Abstract
Objective
The impact of systematic retroperitoneal lymphadenectomy (SRL) remains controversial in patients with advanced ovarian clear-cell carcinoma (CCC) who are optimally debulked.
Methods
Between 1986 and 2017, a total of 3,227 women with epithelial ovarian carcinoma were analyzed in a multi-institutional study. Among them, 166 optimally debulked women with stage IIB–IV CCC were collected (residual tumor of <1 cm). All patients were divided into 2 groups: 1) Group I (n=112): underwent standard radical surgery with SRL, 2) Group II (n=54): underwent non-staging limited surgery. The pathological slides were assessed based on central pathological review. Oncologic outcomes were compared between the two groups using a propensity score (PS)-matching technique to adjust for various clinicopathologic factors.
Results
The median follow-up duration of all surviving women was 52.8 (1.6–184.2) months. Overall, 88 patients (53.0%) experienced recurrence and 68 patients (41.0%) died of the disease. In the original cohort, the 5-year overall survival (OS) rates of groups I and II were 57.9 and 64.9%, respectively (log-rank p=0.415). In the PS-adjusted cohort, the 5-year OS rates were 64.9 and 58.8% in women in groups I and II, respectively (p=0.453). Furthermore, in the PS-matched cohort after adjustment for multiple clinicopathologic factors, there was no significant difference in OS between the 2 groups (group I vs. group II; hazard ratio=1.170; 95% confidence interval=0.633–2.187; p=0.615).
Conclusions
This study suggests that the performance of SRL including radical surgery may not lead to a significant improvement in the oncologic outcome of advanced CCC patients with optimal cytoreduction.
Keywords: Adenocarcinoma, Clear Cell; Lymphadenectomy; Recurrence; Survival; Propensity Score
INTRODUCTION
Ovarian clear-cell carcinoma (CCC) is a relatively rare subtype of epithelial ovarian cancer (EOC), accounting for less than 10% of EOC diagnosed in Western countries. On the other hand, this tumor was reported to be a frequent histological type of all EOC in Asian countries [1,2,3]. Based on prior studies, CCC is diagnosed at an earlier stage, frequently presents with unilateral occurrence, exhibits complication with thromboembolism, and is associated with endometriosis [4,5,6,7]. Moreover, earlier larger-scale studies demonstrated potential chemoresistance to conventional platinum-based compounds, resulting in an unfavorable oncologic outcome of women with advanced-stage CCC in comparison with those with serous carcinoma [8,9]. The standard surgery for women with CCC includes hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and comprehensive surgical staging including retroperitoneal lymphadenectomy. Needless to say, complete tumor debulking without any residual tumor is of marked importance. Nevertheless, the major argument is whether such systematic removal of retroperitoneal lymph nodes contributes to the complete elimination of occult clones and subsequent survival benefit, regardless of its feasibility in clinical practice.
Here, we accumulated 166 women with advanced-stage CCC with optical cytoreduction from multi-institutions. The question of whether the performance of systematic retroperitoneal lymphadenectomy (SRL) influences the long-term oncologic outcome of patients with this tumor was investigated. In the present study, we investigated the clinical significance of SRL for advanced CCC to help make an appropriate therapeutic decision for women with advanced CCC.
