To the editor: We read with interest the recent article “PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma” [1]. Shen et al examined PD-L1 expression by immunohistochemistry in 95 systemic anaplastic large cell lymphomas (ALCLs) and concluded that PD-L1 expression has no prognostic significance in ALCL, including ALK-negative ALCL.
In a previous study that examined PD-L1 expression in 152 ALCLs, we found that ALK-ALCLs with DUSP22 rearrangements expressed minimal PD-L1 and pSTAT3Tyr705 [2], a finding subsequently replicated [3]. We hypothesized that the lack of PD-L1 might be one component of an immunogenic phenotype contributing to the excellent outcomes usually seen in DUSP22-rearranged ALCLs [4, 5]. In light of the data of Shen et al, we examined the association of PD-L1 expression and outcome in our previous series [2]. Of the 152 ALCLs stained for PD-L1, 62 were systemic ALCLs with available outcome data (18 ALK-positive, 44 ALK-negative; M:F, 38:24; mean age, 55 y; median follow-up, 43 months). Using the cutoff of 5% for PD-L1 positivity employed by Shen et al, we found that PD-L1 positivity was associated with inferior outcomes in ALK-negative ALCL (median survival, 38 months vs. 262 months in PD-L1-negative cases; P=0.03, log-rank test; Fig. 1A).
Fig. 1.
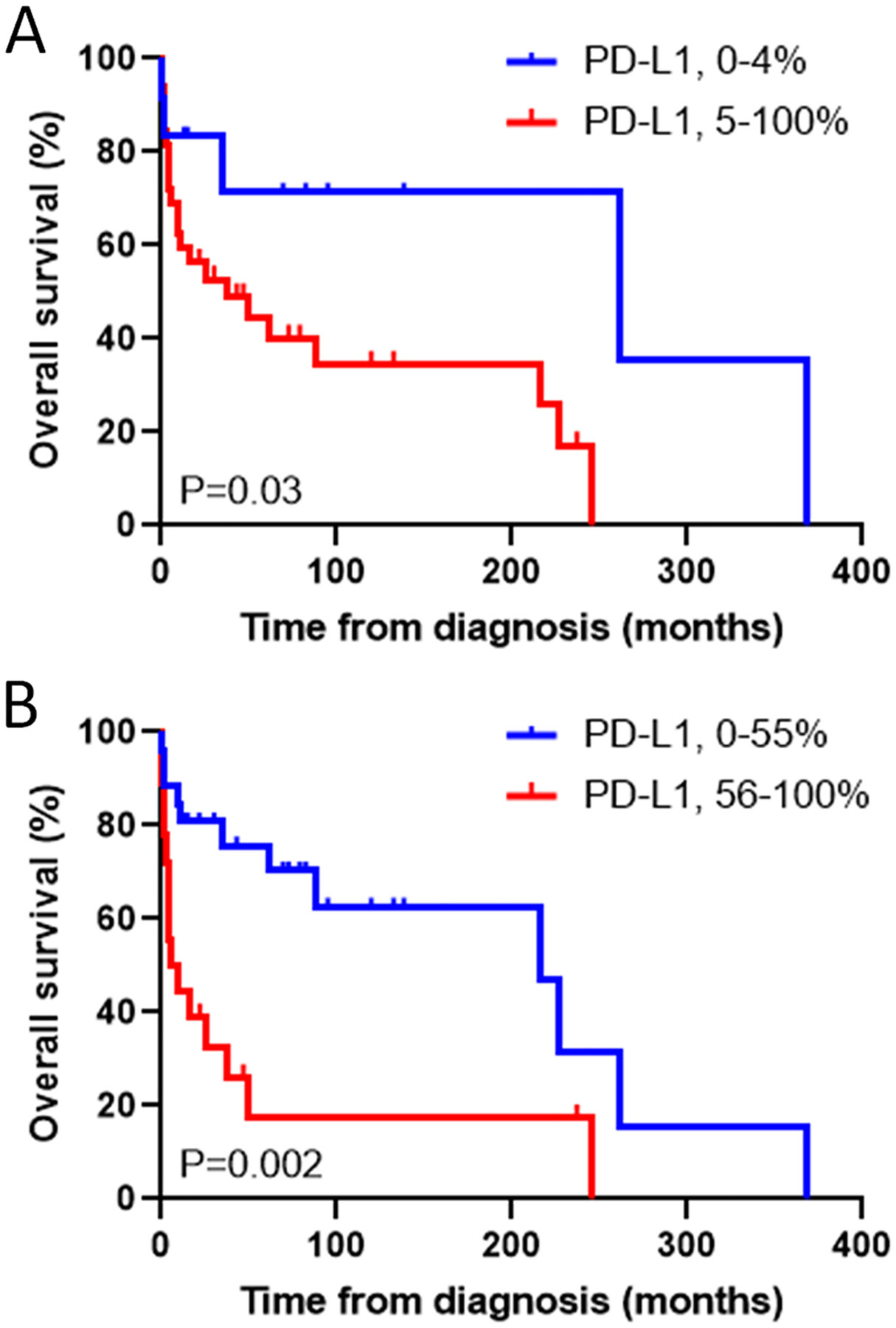
Overall survival in systemic ALK-negative anaplastic large cell lymphoma, stratified by PD-L1 expression. (A) Based on a cutoff for positivity of 5% of the neoplastic cells staining, as per Shen et al [1]. (B) Based on a cutoff of 55%, the median PD-L1 value in our series.
Several factors might account for the different results in our series and that of Shen et al. While used in several studies, the 5% cutoff for PD-L1 positivity may have difficulties with reproducibility, potentially accentuated by antibody/protocol differences ([2]; not specified in Shen et al). Only 27% of our ALK-negative ALCLs were PD-L1-negative using this cutoff, compared to 58% in Shen et al. Analyzing our data with a cutoff based on the median PD-L1 value of 55%, we found an even stronger association of PD-L1 positivity with outcome (median survival, 7 months vs. 216 months in PD-L1-negative cases; P=0.002; Fig. 1B). Differences in the composition of the study groups may also contribute to the different results. Although median follow-up was only 20 months in Shen et al and 