Abstract
It is increasingly recognized that chronic opioid use leads to maladaptive changes in the composition and localization of gut bacteria. Recently, this “opioid-induced dysbiosis” (OID) has been linked to antinociceptive tolerance development in preclinical models and may therefore identify promising targets for new opioid-sparing strategies. Such developments are critical to curb dose escalations in the clinical setting and combat the ongoing opioid epidemic. In this article, we review the existing literature that pertains to OID, including the current evidence regarding its qualitative nature, influence on antinociceptive tolerance, and future prospects.
Keywords: analgesia, antibiotics, antinociception, dorsal root ganglia, dysbiosis, microbiome, morphine, opioid, tolerance
INTRODUCTION
In October 2017, the United States government declared a state of public health emergency in response to the growing prescription opioid epidemic. Social, political, and medical rhetoric has recently demanded improvements in clinical pain control, prompting the publication of the “Guideline for Prescribing Opioids for Chronic Pain” by the Centers for Disease Control and Prevention (CDC) in March, 2016.31 Over the last decade, opioid use in the US has risen dramatically, with the number of prescriptions written by health care providers in 2012 (~259 million) exceeding the total adult population (~240 million). Opioid-related emergency department visits and mortality rates have concurrently increased, with over 165,000 deaths by overdose during 1999–2014. Despite this hardship, opioid analgesics remain an essential component of modern healthcare as the gold standard of therapy for moderate to severe pain. Since complete elimination of opioids would be impractical and even detrimental, there has been a great push to identify new opioid-sparing strategies, such as reducing analgesic tolerance development4,27. With mounting evidence suggesting that opioids alter the composition and localization of gut microbes, questions have been raised of whether opioid-induced dysbiosis (OID) influences analgesic tolerance development. Indeed, the physiological influence of gut microbes is both robust and widespread – prominent examples include gastrointestinal disorders such as irritable bowel syndrome (IBS)70 and inflammatory bowel diseases (IBD)64,116, metabolic and endocrine states such as obesity122 and type I/II diabetes mellitus2,88, and even maladies of central origin such as anxiety and depression71,106.
OPIOID-INDUCED DYSBIOSIS
Composition
Qualitative analyses of opioid-induced dysbiosis (OID) are still developing, but numerous independent investigators have found that rodents with chronic morphine exposure display significant shifts in the composition of commensal bacteria (Table 1). Meng et al. (2015) found a significant enhancement in the abundance of Gram-positive Staphylococcus and Enterococcus in mice75, while an order analysis by Kang et al. (2017) further indicated a reduction of Bacteroidetes (Bacteroidales) and Firmicutes (Clostridiales and Lactobacilliales) with enrichment of Proteobacteria (Enterobacteriales).56 Many actions of morphine likely promote these shifts, including inhibition of gut mucus and bicarbonate secretion95, prolongation of intestinal transit time8,110, inhibition of bile acid secretion118, and suppression of immune surveillance17. In addition to compositional changes, morphine has also been noted to modify the characteristics of individual genera or species. For example, a focused study by Wang et al. (2018) observed enrichment of specific pathogenic genera of Flavobacterium, Enterococcus, Fusobacterium, Sutterella, and Clostridium129, while Banerjee et al. (2016) noted a net expansion in the diversity of Firmicutes (Enterococcaceae, Staphylococcaceae, Bacillaceae, Streptococcaceae, and Erysipelotrichaceae) and a reduction of bile-deconjugating bacterial strains9. An additional study by Babrowski et al. (2012) indicated that chronic morphine activates virulence in Pseudomonas aeruginosa.7 These findings present a sound initial framework, but further studies are certainly warranted to enhance the resolution of these findings and assess whether they are consistent between different opioids. Particular attention should be paid to the treatment protocol in future studies as Lee et al. (2018) observed that escalating intermittent morphine increased abundance of Ruminococcus and decreased Lactobacillus in mouse fecal matter, while continuous exposure instead increased Clostridium and Rikenellaceae.63 Nonetheless, the consensus among existing findings is encouraging and provides some insight about how the biochemical milieu of the gut might be influenced by chronic opioid exposure.
Table 1: Preclinical studies in mice indicate that chronic morphine exposure alters the composition of gut microbiota.
Treatment protocols indicate the route (s.c. pellet implantation vs. i.p. injection), dosage, frequency, and duration of morphine exposure.
| Finding | Treatment protocol | Sample | Strain | Reference |
|---|---|---|---|---|
| ↑ Pathogenic genera (Flavobacterium, Enterococcus, Fusobacterium, Sutterella, Clostridium) |
25 mg pellet (3 days) |
Fecal DNA | C57BL/6J | Wang et al., 2018 |
| ↑ Ruminococcus | 10 mg/kg i.p. | Fecal DNA | C57BL/6J | Lee et al., 2018 |
| ↓ Lactobacillus | (every 12 hours, 4 days) | |||
| ↑ Clostridium | 25 mg pellet | |||
| ↑ Rikenellaceae | (4 days) | |||
| ↑ Proteobacteria (Enterobacteriales) | 75 mg pellet | Fecal DNA | Swiss Webster | Kang et al., 2017 |
| ↓ Bacteroidetes (Bacteroidales) ↓ Firmicutes (Clostridiales, Lactobacilliales) |
(5 days) | |||
| ↑ Diversity of Firmicutes (Enterococcaceae, Staphylococcaceae, Bacillaceae, Streptococcaceae, Erysipelotrichaceae) ↓ Bile-deconjugating bacterial strains |
25 mg pellet (5–6 days) |
Fecal DNA | C57BL/6J | Banerjee et al., 2016 |
| ↑ Staphylococcus ↑ Enterococcus |
25 mg pellet (≤4 days) |
Fecal DNA | C57BL/6 | Meng et al., 2015 |
| ↑ Virulence of Pseudomonas aeruginosa | 25 mg pellet (36 hours) | Cecal tissue DNA | C57BL/6 | Babrowski et al., 2012 |
Gram-negative Bacteroides are the most abundant microbes in the distal ileum and colon. They are generally considered to be gut-protective, in part due to modulation of immune reponses.102 They are broadly proficient digesters of carbohydrates and fiber, yielding short-chain fatty acids (SCFAs) that quell inflammation109 and promote impermeability of the epithelial barrier87. However, individual Bacteroides species demonstrate additional mechanisms of immunomodulation. For example, polysaccharide A (PSA) from Bacteroides fragilis has been observed to enhance the abundance of helper T cells and reduce inflammation73,74. This resonates with findings that the relative abundance of B. fragilis is reduced in patients with IBD50,80. Bacteroides thetaiotaomicron also displays gut-protective properties, having been noted to alter mucosal gene expression in a manner promoting barrier impermeability, nutrient absorption, cellular maturation, and angiogenesis.47,93 Collectively, these findings are consistent with studies of OID in which chronic morphine induced a relative reduction of Bacteroidetes that correlated with an increase in pro-inflammatory cytokine release and breakdown of the epithelial barrier.56
Gram-positive Firmicutes (Clostridiales and Lactobacilliales) are also of reduced abundance in mice with chronic morphine exposure. Clostridia, although better known for their pathogenic species (e.g., Clostridium difficile, tetani, botulinum, and perfringens), are also highly abundant in the healthy distal ileum and colon.102 Commensal Clostridia demonstrate a significant level of anti-inflammatory activity and have significantly reduced abundance in patients with IBD.102 This anti-inflammatory action has primarily been attributed to fiber metabolism, SCFA production (especially butyrate), and induction of regulatory T cells that produce TGFβ and IL-10.6,39 Lactobacillus species are typically less abundant than both Bacteroides and Clostridia, but are among the most thoroughly tested and commonly recommended probiotic agents. Their anti-inflammatory action and therapeutic potential in IBD have been extensively documented and reviewed.84 These findings suggest that the reduced abundance of Firmicutes with chronic morphine may also contribute to pro-inflammatory action in the gut.
