Abstract
Objective
To examine outcomes in a modern treatment era for stage III uterine serous carcinoma (USC).
Methods
Fifty women were retrospectively identified as 2009 International Federation of Gynecology and Obstetrics stage III USC patients who received radiotherapy (RT) at our institution between 1/2003–5/2018. The patients were divided into 2 cohorts: 20 in the early era (2003–2010) and 30 in the modern era (2011–2018). Patient characteristics were compared using χ2 tests for categorical variables and t-tests for continuous variables. Recurrence free survival (RFS) and overall survival (OS) were analyzed with Kaplan-Meier estimates, the log-rank test, and Cox proportional hazards.
Results
The modern era differed from the early era in the increased use of volume-directed external beam RT (EBRT) as opposed to vaginal brachytherapy (VB) alone (33.3% vs 5.0%, p=0.048), minimally invasive surgery (56.7% vs. 25%, p=0.027), sentinel node sampling (26.7% vs. 0%, p=0.012), computed tomography imaging in the perioperative period (63.3% vs. 30%, p=0.044), and human epidermal growth factor receptor 2/neu testing (96.7% vs. 55%, p=0.001). Median follow-up for early and modern eras was 37.27 and 33.23 months, respectively. The early vs. modern 3-year RFS was 33% and 64% (p=0.039), respectively, while the 3-year OS was 55% and 90% (p=0.034). Regional nodal recurrence more common among the patients who received VB only (p=0.048).
Conclusion
Modern era treatment was associated with improved RFS and OS in patients with stage III USC. Regional nodal recurrences were significantly reduced in patients who received EBRT.
Keywords: Endometrium, Carcinoma, Brachytherapy, Radiotherapy
INTRODUCTION
Uterine serous carcinoma (USC) is a rare subtype of endometrial cancer (EC) known to be highly aggressive [1,2,3]. Patients with USC present with more advanced-stage disease and are at greater risk for distant metastases compared to their endometrioid counterparts [2,4,5,6,7]. Though USC patients make up only 10% of all ECs, they account for up to 50% of EC-associated deaths [3,8]. Because USC patients are at risk for both regional and distant recurrence, therapeutic guidelines recommend multimodality treatment including complete surgical staging, chemotherapy, and radiotherapy (RT) [2,8,9]. However, treatment regimens continue to vary widely amongst institutions [2,5,9,10,11]. While some centers offer combination chemoradiation, others choose to forgo adjuvant RT given the thought that USC patients have greater tendencies to recur distantly. Thus, a common concern is that the associated toxicities of RT may not be worth the benefit.
Adjuvant RT for USC has evolved over time. USC was established as a distinct entity from endometrioid EC in 1982, at which time whole abdominal RT was considered as adjuvant treatment [2,12]. This treatment resulted in significant toxicities in addition to persistent locoregional and distant recurrences [12]. In recent years, contemporary techniques involving vaginal brachytherapy (VB) with chemotherapy have been shown to benefit stage I–II USC patients [1,13,14,15], though high rates of failure in advanced disease remain a concern [3,5]. However, very few studies to date have focused on outcomes of advanced stage USC patients, with conflicting findings regarding the benefit of RT. The recently published randomized controlled trial by the PORTEC-3 group, which supports chemotherapy and RT in the management of stage III EC, included only a minority of USC patients, of which only 39 patients were stage III [16]. Thus, definitive recommendations are difficult to form based on these data.
At our institution, beginning around 2003, all USC patients, including those with stage III disease, were traditionally treated with 6 cycles of adjuvant carboplatin and paclitaxel and concurrent VB following surgical staging [5]. Due to a theoretical concern for additive toxicity with chemotherapy, such as bone marrow suppression, pelvic external beam RT (EBRT) was not favored in this early time period. VB, however, was thought to be a minimal field that would not increase toxicity significantly while also providing protection against recurrence in the vagina, known to be a common site of failure in all stages of USC [17]. Therefore, treating the vagina preventatively remained a clinical priority even in the setting of chemotherapy. The stage III USC cohort of patients treated from 2003–2010 was reported previously by Young et al., [5] with a finding that 66.7% of these patients experienced failures. Beginning around 2011, while surgery and chemotherapy remain the backbone of treatment, our institution has built upon and intensified this existing treatment paradigm for advanced stage USC patients. Changes have included an increased use of computed tomography (CT) imaging in the perioperative period to define baseline disease extent, routine testing for human epidermal growth factor receptor 2 (HER2)/neu with tailored use of trastuzumab for HER2/neu positive USC patients [4], and lastly, in addition to VB and chemotherapy, routine consideration of EBRT for all stage III patients. Further, there has been increased use of minimally invasive surgical methods to improve post-operative recovery.
Given these contemporary changes in treatment for advanced stage USC patients, the purpose of this study is to examine outcomes, tolerability, and toxicities in the modern treatment era (defined as years 2011–2018) and to compare these findings with the prior treatment era (years 2003–2010).
MATERIALS AND METHODS
A retrospective review was conducted of patients with International Federation of Gynecology and Obstetrics (FIGO) stage III USC who received radiation at our institution from January 2003 to May 2018. Treatment eras were delineated based on a previous analysis at this institution by Young et al., [5] as treatment for USC patients was standardized until 2011. All patients diagnosed before December 2010 were placed in the early cohort. This study was approved by the Institutional Review Board (approved No.1302011584).