MATERIALS AND METHODS
1. Patient cohort
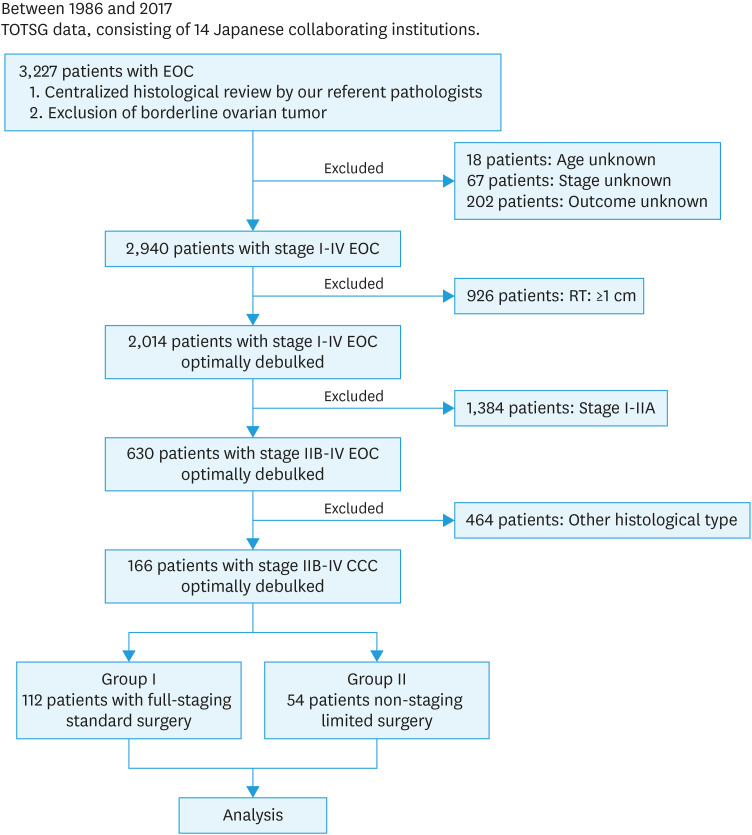
Between 1986 and 2017, a total of 3,227 women with EOC were collected by the Tokai Ovarian Tumor Study Group, consisting of Nagoya University Hospital and 13 affiliated institutions cooperating under a central pathological review system. Patients were eligible if they fulfilled the following: 1) diagnosed with stage IIB-IV pure type CCC due to typical hobnail or clear cells growing in a tubulocystic, solid, or papillary pattern in more than 90% of all pathological specimens, based on a central pathologic review system, 2) had sufficient clinical data, including the details of initial surgery and oncologic outcome, and 3) underwent optimal cytoreduction (residual tumor of <1 cm) in the initial surgery. Overall, 287 women without sufficient data on the residual tumor were excluded (18: age unknown, 67: stage unknown, and 202: outcome unknown). Furthermore, 1,384 with stage I–IIA and 926 patients with residual tumors of ≥1 cm were excluded from this study. From these data, consequently, 166 patients with stage IIB–IV CCC who were optimally debulked were finally extracted and analyzed, including 112 patients who had received standard surgery with complete SRL (group I) and 54 who had undergone non-staging limited surgery (group II) (Fig. 1). Patient allocation to each group was based on the comprehensive clinical decision by surgeon's and/or institution's discretion.
Fig. 1. Patient flowchart.
CCC, clear-cell carcinoma; EOC, epithelial ovarian carcinoma; TOTSG, Tokai Ovarian Tumor Study Group.
Data were collected from medical records and clinical follow-up visits. The clinical stage was assigned based on the International Federation of Gynecology and Obstetrics (FIGO 1988). The histological cell types were assigned according to the criteria of the World Health Organization (WHO) staging system [10]. Histological slides were reviewed by gynecologic pathologists under a central pathological review system with no knowledge of the patients' clinical data. This study was approved by the Ethics Committee of Nagoya University (approval number: 357-3).
2. Treatments
The standard operation in patients who underwent the standard surgery with complete SRL (group I) was standard radical surgery, principally including hysterectomy and bilateral salpingo-oophorectomy with complete staging surgery. Complete staging surgery was defined as peritoneal staging and systematic pelvic and para-aortic lymphadenectomy. Exploration of regional lymph nodes included systemic lymphadenectomy, the removal of palpable nodes, or the removal of all lymphatic tissue surrounding the retroperitoneal vessels, in the absence of clinically obvious disease. Para-aortic lymph node dissection was performed from the bifurcation of the aorta to the origin of the renal vessels. Pelvic node dissection was done from the common, internal and external iliac, and obturator vessels to the femoral ring. On the other hand, the standard operation in patients belonging to group II was uni- or bilateral salpingo-oophorectomy, with or without hysterectomy and omentectomy, and without SRL. However, in those group II patients, the locally swollen lymph nodes more than 1 cm in diameter confirmed by a preoperative computed tomography (CT) were appropriately resected. In all patients, peritoneal staging, defined as peritoneal exploration, cytology, biopsy, and/or omentectomy was routinely carried out. Of all patients, 160 (96.4%) were treated postoperatively with adjuvant chemotherapy. Six women (3.6%) did not undergo adjuvant chemotherapy for individual reasons. Details of each prior first-line chemotherapy regimen were as follows: CAP (cyclophosphamide [300 mg/m2], adriamycin [30 mg/m2], and cisplatin [70 mg/m2]) (1986–1989); CAP or PVB (cisplatin [70 mg/m2], vinblastine [6 mg/m2], and bleomycin [12 mg/m2]) (1989–1991); PVB or PP (carboplatin [300 mg/m2] and cisplatin [70 mg/m2]) (1992–2000); TC (paclitaxel [180 mg/m2] and carboplatin [area under curve; AUC=5–6]) (2000–2002); TC or DC (docetaxel [70 mg/m2] and carboplatin [AUC=5–6]) (2003–2013); TC or DC with or without bevacizumab (15 mg/kg) (2013–) [11].
