Abstract
Background
Many studies employed cue exposure paradigms to investigate the neural processes underlying cue-elicited alcohol craving. Cue exposure elicits robust autonomic reactivity. However, whether or how cue-elicited autonomic response relates to the severity of alcohol misuse and the neural bases underlying the potential relationship remain unclear.
Methods
We examined cue-related brain activations in association with heart rate variability (HRV), as indexed by the Root Mean Square of the Successive Differences (RMSSD), during alcohol vs. neutral cue blocks in 50 adult alcohol drinkers (24 men). Imaging and HRV data were collected and processed with published routines. Mediation analyses were conducted to examine the inter-relationship between regional activities, cue-elicited changes in RMSSD, and the severity of problem alcohol use, as assessed with the Alcohol Use Disorder Identification Test (AUDIT).
Results
The results showed higher RMSSD during alcohol than during neutral cue exposures, with alcohol (vs. neutral) cue-evoked RMSSD positively correlated with AUDIT score. Further, alcohol (vs. neutral) cue-elicited activity in the ventromedial prefronal cortex (vmPFC) was negatively correlated both with increases in RMSSD and with AUDIT score. Mediation analyses suggested that the RMSSD mediated the relationship between vmPFC cue activity and AUDIT score.
Conclusions
These findings substantiate the neural correlates of the presumably parasympathetic response during alcohol cue exposure and the inter-relationship between vmPFC activity, autonomic response and problem alcohol use.
Keywords: alcohol, cue, autonomic, HRV, imaging, ventromedial PFC
Introduction
Drug craving is associated with changes in interoceptive responses and physiological arousal (1–10). Many imaging studies have examined the neural correlates of craving (11) and of physiological arousal during cognitive and affective challenges (12). For instance, as compared to light drinkers, individuals abusing alcohol demonstrated greater cue-related activation of the parietal and temporal cortices (13). We recently showed higher alcohol vs. neutral cue responses in the occipital, retrosplenial, and medial orbitofrontal cortex and left caudate head in non-dependent drinkers (14). A study of male drinkers demonstrated a positive correlation between cue-evoked craving as well as thalamic cue activities and skin conductance response (SCR) (15). Further, mediation analyses suggested that thalamic activity mediated the correlation between craving and SCR. The latter findings suggested higher physiological arousal during cue-evoked craving and a specific role of the thalamus in supporting physiological changes during cue-evoking craving.
Skin conductance manifests via sympathetic innervations of the sweat glands and SCR is elevated during heightened arousal as one would experience during exposure to stress or a salient environment (16, 17). Another aspect of autonomic responses, as supported by the parasympathetic system, can be evaluated by heart rate variability (HRV). Heart rate is controlled by the balancing acts of the sympathetic and parasympathetic systems, each serving to accelerate and decelerate heart beat. HRV quantifies the variation of inter-beat-intervals and, as with heart rate, is regulated by the autonomic system. The sympathetic and parasympathetic systems modulate HRV each at the low (0.04–0.15 Hz) and higher (0.15–0.4 Hz) frequency range of the heart rate (18). Heightened sympathetic and parasympathetic tone decreases and increases HRV, respectively. Decreases in HRV are known to co-exist with or precede a number of medical conditions, including impending myocardial infarction (19), and have been associated with diminished cognitive performance (20).
Cerebral control of cardiovascular responses is supported by the limbic circuits. In particular, the medial prefrontal cortex (mPFC) has been implicated in numerous studies in the regulation of autonomic and cardiovascular responses (21–27). For instance, an fMRI study showed that functional connectivity of the perigenual anterior cingulate cortex covaried with individual variation in high-frequency HRV during resting state (22). During isometric hand grips the time course of heart rate was associated with decreases in MPFC activation and individuals with higher physical fitness demonstrated greater deactivation of the MPFC (28). An earlier study showed that heart rate responses to evaluation of social threat were mediated by opposing signals in two distinct sub-regions of the MPFC – activations in rostral dorsal anterior cingulate cortex and de-activations in ventromedial prefrontal cortex (29).
The root mean squared difference of successive intervals (RMSSD) has been widely used as a time-domain HRV measure to reflect parasympathetic activity (30) in basic neurophysiological studies and investigations involving clinical populations, including those with substance misuse. A recent meta-analysis showed that HRV, as indexed by the RMSSD, was significantly lower in individuals with alcohol use disorders, as compared to healthy controls (31). Another review also showed decreased HRV in association with heavy drinking (32). Further, heavy drinkers showed higher HRV in response to alcohol or emotional cues, when compared to controls, and the reactivity was associated with craving, negative mood, and faster relapse (32). A number of studies have specifically examined HRV as a physiological marker of cue exposure in drug addicted individuals (33–40). Alcohol attentional bias was positively associated with parasympathetically mediated HRV (39). Alcohol-dependent patients who relapsed exhibited a significantly greater high-frequency HRV in response to alcohol cues, as compared to those who did not relapse (38). Overall, previous studies have consistently reported increases in HRV during alcohol cue exposures in heavy drinkers. On the other hand, no studies to our knowledge have examined the cerebral processes underlying the changes in HRV or whether these neural processes may relate to the severity of alcohol use.
Here, fifty adult drinkers participated in clinical assessments and an fMRI study of alcohol cue reactivity in conjunction with physioglical recordings. We addressed the following aims. Firstly, is HRV altered during alcohol vs. neutral cue exposure and, if yes, are changes in HRV related to individual differences in the severity of alcohol use? As described earlier, the literature has largely supported cue-elicited increases in HRV and its relationship with the extent of alcohol misuse and treatment outcomes, and we expected to replicate these findings. Secondly, what are the neural processes underlying cue-elicited changes in HRV and their relationship with problem alcohol use? Considering the imaging literature implicating the mPFC in autonomic control, we hypothesized cue-evoked mPFC responses in association with changes in HRV. We also explored the inter-relationships between cue-related mPFC activity and HRV and the severity of alcohol use. As studies have reported gender and age effects on HRV (41–44), we analyzed the data with age and gender as covariates.