All included patients had USC histology (pure or mixed with 5% or greater serous component) and were stage III USC as defined by the 2009 FIGO staging system. Patients who did not receive RT were not captured. Patients diagnosed before 2009 were re-staged. Patients were generally managed with total hysterectomy, bilateral salpingoophorectomy, and extended surgical staging, including pelvic and para-aortic nodal dissections, peritoneal fluid cytology, and omental sampling. Several patients were treated with sentinel lymph node biopsy with or without selective lymphadenectomy. Surgical methods were total abdominal, laparoscopic, or robotic. All pathological specimens were reviewed by our institution's gynecological pathologists.
All patients underwent adjuvant chemotherapy with an intended regimen of carboplatin (area under the curve=5–7) and paclitaxel (175 mg/m2) given every 3 weeks, typically for 6 cycles. Adjuvant RT consisted of VB, EBRT, or both. VB was used almost exclusively in the early era, whereas pelvic RT ± VB for all stage III patients (with inclusion of para-aortic nodal coverage for IIIC2 patients) was considered in the modern era. However, reasons for exclusion of EBRT in the modern era may have included patient refusal, poor performance status or multiple comorbidities, or enrollment in clinical trials not permissive of EBRT; thus, RT in the modern era was less standardized. Among those who received EBRT, the dose was 45 Gy in 25 fractions either in a “sandwich” regimen between cycles 3 and 4 of chemotherapy or following completion of all 6 cycles. Intensity modulated radiation therapy (IMRT), typically with volumetric arc therapy, utilizing 2–4 arcs with 10 MV energy, was the chosen technique in all EBRT cases. The bowel bag was constrained to V40 less than 30% and V45 <200 mL. Bone marrow constraints were not used. VB was performed with a single channel vaginal cylinder and a high-dose-rate Ir-192 source. When performed as a boost to external beam, VB was delivered as 6 Gy × 2 fractions prescribed at the vaginal surface, and when prescribed as sole adjuvant treatment, was given as 6–7 Gy × 2–3 fractions prescribed at depth of 5 mm. The active treatment length varied from 5–6 cm.
Early toxicities were those recorded during treatment or within 3 months of treatment completion. Late toxicities were those documented more than 90 days after treatment. All toxicities were graded with the CTCAE version 4.0.
Patients were followed with routine clinical examination, tumor markers, and vaginal cytology. In the early treatment era, contrast-enhanced CT imaging of the chest, abdomen, and pelvis was performed following completion of all adjuvant treatment, while in the modern treatment era, additional CT imaging may have been performed before surgery or within 30 days of surgery prior to adjuvant treatment. Recurrences were identified by imaging and biopsy and were identified as pelvic, para-aortic, or distant.
Statistical methods
Descriptive statistics on patient, tumor, and treatment characteristics were compared between treatment eras using χ2 tests for categorical variables and t-tests for continuous variables.
Overall survival (OS) and recurrence free survival (RFS) curves were estimated using the Kaplan-Meier methods. RFS was counted from the surgery date until the date of first recurrence or date of last follow-up. Similarly, OS was defined as the time from surgery date until the date of death or date of last follow-up. RFS and OS were compared across treatment periods using the log-rank test. Univariate Cox proportional hazards regression was performed to identify any predictors of RFS and OS.
Statistical analyses were performed using Stata version 13.1.
RESULTS
1. Patient, tumor, and treatment characteristics
Fifty patients were identified with stage III USC: 20 patients (40%) treated between 2003–2010, and 30 (60%) treated between 2011–2018. Table 1 summarizes patient, tumor, and treatment characteristics, as well as the balance of covariates across the treatment eras. The majority of patients in both eras had extended surgical staging, with a median of 16 pelvic lymph nodes removed (interquartile range of 10–21). Only 12 patients had fewer than 10 pelvic nodes dissected, of whom 8 had undergone sentinel lymph node procedures. The median number of para-aortic removed was 3 (interquartile range of 1–7). Peritoneal fluid cytology was examined for 46 patients (92%). Omental sampling was completed for 44 patients (88%).
Table 1. Patient, tumor, and treatment characteristics across treatment eras.