Gram-positive Proteobacteria (Enterobacteriales) demonstrate enhanced abundance in mice with chronic morphine exposure. These microbes are notably enriched in a number of disease states (both intra- and extra-intestinal), and have been suggested as biological indicators of dysbiosis.107 Inflammation appears to be a fundamental component of Proteobacteria-related diseases94, and an enhanced relative abundance is observed in patients with IBD38,43,101. A recent study indicated that intestinal biopsy samples possess a greater abundance of Proteobacteria than fecal samples41,80, suggesting that their expansion favors regions with close mucosal proximity. This enhances the potential for functional impacts on mucosal tissues, and may be an important component of the pro-inflammatory activity occurring with chronic morphine exposure.
Genomic and proteomic analyses with greater depth and coverage (e.g., bacteria, fungi, viruses, etc.) are undoubtedly necessary to capture the full breadth of OID. Nonetheless, the current evidence in mice suggests a shift in GI microflora toward a phenotype that is pro-inflammatory and deleterious to epithelial barrier integrity. It remains unclear whether these findings will translate to humans. In terms of composition, the gut microbiomes of mice and humans are broadly similar with Bacteroidetes and Firmicutes (Clostridiales and Lactobacilliales) dominating over numerous other minor phyla.66,123,134 These minor phyla, however, have demonstrated differences in composition and interactions with host tissues.81 For example, Gram-positive segmented filamentous bacteria (SFB) with pro-inflammatory properties are enriched in the terminal ileum of rodents51,59, and 85% of the other minor phyla in mice are completely absent in humans65. Whether the minor differences will transcend the overarching similarities remains to be determined, and studies to date have not been designed to address this question. Dysbiosis has been noted in patients with substance use disorders (heroin among them)135, but no study has examined for a main effect of opioid use. Such a design, however valuable, may be complicated by the frequency of polysubstance use in opioid abusers. A better controlled design may involve patients with clinical opioid use. It has been speculated that opioids are linked to enhanced microbial virulence in critically ill ICU patients, but further investigations are needed22 and may be limited by the duration of use. Chronic outpatient users may provide an ideal study group (see Experimental Paradigms, Colon Tissue Supernatants).
Translocation
Recent findings have also linked opioid exposure to the translocation of gut microbes. Chronic morphine has been noted to produce a “leaky” gut in mice by compromising the integrity of epithelial tight junctions through mechanisms involving TLR-2/4 activation, IL-17A release (driven by IL-1β and IL-23), and redistribution of tight junction protein zonula occludens-1 (ZO-1).56,75 The resulting increase in epithelial permeability allows translocation of luminal bacteria to the gut wall, mesenteric lymph nodes (MLN), blood, liver, and spleen. The intestinal histology in these mice reveals neutrophilic infiltration (i.e., inflammation) and structural disruption of the mucosal crypts and lamina propria. Further investigation is certainly necessary, but similar tissue destruction has been noted in the intestines of human opioid abusers (Meng et al., 2015, Figure 6J).75 Future investigations might also consider the antimicrobial peptides and local immune responses in Peyer’s patches that are well known to contribute to the physical barrier of the gut.
Immunomodulation
Regulation of immune responses by opioids is exceptionally complex, stimulating some pathways, inhibiting others, and often demonstrating dual effects. The immunosuppressive action of opioids on T-cells, B-cells, and macrophages is well documented82,98,125, yielding reductions in peripheral inflammatory mechanisms such as neutrophil chemotaxis and extravasation68,78, macrophage recruitment, and TNF-α release from mast cells98. This is a widely accepted postulate, although it has been noted that many characterization studies are performed in vitro or utilize an acute morphine paradigm that may limit their translation to chronic models in vivo.68 Perhaps paradoxically, mice with chronic morphine exposure actually demonstrate increased activation of innate immune responses in the gut, including neutrophil infiltration and expression of IL-1β.56,76 This suggests that any immunosuppressive effects of morphine in the gut are overshadowed by local pro-inflammatory stimuli. This concurs with findings by Zhang et al. (2019) that chronic morphine exposure in mice enhances expression of IL-6, IL-1β, and TNF-α.138 Pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6, and IL-8) has also been noted in patients on methadone maintenance therapy, with TNF-α and IL-6 concentrations that correlate with methadone usage.21
A potentially important source of this cytokine release is glial cells (including microglia and astrocytes). Glial cell activation by morphine has been described in the dorsal horn of the spinal cord49,53,131, in enteric glia14, and in satellite glia of the dorsal root ganglia13,52. Glial activation mechanisms are thought to contribute to the pathogenesis of analgesic tolerance25,32,44, and are discussed further in the corresponding section below (see Analgesic tolerance).