Materials and methods
Experimental procedures are largely identical to those described in our previous study (15).
Subjects and assessments
Candidates were recruited from the greater New Haven, CT area. Fifty adult drinkers met eligibility criteria and participated in this study. All subjects were required to be physically healthy with no major medical conditions. Those with current use of prescription medications or with a history of head injury or neurological illness were excluded. Other exclusion criteria included current or past dependence on a psychoactive substance (except alcohol and nicotine) and current or history of Axis I disorders according to the Structured Clinical Interview for DSM-IV [SCID; (45)]. Four men and four women met the DSM criteria for an alcohol use disorder and none met criteria for a nicotine use disorder. Candidates who reported current use of illicit substances or tested positive for cocaine, methamphetamine, opioids, marijuana, barbiturates, or benzodiazepines were not invited to participate. The Human Investigation committee at Yale University School of Medicine approved the study procedures. All participants signed an informed consent prior to the study.
Participants were evaluated with Alcohol Use Disorders Identification Test [AUDIT; (46)], an instrument widely used to assess alcohol use behavior and related problems. Participants were assessed with the Fagerström Test for Nicotine Dependence [FTND; (47)] and averaged 1.1 ± 2.4 (mean ± SD) in FTND score, suggesting low dependence. Table 1 summarizes the demographic and clinical characteristics of the participants. Men and women did not differ in any of the demographic and clinical characteristics or outcome measures except for cue-elicited craving. However, cue-elicited craving was not correlated with AUDIT score or with physiological responses to cues (see Results). Thus, we noted these results on sex differences and, considering the moderate sample size, combined men and women in data analyses.
Table 1:
Demographics and clinical measures of male and female participants
| Subject characteristic | Men (n=24) | Women (n=26) | P value* |
|---|---|---|---|
| Age (yrs) | 39.7 ± 12.7 | 40.4 ± 15.2 | 0.85 |
| AUDIT score | 11.8 ± 12.2 | 10.0 ± 11.0 | 0.57 |
| Duration of alcohol use (yrs) | 22.1 ± 13.5 | 21.8 ± 15.8 | 0.94 |
| # of drinking days/mo, prior yr | 9.2 ± 5.4 | 9.3 ± 6.3 | 0.95 |
| # of drinks/per occasion | 3.7 ± 2.5 | 3.2 ± 2.5 | 0.50 |
| # of drinks/mo, prior yr | 39.3 ± 42.4 | 38.9 ± 46.2 | 0.97 |
| FTND score | 0.7 ±2.0 | 1.5 ± 2.6 | 0.26 |
| Current smoker (yes/no) | 5/24 | 10/26 | 0.32 |
Note: values are mean ± S.D.;
two-tailed two-sample t test, except for smoker status which was based on Chi-square test; AUDIT: Alcohol Use Disorder Identification Test; FTND: Fagerström Test for Nicotine Dependence.
Behavioral tasks
We employed a cue-induced alcohol craving task for fMRI in the current study. Participants viewed alcohol-related or neutral pictures and reported alcohol craving in alternating blocks. Briefly, a cross was used to engage attention at the beginning of each block. After 2 s, six pictures displaying alcohol related cues (alcohol block) or neutral visual scenes (neutral block) were shown for 6 s each. Participants were asked to view the pictures and ponder how they might relate to the scenes. The pictures were collected from the Internet and independently reviewed by two investigators. Alcohol pictures included bar scenes, individuals or a group of people holding or drinking alcoholic beverages, and images of a variety of alcoholic drinks, such as beer, wine, and vodka. Neutral pictures comprised natural sceneries. Participants were asked at the end of each block to report how much they craved for alcohol from 0 (no craving) to 10 (highest craving ever experienced) on a visual analog scale. Each block lasted about 45 s, including time for craving rating. A total of 6 alcohol and 6 neutral blocks took approximately 9 m to complete. Each participant completed two runs of the task.
Heart rate variability: data acquisition and analysis
The photoplethysmography (PPG) was a non-restraining and non-invasive method widely used to detect pulse and oxygen saturation (48). The PPG of heart rate was collected during fMRI scan using a pressure transducer which adhered to the participant’s left thumb (all used right hand to respond). A Biopac MP150 pressure transduction system, running AcqKnowledge 4.1 software (Biopac Systems, USA), was used for acquiring heart rate data at a sampling rate of 1000 Hz (49). With AcqKnowledge 5.0.2 software (Biopac Systems, USA), the raw signal was bandpass filtered at 0.5–35 Hz. Independent component analysis was applied to separate the PPG signal from respiration and scanner signals. A template-correlation function was then applied to identify the waveform that matched the time course of the template, a “good” heart beat (27). Peaks were identified from the waveforms that closely matched the template to define the inter-beat or R-R intervals (30).
We estimated mean heart rate (HR) on the basis of the R–R intervals each for the alcohol and neutral cue blocks using the Kubios software (Kubios.com), We distinguished low- (0.04–0.15 Hz; LF-HRV) and high- (0.15–0.4 Hz; HF-HRV) frequency components of the HRV. The root mean squared difference of successive intervals (RMSSD) reflects the high-frequency dynamics of HRV (30, 50) and is considered a reliable measure of vagal parasympathetic activity (27, 30). The RMSSD was computed as follows. Assuming the successive differences are RR1 - RR2 = D1; RR2 - RR3 = D2; …, RRn-1 - RRn = Dn-1,
where i = interval index, n = the total number of intervals.