3. Follow-up and analysis
Patients' follow-up protocol was described previously [12]. Briefly, all patient follow-up was conducted at an outpatient clinic at each institution from the end of treatment. In principle, it was done every 1–3 months during the first-second year, every 3–6 months during the third to fifth years, and annually thereafter. Follow-up procedures included serum cancer antigen 125 (CA125) evaluation, gynecologic examination, and ultrasonography. In principle, evaluation with CT was repeated every 6–12 months during the first 2 years and once a year thereafter, and/or when the physician considered it necessary. Progression-free survival (PFS) was defined as between the date of initial treatment and the last date of follow-up or recurrence. Overall survival (OS) was defined as the time interval between the date of initial treatment and the last date of follow-up or death from any cause.
To balance the clinicopathologic characteristics between the 2 groups, propensity score (PS) matching was performed [13]. PS was calculated by multivariate logistic regression models for the probability of the aforementioned standard surgery with SRL adjusting for the age, stage (II vs. III–IV), preoperative CA125 value (≤35 vs. >35 U /mL), ascites volume (<100 vs. ≥100 mL), and type of chemotherapy (taxane plus platinum [TP] vs. non-TP). Patients in group I were matched with those in group II based on PS, resulting in an even distribution of potential confounding factors in both groups. Then, outcomes were analyzed by PS-matched cohorts to balance covariates that might confound the effect of surgical modalities on OS and PFS. Patients who received full-staging surgery were matched to those who received non-full-staging surgery by nearest neighbor matching.
Within the original (unmatched) and PS-matched cohorts, Kaplan-Meier survival curves were generated. The survival curves were compared using the Log-rank test. A Cox proportional hazards regression model was employed to investigate associations between the surgical modality (group I vs. group II) and OS/PFS. The distributions of clinicopathologic factors were evaluated using the χ2 test. The p<0.05 was considered significant.
RESULTS
1. Patients' characteristics
Patients' characteristics are shown in Table 1. In total, 166 women were identified for the current analysis. There were 112 patients (67.5%) who underwent full-staging standard surgery with SRL (group I) and 54 patients (32.5%) who received non-staging limited surgery (group II). All patients excluding two cases in the Group I underwent complete surgery (no residual tumor). The median follow-up duration of all surviving women was 52.8 (1.6–184.2) months. The median follow-up of patients in the group I and II groups was 54.0 (5.1–184.2) months and 50.4 (1.6–159.8) months; respectively (p=0.393). The median±standard deviation ages of patients in Groups I and II were 55.0±9.6 and 56.5±12.3 years, respectively. There was no significant difference in the age distribution between patients who belonged to group I and those in group II (p=0.350). Among the 112 women in group I, 53 (47.3%) had stage II disease, and 52 (46.4%) had stage III disease. Among the total of 54 patients in group II, 25 patients (46.3%) had stage II disease, and 23 (42.6%) had stage III disease. Our current study included 13 (7.8%) stage IV patients with positive pleural effusion and/or a limited metastasis to the parenchymal organ. Those who had a limited metastasis in the parenchymal organ received complete resection. With regard to the distribution of the stage, the preoperative CA125 value, volume of ascites, and type of chemotherapy, no differences between the two cohorts were identified (Table 1).