Experimental paradigms
Axenic animals
Many recent studies of the microbiome have used animals reared in germ-free environments as a model of bacterial clearance. These paradigms should be implemented cautiously as numerous developmental anomalies have been documented in these subjects that may confound the outcomes. The “natural” relationship with bacteria begins during birth, is pervasive throughout life, and serves numerous key physiological roles. These include 1) production of short-chain fatty acids (SCFA), vitamins, essential amino acids, and neurotransmitters, 2) modulation of gut epithelial gene expression, and 3) transformation of bile acids. Animals raised in germ-free environments exhibit profound developmental immaturity of virtually every body system108, and a short list of the dietary and metabolic impacts include the requirement of exogenous vitamin K, decreased body fat, increased cholesterol, decreased total blood volume, and decreased basal metabolic rate. Immune insufficiency is particularly prominent in these animals, with some of the greatest immune deficits being noted in the gut (e.g., decreased immune tissue density, small and sparse Peyer’s patches, and small mesenteric lymph nodes that lack germinal centers). These findings offer high face validity given that the microbiome is in intimate contact with gut tissues in the native state. Indeed, gastrointestinal immaturity is a general theme in axenic animals, with eminent examples found in the small intestine (reduced osmolarity, elevated oxygen tension and electripotential, decreased total mass and surface area for absorption, narrowing of villi, and truncation of ileal villi) and cecum (increased volume, reduced wall thickness, decreased spontaneous contraction, and increased alkalinity). While germ-free mice may provide some utility in studies of OID, investigators should be advised to interpret results cautiously and correct for confounding variables as able.
Antibiotics
Bacterial clearance can alternatively be achieved through the use of antibiotics. This allows subjects to achieve developmental adulthood prior to testing, and circumvents the issues noted in axenic animals. It allows targeted elimination of bacteria based on the coverage spectrum of the antibiotic(s) and creates potential for within-subjects comparisons of responses before and after bacterial clearance. However, the potential for drug-drug and other metabolic interactions should always be considered when using antibiotics, and investigators should attempt to minimize these factors when able. In this regard, antibiotics with low oral bioavailability offer some advantage, but it remains important to consider how opioid-induced breakdown of the gut epithelial barrier may influence drug uptake. Selecting antibiotics with negligible production of metabolites and off-target binding can minimize the impact of inadvertent absorption.
An example that meets the aforementioned criteria is vancomycin. Vancomycin has virtually 0% oral bioavailability and is eliminated unchanged in the urine even if small amounts enter the bloodstream. Importantly, it does not demonstrate significant conversion to metabolites in vivo.72 It bactericidal action is restricted to Gram-positive organisms by its mechanism of action, binding the D-Ala-D-Ala component of the cell wall precursor peptidoglycan. Mammalian cells do not possess this D-Ala-D-Ala component or have cell walls, and evidence is lacking for significant off-target binding.99 Though some impacts on gut physiology have been noted with vancomycin administration (e.g., modulation of permeability121), much of this can be attributed to reductions in Gram-positive bacteria.
Fecal matter transplant (FMT)
The introduction of foreign fecal matter into the gut has emerged as a robust method for modifying floral composition in vivo. This method is well known for its efficacy in treating pseudomembranous colitis caused by Clostridium difficile infection57,58, but significant benefits have also been noted for IBD, IBS, and constipation.3,5,15,16,89 A recent investigation by Banerjee et al. (2016) demonstrated that the microbial dysbiosis and gut barrier disruption associated with chronic morphine exposure could be prevented by FMT from placebo-treated counterparts.9 More recently, Zhang et al. (2019) noted that fecal matter from mice with chronic morphine exposure induces morphine tolerance in germ-free mice.138
Probiotics
A less robust but easily translated model of microbial restructuring is probiotic treatment. Numerous bacterial genera have demonstrated efficacy in this regard, but Lactobacillus and Bifidobacteria are among the most commonly utilized. Their anti-inflammatory action and therapeutic potential in IBD have been extensively documented84, and recent evidence suggests that they may be able to reduce visceral pain associated with conditions like IBS96,117. Probiotic supplementation (e.g., Lactobacillus rhamnosus GG) may therefore be a viable method of rescuing the deleterious shifts in microbial composition that occur with chronic morphine exposure. Recent investigations demonstrate that probiotic treatment is sufficient to reverse tolerance development138, and have the major advantage of being readily translated to a human patient population.
Colon tissue supernatants
The use of supernatants from colon tissue samples has arisen as an innovative and powerful paradigm for investigating the mechanisms of peripheral nociception in both mice and human patients.1,10,20,77,124 Full-circumference colon tissue segments are resected and incubated in culture medium such that biochemical mediators in the gut wall (e.g., bacterial products and pro-inflammatory cytokines) leach out into the culture medium. The liquid supernatant may then be transferred to a target cell population (e.g., naïve DRG cultures) to assess the impact of gut-localized mediators on neuronal excitability. A recent study observed that colon tissue supernatants from mice with chronic morphine exposure induced tolerance and hyperexcitability in naïve mouse DRG neurons.77 Oral vancomycin treatment in this paradigm was sufficient to block tolerance development, but not hyperexcitability. This evidence suggests that tolerance development in vivo involves a peripheral, gut-mediated component in addition to the central mechanisms that have been extensively described.
A major cited advantage of using colon tissue supernatants is the ease with which translational relevance can be established. To this end, Valdez-Morales et al. (2013) demonstrated that supernatants (and thus gut-derived mediators) from human mucosal biopsy samples could be applied to mouse DRG neurons to examine the influence on neuronal excitability. In the absence of human DRG donors, this offers a practical method to establish translational relevance prior to the rigorous and costly process of identifying the underlying biochemical mechanisms involved. In a similar fashion, naïve mouse DRG neurons may be exposed to supernatants from human colon biopsy samples in patients with a history of chronic opioid use to examine the influence on tolerance development. Patients with constipation-predominant irritable bowel syndrome (IBS-C) represent an ideal population for such an investigation for numerous reasons: 1) the prevalence of opioid use for clinical pain is likely to be significant, 2) colonoscopy with biopsy is commonly performed in these patients for pathological assessments and the samples often lack confounding pathology (e.g., malignancy or IBD), 3) evidence indicates that control IBS-C colon supernatants will not impact excitability in DRG neurons (Valdez-Morales et al., 2013), and 4) the incidence of IBS-C diagnoses at most major medical centers is likely to be high. Such an experiment should include standardization and/or stratification of patients to the extent possible, and sources of variation should be considered and compensated for. This may include, but is not limited to, the opioid regimen (specific drug, dosage, presence of additives, rate of release, and duration of use), past medical history (e.g., recent antibiotic administration), and demographics (e.g., age, sex, and race). With regard to variability among opioids, it is notable that many produce cross-tolerance with morphine and modulation of tolerance may be observed even if stratification is not possible.