Imaging protocol and data analyses
Brain images were collected using multiband imaging (multiband factor = 3) with a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany). We acquired conventional T1-weighted spin echo sagittal anatomical images for slice localization. Anatomical 3D MPRAGE image were obtained with spin echo imaging in the transverse plane parallel to the anteror commissure – posterior commissure or AC–PC line with TR = 1900 ms, TE = 2.52 ms, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional, blood oxygenation level-dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. A total of 51 transverse slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, and slice thickness = 2.5 mm with no gap.
We analyzed the imaging data with Statistical Parametric Mapping or SPM12 (Wellcome Department of Imaging Neuroscience, University College London, U.K.). We aligned (motion corrected) and corrected for slice timing of images of each individual subject, and constructed a mean functional image volume for each subject per run from the realigned volumes. We co-registered these mean images with the high-resolution structural image and segmented the images for normalization with affine registration and nonlinear transformation (51–53). We applied the normalization parameters as determined for the structure volume to the corresponding functional images for each subject. The resampled voxel size was 3 × 3 × 3 mm3. Finally, we applied a Gaussian kernel of 8 mm at Full Width at Half Maximum for smoothing.
In modeling of imaging data, we distinguished alcohol and neutral cue blocks for each individual subject using a general linear model (GLM) that included the realignment parameters in all six dimensions. We corrected for serial autocorrelation caused by aliased cardiovascular and respiratory effects by a first-degree autoregressive model. The GLM was used to estimate the component of variance explained by each of the regressors. We constructed for individual subjects a contrast of alcohol vs. neutral blocks to evaluate regional activities that differentiated viewing of alcohol and neutral pictures. We used the contrast or con (difference in β) images of the subject-level analysis for group-level, random-effects analyses.
We performed a linear regression of con images against the differences in RMSSD between alcohol and neutral blocks across subjects, with age and sex as covariates. In a separate model, we performed a linear regression of the con images against individual AUDIT score, with age and sex as covariates. To identify the neural correlates that overlapped between the two regressions, we employed a p<0.05, uncorrected to cover brain regions as extensively as possible. Shared correlates were designated as the regions of interest (ROI). In ROI analysis, we used MarsBaR (http://marsbar.sourceforge.net/) to derive for each individual subject the β contrast or alcohol – neutral cue activity for the ROIs. We showed all voxel activations in the Montreal Neurological Institute (MNI) coordinates.
Mediation analyses
For shared correlates, we performed mediation analyses to evaluate the inter-relationship of cue activity (β contrast), differences in RMSSD (alcohol – neutral) and AUDIT score.
We performed mediation analyses (54) with the toolbox M3 (http://wagerlab.colorado.edu/tools), as in our previous work (55). In a mediation analysis, one examines if the relation between the independent variable X and dependent variable Y (i.e. X→Y) is significantly mediated by a variable M. Three regression equations were performed for the mediation test (54):
where a represents X→M, b represents M→Y (controlling for X), c’ represents X→Y (controlling for M), and c represents X→Y, the constants i1, i2, i3 are the intercepts, and e1, e2, e3 represent the residual errors. In the literature, a, b, c and c’ are referred to as path coefficients or simply paths (54, 56). We followed this notation. Variable M is said to be a mediator of the correlation X→Y if (c – c’), mathematically equivalent to the product a*b of the path a and b, differs significantly from zero (54). If the product a*b and the paths a and b are both significant, one concludes that X→Y is mediated by M. Further, if path c’ is not significant, one concludes that there is no direct connection from X to Y and that X→Y is completely mediated by M.
Results
Cue-elicited craving
Subjects reported higher craving during alcohol (3.6 ± 3.0) as compared to neutral (2.4 ± 2.4) cue blocks (t=5.16, p<0.001, two-tailed paired sample t test). Cue-elicited craving (alcohol – neutral) was not significantly correlated with AUDIT score without covariates (r=0.10; p=0.4788) or with age and gender as covariates (r=0.09; p=0.5647). Compared to women (0.73 ± 0.98), men (1.74 ± 2.07) showed higher cue-elicited craving (t=2.22, p=0.031; two-tailed independent sample t test).