| Characteristics | All patients (n=50) | 2003–2010 (n=20) | 2011–2018 (n=30) | p-value | |
|---|---|---|---|---|---|
| Age | 70 (28–85) | 70.5 (28–83) | 70 (40–85) | 0.606 | |
| Race | |||||
| Non-hispanic white | 34 (68) | 12 (60) | 22 (73.3) | 0.495 | |
| Non-hispanic black | 13 (26) | 7 (35) | 6 (20) | - | |
| Hispanic | 3 (6) | 1 (5) | 2 (6.7) | - | |
| BMI | 0.763 | ||||
| <30 | 25 (50) | 9 (45) | 16 (53.3) | ||
| 30–40 | 17 (34) | 8 (40) | 9 (30) | ||
| >40 | 8 (16) | 3 (15) | 5 (16.7) | ||
| Cancer history | |||||
| Breast cancer | 8 (16) | 3 (15) | 5 (16.7) | 0.451 | |
| Renal cell carcinoma | 3 (6) | 1 (5) | 2 (6.7) | - | |
| Lung cancer | 2 (4) | 1 (5) | 1 (3.3) | - | |
| AML | 2 (4) | 1 (5) | 1 (3.3) | - | |
| Other | 3 (6) | 1 (5) | 2 (6.7) | - | |
| Tamoxifen use | 3 (6) | 1 (5) | 2 (6.7) | 0.831 | |
| Hormone replacement therapy use | 4 (8) | 2 (10) | 2 (6.7) | 0.505 | |
| Gravidity | 2 (0–9) | 2 (0–9) | 2 (0–5) | 0.204 | |
| Parity | 2 (0–8) | 2 (0–8) | 2 (0–5) | 0.171 | |
| Subtype | |||||
| UPSC | 34 (68) | 14 (70) | 20 (66.7) | 0.804 | |
| Mixed | 16 (32) | 6 (30) | 10 (33.3) | - | |
| Pathologic stage | |||||
| IIIA | 14 (28) | 6 (30) | 8 (26.7) | 0.655 | |
| IIIB | 2 (4) | 0 (0) | 2 (6.7) | - | |
| IIIC1 | 18 (36) | 8 (40) | 10 (30) | - | |
| IIIC2 | 16 (32) | 6 (30) | 10 (33.3) | - | |
| Percentage of myometrial invasion | |||||
| <50% | 19 (38) | 8 (40) | 11 (36.7) | 0.812 | |
| ≥50% | 31 (62) | 12 (60) | 19 (63.3) | - | |
| Percentage depth of myometrial invasion | 65 (0–100) | 63.5 (0–100) | 65.5 (0–100) | 0.779 | |
| Lower uterine segment involved | |||||
| No | 17 (34) | 5 (25) | 12 (40) | 0.236 | |
| Yes | 32 (64) | 15 (75) | 17 (56.7) | - | |
| Unknown | 1 (2) | 0 (0) | 1 (3.3) | - | |
| Cervical involvement | |||||
| No | 31 (62) | 13 (65) | 18 (60) | 0.721 | |
| Yes | 19 (38) | 7 (35) | 12 (40) | - | |
| Lymphovascular space invasion | |||||
| No | 18 (36) | 9 (45) | 9 (30) | 0.279 | |
| Yes | 32 (64) | 11 (55) | 21 (70) | - | |
| Uterine serosa involvement | |||||
| No | 40 (80) | 17 (85) | 23 (76.7) | 0.613 | |
| Yes | 9 (18) | 3 (15) | 6 (20) | - | |
| Unknown | 1 (2) | 0 (0) | 1 (3.3) | - | |
| Fallopian tube involvement | |||||
| No | 35 (70) | 12 (60) | 23 (76.7) | 0.208 | |
| Yes | 15 (30) | 8 (40) | 7 (23.3) | - | |
| Ovary involvement | |||||
| No | 39 (78) | 15 (75) | 24 (80) | 0.676 | |
| Yes | 11 (22) | 5 (25) | 6 (20) | - | |
| Pelvic nodes removed | |||||
| No | 1 (2) | 0 (0) | 1 (3.3) | 0.409 | |
| Yes | 49 (98) | 20 (100) | 29 (96.7) | - | |
| No. of pelvic nodes removed | |||||
| 0 | 1 (2) | 0 (0) | 1 (3.3) | 0.157 | |
| 1–10 | 12 (24) | 4 (20) | 8 (26.7) | - | |
| 11–20 | 23 (46) | 7 (35) | 16 (53.3) | - | |
| 21+ | 14 (28) | 9 (45) | 5 (16.7) | - | |
| Sentinel pelvic node biopsy performed | 0.012 | ||||
| No | 42 (84) | 20 (100) | 22 (73.3) | ||
| Yes | 8 (16) | 0 (0) | 8 (26.7) | ||
| Pelvic nodes involved | |||||
| No | 23 (46) | 10 (50) | 13 (43.3) | 0.643 | |
| Yes | 27 (54) | 10 (50) | 17 (56.7) | - | |
| Para-aortic nodes removed | |||||
| No | 11 (22) | 2 (10) | 9 (30) | 0.094 | |
| Yes | 39 (78) | 18 (90) | 21 (70) | - | |
| No. of para-aortic nodes removed | |||||
| 0 | 11 (22) | 2 (10) | 9 (30) | 0.143 | |
| 1–5 | 25 (50) | 10 (50) | 15 (50) | - | |
| 6+ | 14 (28) | 8 (40) | 6 (20) | - | |
| Para-aortic nodes involved | |||||
| No | 34 (68) | 13 (65) | 21 (70) | 0.710 | |
| Yes | 16 (32) | 7 (35) | 9 (30) | - | |
| Gross tumor size (cm) | 3.75 (0–15) | 4 (3–6) | 3.5 (2–5) | 0.801 | |
| Pelvic washing with malignant cells | |||||
| No | 35 (70) | 17 (85) | 18 (60) | 0.214 | |
| Yes | 11 (22) | 3 (15) | 8 (26.7) | - | |
| Unknown | 4 (8) | 0 (0) | 4 (13.3) | - | |
| Surgery type | |||||
| Open | 28 (56) | 15 (75) | 13 (43.3) | 0.027 | |
| Minimally invasive | 22 (44) | 5 (25) | 17 (56.7) | - | |
| Days to imaging | |||||
| Less than 30 days after surgery | 24 (48) | 6 (30) | 19 (63.3) | 0.044 | |
| More than 30 days after surgery | 24 (48) | 12 (60) | 11 (36.67) | - | |
| Unknown | 2 (4) | 2 (10) | 0 (0) | - | |
| HER2/neu status | |||||
| Positive | 31 (62) | 10 (50) | 21 (70) | 0.001 | |
| Negative | 9 (18) | 1 (5) | 8 (26.7) | - | |
| Unknown | 10 (20) | 9 (45) | 1 (3.3) | - | |
| Herceptin use | 4 (8) | 0 (0) | 4 (13.3) | 0.083 | |
| RT modality | |||||
| VB | 39 (78) | 19 (95) | 20 (66.7) | 0.048 | |
| EBRT | 5 (10) | 1 (5) | 4 (13.3) | - | |
| EBRT + VB | 6 (12) | 0 (0) | 6 (20) | - | |
Values are presented as number (%).