ANALGESIC TOLERANCE
Behavioral tolerance
With mounting evidence supporting the notion of OID, questions have been raised of how the liberated bacterial products and secondary pro-inflammatory responses (particularly in the gut wall) may influence the adaptive cellular processes associated with antinociceptive tolerance development. Indeed, bacterial N-formylated peptides have been noted to directly activate sensory neurons24 and inflammation is well established to sensitize primary afferent pain fibers and modulate nociceptor signaling/gene expression in various paradigms.12,33,42,67,114,128 Inflammation has also been shown to result in significant plasticity of opioid signaling, including receptor expression, G protein signaling, and receptor trafficking.139 In fact, a recent study found that TNBS-induced colitis hastens the development of antinociceptive tolerance in mice with chronic morphine exposure.61 This certainly resonates with the qualitative evidence of OID, and is supported by years of clinical evidence demonstrating a synergistic relationship in the analgesic properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids.60,137
Investigators have now begun to assess the role of OID in antinociceptive tolerance by utilizing the variety of experimental paradigms described above (see Experimental paradigms). Kang et al. (2017) noted that selective elimination of Gram-positive organisms via oral vancomycin attenuated morphine tolerance development in vivo.56 This effect was only modestly enhanced using broad-spectrum antibiotics with coverage for Gram-negative bacteria; so while both may have some impact on tolerance development, Gram-positive organisms appear to be the primary offenders. This resonates with evidence that chronic morphine exposure in mice selectively promotes Gram-positive sepsis.75 A study by Zhang et al. (2019) also noted attenuation of tolerance using a non-absorbable broad-spectrum antibiotic cocktail and a tolerance paradigm of escalating-dose morphine injections. This study further noted attenuation of tolerance in germ-free mice, induction of tolerance via FMT from mice with chronic morphine exposure, and reversal of tolerance via probiotic treatment.138
The implication of Gram-positive microbes certainly warrants further investigation. Gram-positive and Gram-negative bacteria are, by definition, differentiated based on the retention of crystal violet dye, which depends on the physical and chemical composition of the cell wall. Lipoteichoic acid (LTA) is a major constituent of the Gram-positive cell wall that is known to bind TLR-2, inducing NF-κB expression and downstream pro-inflammatory activity. The Gram-negative correlate of LTA is lipopolysaccharide (LPS, endotoxin).112,126 LPS is present in the outer membrane of the cell wall and binds TLR-4 to induce NF-κB signaling. Despite their similarities, nuances have been noted in the responses elicited by LTA and LPS, including cytokine induction profiles, leukocyte epithelial adhesion, and reorganization of tight junction proteins.19,37,54 TLR-2 is the only Toll-like receptor that has been observed to modify the arrangement of tight junction proteins in the gut epithelial barrier,18 and may therefore contribute to the compromise of epithelial barrier integrity that is noted with chronic morphine. Indeed, this finding was mitigated in studies using vancomycin. Still, the interactive effect with Gram-negative microbes is apparent, and may involve LPS-induced cytokine release from enteric and/or DRG satellite glia.14
Cellular tolerance and signaling
A critical future direction is to identify the cell populations and signaling mechanisms that underlie the behavioral findings. Primary afferent neurons are the first-order components of nociceptive signaling and an immediately accessible component of the pain pathway for translocating bacteria. These cells have soma localized to the DRG and terminal processes that innervate peripheral regions including the gut wall. The importance of DRG nociceptors in antinociceptive tolerance was recently demonstrated by Corder et al. (2017) utilizing a mouse model with TRPV1 promoter-driven Cre recombinase and a loxP-flanked OPRM1 gene that conditionally deleted μ-OR expression in neurons of TRPV1 lineage (i.e., nociceptors).28 When exposed to morphine, these mice exhibited reductions in tolerance and opioid-induced hypernociception (OIH) development, suggesting that these effects are driven by μ-OR activation on DRG nociceptors. Peripheral mechanisms play a key role in nociceptive sensation, and opioid receptors on DRG neurons have been suggested to account for 50–100% of the antinociceptive effect of systemic opioids.111 Indeed, even peripherally restricted opioids like loperamide demonstrate significant antinociceptive properties along with tolerance and OIH development.46,55,127 With regard to OID, Kang et al. (2017) noted that oral vancomycin prevented morphine tolerance in DRG neurons isolated from lumbosacral spinal levels. This theme persisted in a further study examining the inactivation kinetics of tetrodotoxin-resistant (TTX-R) Na+ channels (Nav1.8/1.9).77 Of note, these observations in peripheral afferents do not dispute central mechanisms of opioid tolerance, but rather imply that additional mechanisms are contributing.
An appealing target for further investigation of TTX-R Na+ channels is β-arrestin2 (β-Arr2). μ-ORs are classical Gi/o-coupled receptors that dissociate Gα and Gβγ subunits when activated. The Gα subunit inhibits the adenylyl cyclase (AC)/cAMP/PKA pathway. The Gβγ component has a number of established effects, including 1) activation of GIRK channels100,119,120, 2) inhibition of voltage-gated Ca2+ channels79,104, 3) recruitment of GRK2/3, 4) activation of the MAPK cascade (Ras, Raf, MEK, ERK1/2, JNK, p38) in a PI3K-/c-Src-dependent manner90,91,133, 5) activation of the PLC/PKC pathway, and 6) stimulation of CaMKII92. GRK2/3 recruitment to the μ-OR results in phosphorylation of the agonist-bound receptor, inducing a μ-OR conformation with greater affinity for β-Arr2. β-arrestins are ubiquitously expressed proteins that function to desensitize (or “arrest”) the vast majority of G protein-coupled receptors.30 Binding of β-Arr2 creates steric hindrance that prevents μ-OR coupling with G proteins and targets the μ-OR to clathrin-coated pits for internalization. These canonical actions play key roles in morphine desensitization and tolerance development. Recruitment of β-Arr2 to the μ-OR modulates channel activity by diverting β-Arr2 away from other molecular targets. This effect has been noted to enhance the sensitivity of TRPV1 channels.97 Whether this mechanism also impacts TTX-R Na+ currents, directly or indirectly, remains to be evaluated. Recent evidence suggests that β-Arr2 also serves as a scaffolding protein for activation of various intracellular kinase cascades, including MAPK.30,34 MAPK can phosphorylate multiple cytoplasmic and nuclear targets (e.g., CREB) to modulate transcriptional events. It is therefore probable that this β-Arr2/MAPK pathway is involved in the cellular adaptive processes that take place with chronic opioid exposure to modulate excitability and tolerance. Finally, β-Arr2 appears to play a role in inflammation and immunity.11,35,45 In this regard, it has been noted to inhibit the NF-κB pathway by preventing the phosphorylation and degradation of IκBα40,113, interfering with LPS-induced NF-κB activation, and reducing TRAF6 autoubiquitination.130 This may have important implications for cytokine production pathways that involve nuclear translocation of NF-κB (e.g., TLR/MyD88), and may play a role in morphine tolerance development.56,75,76
Glial activation
The influence of gut dysbiosis on tolerance in primary afferent neurons raises questions regarding the involvement of glia. Opioid-induced glial activation has been described in the dorsal horn of the spinal cord49,53,131, enteric glia14, and satellite glia of dorsal root ganglia.13,52 Glial activation releases numerous cytokines, chemokines, and neurotropic factors into the surrounding environment26,36,62,85 and is thought to contribute to analgesic tolerance development25,32,44. Indeed, studies using non-selective glial inhibitors (e.g., minocycline29, fluorocitrate132, and propentofylline115) have noted attenuation of tolerance in vivo.48 Bacterial products like LTA and LPS are well established to induce glial activation and inflammation.14,69,83,136 Given that OID involves bacterial translocation, the release of bacterial products may contribute to tolerance development via glial activation. Satellite and spinal glia have direct contacts with somatic afferents in pain pathways, and enteric glia could sensitize somatic afferents indirectly via visceral afferents and satellite glia. Future investigations should note that opioid receptor activation per se has been linked to expression of pro-inflammatory mediators.26,85,140 This mechanism should be regarded as a potential confounder.