Heart rate and heart rate variability
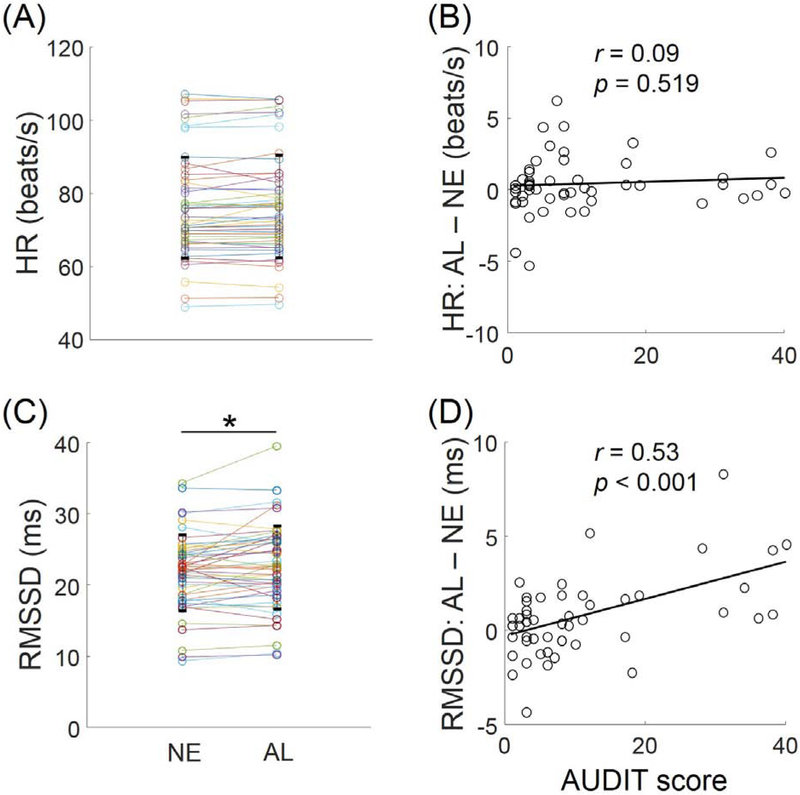
Men and women did not differ in alcohol vs. neutral cue-elicited HR (t=0.93, p=0.359). Across all subjects, the HR was not significantly different during alcohol and neutral cue blocks (29 of the 50 showed an increase: mean ± SD increases: 0.41 ± 1.96 beats/s; t=1.48, p=0.1442, two-tailed paired sample t test). The mean heart rate (HR) during alcohol and neutral cue blocks are shown in Figure 1A. The block differences in HR were not signifiantly correlated with the AUDIT score (r=0.09, p=0.519, without covariates; r=0.08, p=0.585, with age and gender covariates; Figure 1B). The difference in cue-induced craving was not correlated with the difference in HR between alcohol and neutral cue blocks (r=0.24, p=0.0972, without covariates; r=0.26, p=0.0751, with age and gender as covariates).
Figure 1.
Heart rate (HR) and heart rate variability. (A) HR did not show differences between alcohol (AL) and neutral cue (NE) blocks. Each pair of connected data points represents one subject. (B) The differences in HR (AL – NE) were not correlated with AUDIT score across subjects. (C) RMSSD showed a small but significant increase during AL as compared to NE blocks. *p<0.05. Each pair of connected data points represents one subject. (D) The differences in RMSSD (AL – NE) were positivelyc correlated with AUDIT score across subjects (p<0.001).
Men and women did not differ in alcohol vs. neutral cue-elicited RMSSD (t=0.47, p=0.643). The RMSSD showed a small but significant increase during alcohol as compared to neutral cue blocks across participants (32 of the 50 showed an increase: mean ± SD increases: 0.738 ± 2.1ms; t=2.49, p=0.0164, two-tailed paired sample t test). The RMSSD during alcohol and neutral cue blocks are shown in Figure 1C. The differences in RMSSD between the alcohol and neutral cue blocks was positively correlated with the AUDIT score (r=0.53, p<0.001, without covariates, Figure 1D; or with age and gender covariates). The difference in cue-induced craving was not correlated with the difference in RMSSD between alcohol and neutral cue blocks (r=0.11, p=0.4438, without covariates; r=0.03, p=0.8423, with age and gender as covariates).
Cue-elicited regional responses in relation to AUDIT score and changes in RMSSD
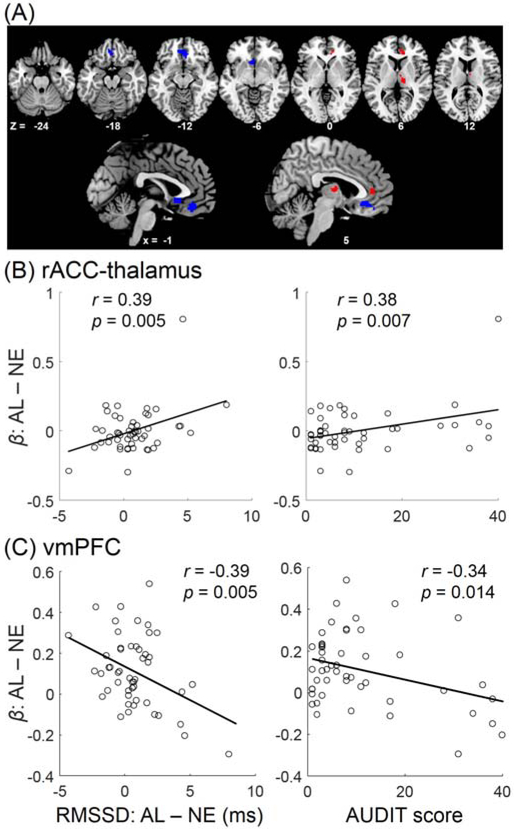
The AUDIT score and RMSSD (alcohol – neutral) was positively correlated across subjects. We performed regression analyses to identify shared cue-elicited activities - activities in correlation with both AUDIT score and RMSSD (alcohol – neutral). We evaluated the results at a liberal threshold (p<0.05, uncorrected) in order to identify all shared voxels. In whole-brain regressions of alcohol – neutral blocks each with the AUDIT score and RMSSD (alcohol – neutral) as predictors and age and sex as covariates, two distinct clusters were positively correlated with both predictors, each in the right rostral anterior cingulate cortex or rACC (mean coordinates: x=11, y=39, z=4, 267 mm3) and right thalamus (x=11, y=−17, z=8, 342 mm3). A cluster in the ventromedial prefrontal cortex or vmPFC (x=−2, y=34, z=−12, 933 mm3) showed negative correlation with both predictors. These clusters are shown in Figure 2A.
Figure 2.
AUDIT score and cue-elicited changes in HRV shared correlates in the medial prefrontal cortex and thalamus. (A) A cluster in the rostral anterior cingulate cortex (rACC) and right thalamus (red) and vmPFC (blue) showed cue resposes (AL-NE) each in positive and negative correlation both with AUDIT score and with differences in RMSSD (AL-NE). These correlations are shown here for the (B) rACC-thalamus and (C) vmPFC.