AML, acute myeloid leukemia; BMI, body mass index; EBRT, external beam radiotherapy; HER2, human epidermal growth factor receptor 2; RT, radiotherapy; UPSC, uterine papillary serous carcinoma; VB, vaginal brachytherapy.
There were no differences in any clinicopathologic characteristics between patients treated in the early and modern era. However, several differences in treatment paradigm are evident in Table 1. Early era patients more often underwent open surgery (75%) compared to patients in the modern era (43.4%) (p=0.027). No patients in the early era underwent sentinel node biopsy compared to 8 women (26.7%) in the modern era who underwent sentinel node biopsy with or without selective lymphadenectomy (p=0.012). Nine patients (45%) in the early era did not undergo HER2/neu testing, while only 1 patient (3.3%) in the modern era did not receive HER2/neu testing (p=0.001). With regards to CT imaging, only 6 women (30%) in the early era underwent imaging in the perioperative period, while 19 women (63.3%) in the modern era received CT imaging within 30 days of surgery (p=0.044).
Lastly, in terms of adjuvant RT, 19 patients (95%) in the early era received VB only, and 1 patient (5%) received EBRT only as a part of a clinical trial protocol. In the modern era, 20 women (66.7%) were treated with VB only, while 10 (33.3%) received EBRT with (n=6) or without (n=4) a VB boost. Of the 11 patients who received EBRT, 6 patients underwent a “sandwich” regimen, in which EBRT was given after 3 cycles of chemotherapy, while 5 patients completed all 6 cycles of chemotherapy before starting EBRT. Seven patients were treated to the pelvis only, while 4 were treated to both pelvic and para-aortic nodal regions. Although EBRT was offered to all patients treated in the modern era, it was not administered in all cases due to patient refusal and/or clinician preference (n=5), poor performance status and/or multiple comorbidities (n=10), or enrollment on clinical trial protocols not permissive of EBRT (n=5). Of note, in the modern era, 2 of the 10 node-negative patients (20%) received EBRT, while 9 of the 20 node-positive patients (45%) received EBRT. Reasons for VB exclusion in patients treated with EBRT were related to patient preference.
Overall, treatment was tolerated well. In the early era, 17 patients (85%) received full doses of all planned chemotherapy cycles. Three patients underwent only 3 cycles due to poor toleration. In the modern era, 25 patients (83.3%) received the full doses. Chemotherapy for 3 patients (10%) was stopped because it was not well tolerated, while the dosage was reduced for 2 patients (6.7%) due to side effects. Among the 11 EBRT patients, 1 patient had a grade 3 toxicity of diarrhea. Otherwise, EBRT patients only had grade 1 or 2 toxicities including lymphedema, vaginal dryness, fatigue, diarrhea, proctitis, urinary incontinence, and dyspareunia. All EBRT patients completed planned chemotherapy regimens.
2. Patterns of recurrence
Table 2 describes the locations of first recurrence based on variables that were significantly different between treatment eras. In the early treatment era, 13 women (65%) recurred; of those, 10 patients had a regional nodal component of their recurrence (defined as pelvic ± para-aortic nodes with/without distant), and 3 had distant recurrence only. In the modern treatment era, a total of 10 women (33.3%) recurred; of those, 7 patients had a regional nodal recurrence as a component (with or without distant disease), and 3 had distant recurrence only. There were no vaginal recurrences in either time period.
Table 2. Sites of initial recurrence by time period, treatment modality, imaging, and surgery type.
| Treatment variable | No recurrence | Regional only | Distant only | Regional and distant | Total number who recurred | |
|---|---|---|---|---|---|---|
| Total (n=50) | 27 | 13 | 6 | 4 | 23 | |
| Time period | ||||||
| 2003–2010 (n=20) | 7 | 8 | 3 | 2 | 13 | |
| 2011–2018 (n=30) | 20 | 5 | 3 | 2 | 10 | |
| RT modality | ||||||
| VB only (n=39) | 20 | 12 | 3 | 4 | 19 | |
| EBRT ± VB (n=11) | 7 | 1 | 3 | 0 | 4 | |
| Herceptin use | ||||||
| Yes (n=4) | 4 | 0 | 0 | 0 | 0 | |
| No (n=46) | 23 | 13 | 6 | 4 | 23 | |
| Days to imaging | ||||||
| Less than 30 days after surgery (n=24) | 13 | 7 | 2 | 2 | 11 | |
| More than 30 days after surgery (n=24) | 13 | 6 | 4 | 1 | 11 | |
| Unknown (n=2) | 1 | 0 | 0 | 1 | 1 | |
| Surgery type | ||||||
| Open (n=28) | 13 | 9 | 4 | 2 | 15 | |
| Minimally invasive (n=22) | 14 | 4 | 2 | 2 | 8 | |
EBRT, external beam radiotherapy; RT, radiotherapy; VB, vaginal brachytherapy.
As shown in Table 2, regional nodal recurrence was rare among the 11 patients who received EBRT (n=1, 9%), whereas it was significantly more common (n=16, 41%) among the 39 patients who received VB only (p=0.048).