CONCLUDING REMARKS
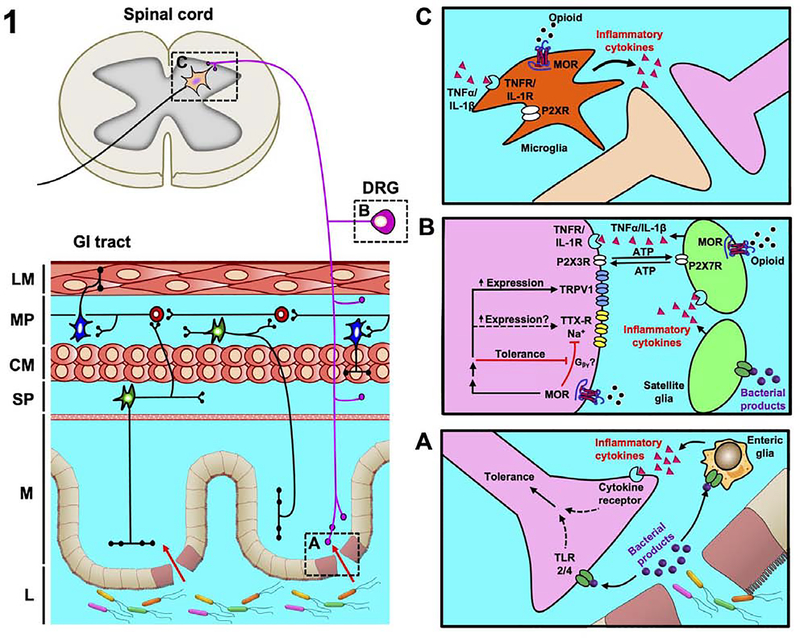
The role of opioid-induced dysbiosis (OID) in modulation of pain control is increasingly evident. Further characterization studies are necessary, particularly to improve the resolution of compositional analyses (e.g., advanced genomics and proteomics). This will greatly facilitate the identification of causal mechanisms in tolerance development. Nonetheless, the consensus of present literature points to a few prevailing themes regarding OID: 1) a shift in gastrointestinal microflora toward a phenotype that is pro-inflammatory and deleterious to epithelial barrier integrity, 2) translocation of microbes to the gut wall and other extra-intestinal sites, and 3) a pervasive correlation between dysbiosis, activation of pro-inflammatory cascades, and development of antinociceptive tolerance. Current findings further suggest that peripheral components of the pain pathway (i.e., primary afferent neurons) significantly contribute to this process (Figure 1). This presents with great face validity given the close proximity of the microbiome with peripheral tissues; however, it does not dispute the potential co-involvement of central mechanisms of tolerance, and further evaluation of this topic is necessary.
Figure 1: Opioid-induced dysbiosis (OID) modulates tolerance development in primary afferent neurons.
A representative schematic depicts the interactions between dysbiotic gut microbes and gut-innervating primary afferent neurons. This exhibit captures the mechanisms discussed in this article, and is not intended to be comprehensive. Layers of the gastrointestinal (GI) tract have been labeled for clarity – (L) lumen, (M) mucosa, (SP) submucosal plexus, (CM) circular muscle layer, (MP) myenteric plexus, (LM) longitudinal muscle layer. Chronic opioid exposure alters the composition of gut microbes and compromises epithelial barrier integrity, promoting bacterial translocation to the gut wall and other extra-intestinal sites. Studies of OID demonstrate a pervasive correlation between the subsequent release of biochemical mediators (e.g., bacterial products, pro-inflammatory cytokines) and the development of antinociceptive tolerance. The dorsal root ganglion (DRG) nerve terminals (A), cell soma (B), and dorsal horn synapses (C) are all sites that have been implicated in this process and/or demonstrate promising prospects for further investigation.
Beyond antinociceptive tolerance, it is also worth noting that the mechanisms of opioid-induced hypernociception (OIH) are also thought to contribute to dose escalations in the clinical setting.4,27 Even in the absence of bona fide hyperalgesia, the mechanisms involved in OIH may be activated, reducing the analgesic potency of opioids.23,25,103 “Tolerance” is often used as a catch-all term in instances where a diminished response is noted, but the underlying mechanisms are unique and should be approached accordingly. At present, data are insufficient to determine whether OID impacts the mechanisms of OIH, but studies using vancomycin to eliminate Gram-positive organisms have not observed any influence in the setting of chronic morphine exposure.77
Regarding the opioid epidemic, the need for novel opioid-sparing strategies is self-evident. New therapeutic methods that reduce the dose and duration of opioid use are essential, as each is predictive of physical dependence, addiction, and overdose.86,105 Elucidating the mechanisms of OID in pain control, while complex, will advance our understanding of opioid pharmacology and take strides toward improving patient outcomes.
Highlights.
Chronic opioid exposure results in opioid-induced dysbiosis (OID) of gut bacteria
OID is characterized by pro-inflammatory gut bacteria and leaky epithelial barriers
OID correlates with antinociceptive tolerance development
Peripheral mechanisms contribute to OID-related tolerance development
Perspective.
This article reviews the current literature on opioid-induced dysbiosis (OID) of gut bacteria, including its qualitative nature, influence on antinociceptive tolerance, and future prospects. This work may help identify targets for new opioid-sparing strategies.