We plotted the linear regressions between the regional activities (β contrast) and predictor to visualize the correlations. The β contrasts of the rACC and thalamus clusters were highly correlated (r=0.48, p<0.001), we thus combined the two clusters as a single region of interest. As expected, the β contrast of the rACC-thalamus cluster was positively correlated both with the differences in RMSSD between the alcohol and neutral cue blocks (r=0.39, p=0.005; r=0.39, p=0.006 with age and gender as covariates) and with AUDIT scores (r=0.38, p=0.007; r=0.37, p=0.009 with age and gender as covariates) (Figure 2B). Likewise, the β contrast of vmPFC was negatively correlated both with the differences in RMSSD between the alcohol and neutral cue blocks (r=−0.39, p=0.0050; r=−0.41, p=0.0038, with age and gender as covariates) and with the AUDIT scores (r=−0.34, p=0.0142; r=−0.36, p=0.0109, with age and gender as covariates) (Figure 2C).
Mediation analysis:
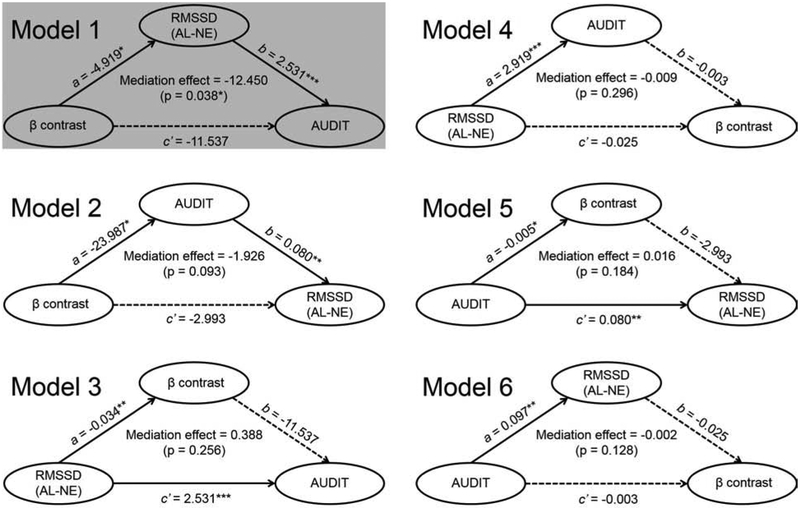
AUDIT score, rACC-thalamus cue response, and RMSSD were pair-wise correlated across participants. We performed mediation analyses for all six models with age and gender as covariates and none of the mediation effects were significant (all p’s > 0.062). AUDIT score, vmPFC cue response, and RMSSD were also pair-wise correlated across participants. Thus, we examined their inter-relationships using mediation analyses with age and gender as covariates. As shown in Figure 3, of the six models, model 1 showed that the RMSSD significantly mediated the correlation between vmPFC cue response and AUDIT score (p<0.05). None of the other five models showed significant mediation.
Figure 3.
Mediation analyses of vmPFC cue response (β contrast), differences in RMSSD across alcohol (AL) and neutral (NE) blocks, and AUDIT score. All six models of mediation were tested with age and gender as covariates. Model 1 showed a significant and complete mediation: vmPFC activity → RMSSD (AL – NE) → AUDIT score. Solid lines represent significant correlation, dotted lines represent insignificant correlation, *p<0.05, **p<0.01, ***p<0.001.
Although all 6 models are presented in Figure 3, the results of the mediation analyses can be more specifically evaluated for the role of the vmPFC in regulating autonomic functions. Thus, with the rationale that neural activities precede autonomic responses, only Models 1, 2 and 5 were physiologically plausible. The hypothesis was to test whether vmPFC activities contributed to problem alcohol use (AUDIT score) via cue-elicited changes in HRV (Model 1), whether vmPFC cue activities contributed to problem alcohol use and, in turn, to cue-elicited changes in HRV (Model 2), or whether problem alcohol use contributed to vmPFC cue activities and, in turn, cue-elicited HRV (Model 5). In particular, a contrast between Model 1 and 5 would provide evidence to address whether cue-evoked vmPFC activities and HRV are a result of problem alcohol use or whether problem alcohol use leads to these neural and physiological changes.
Discussion
Alcohol drinkers showed an increase in high-frequency heart rate variability (HRV), as reflected by the root-mean-square of successive differences (RMSSD) in heart beat, when exposed to alcohol as compared to neutral cues. This increase was correlated with the severity of problem alcohol use, as characterized by the AUDIT score, and decreases in ventromedial prefrontal cortical activity during alcohol vs. neutral cue exposure. Further, mediation analysis showed that decreases in cue-elicited vmPFC activities contributed to higher RMSSD during alcohol cue exposure and, in turn, higher AUDIT score. Together, these results substantiate the role of HRV in cue reactivity and problem drinking as well as the neural processes associating cue-evoked changes in HRV and the severity of alcohol misuse. We highlighted the main findings in the below.
Autonomic and neural responses to cue exposure
As described earlier, a number of studies have consistently reported increases in HRV during alcohol cue exposures in alcohol dependent or heavy drinking individuals. Here, we replicated this finding in adults with varying severity of problem alcohol use and showed that cue-evoked increases in HRV were positively correlated with the severity of alcohol misuse. This finding suggests HRV as an important physiological marker of habitual and heavy alcohol consumption.