3. Treatment outcomes
Median follow-up time for the early era and modern era was 37.27 months (range: 13.78–158.56) and 33.23 months (range: 5.85–82.29), respectively. Amongst patients treated in the early era, 13 (65%) had a recurrence with a median time to first recurrence of 13.08 months (range: 4.77–32.09). In the modern era, 10 patients (33.3%) had a recurrence with a median time to first recurrence of 20.32 months (range: 5.72–38.04). The median time to death for early and modern eras was 34.85 months (range: 13.78–102.84) and 37.51 months (range: 24.89–52.34), respectively. Of the 22 deaths recorded, 14 were due to EC, 3 were due to another primary cancer, and 5 deaths occurred in patients who were disease-free at their last follow-up but whose records were insufficient to determine their ultimate cause of death.
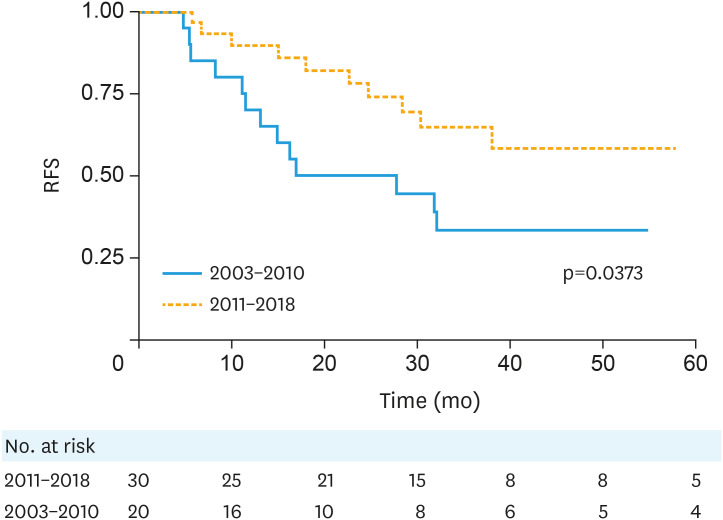
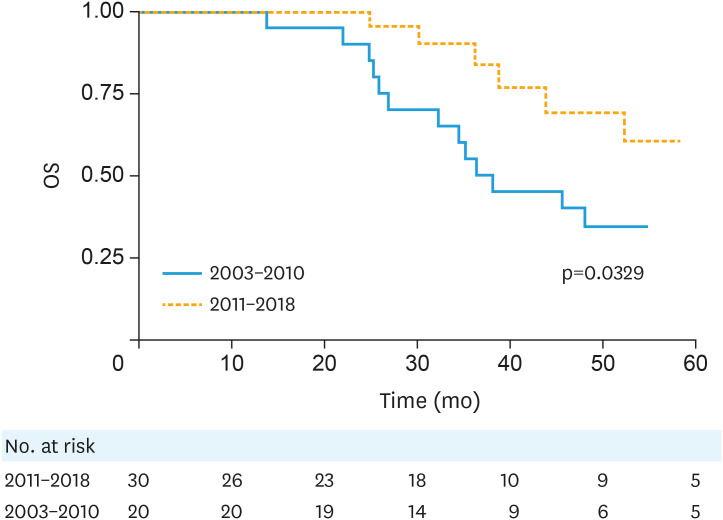
Table 3 summarizes unadjusted hazard ratios for RFS and OS. Of all variables examined, only later treatment era predicted for both improved RFS (p=0.044) and OS (p=0.032). The early vs. modern treatment era 3-year RFS was 33% and 64% (p=0.039), respectively, while the 3-year OS was 55% and 90% (p=0.034) (Figs. 1 and 2).
Table 3. Univariate analysis for factors associated with RFS and OS.
| Characteristics | RFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | 0.981 (0.939–1.026) | 0.431 | 0.984 (0.942–1.027) | 0.489 | |
| Race | |||||
| Non-hispanic black vs. non-hispanic white | 0.722 (0.267–0.967) | 0.511 | 1.138 (0.439–2.943) | 0.792 | |
| BMI | 0.963 (0.907–1.022) | 0.184 | 1.011 (0.960–1.065) | 0.690 | |
| Surgery type | |||||
| Open vs. laparoscopic | 1.302 (0.555–3.102) | 0.543 | 1.617 (0.601–4.442) | 0.327 | |
| Subtype | |||||
| UPSC vs. mixed | 1.170 (0.492–2.757) | 0.719 | 1.036 (0.430–2.494) | 0.937 | |
| Pathologic stage | |||||
| IIIC1/C2 vs. IIIA/IIIB | 0.972 (0.410–2.281) | 0.948 | 0.934 (0.384–2.275) | 0.882 | |
| Days to imaging | |||||
| More than 30 days after surgery vs. less than 30 days after surgery | 1.160 (0.498–2.656) | 0.729 | 1.078 (0.446–2.649) | 0.868 | |
| Percentage of myometrial invasion | |||||
| ≥50% vs. <50% | 1.641 (0.674–3.993) | 0.256 | 1.313 (0.547–3.176) | 0.540 | |
| Lower uterine segment involved | 1.543 (0.633–3.764) | 0.328 | 2.075 (0.756–5.689) | 0.134 | |
| Cervical involvement | 0.838 (0.357–1.988) | 0.684 | 1.768 (0.748–4.229) | 0.202 | |
| Lymphovascular space invasion | 1.130 (0.485–2.604) | 0.775 | 0.945 (0.401–2.201) | 0.897 | |
| Uterine serosa involvement | 0.837 (0.285–2.477) | 0.742 | 1.050 (0.381–2.910) | 0.926 | |
| Fallopian tube involvement | 1.568 (0.680–3.652) | 0.304 | 1.743 (0.743–4.090) | 0.208 | |
| Ovary involvement | 1.150 (0.428–3.120) | 0.786 | 1.655 (0.593–4.592) | 0.355 | |
| Pelvic nodes removed | 0.754 (0.332–0.712) | 0.500 | 1.006 (0.584–3.539) | 0.727 | |
| Pelvic nodes involved | 1.408 (0.617–3.217) | 0.414 | 1.452 (0.605–3.488) | 0.397 | |
| Para-aortic nodes removed | 0.763 (0.300–1.940) | 0.172 | 0.907 (0.807–1.019) | 0.077 | |
| Para-aortic nodes involved | 0.845 (0.578–1.236) | 0.332 | 0.425 (0.142–1.269) | 0.100 | |
| Gross tumor size (cm) | 1.297 (0.560–3.006) | 0.547 | 1.318 (0.541–3.213) | 0.546 | |
| Pelvic washing with malignant cells | 1.545 (0.551–4.318) | 0.424 | 1.896 (0.621–5.789) | 0.291 | |
| HER2/neu status | 0.998 (0.603–1.658) | 0.996 | 0.956 (0.585–1.561) | 0.857 | |
| RT modality | |||||
| VB vs. EBRT ± VB | 1.440 (0.493–4.280) | 0.492 | 1.359 (0.393–4.645) | 0.614 | |
| Time period | |||||
| 2011–2018 vs. 2003–2010 | 0.429 (0.187–0.974) | 0.044 | 0.369 (0.141–0.956) | 0.032 | |
BMI, body mass index; CI, confidence interval; EBRT, external beam radiotherapy; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; RFS, recurrence free survival; RT, radiotherapy; UPSC, uterine papillary serous carcinoma; VB, vaginal brachytherapy.