5-year overall survival in ALK-negative ALCL is not provided, from their Fig. 4, it appears to be ~25%, which is considerably poorer than the 52% in our data and references they cite (e.g., [6], 49%). Possibly, the more clinically aggressive disease in the series of Shen et al masked contributions of PD-L1. Though not reported, the series by Shen et al might have fewer DUSP22-rearranged ALCLs than ours (24%), or might be enriched for “high-risk” DUSP22-rearranged ALCLs as recently described [3].
Based on these discrepancies and the features of the series analyzed by Shen et al including limited follow-up, we believe the conclusion that PD-L1 is not associated with outcome in ALK-negative ALCL is premature. The collective literature is in agreement that ALCLs demonstrate variable expression of PD-L1. As Shen et al note, the most important implication of this variable expression is the hypothesis that PD-L1 functionally promotes immune escape in some ALCLs but not others and might serve as a biomarker to individualize immunotherapeutic approaches. Our data showing poorer outcomes in PD-L1-positive ALK-negative ALCLs are consistent with this hypothesis and with data from other cancers as reviewed by Shen et al. Future work should examine the functional role of PD-L1 in ALCL and its relationship to immunotherapy response in pre-clinical models and clinical trials.
Acknowledgment:
This work was supported by Award Number R01 CA177734 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Shen J, Li S, Medeiros LJ, et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Modern Pathology 2019. [DOI] [PubMed] [Google Scholar]
- 2.Luchtel RA, Dasari S, Oishi N, et al. Molecular profiling reveals immunogenic cues in anaplastic large cell lymphomas with DUSP22 rearrangements. Blood 2018;132:1386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hapgood G, Ben-Neriah S, Mottok A, et al. Identification of high-risk DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. Br J Haematol 2019;186:e28–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014;124:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen MB, Hamilton-Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood 2017;130:554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage KJ, Harris NL, Vose JM, et al. ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–504. [DOI] [PubMed] [Google Scholar]