Cue responses of the rostral anterior cingulate cortex (rACC) and thalamus showed a positive correlation both with differences in RMSSD and with AUDIT score across subjects, although their relationships could not be confirmed with mediation analyses. Another cluster in the ventromedial prefrontal cortex (vmPFC), in the area of the medial orbitofrontal frontal cortex showed a negative correlation both with differences in RMSSD and with AUDIT score across subjects. In mediation analysis, the model where vmPFC cue activities contributed to differences in RMSSD and, in turn, AUDIT score showed significant and complete mediation. Thus, the vmPFC may play a critical role in supporting cue-elicited differences in HRV in relation to the severity of alcohol misuse. Interestingly, the contrasting patterns of correlation between the rACC and vmPFC accorded with their opposing roles in regulating the cardiovascular and behavioral responses to emotional and other salient stimuli (29, 57).
The current findings are broadly consistent with many previous studies reporting vmPFC activity in relation to HRV or other autonomic responses (58–60). For instance, in a study of combat veterans exposed to alternating blocks of threats of electric shocks and safety, HRV was inversely correlated with the severity of PTSD symptoms (61). Further, an area in the vmPFC (though anterior to the vmPFC cluster in the current study) showed anticipatory activation (threat – safety) in negative correlation with the HRV. A study of aging showed age-related decreases in resting HRV and an age-dependent association between resting HRV and eigenvector centrality, a graph-theoretic measure of functional connectivity, in bilateral vmPFC (62). An earlier study demonstrated that patients with PTSD showed lower HRV and altered vmPFC connectivities with regions of emotional reactivity and motor readiness, as compared to healthy controls (63). Another study showed that HRV explained a significant portion of the individual variability in dietary self-control, and those with higher HRV were better able to downregulate their cravings in the face of taste temptations (64). Further, individuals with higher HRV showed attenuated taste representations in the vmPFC during self-control. The current findings thus extend this literature by showing an association of vmPFC activities with cue-elicited increases in HRV in adult drinkers. On the other hand, we did not observe a significant correlation between HRV and craving rating across alcohol and neutral blocks. Thus, the functional implications of the changes in HRV remained unclear.
Craving and autonomic response
We observed a small but significant increase in HRV during alcohol than during neutral blocks, consistent with previous reports of a moderate effect size of cue-evoked physiological responses (65). However, the differences in HRV and craving rating were not correlated, suggesting that subjective craving was not reflected by physiological reactivity (66, 67). Indeed, whereas some studies have reported a relationship between cue-induced craving and physiological reactivity, including changes in heart rate and HRV (68, 69), others did not reveal or examine such a relationship (67, 70–72). Other studies have reported the influences of various psychological factors on cue-elicited drug craving and autonomic reactivity. For instance, both adverse childhood experiences and duration of opioid use redicted blunted HRV during negative emotion regulation and increased negative emotional cue-elicited craving in women with chronic pain (34). Multi-level analysis suggested that childhood abuse occasioned emotion dysregulation and appetitive responding toward opioids in negative affective contexts. It was not clear, however, whether cue-evoked differences in HRV and craving were correlated (34). Together, the literature appears less than consistent regarding the autonomic correlates of drug-induced craving. Substances of abuse, specific experimental manipulations and physiological measures need to be considered in reviewing this body of work and in conducting new studies to address this issue.
Even fewer studies have examined the neural processes linking craving and autonomic reactivity. In a recent study we showed in male but not female drinkers a positive correlation between cue-evoked craving as well as thalamic cue activities and skin conductance response (15). A previous work demonstrated higher cue-induced arousal, as indicated by a significant increase in skin conductance and a larger late positivity of visual event-related brain potential, although the physiological and neural responses were not correlated, in cannabis users (73). Likewise, more studies are needed to investigate this issue and experiments encompassing a variety of physiological measures would provide an opportunity to identify neural responses shared by physiological changes and a subjective psychological state.
Limitations of the study and conclusions
A number of limitations need to be considered for the study. First, the study comprised a moderate sample size. Although whole-brain analyses in correlation with AUDIT score and differences in RMSSD identified shared correlates in the mPFC and thalamus, a larger sample size may reveal other cortical and subcortical structures to support responses to cue-elicited changes in HRV and in link with the severity of alcohol misuse. Likewise, a larger sample would allow a direct comparison of women and men in the autonomic and neural markers of cue reactivity. Second, cue reactivity is a complex process, involving a multitude of other psychological processes. It remains unclear how alcohol cues may interact with other psychological constructs in eliciting changes in HRV or in determining future alcohol use. Further, other than rating their craving, the participants were passively involved in the task, which may have influenced the craving and imaging findings. A behavioral paradigm that better engages participants’ attention (e.g., visual discrimination) would potentially be more powerful in eliciting craving and cue responses. Third, many other clinical variables, including depression and anxiety, weight and/or BMI, may influence cue-evoked regional activities and HRV. These variables were not accounted for here and more research is needed to address their potential confounding effects. Fourth, we employed a liberal threshold to explore how cue-evoked activities may relate to HRV and the severity of alcohol misuse for the whole brain. Notably, even at voxel p<0.05 uncorrected, no regions other than the vmPFC showed cue activities that were correlated with both the HRV and AUDIT score. This finding may suggest a unique role of the vmPFC in supporting the changes in HRV during cue exposure and problem alcohol use, as substantiated by the mediation analysis. Further, we did not correct for the number of models tested in evaluating the results of mediation analyses. Contrasting the 3 different models is akin to model selection – Model 1 was significant but Model 2 and 5 was not. Nonetheless, more research is needed to replicate this finding and to investigate whether cue-elicited vmPFC activities and changes in HRV dispose individuals to problem drinking in a longitudinal setting.
To conclude, we showed that cue-evoked differences in RMSSD was negatively correlated with vmPFC activity and mediated the correlation between vmPFC cue activity and the severity of alcohol misuse. These findings add to the literature of the autonomic and neural markers of cue reactivity and substantiate the interacting roles of HRV and vmPFC activity during alcohol cue expsoures.
