specialty hospitals during 2014 [Internet] Washington, D.C.: America's Health Insurance Plans; 2017. [cited 2019 Dec 25]. Available from: https://www.ahip.org/specialty-hospitals-report. [Google Scholar]
- 28.Lobb R, Ayanian JZ, Allen JD, Emmons KM. Stage of breast cancer at diagnosis among low-income women with access to mammography. Cancer. 2010;116:5487–5496. doi: 10.1002/cncr.25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalkidou K, Marquez P, Dhillon PK, Teerawattananon Y, Anothaisintawee T, Gadelha CA, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol. 2014;15:e119–e131. doi: 10.1016/S1470-2045(13)70547-3. [DOI] [PubMed] [Google Scholar]
- 30.Bukowski A, Chávarri-Guerra Y, Goss PE. The potential role of patient navigation in low- and middle-income countries for patients with cancer. JAMA Oncol. 2016;2:994–995. doi: 10.1001/jamaoncol.2016.0766. [DOI] [PubMed] [Google Scholar]
- 31.Urban RR, He H, Alfonso R, Hardesty MM, Goff BA. The end of life costs for Medicare patients with advanced ovarian cancer. Gynecol Oncol. 2018;148:336–341. doi: 10.1016/j.ygyno.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Margolis B, Chen L, Accordino MK, Clarke Hillyer G, Hou JY, Tergas AI, et al. Trends in end-of-life care and health care spending in women with uterine cancer. Am J Obstet Gynecol. 2017;217:434.e1–434.e10. doi: 10.1016/j.ajog.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ, Teitelbaum AH. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s–80s. doi: 10.1200/JOP.2011.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 35.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol. 2012;127:75–82. doi: 10.1016/j.ygyno.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20:2006–2014. doi: 10.1158/1055-9965.EPI-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20:2006–2014. doi: 10.1158/1055-9965.EPI-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harlow SD, Linet MS. Agreement between questionnaire data and medical records. The evidence for accuracy of recall. Am J Epidemiol. 1989;129:233–248. doi: 10.1093/oxfordjournals.aje.a115129. [DOI] [PubMed] [Google Scholar]
- 40.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]