Table 1. Patients' characteristics, original cohort.
| Characteristics | Total | Group I, No. (%) | Group II, No. (%) | p-value | |
|---|---|---|---|---|---|
| No. of patients | 166 | 112 | 54 | ||
| Age (median/mean/standard deviation) | 55/53.8/9.6 | 56.5/55.5/12.3 | 0.350 | ||
| FIGO substage (1988) | |||||
| IIB | 8 | 8 (7.1) | 0 | 0.163 | |
| IIC | 70 | 45 (40.2) | 25 (46.3) | ||
| IIIA | 14 | 8 (7.1) | 6 (11.1) | ||
| IIIB | 9 | 5 (4.5) | 4 (7.4) | ||
| IIIC (T3N0) | 31 | 29 (25.9) | 2 (3.7) | ||
| IIIC (TXN1) | 21 | 10 (8.9) | 11 (20.4) | ||
| IV | 13 | 7 (6.3) | 6 (11.1) | ||
| Initial surgery | <0.001 | ||||
| TH+BSO+OM+SRL | 112 | 112 (100.0) | 0 | ||
| TH+BSO±OM | 41 | 0 | 41 (75.9) | ||
| TH+USO±OM | 3 | 0 | 3 (5.6) | ||
| BSO±OM | 2 | 0 | 2 (3.7) | ||
| USO±OM+RPN | 1 | 0 | 1 (1.9) | ||
| USO±OM | 4 | 0 | 4 (7.4) | ||
| Tumorectomy±OM | 3 | 0 | 3 (5.6) | ||
| Ascites volume (mL) | 0.682 | ||||
| <100 | 126 | 84 (75.0) | 42 (77.8) | ||
| 100–499 | 22 | 17 (15.2) | 5 (9.3) | ||
| 500–1,000 | 6 | 4 (3.6) | 2 (3.7) | ||
| >1,000 | 12 | 7 (6.3) | 5 (9.3) | ||
| Preoperative CA125 value (U/mL) | 0.363 | ||||
| ≤35 | 36 | 22 (19.6) | 14 (25.9) | ||
| >35 | 130 | 90 (80.4) | 40 (74.1) | ||
| Chemotherapy | 0.589 | ||||
| None | 6 | 3 (2.7) | 3 (5.6) | ||
| TP | 122 | 82 (73.2) | 40 (74.1) | ||
| Non-TP | 38 | 27 (24.1) | 11 (20.4) | ||
Group I is patients with full-staging standard surgery and group II is patients with non-staging limited surgery.
BSO, bilateral salpingo-oophorectomy; CA125, cancer antigen 125; FIGO, Internatinal Federation of Gynecology and Obstetrics; NA, not applicable; OM, omentectomy; RPN, retroperitoneal lymphadenectomy; SRL, systematic retroperitoneal lymphadenectomy; TH, total hysterectomy; TP, taxane plus platinum; USO, unilaretal salpingo-oophorectomy.
2. Survival analyses using the unmatched cohort
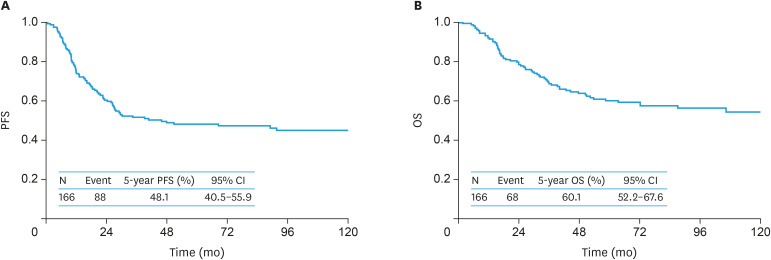
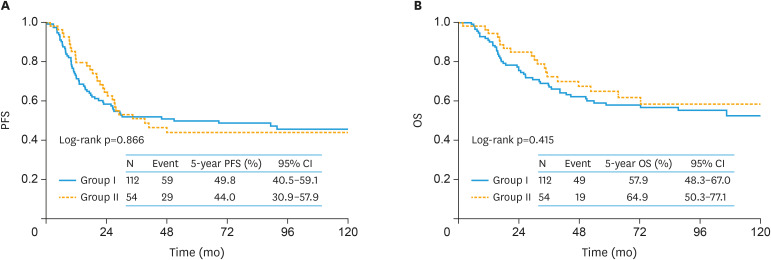
During the follow-up of a total of 166 women, 88 (53.0%) experienced recurrence. Consequently, 68 (41.0%) died of the disease. Recurrence was observed in 59 (52.7%) women in group I and 29 (53.7%) women in Group II. Death was noted in 49 (43.7%) women in group I and 19 (35.1%) women in group II. In the original cohort, the 5-year OS and PFS (95% confidence interval [CI]) rates of all enrolled women were 60.1% (52.2–67.6) and 48.1% (40.5–55.9), respectively (Fig. 2). On stratification by patient group, the 5-year PFS (95% CI) rates of groups I and II were 49.8% (40.5–59.1) and 44.0% (30.9–57.9), respectively. There was no significant difference in PFS between the 2 groups (log-rank p=0.866) (Fig. 3A). Furthermore, the 5-year OS (95% CI) rates of Groups I and II were 57.9% (48.3–67.0) and 64.9% (50.3–77.1), respectively (Fig. 3B) (p=0.415).