Acknowledgements and disclosures
The study was supported by NIH grant AA021449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao M, Fan C, Du J, Jiang H, Chen H, Sun H (2012): Cue-induced craving and physiological reactions in recently and long-abstinent heroin-dependent patients. Addict Behav. 37:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trotzke P, Starcke K, Pedersen A, Brand M (2014): Cue-induced craving in pathological buying: empirical evidence and clinical implications. Psychosomatic medicine. 76:694–700. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM (2009): Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 34:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP (2000): Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug Alcohol Depen. 59:33–42. [DOI] [PubMed] [Google Scholar]
- 5.Pachas GN, Gilman J, Orr SP, Hoeppner B, Carlini SV, Loebl T, et al. (2015): Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology. 232:1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, et al. (2015): Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 20:513–522. [DOI] [PubMed] [Google Scholar]
- 7.Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, et al. (2012): Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 26:958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childress A, Ehrman R, McLellan A, O’Brien C (1988): Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 81:74–80. [PubMed] [Google Scholar]
- 9.Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, et al. (2009): Generalized craving, self-report of arousal, and cue reactivity after brief abstinence. Nicotine & Tobacco Research. 11:823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner JD, Zvolensky MJ, Ecker AH, Jeffries ER (2016): Cannabis craving in response to laboratory-induced social stress among racially diverse cannabis users: The impact of social anxiety disorder. J Psychopharmacol. 30:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014): Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley HD, Nagai Y, Gray MA, Mathias CJ (2011): Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci. 161:34–42. [DOI] [PubMed] [Google Scholar]
- 13.Schacht JP, Anton RF, Myrick H (2013): Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le T, et al. (2018): Alcohol expectancy and cerebral responses to cue-elicited craving in adult non-dependent drinkers. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Zhornitsky S, Le TM, Dhingra I, Zhang S, Krystal JH, et al. (2019): Cue-elicited craving, thalamic activity, and physiological arousal in adult non-dependent drinkers. J Psychiatr Res. 116:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ (2002): Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage. 16:909–919. [DOI] [PubMed] [Google Scholar]
- 17.Naqvi N, Shiv B, Bechara A (2006): The role of emotion in decision making: A cognitive neuroscience perspective. Current directions in psychological science. 15:260–264. [Google Scholar]
- 18.Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS (2006): Heart rate variability: a review. Medical and biological engineering and computing. 44:1031–1051. [DOI] [PubMed] [Google Scholar]
- 19.Mulcahy JS, Larsson DEO, Garfinkel SN, Critchley HD (2019): Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. Neuroimage. 202:116072. [DOI] [PubMed] [Google Scholar]
- 20.Forte G, Favieri F, Casagrande M (2019): Heart Rate Variability and Cognitive Function: A Systematic Review. Front Neurosci. 13:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansel A, von Kanel R (2008): The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc Med. 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennings JR, Sheu LK, Kuan DC, Manuck SB, Gianaros PJ (2016): Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 53:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoemaker JK, Goswami R (2015): Forebrain neurocircuitry associated with human reflex cardiovascular control. Front Physiol. 6:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M (2016): Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 139:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thayer JF, Koenig J (2019): Resting Cerebral Blood Flow and Ethnic Differences in Heart Rate Variability: Links to Self-Reports of Affect and Affect Regulation. Neuroimage. 202:116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK (2007): Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 35:698–708. [DOI] [PubMed] [Google Scholar]
- 27.Yoo HJ, Thayer JF, Greening S, Lee TH, Ponzio A, Min J, et al. (2018): Brain structural concomitants of resting state heart rate variability in the young and old: evidence from two independent samples. Brain Struct Funct. 223:727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood KN, Luchyshyn TA, Shoemaker JK (2017): High cardiorespiratory fitness in early to late middle age preserves the cortical circuitry associated with brain-heart integration during volitional exercise. J Neurophysiol. 117:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009): Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik M, Camm AJ, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. (1996): Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European heart journal. 17:354–381. [PubMed] [Google Scholar]
- 31.Cheng YC, Huang YC, Huang WL (2019): Heart rate variability as a potential biomarker for alcohol use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 204:107502. [DOI] [PubMed] [Google Scholar]
- 32.Ralevski E, Petrakis I, Altemus M (2019): Heart rate variability in alcohol use: A review. Pharmacol Biochem Behav. 176:83–92. [DOI] [PubMed] [Google Scholar]
- 33.Wang YG, Liu MH, Shen ZH (2019): A virtual reality counterconditioning procedure to reduce methamphetamine cue-induced craving. J Psychiatr Res. 116:88–94. [DOI] [PubMed] [Google Scholar]
- 34.Garland EL, Reese SE, Bedford CE, Baker AK (2019): Adverse childhood experiences predict autonomic indices of emotion dysregulation and negative emotional cue-elicited craving among female opioid-treated chronic pain patients. Dev Psychopathol. 31:1101–1110. [DOI] [PubMed] [Google Scholar]
- 35.Garland EL, Bryan CJ, Kreighbaum L, Nakamura Y, Howard MO, Froeliger B (2018): Prescription opioid misusing chronic pain patients exhibit dysregulated context-dependent associations: Investigating associative learning in addiction with the cue-primed reactivity task. Drug Alcohol Depend. 187:13–21. [DOI] [PubMed] [Google Scholar]