Fig. 1. RFS by treatment era, Kaplan-Meier curves.
RFS, recurrence-free survival.
Fig. 2. OS by treatment era, Kaplan-Meier curves.
OS, overall survival.
DISCUSSION
This study examines trends in treatment and compares the outcomes of 50 patients with stage III USC treated in early and modern treatment eras. Follow-up times were comparable between groups. Though the patient populations had similar treatment characteristics, we found several significant differences. Patients treated in the modern era more often underwent minimally invasive surgery, CT imaging within the perioperative period, HER2/neu testing, and EBRT. Our results demonstrate improvement in RFS and OS for those treated in the modern era, suggesting that some combination of these treatment characteristics provides benefit to this population. Furthermore, despite intensification of treatment in the modern era, we did not see an increase in toxicities or decrease in tolerability of recommended regimens.
There are several notable findings from our study. First, minimally invasive surgical methods have increased over the past 7 years with no detriment to outcomes. This trend is mirrored nationally, as the number of EC patients who underwent minimally invasive hysterectomy increased from 22% to 50% between 2007 and 2011 [18]. A landmark randomized controlled trial by the Gynecologic Oncology Group (GOG) comparing laparoscopic and open hysterectomies found that minimally invasive hysterectomies result in improved short-term surgical outcomes [19,20]. Because USC is a disease of older women, many of whom have other comorbidities, improved short-term surgical outcomes may result in improved OS. The modern era was also characterized by increased use of sentinel lymph node biopsy with or without selective lymphadenectomy. Complete lymphadenectomy has been the gold standard for detecting EC metastases [21] and has also been utilized as a tool for detecting microscopic and macroscopic disease [22]. Recently, sentinel node biopsies have been shown to have high diagnostic accuracy for metastases while exposing to patients to less risks [23].
Second, the use of peri-operative CT imaging was not standardized for high risk EC at our institution until recently. In the early era of our study, CT imaging was viewed as a tool for follow-up surveillance upon completion of adjuvant therapies. However, because imaging can be used to identify lymph node metastases and distant spread, it can be a helpful tool in assessing baseline extent of disease before adjuvant treatment is administered, even in the setting of extensive staging surgery. As patients with early metastatic disease are not appropriate for adjuvant EBRT, CT imaging should be considered for all EC patients with high-risk histology, especially in the setting of node-positive disease, and is supported by national guidelines [24].
Third, the modern treatment of EC is characterized by novel therapeutic strategies based on molecular characteristics of the disease [4,25]. For advanced USC, one such therapy is trastuzumab, a humanized monoclonal antibody against HER2/neu. Dysregulation of HER2/neu occurs in about 30% of USC patients [4]. Almost all patients (96.7%) treated in the modern era underwent immunohistochemistry testing for HER2/neu, while only 11 (55%) were tested in the earlier era. While only 4 HER2/neu-positive patients received trastuzumab, all 4 of these women remained recurrence free. A recent randomized, phase II trial comparing carboplatin-paclitaxel with or without trastuzumab in HER2/neu positive USC patients reported improved progression-free survival [4]. While the numbers in this study are small, the addition of consistent HER2/neu testing and the use of trastuzumab may have contributed to improved outcomes in the modern era.