Fig. 2. The original cohort. Kaplan-Meier-estimated PFS (A) and OS (B) curves of all enrolled patients (n=166).
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
Fig. 3. The original cohort. Kaplan-Meier-estimated survival curves on stratifying by the surgical type (group I [n=112] vs. group II [n=54]).
Group I is patients who received standard surgery with complete SRL and group II is patients who underwent non-staging limited surgery.
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
3. Survival analyses using the PS-matched cohort
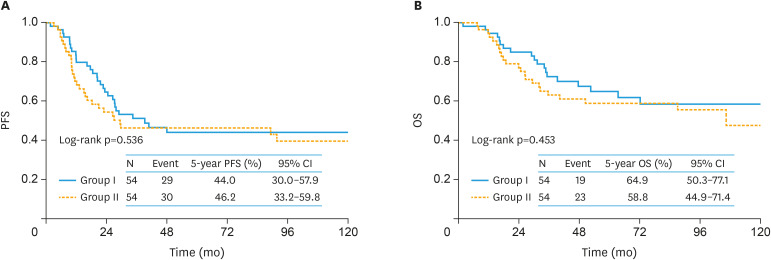
Overall, 108 matched pairs were generated using PS-matching. Supplementary Table 1 summarizes patients' characteristics after matching. All variables were well-balanced after PS-matching. Kernel density plots demonstrated that the distribution of PS was also well-balanced between the two subgroups after the matching (Supplementary Fig. 1). In the PS-matching cohort, the 5-year PFS (95% CI) rate was 44.0% (30.0–57.9) in group I patients and 46.2% (33.2–59.8) in group II patients (Fig. 4A). Thus, after the PS-matching, no significant difference was seen in PFS between the 2 surgical groups (log-rank p=0.536). Similarly, in the PS-adjusted cohort, the 5-year OS rates were 64.9 and 58.8% in women in groups I and II, respectively. There was no significant difference in OS between them (log-rank p=0.453) (Fig. 4B).
Fig. 4. The PS-matching cohort. Kaplan-Meier-estimated survival curves on stratifying by the surgical type (group I [n=54] vs. group II [n=54]).
Group I is patients who received standard surgery with complete SRL and group II is patients who underwent non-staging limited surgery.
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
Moreover, in Cox multivariable analysis for OS with or without PS adjustment, including the type of surgery, age (≤55 vs. >55 years), FIGO stage (II vs. III–IV), type of chemotherapy, preoperative CA125 value (≤35 vs. >35 U/mL), and surgical modality were not significant predictors of the risk of mortality (original cohort: group II vs. group I, adjusted HR=0.846; 95% CI=0.703–2.064; p=0.538 and PS-matching cohort: group II vs. group I, adjusted HR=1.170; 95% CI=0.633–2.187; p=0.615) (Table 2).