ACKNOWLEDGEMENTS
R.A.M. and H.I.A. intellectually conceived the manuscript. R.A.M. and K.H.M. produced the manuscript, which H.I.A. edited and all authors approved.
DISCLOSURES
The authors are supported by the National Institutes of Health grants F30 DA042542 to R.A.M., R01 DA036975 to H.I.A. and W.L.D., P30 DA033934, and T32 DA007027. All authors declare no conflict of interest or competing financial interests.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- β-Arr2
β-arrestin2
- Ca2+
divalent calcium
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- cAMP
cyclic adenosine monophosphate
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- DRG
dorsal root ganglion
- ERK
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorting
- GIRK
G protein-coupled inwardly-rectifying potassium
- GRK
G protein-coupled receptor kinase
- JNK
c-Jun N-terminal kinase
- K+
monovalent potassium
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- mRNA
messenger ribonucleic acid
- Na+
monovalent sodium
- NMDA
N-Methyl-D-aspartate
- OIH
opioid-induced hypernociception
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- s.c.
subcutaneous
- TEA
tetraethylammonium
- TRPV1
transient receptor potential cation channel subfamily V member 1
- TTX-R
tetrodotoxin-resistant
- TTX-S
tetrodotoxin-sensitive
- Vt
action potential threshold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S: Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57:923–9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, Frank DN, Zipris D: Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64:3510–20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P, Borody T, Shortis N, Thompson S: Bacteriotherapy for chronic constipation - a long term follow-up. Gastroenterology 108:A563, 1995. [Google Scholar]
- 4.Angst MS, Clark JD: Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 104:570–87, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Aroniadis OC, Brandt LJ: Fecal microbiota transplantation: Past, present and future. Curr Opin Gastroenterol 29:79–84, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K: Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–41, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC: Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg 255:386–93, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I: Bacterial translocation: Overview of mechanisms and clinical impact. J Gastroenterol Hepatol 22:464–71, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T: Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9:612–24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R: Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology 126:693–702, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Barlic J, Andrews JD, Kelvin a a, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ: Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol 1:227–33, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Basbaum AI, Bautista DM, Scherrer G, Julius D: Cellular and Molecular Mechanisms of Pain. Cell 139:267–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berta T, Liu T, Liu YC, Xu ZZ, Ji RR: Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9. Mol Pain 8:18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI: Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J 31:2649–60, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borody T, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D: Bowel-flora alteration: A potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust 150:604, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Borody TJ, Paramsothy S, Agrawal G: Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr Gastroenterol Rep 15:337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosnahan A, Jones B, Dvorak C, Brown D: Morphine Attenuates Apically-Directed Cytokine Secretion from Intestinal Epithelial Cells in Response to Enteric Pathogens. Pathogens 3:249–57, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cario E: Toll-like receptors in inflammatory bowel diseases: A decade later. Inflamm Bowel Dis 16:1583–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cario E, Gerken G, Podolsky DK: Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127:224–38, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JGP, Shaffer E, Vergnolle N: Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117:636–47, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY: Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res 226:230–4, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Chapple LA, Deane A: From dysmotility to virulent pathogens: Implications of opioid use in the ICU. Curr Opin Crit Care 24:118–23, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Chen MD, Sein L, MD M, Vo BAT, Amhmed MDS, Zhang MDY, St Hilaire BAK, Houghton BAM, Mao MD, PhD J: Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 10:383–93, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Wardenburg JB, Hwang SW, Carroll MC, Woolf CJ: Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu LF, Angst MS, Clark D: Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain 24:479–96, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Coller JK, Hutchinson MR: Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134:219–45, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Collett BJ: Opioid tolerance: the clinical perspective. Br J Anaesth 81:58–68, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G: Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 23:164–73, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ: A novel role of minocycline: Attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22:114–23, 2008. [DOI] [PubMed] [Google Scholar]
- 30.DeWire S, Ahn S, Lefkowitz R, Shenoy S: Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Dowell D, Haegerich TM, Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Reports 65:1–49, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Drossman D, Szigethy E: The Narcotic Bowel Syndrome: A Recent Update. Am J Gastroenterol Suppl 2:22–30, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Dubin AE, Patapoutian A: Nociceptors: The sensors of the pain pathway. J Clin Invest 120:3760–72, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichel K, Jullié D, Von Zastrow M: β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol 18:303–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA: β-Arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol 44:3092–9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrini F, Trang T, Mattioli TAM, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y: Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl-homeostasis. Nat Neurosci 16:183–92, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finney S, Leaver S, Evans T, Burke-Gaffney A: Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med 38:324–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR: Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci 104:13780–5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H: Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–50, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G: Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14:303–17, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, González A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ: The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15:382–92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold MS, Gebhart GF: Nociceptor sensitization in pain pathogenesis. Nat Med 16:1248–57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen Van Zanten SJO: Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol 44:4136–41, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunkemeier DMS, Cassara JE, Dalton CB, Drossman DA: The Narcotic Bowel Syndrome: Clinical Features, Pathophysiology, and Management. Clin Gastroenterol Hepatol 5:1126–39, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurevich V: Arrestins – Pharmacology and Therapeutic Potential. Springer-Verlag Berlin Heidelb; 2014. [Google Scholar]