Lastly, optimal multimodality adjuvant treatment remains murky for advanced stage USC patients due to paucity of trials focused specifically on this population. The importance of chemotherapy is frequently emphasized. Our study, as well as several prior retrospective studies, suggest more favorable outcomes with combination chemotherapy and EBRT [7,8,26,27]. In an institutional review of 135 patients with stage I–IV USC patients, of which 51 were stage III, Viswanathan et al. [2] demonstrated an improved relapse-free survival for patients treated with RT compared to those whose treatment did not include RT. A Surveillance, Epidemiology, and End Results study of stage III USC patients reported improved 5-year OS (38% vs. 27%) and RFS (38% vs. 27%) for combination chemotherapy and pelvic radiation ± brachytherapy compared to chemotherapy alone [7]. Patterns of failure differ for patients treated with adjuvant chemotherapy or RT. One series studying high-risk EC patients treated with only adjuvant chemotherapy reported high rates of pelvic recurrence [11], while another studying stage III EC patients treated with only regional RT reported high rates of distant and local recurrence [28]. In our analysis of sites of recurrence, we found fewer recurrences with any regional component in the modern era compared to the earlier era (23.3% vs. 50%). Of the 11 patients who received EBRT, only 1 patient recurred regionally (9%), compared to 16 of the 39 patients who received VB only (41%). Of the regional recurrences in the VB only group, a majority (12/16) were isolated regional failures, which may have been prevented with use of volume-directed EBRT. Furthermore, EBRT was generally well-tolerated with few grade 3–4 toxicities, apart from one patient who experienced grade 3 diarrhea. These results agree with several studies that indicate acceptably low toxicities in advanced stage EC patients treated with EBRT in either the sequential or “sandwich” method [29,30,31], especially with IMRT techniques [32]. Because of the small number of patients who received EBRT in our study, it is difficult to draw definitive conclusions. However, these pattern of failure results suggest that EBRT may be indicated for USC patients who present with advanced stage and are at high risk for regional recurrences. These conclusions are further bolstered by recent randomized data of advanced or high risk EC, including the published PORTEC-3 trial [16], which supports both chemotherapy and EBRT in this population, and the GOG 258, which found improved locoregional failure rates in the group of patients who received both chemotherapy and EBRT as opposed to chemotherapy alone [33].
This study has several important limitations. First, this study analyzes a small cohort of patients at a single institution, all of whom received radiation, and it is thus difficult to generalize conclusions. In addition, though we have highlighted several distinctions between treatment eras, there may be other unstudied factors that may also account for the observed difference in treatment outcomes. Since this study reviews outcomes over a span of time, stage migration is also a possible limitation. Furthermore, this was a retrospective study, and while adjuvant treatment was relatively standardized in the early treatment era, there may have been some treatment bias in the modern era (i.e. which patients received EBRT, etc.). While we show an association between modern treatment approaches with better outcomes compared to our historic cohort, we cannot prove causation by any one or some of these factors. This could only be accomplished with randomization and larger numbers. However, clinical trials focused specifically on advanced stage USC are rare and prospective studies for this rare disease are challenging.
Despite these limitations, this study is the first to our knowledge that directly compares VB and EBRT in conjunction with chemotherapy in stage III USC patients and thus provides unique information unavailable in recent trials. When compared to published data on stage III patients treated with chemotherapy alone, the patients treated with VB in our study did not appear to have a reduced risk of regional failure; however, there was likely a benefit in terms of reduced vaginal failure. For example, in the recently published GOG 258 trial, the 5-year incidence of vaginal recurrence was 7% in the chemotherapy alone arm (compared with 2% in the chemoradiotherapy arm and compared with no vaginal recurrences in the present study) [33]. Further, this is one of very few studies that focuses specifically on outcomes of advanced stage USC patients; as such, this study is strengthened by the stage homogeneity of its cohort. In addition, given the rarity of this subtype, our study includes a relatively large cohort of stage III USC patients, comparable to the number of stage III USC patients included in prior large institutional series [2,3]. Our study is further strengthened by its grounding at a high volume, tertiary care center with a long history of studying and treating this rare disease.
In summary, patients with stage III USC have improved RFS and OS in the modern treatment era that includes minimally invasive surgery, earlier use of CT imaging in monitoring disease, molecular and genomics driven care, and EBRT for locoregional control of disease. Larger multi-institutional and prospective studies are needed to better delineate these patterns towards improved RFS and OS.
Footnotes
Funding: This research was supported by the Yale School of Medicine Medical Student Fellowship.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Data curation: L.J.Y., Y.M.R.
- Formal analysis: L.J.Y.
- Supervision: D.S.
- Writing - original draft: L.J.Y.
- Writing - review & editing: L.J.Y., Y.M.R., H.G., L.B., S.A., S.P.E., D.S.