Table 2. Multivariable Cox hazard model in relation to OS.
| Variables | Original cohort | PS-matching cohort | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age (yr) | |||||||
| ≤55 | Reference | 0.464 | Reference | 0.194 | |||
| >55 | 0.835 | 0.514–1.350 | 0.664 | 0.355–1.228 | |||
| Surgery | |||||||
| Group I | Reference | 0.538 | Reference | 0.615 | |||
| Group II | 0.846 | 0.703–2.064 | 1.170 | 0.633–2.187 | |||
| FIGO stage | |||||||
| II | Reference | 0.035 | Reference | 0.674 | |||
| III–IV | 1.706 | 1.044–2.854 | 1.142 | 0.616–2.167 | |||
| CA125 value (U/mL) | |||||||
| ≤35 | Reference | 0.168 | Reference | 0.175 | |||
| >35 | 1.577 | 0.834–3.304 | 1.761 | 0.792–4.673 | |||
| Chemotherapy | |||||||
| Non-TP | Reference | 0.497 | Reference | 0.242 | |||
| TP | 0.832 | 0.500–1.433 | 0.672 | 0.357–1.325 | |||
Group I is patients with full-staging standard surgery and group II is patients with non-staging limited surgery.
CA125, cancer antigen 125; CI, confidence interval; HR, hazard ratio; FIGO, Internatinal Federation of Gynecology and Obstetrics; OS, overall survival; PS, propensity score; TP, taxane plus platinum.
DISCUSSION
Involvement of retroperitoneal lymph nodes occurs in approximately more than 50% of patients with advanced EOC [14]. Whether the removal of all nodes by lymphadenectomy eliminates occult metastasis is controversial, despite the possibility that they may be seeds of recurrence. Several prior reports demonstrated a potential survival benefit of systematic pelvic and para-aortic lymphadenectomy in women with macroscopically completely resected advanced EOC [15,16,17,18]. In contrast, Panici et al. [19] conducted a randomized controlled trial (RCT) of women with optimally debulked advanced EOC, investigating whether SRL improved PFS and OS, compared with resection of bulky nodes only. In their study, in spite of the fact that the performance of SRL improves PFS (median: 29.4 months [SRL group] and 22.4 months [control group], 95% CI=1.0–14.4 months, respectively), it did not contribute to significantly improved OS (median: 58.7 months [SRL group] and 56.3 months [control group], 95% CI=−11.8 to 21.0 months, respectively) [19]. Furthermore, recently, Harter and colleagues conducted a randomized trial involving women with macroscopically complete resection of advanced EOC and clinically negative lymph nodes (LION study). In that study, a total of 647 patients underwent randomization, were assigned to the lymphadenectomy group (n=323) or non-lymphadenectomy group (n=324). The median OS was 69.2 months in the non-lymphadenectomy group and 65.5 months in the lymphadenectomy group, and median PFS was 25.5 months in both groups (both analyses: no significant). Therefore, they concluded that SRL was not associated with better oncologic outcomes than no lymphadenectomy, but was associated with a higher incidence of postoperative complications [20]. Nevertheless, one of the weaknesses of this evidence comes from the ununiformity of the tumors' histological type. The heterogeneity of EOC is now the biggest challenge in all relevant studies. Particularly, in the LION study, the number of patients with CCC was very limited (lymphadenectomy group vs. non-lymphadenectomy group, 7 [2.2%] vs. 7 [2.2%]) [20]. Despite the existence of a number of above-mentioned studies regarding SRL for EOC, no study has focused on the clinical outcome in women with advanced CCC receiving SRL.
One of the most fundamental questions was whether choosing SRL alters the oncologic outcome of patients with advanced CCC receiving optimal cytoreduction; in other words, whether aggressive radical surgery with SRL contributes to prolongation of the survival time. In this retrospective investigation, we examined a large number of advanced-stage CCC women with optimal cytoreduction to investigate the benefit of SRL. We demonstrated that the 5-year PFS/OS rates in the two cohorts were 44.0%/64.9% (SRL group I) and 58.8%/46.2% (non-SRL group), showing no significant difference (log-rank p=0.453 and p=0.536 for OS and PFS, respectively). Indeed, complete lymphadenectomy may contribute to improvement of the survival time by removing occult nodal metastasis that was hardly detected on routine pathologic analyses. However, this surgical procedure itself may not be a prognostic indicator of the oncologic outcome. It is possible that there are further micrometastases expanding to other lymph nodes or distant organs via numerous lymph vessels, even when we identify solitary nodal metastasis. On the other hand, according to an earlier retrospective study, multivariable analysis demonstrated that the existence of a residual tumor was an independent prognostic indicator in women with advanced-stage CCC [21]. Actually, due to potential chemoresistance to conventional platinum-based compounds of CCC [8,9], it is extremely difficult to treat patients with this tumor by chemotherapy alone. Thus, we think that, to remove the residual tumor as thoroughly possible, the resection of bulky nodal metastasis is of importance. In this context, we did not refute the utility of surgical resection of bulky enlarged nodes aiming for complete or optimal cytoreduction.