- 36.Garland EL, Froeliger B, Howard MO (2014): Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl). 231:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witteman J, Post H, Tarvainen M, de Bruijn A, Perna EDSF, Ramaekers JG, et al. (2015): Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology. 232:3685–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garland EL, Franken IH, Howard MO (2012): Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 222:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garland EL, Franken IH, Sheetz JJ, Howard MO (2012): Alcohol attentional bias is associated with autonomic indices of stress-primed alcohol cue-reactivity in alcohol-dependent patients. Exp Clin Psychopharmacol. 20:225–235. [DOI] [PubMed] [Google Scholar]
- 40.Rajan I, Murthy PJ, Ramakrishnan AG, Gangadhar BN, Janakiramaiah N (1998): Heart rate variability as an index of cue reactivity in alcoholics. Biol Psychiatry. 43:544–546. [DOI] [PubMed] [Google Scholar]
- 41.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P (1999): Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 277:H2233–2239. [DOI] [PubMed] [Google Scholar]
- 42.Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, et al. (2003): Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 14:791–799. [DOI] [PubMed] [Google Scholar]
- 43.Wong SW, Kimmerly DS, Masse N, Menon RS, Cechetto DF, Shoemaker JK (2007): Sex differences in forebrain and cardiovagal responses at the onset of isometric handgrip exercise: a retrospective fMRI study. J Appl Physiol (1985). 103:1402–1411. [DOI] [PubMed] [Google Scholar]
- 44.Macey PM, Rieken NS, Kumar R, Ogren JA, Middlekauff HR, Wu P, et al. (2016): Sex Differences in Insular Cortex Gyri Responses to the Valsalva Maneuver. Front Neurol. 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.First MS R; Williams J; Gibbon M (1995): Structured Clinical Interview for DSM-IV (SCID). Washington DC: American Psychiatric Association. [Google Scholar]
- 46.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001): The alcohol use disorders identification test. Geneva: World Health Organization, Department of Mental Health and Substance Dependence. [Google Scholar]
- 47.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991): The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 48.Webster JG (1997): Design of pulse oximeters. CRC Press. [Google Scholar]
- 49.Libby DJ, Worhunsky PD, Pilver CE, Brewer JA (2012): Meditation-induced changes in high-frequency heart rate variability predict smoking outcomes. Frontiers in human neuroscience. 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsvetanov KA, Henson RN, Tyler LK, Davis SW, Shafto MA, Taylor JR, et al. (2015): The effect of ageing on fMRI: correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human brain mapping. 36:2248–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashburner J, Friston KJ (1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 7:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS (1994): Statistical parametric maps in functional imaging: a general linear approach. Human brain mapping. 2:189–210. [Google Scholar]
- 53.Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R (1995): Spatial registration and normalization of images. Hum Brain Mapp 2:165–189. [Google Scholar]
- 54.MacKinnon DP, Fairchild AJ, Fritz MS (2007): Mediation analysis. Annu Rev Psychol. 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Chiang-shan RL (2017): Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: a structural brain imaging study. Neuroimage: clinical. 14:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallis CU, Cardinal RN, Alexander L, Roberts AC, Clarke HF (2017): Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc Natl Acad Sci U S A. 114:E4075–E4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubio A, Van Oudenhove L, Pellissier S, Ly HG, Dupont P, Lafaye de Micheaux H, et al. (2015): Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system--a fMRI study in healthy volunteers. Neuroimage. 107:10–22. [DOI] [PubMed] [Google Scholar]
- 59.Shoemaker JK, Usselman CW, Rothwell A, Wong SW (2012): Altered cortical activation patterns associated with baroreflex unloading following 24 h of physical deconditioning. Exp Physiol. 97:1249–1262. [DOI] [PubMed] [Google Scholar]
- 60.Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, et al. (2009): Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 106:8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grupe DW, Imhoff-Smith T, Wielgosz J, Nitschke JB, Davidson RJ (2019): A common neural substrate for elevated PTSD symptoms and reduced pulse rate variability in combat-exposed veterans. Psychophysiology.e13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumral D, Schaare HL, Beyer F, Reinelt J, Uhlig M, Liem F, et al. (2019): The age-dependent relationship between resting heart rate variability and functional brain connectivity. Neuroimage. 185:521–533. [DOI] [PubMed] [Google Scholar]
- 63.Thome J, Densmore M, Frewen PA, McKinnon MC, Theberge J, Nicholson AA, et al. (2017): Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Hum Brain Mapp. 38:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maier SU, Hare TA (2017): Higher Heart-Rate Variability Is Associated with Ventromedial Prefrontal Cortex Activity and Increased Resistance to Temptation in Dietary Self-Control Challenges. J Neurosci. 37:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carter BL, Tiffany ST (1999): Meta-analysis of cue-reactivity in addiction research. Addiction. 94:327–340. [PubMed] [Google Scholar]
- 66.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1997): Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 22:157–167. [DOI] [PubMed] [Google Scholar]
- 67.Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, et al. (2010): Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse. 36:106–113. [DOI] [PubMed] [Google Scholar]
- 68.Baker AK, Garland EL (2019): Autonomic and affective mediators of the relationship between mindfulness and opioid craving among chronic pain patients. Exp Clin Psychopharmacol. 27:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ordonana JR, Gonzalez-Javier F, Gomez-Amor J (2012): Psychophysiological reactivity to environmental tobacco smoke on smokers and non-smokers. Addict Behav. 37:838–843. [DOI] [PubMed] [Google Scholar]
- 70.Fox HC, Hong KI, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, et al. (2009): Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 44:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heishman SJ, Lee DC, Taylor RC, Singleton EG (2010): Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of tobacco deprivation and sex. Exp Clin Psychopharmacol. 18:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skinner MD, Coudert M, Berlin I, Passeri E, Michel L, Aubin HJ (2010): Effect of the threat of a disulfiram-ethanol reaction on cue reactivity in alcoholics. Drug Alcohol Depend. 112:239–246. [DOI] [PubMed] [Google Scholar]
- 73.Wolfling K, Flor H, Grusser SM (2008): Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 27:976–983. [DOI] [PubMed] [Google Scholar]