- 46.Hagiwara K, Nakagawasai O, Murata A, Yamadera F, Miyoshi I, Tan-No K, Tadano T, Yanagisawa T, Iijima T, Murakami M: Analgesic action of loperamide, an opioid agonist, and its blocking action on voltage-dependent Ca2+channels. Neurosci Res 46:493–7, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI: Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–4, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Horvath RJ, DeLeo JA: Morphine Enhances Microglial Migration through Modulation of P2X4 Receptor Signaling. J Neurosci 29:9987–1005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR: Opioid-Induced Glial Activation: Mechanisms of Activation and Implications for Opioid Analgesia, Dependence, and Reward. Sci World J 7:98–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huttenhower C, Kostic AD, Xavier RJ: Inflammatory bowel disease as a model for translating the microbiome. Immunity 40:843–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR: Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 139:485–98, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji RR, Berta T, Nedergaard M: Glia and pain: Is chronic pain a gliopathy? Pain 154:S10–28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston IN: A Role for Proinflammatory Cytokines and Fractalkine in Analgesia, Tolerance, and Subsequent Pain Facilitation Induced by Chronic Intrathecal Morphine. J Neurosci 24:7353–65, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jorgensen PF, Wang JE, Almlof M, Thiemermann C, Foster SJ, Solberg R, Aasen AO: Peptidoglycan and Lipoteichoic Acid Modify Monocyte Phenotype in Human Whole Blood. Clin Vaccine Immunol 8:515–21, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joseph EK, Reichling DB, Levine JD: Shared Mechanisms for Opioid Tolerance and a Transition to Chronic Pain. J Neurosci 30:4660–6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, Akbarali HI: The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 7:42658, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A, Gordon S, Gluck M, Hohmann EL, Kao D, Kao JY, McQuillen DP, Mellow M, Rank KM, Rao K, Ray A, Schwartz MA, Singh N, Stollman N, Suskind DL, Vindigni SM, Youngster I, Brandt L: Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109:1065–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khoruts A, Sadowsky MJ, Hamilton MJ: Development of Fecal Microbiota Transplantation Suitable for Mainstream Medicine. Clin Gastroenterol Hepatol 13:246–50, 2015. [DOI] [PubMed] [Google Scholar]
- 59.Klaasen HLBM, Koopman JP, Van Den Brink ME, Bakker MH, Poelma FGJ, Beynen AC: Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab Anim 27:141–50, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Kolesnikov YA, Wilson RS, Pasternak GW: The Synergistic Analgesic Interactions between Hydrocodone and Ibuprofen. Anesth Analg 97:1721–3, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Komla E, Stevens DL, Zheng Y, Zhang Y, Dewey WL, Akbarali HI: Experimental colitis enhances the rate of antinociceptive tolerance to morphine via peripheral opioid receptors. J Pharmacol Exp Ther 370:504–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leduc-Pessah H, Weilinger NL, Fan CY, Burma NE, Thompson RJ, Trang T: Site-Specific Regulation of P2X7 Receptor Function in Microglia Gates Morphine Analgesic Tolerance. J Neurosci 37:10154–72, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW: The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43:2606–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lepage P, Hösler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, Schreiber S: Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141:227–36, 2011. [DOI] [PubMed] [Google Scholar]
- 65.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI: Obesity alters gut microbial ecology. Proc Natl Acad Sci 102:11070–5, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI: Evolution of mammals and their gut microbes. Science 320:1647–51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q: Sensitization of Primary Afferent Nociceptors Induced by Intradermal Capsaicin Involves the Peripheral Release of Calcitonin Gene-Related Peptide Driven by Dorsal Root Reflexes. J Pain 9:1155–68, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang X, Liu R, Chen C, Ji F, Li T: Opioid system modulates the immune function: A review. Transl Perioper Pain Med 1:5–13, 2016. [PMC free article] [PubMed] [Google Scholar]
- 69.Lim H, Kim D, Lee SJ: Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J Biol Chem 288:7572–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT: Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis 49:331–7, 2017. [DOI] [PubMed] [Google Scholar]
- 71.Luna RA, Foster JA: Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr Opin Biotechnol 32:35–41, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Matzke GR, Zhanel GG, Guay DRP: Clinical Pharmacokinetics of Vancomycin. Clin Pharmacokinet 11:257–82, 1986. [DOI] [PubMed] [Google Scholar]
- 73.Mazmanian SK: Capsular polysaccharides of symbiotic bacteria modulate immune responses during experimental colitis. J Pediatr Gastroenterol Nutr 46 Suppl 1:E11–2, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Mazmanian SK, Round JL, Kasper DL: A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453:620–5, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S: Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17A neutralization. Sci Rep 5:, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S: Morphine Induces Bacterial Translocation in Mice by Compromising Intestinal Barrier Function in a TLR-Dependent Manner. PLoS One 8:e54040, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mischel RA, Dewey WL, Akbarali HI, Mischel RA, Dewey WL, Akbarali HI: Tolerance to Morphine-Induced Inhibition of TTX-R Sodium Channels in Dorsal Root Ganglia Neurons Is Modulated by Gut-Derived Mediators. iScience 2:1–17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyagi T, Chuang LF, Lam KM, Kung HF, Wang JM, Osburn BI, Chuang RY: Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes - An instant response. Immunopharmacology 47:53–62, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Moises HC, Rusin KI, Macdonald RL: mu-Opioid receptor-mediated reduction of neuronal calcium current occurs via a G(o)-type GTP-binding protein. J Neurosci 14:3842–51, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward D V, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C: Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13:R79, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen TLA, Vieira-Silva S, Liston A, Raes J: How informative is the mouse for human gut microbiota research? Dis Model Mech 8:1–16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ninković J, Roy S: Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 45:9–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obata Y, Pachnis V: The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 151:836–44, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandey KR, Naik SR, Vakil B V.: Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol 52:7577–87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parsadaniantz SM, Rivat C, Rostène W, Réaux-Le Goazigo A: Opioid and chemokine receptor crosstalk: A promising target for pain therapy? Nat Rev Neurosci 16:69–78, 2015. [DOI] [PubMed] [Google Scholar]
- 86.Paulozzi L, Baldwin G, Franklin G, Kerlikowske R, Jones C, Ghiya N, Popovic T: CDC Grand Rounds: Prescription Drug Overdoses - A U.S. Epidemic. MMWR Morb Mortal Wkly Rep 61:10–3, 2012. [PubMed] [Google Scholar]
- 87.Peng L, Li Z-R, Green RS, Holzman IR, Lin J: Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J Nutr 139:1619–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI: Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534:213–7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinn DM, Aroniadis OC, Brandt LJ: Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil 27:19–29, 2015. [DOI] [PubMed] [Google Scholar]
- 90.Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ: A mitogen-activated protein kinase pathway is required for μ-opioid receptor desensitization. J Biol Chem 273:12402–6, 1998. [DOI] [PubMed] [Google Scholar]
- 91.Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ:??-Opioid Receptor Activates Signaling Pathways Implicated in Cell Survival and Translational Control. J Biol Chem 273:23534–41, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Raehal KM, Schmid CL, Groer CE, Bohn LM: Functional Selectivity at the u-Opioid Receptor: Implications for Understanding Opioid Analgesia and Tolerance. Pharmacol Rev 63:1001–19, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rawls JF, Samuel BS, Gordon JI: Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci 101:4596–601, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A: Proteobacteria: A common factor in human diseases. Biomed Res Int 2017:9351507, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogers DF, Barnes PJ: Opioid Inhibition of Neurally Mediated Mucus Secretion in Human Bronchi. Lancet 333:930–2, 1989. [DOI] [PubMed] [Google Scholar]