References
- 1.Damast S, Higgins SA, Ratner E, De Leon MC, Mani S, Silasi DA, et al. High-dose-rate vaginal brachytherapy with chemotherapy for surgically staged localized uterine serous carcinoma. J Contemp Brachytherapy. 2015;7:35–40. doi: 10.5114/jcb.2015.48539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viswanathan AN, Macklin EA, Berkowitz R, Matulonis U. The importance of chemotherapy and radiation in uterine papillary serous carcinoma. Gynecol Oncol. 2011;123:542–547. doi: 10.1016/j.ygyno.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Holman LL, Pal N, Iglesias DA, Soliman PT, Balakrishnan N, Klopp A, et al. Factors prognostic of survival in advanced-stage uterine serous carcinoma. Gynecol Oncol. 2017;146:27–33. doi: 10.1016/j.ygyno.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 5.Young MR, Higgins SA, Ratner E, Yu JB, Mani S, Silasi DA, et al. Adjuvant carboplatin, paclitaxel, and vaginal cuff brachytherapy for stage III endometrial cancer: analysis of outcomes and patterns of recurrence based on pathologic characteristics. Int J Gynecol Cancer. 2015;25:431–439. doi: 10.1097/IGC.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehrig PA, Morris DE, Van Le L. Uterine serous carcinoma: a comparison of therapy for advanced-stage disease. Int J Gynecol Cancer. 2004;14:515–520. doi: 10.1111/j.1048-891x.2004.14313.x. [DOI] [PubMed] [Google Scholar]
- 7.Mahdi H, Nutter B, Abdul-Karim F, Amarnath S, Rose PG. The impact of combined radiation and chemotherapy on outcome in uterine papillary serous carcinoma compared to chemotherapy alone. J Gynecol Oncol. 2016;27:e19. doi: 10.3802/jgo.2016.27.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Zhou J, Cheng Y, Zhao L, Yang Y, Wang J. Comparison of survival benefits of combined chemotherapy and radiotherapy versus chemotherapy alone for uterine serous carcinoma: a meta-analysis. Int J Gynecol Cancer. 2017;27:93–101. doi: 10.1097/IGC.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee LJ, Bu P, Feltmate C, Viswanathan AN. Adjuvant chemotherapy with external beamradiation therapy for high-grade, node-positive endometrial cancer. Int J Gynecol Cancer. 2014;24:1441–1448. doi: 10.1097/IGC.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 10.Rauh-Hain JA, Diver E, Meyer LA, Clemmer J, Lu KH, Del Carmen MG, et al. Patterns of care, associations and outcomes of chemotherapy for uterine serous carcinoma: analysis of the National Cancer Database. Gynecol Oncol. 2015;139:77–83. doi: 10.1016/j.ygyno.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I--IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145–1153. doi: 10.1016/s0360-3016(01)01566-8. [DOI] [PubMed] [Google Scholar]
- 12.Frank AH, Tseng PC, Haffty BG, Papadopoulos DP, Kacinski BM, Dowling SW, et al. Adjuvant whole-abdominal radiation therapy in uterine papillary serous carcinoma. Cancer. 1991;68:1516–1519. doi: 10.1002/1097-0142(19911001)68:7<1516::aid-cncr2820680709>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Turner BC, Knisely JP, Kacinski BM, Haffty BG, Gumbs AA, Roberts KB, et al. Effective treatment of stage I uterine papillary serous carcinoma with high dose-rate vaginal apex radiation (192Ir) and chemotherapy. Int J Radiat Oncol Biol Phys. 1998;40:77–84. doi: 10.1016/s0360-3016(97)00581-6. [DOI] [PubMed] [Google Scholar]
- 14.Kelly MG, O'malley DM, Hui P, McAlpine J, Yu H, Rutherford TJ, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N, Yamada SD, Rotmensch J, Mundt AJ. Outcome and pattern of failure in pathologic stage I-II papillary serous carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003;57:1004–1009. doi: 10.1016/s0360-3016(03)00753-3. [DOI] [PubMed] [Google Scholar]
- 16.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood BM, Jones J, Gupta S, Khabele D, Guha C, Runowicz C, et al. Patterns of failure after the multimodality treatment of uterine papillary serous carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:208–216. doi: 10.1016/s0360-3016(03)00531-5. [DOI] [PubMed] [Google Scholar]
- 18.Stewart KI, Fader AN. New developments in minimally invasive gynecologic oncology surgery. Clin Obstet Gynecol. 2017;60:330–348. doi: 10.1097/GRF.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilgore LC, Partridge EE, Alvarez RD, Austin JM, Shingleton HM, Noojin F, 3rd, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 22.Katz LA, Andrews SJ, Fanning J. Survival after multimodality treatment for stage IIIC endometrial cancer. Am J Obstet Gynecol. 2001;184:1071–1073. doi: 10.1067/mob.2001.115225. [DOI] [PubMed] [Google Scholar]
- 23.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 25.Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 26.Lin JF, Muñiz K, Sukumvanich P, Gehrig P, Beriwal S, Kelley JL, et al. Survival advantage associated with multimodal therapy in women with node-positive (stage-IIIC) uterine papillary serous carcinoma: a National Cancer Database study. BJOG. 2016;123:1846–1852. doi: 10.1111/1471-0528.13726. [DOI] [PubMed] [Google Scholar]
- 27.Block AM, Small W., Jr Combined modality therapy in the adjuvant treatment of uterine serous carcinoma. J Gynecol Oncol. 2016;27:e13. doi: 10.3802/jgo.2016.27.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundt AJ, Murphy KT, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Surgery and postoperative radiation therapy in FIGO stage IIIC endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1154–1160. doi: 10.1016/s0360-3016(01)01590-5. [DOI] [PubMed] [Google Scholar]
- 29.Lu SM, Chang-Halpenny C, Hwang-Graziano J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol Oncol. 2015;137:28–33. doi: 10.1016/j.ygyno.2015.01.546. [DOI] [PubMed] [Google Scholar]
- 30.Einstein MH, Frimer M, Kuo DY, Reimers LL, Mehta K, Mutyala S, et al. Phase II trial of adjuvant pelvic radiation “sandwiched” between combination paclitaxel and carboplatin in women with uterine papillary serous carcinoma. Gynecol Oncol. 2012;124:21–25. doi: 10.1016/j.ygyno.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2008;108:201–206. doi: 10.1016/j.ygyno.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol. 2018;36:2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317–2326. doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]