Even if displaying no significant difference in oncologic outcomes, clinicopathologic backgrounds were inconsistent between the 2 populations. Recently, abundant evidence demonstrated the effectiveness of a PS-matching technique as an alternative to an RCT [13,22,23,24,25]. Thus, our current study evaluated the effect of aggressive standard surgery with full staging, comparing two cohorts using the PS-matching technique. As a result of the fact that those confounders were well-balanced between the two surgical cohorts, comparison between the 2 surgical cohorts revealed no significant difference in OS rates (log-rank p=0.453). Accordingly, the current PS-matching study provides evidence that the performance of SRL did not necessarily contribute to better OS. At least, on considering clinical information on how the oncologic outcome is influenced with or without SRL, our current work may be helpful for physicians and patients to estimate risk-and-benefit before adopting this surgical procedure. We need to investigate this in a multi-institutional study recruiting a larger population of patients.
The current study includes several limitations, since it was essentially retrospective, various clinicopathologic factors relevant to clinical decisions were not as strictly controlled as they would be in an RCT. Next, because it was a long-term multi-institutional study, the composition of the study subjects might have been influenced by referral bias. Lastly, several crucial data were not provided, including the completeness of SRL e.g., the number of resected lymph nodes, which may have affected the reliability of the estimated PS. In addition, the absence of a significant difference may be merely attributable for lack of power caused by the limited patient number. We need to investigate this in a multi-institutional study recruiting a larger population of patients. In contrast, as the strengths of our study: firstly, the practice of central pathological review by expert gynecologic pathologists, and secondly, the same chemotherapeutic protocol and criteria as for the identical study group. Although our current work is a hypothesis-generating study, including many limitations, the main clinical utility of our study may be in the area of preoperative counseling regarding surgical aggressiveness and expected prognosis in the future. Thus, we do not think that the data will immediately result in the omission of SRL. We hope to reassess and verify the present results in a future trial to shed further light on the appropriate strategy to treat patients with CCC.
In summary, we examined the fundamental question of how much less radical limited surgery is associated with recurrence. We hypothesized that advanced-stage CCC women who do not receive SRL may not show a prognosis any less favorable than that of those undergoing this surgical procedure. However, our current study had several limitations, including a non-prospective, exploratory study, lack of sufficient power, heterogeneous treatment modalities, and different follow-up periods. The significance of SRL for these patients should be appropriately assessed in subsequent prospective trials. We hope that our hypothesis will be further supported by collecting a larger number of CCC women through a future patient registry system.
ACKNOWLEDGEMENTS
We sincerely thank Drs. Y. Kimio Mizuno (Nagoya First Red Cross Hospital), K. Sakakibara (Okazaki Municipal Hospital), S. Yamamuro (Nagoya Second Red Cross Hospital), T. Misawa (Nagoya Ekisaikai Hospital), M. Kawai (Toyohashi Municipal Hospital), H. Oguchi (Toyota Memorial Hospital), and T. Suzuki (Anjyo Kosei Hospital) who collaborated in data collection. We sincerely thank Dr. Tetsuro Nagasaka (Nagoya University) who collaborated in central pathological review.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.H.
- Formal analysis: K.H.
- Investigation: K.H.
- Project administration: K.H.
- Resources: S.S., Y.N., T.S.
- Supervision: S.K., K.F.
- Validation: K.H.
- Writing - original draft: K.H.
- Writing - review & editing: K.H.
SUPPLEMENTARY MATERIALS
Patients' characteristics, PS-matching cohort
Frequency and Kernel density plots to depict the pre- (A) and the post- (B) PS-matching adjustment distribution of the PS in each treatment group.