- 96.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P: Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13:35–7, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Rowan MP, Bierbower SM, Eskander MA, Szteyn K, Por ED, Gomez R, Veldhuis N, Bunnett NW, Jeske NA: Activation of mu opioid receptors sensitizes Transient Receptor Potential Vanilloid Type 1 (TRPV1) via β-arrestin-2-mediated cross-talk. PLoS One 9:e93688, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA, Koodie L, Ma J, Meng J, Barke RA: Opioid drug abuse and modulation of immune function: Consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6:442–65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rybak MJ: The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin Infect Dis 42:S35–9, 2006. [DOI] [PubMed] [Google Scholar]
- 100.Sadja R, Alagem N, Reuveny E: Gating of GIRK channels: Details of an intricate, membrane-delimited signaling complex. Neuron 39:9–12, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Sartor RB: Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 134:577–94, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Sartor RB: The intestinal microbiota in inflammatory bowel diseases. Nestle Nutr Inst Workshop Ser 79:29–39, 2014. [DOI] [PubMed] [Google Scholar]
- 103.Schneider MD, PhD JP, Kirsh PhD KL: Defining clinical issues around tolerance, hyperalgesia, and addiction: A quantitative and qualitative outcome study of long-term opioid dosing in a chronic pain practice. J Opioid Manag 6:385–95, 2010. [DOI] [PubMed] [Google Scholar]
- 104.Schroeder JE, Fischbach PS, Zheng D, McCleskey EW: Activation of μ opioid receptors inhibits transient high- and low-threshold Ca2+currents, but spares a sustained current. Neuron 6:13–20, 1991. [DOI] [PubMed] [Google Scholar]
- 105.Shah A, Hayes CJ, Martin BC: Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep 66:265–9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK: The Central Nervous System and the Gut Microbiome. Cell 167:915–32, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin NR, Whon TW, Bae JW: Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33:496–503, 2015. [DOI] [PubMed] [Google Scholar]
- 108.Smith K, McCoy KD, Macpherson AJ: Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19:59–69, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS: The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 341:569–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith TH, Grider JR, Dewey WL, Akbarali HI: Morphine Decreases Enteric Neuron Excitability via Inhibition of Sodium Channels. PLoS One 7:e45251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stein C, Machelska H: Modulation of Peripheral Sensory Neurons by the Immune System: Implications for Pain Therapy. Pharmacol Rev 63:860–81, 2011. [DOI] [PubMed] [Google Scholar]
- 112.Su SC, Hua KF, Lee H, Chao LK, Tan SK, Lee H, Yang SF, Hsu HY: LTA and LPS mediated activation of protein kinases in the regulation of inflammatory cytokines expression in macrophages. Clin Chim Acta 374:106–15, 2006. [DOI] [PubMed] [Google Scholar]
- 113.Sun JJ, Lan JF, Shi XZ, Yang MC, Niu GJ, Ding D, Zhao XF, Yu XQ, Wang JX: β-Arrestins negatively regulate the toll pathway in shrimp by preventing dorsal translocation and inhibiting dorsal transcriptional activity. J Biol Chem 291:7488–504, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sung Y-J, Ambron RT: Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain. Neurol Res 26:195–203, 2004. [DOI] [PubMed] [Google Scholar]
- 115.Sweitzer S, De Leo J: Propentofylline: Glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol 200:235–50, 2011. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Andoh A, Sugimoto M: Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 93:59–65, 2016. [DOI] [PubMed] [Google Scholar]
- 117.Theodorou V, Belgnaoui AA, Agostini S, Eutamene H: Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 5:430–6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thompson DR: Narcotic analgesic effects on the sphincter of Oddi: A review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol 96:1266–72, 2001. [DOI] [PubMed] [Google Scholar]
- 119.Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K: G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22:4328–34, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torrecilla M, Quillinan N, Williams JT, Wickman K: Pre- and postsynaptic regulation of locus coeruleus neurons after chronic morphine treatment: A study of GIRK-knockout mice. Eur J Neurosci 28:618–24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tulstrup MVL, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, Licht TR, Bahl MI: Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class. PLoS One 10:e0144854, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–31, 2006. [DOI] [PubMed] [Google Scholar]
- 123.Umesaki Y, Setoyama H: Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect 2:1343–51, 2000. [DOI] [PubMed] [Google Scholar]
- 124.Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ: Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: A role for PAR2. Am J Gastroenterol 108:1634–43, 2013. [DOI] [PubMed] [Google Scholar]
- 125.Vallejo R, de Leon-Casasola O, Benyamin R: Opioid therapy and immunosuppression: a review. Am J Ther 11:354–65, 2004. [DOI] [PubMed] [Google Scholar]
- 126.Van Amersfoort ES, Van Berkel TJ, Kuiper J: Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. ClinMicrobiolRev 16:379–414, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J: Direct Inhibition of Ih by Analgesic Loperamide in Rat DRG Neurons. J Neurophysiol 97:3713–21, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Villarreal CF, Funez MI, Cunha FDQ, Parada CA, Ferreira SH: The long-lasting sensitization of primary afferent nociceptors induced by inflammation involves prostanoid and dopaminergic systems in mice. Pharmacol Biochem Behav 103:678–83, 2013. [DOI] [PubMed] [Google Scholar]
- 129.Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S: Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8:3596, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G: Association of β-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 7:139–47, 2006. [DOI] [PubMed] [Google Scholar]
- 131.Watkins LR, Hutchinson MR, Rice KC, Maier SF: The “Toll” of Opioid-Induced Glial Activation: Improving the Clinical Efficacy of Opioids by Targeting Glia. Trends Pharmacol Sci 30:581–91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Watkins LR, Maier SF: Glia: A novel drug discovery target for clinical pain. Nat Rev Drug Discov 2:973–85, 2003. [DOI] [PubMed] [Google Scholar]
- 133.Williams JT, Christie MJ, Manzoni O: Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D: Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32:815–27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K: Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep 7:3628, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoon SY, Patel D, Dougherty PM: Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience 221:214–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zelcer S, Kolesnikov Y, Kovalyshyn I, Pasternak DA, Pasternak GW: Selective potentiation of opioid analgesia by nonsteroidal anti-inflammatory drugs. Brain Res 1040:151–6, 2005. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L, Meng J, Ban Y, Jalodia R, Chupikova I, Fernandez I, Brito N, Sharma U, Abreu M, Ramakrishnan S, Roy S: Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci 116:13523–32, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X, Bao L, Li S: Opioid receptor trafficking and interaction in nociceptors. Br J Pharmacol 172:364–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou D, Chen M-L, Zhang Y-Q, Zhao Z-Q: Involvement of Spinal Microglial P2X7 Receptor in Generation of Tolerance to Morphine Analgesia in Rats. J Neurosci 30:8042–7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]