References
- 1.Yamagami W, Aoki D. Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:167–177. doi: 10.1111/jog.12596. [DOI] [PubMed] [Google Scholar]
- 2.Suh DH, Park JY, Lee JY, Kim BG, Lim MC, Kim JW, et al. The clinical value of surgeons' efforts of preventing intraoperative tumor rupture in stage I clear cell carcinoma of the ovary: a Korean multicenter study. Gynecol Oncol. 2015;137:412–417. doi: 10.1016/j.ygyno.2015.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, Liu Y, Wang X, Guan L, Chen W, Jiang H, et al. Clear cell carcinoma of the ovary: Clinicopathologic features and outcomes in a Chinese cohort. Medicine (Baltimore) 2018;97:e10881. doi: 10.1097/MD.0000000000010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Jenison EL, Montag AG, Griffiths CT, Welch WR, Lavin PT, Greer J, et al. Clear cell adenocarcinoma of the ovary: a clinical analysis and comparison with serous carcinoma. Gynecol Oncol. 1989;32:65–71. doi: 10.1016/0090-8258(89)90852-4. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy AW, Biscotti CV, Hart WR, Webster KD. Ovarian clear cell adenocarcinoma. Gynecol Oncol. 1989;32:342–349. doi: 10.1016/0090-8258(89)90637-9. [DOI] [PubMed] [Google Scholar]
- 7.Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007;97:1053–1057. doi: 10.1038/sj.bjc.6603989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 9.Mizuno M, Kikkawa F, Shibata K, Kajiyama H, Ino K, Kawai M, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94:138–143. doi: 10.1002/jso.20251. [DOI] [PubMed] [Google Scholar]
- 10.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Kajiyama H, Shibata K, Ino K, Nawa A, Sakakibara K, et al. Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann Oncol. 2008;19:1284–1287. doi: 10.1093/annonc/mdn059. [DOI] [PubMed] [Google Scholar]
- 12.Kajiyama H, Suzuki S, Utsumi F, Yoshikawa N, Nishino K, Ikeda Y, et al. Comparison of long-term oncologic outcomes between metastatic ovarian carcinoma originating from gastrointestinal organs and advanced mucinous ovarian carcinoma. Int J Clin Oncol. 2019;24:950–956. doi: 10.1007/s10147-019-01438-6. [DOI] [PubMed] [Google Scholar]
- 13.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 14.Morice P, Joulie F, Camatte S, Atallah D, Rouzier R, Pautier P, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003;197:198–205. doi: 10.1016/S1072-7515(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 15.Aletti GD, Dowdy S, Podratz KC, Cliby WA. Role of lymphadenectomy in the management of grossly apparent advanced stage epithelial ovarian cancer. Am J Obstet Gynecol. 2006;195:1862–1868. doi: 10.1016/j.ajog.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Chan JK, Urban R, Hu JM, Shin JY, Husain A, Teng NN, et al. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: a study of 13918 patients. Br J Cancer. 2007;96:1817–1822. doi: 10.1038/sj.bjc.6603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.di Re F, Baiocchi G, Fontanelli R, Grosso G, Cobellis L, Raspagliesi F, et al. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: prognostic significance of node metastases. Gynecol Oncol. 1996;62:360–365. doi: 10.1006/gyno.1996.0249. [DOI] [PubMed] [Google Scholar]
- 18.Scarabelli C, Gallo A, Visentin MC, Canzonieri V, Carbone A, Zarrelli A. Systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer patients with no residual intraperitoneal disease. Int J Gynecol Cancer. 1997;7:18–26. doi: 10.1046/j.1525-1438.1997.00418.x. [DOI] [PubMed] [Google Scholar]
- 19.Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 20.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380:822–832. doi: 10.1056/NEJMoa1808424. [DOI] [PubMed] [Google Scholar]
- 21.Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hullsiek KH, Louis TA. Propensity score modeling strategies for the causal analysis of observational data. Biostatistics. 2002;3:179–193. doi: 10.1093/biostatistics/3.2.179. [DOI] [PubMed] [Google Scholar]
- 23.Mitra N, Indurkhya A. A propensity score approach to estimating the cost-effectiveness of medical therapies from observational data. Health Econ. 2005;14:805–815. doi: 10.1002/hec.987. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med. 1991;115:901–905. doi: 10.7326/0003-4819-115-11-901. [DOI] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients' characteristics, PS-matching cohort
Frequency and Kernel density plots to depict the pre- (A) and the post- (B) PS-matching adjustment distribution of the PS in each treatment group.