Abstract
Background:
The two most commonly used illegal substances by adolescents in the United States are alcohol and cannabis. Alcohol use disorder (AUD) and cannabis use disorder (CUD) have been associated with dysfunction in decision-making processes in adolescents. One potential mechanism for these impairments is thought to be related to abnormalities in reward and punishment processing. However, very little work has directly examined potential differential relationships between AUD and CUD symptom severity and neural dysfunction during decision-making in adolescents.
Methods:
In the present study, 154 youths participated in a passive avoidance (PA) learning task during functional magnetic resonance imaging to investigate the relationship between relative severity of AUD/CUD and dysfunction in processing reward and punishment feedback.
Results:
Increasing AUD Identification Test (AUDIT) scores were associated with reduced neural differentiation between reward and punishment feedback within regions of striatum, posterior cingulate cortex, and parietal cortex. However, increasing CUD Identification Test (CUDIT) scores were not associated with any neural dysfunction during the PA task.
Conclusions:
These data expand on an emerging literature that relative severity of AUD is associated with reduced responsivity to rewards in adolescents and that there are differential associations between AUD and CUD symptoms and neuro-circuitry dysfunction in the developing adolescent brain.
Keywords: Adolescent, Alcohol Use Disorder, fMRI, Instrumental Learning, Reward, Striatum
Introduction
The lifetime prevalence rates of alcohol use disorder (AUD) and cannabis use disorder, (CUD) are 29% and 6%, respectively (1, 2). Epidemiological evidence indicates that the use of these substances during adolescence is associated with an increased risk of developing AUD and/or CUD in adulthood (3). Individuals with AUD and/or CUD who initiated use of these respective substances in adolescence face a more severe disease course and a greater rate of relapse (4). This may be due in part to the putative adverse neurodevelopmental effects of these substances on the adolescent brain (5, 6).
Adolescents with SUDs show impairments in decision-making (7). Behavioral work in adolescents has shown that individuals with co-morbid substance dependence and disruptive behavior disorders (DBDs) make riskier decisions relative to adolescents with only DBDs (7). Deficits in decision-making processes in adolescent substance users may reflect dysfunction in reinforcement processing (8-11). Indeed, one structure that is undergoing development during adolescence and is implicated in reinforcement processing is the striatum (12). There are suggestions that both hyper- and hypo- reward/striatal responsiveness may increase the risk for substance use (13-15). Models suggest that long-term substance use induces reward circuitry hyper-responsiveness to substance-associated cues but reward circuitry hypo-responsiveness to non-drug cues (e.g., money; 16-18). In line with this, neuroimaging work with individuals with AUD has shown increased striatal responsivity to alcohol-related images (19) but reduced striatal responsivity to monetary rewards (14, 19, 20). Similarly, increased striatal responsivity to cannabis-related images is seen in long-term cannabis users (21). However, the literature with respect to striatal responsivity to monetary rewards is mixed. Three studies, including one with adolescent participants, have indicated cannabis use is associated with reduced striatal or orbitofrontal cortex responsivity to monetary rewards (22-24). However, others find no such association (14, 25, 26) including two with adolescent samples (14, 27), while three studies reported increased striatal responsivity in cannabis users (26-28). However, only one of these found increased responsivity to reward (28). The other two, including one with adolescent male regular cannabis users (27), reported this either during anticipation of non-rewards (27) or in response to losses (26).
In addition to the inconsistency with respect to reward responsiveness and cannabis abuse, there are several other gaps in the previous literature. First, most of this literature has used the Monetary Incentive Delay (MID) task (14, 26, 27) where participants respond during the presence of a briefly presented cue in order to win (or avoid losing) money (29). There has also been some using the balloon analogue risk task (BART; 11) where participants pump a virtual balloon in order to win increasing amounts of money, but risk popping the balloon and losing their earnings (30). However, reward responsiveness has not been examined in the context of instrumental learning in this population (31). Instrumental learning depends on two interdependent systems: reward responsiveness and reward learning (32). Reward responsiveness is of particular clinical interest given the role of instrumental learning in the development of addictive behaviors (33). Specifically, it has been suggested that substance use initiates instrumental learning; the substance is a strong reward increasing the reward value of stimuli associated with, and behaviors that elicited, the substance ingestion (33). Notably, if substance abuse compromises striatal reward responsiveness to non-drug rewards then behaviors associated with these previously rewarding (non-drug) rewards will be extinguished leading to an individual who is focused primarily on substance use for their reward gains. In addition, it is worth noting that instrumental tasks identify regions implicated in representing the subjective value of reinforcement; in particular, ventromedial prefrontal and posterior cingulate cortices (vmPFC and PCC; 34, 37). These regions may also show disrupted activity as a function of AUD/CUD symptom severity (14).
Second, very little work has directly examined differential relationships between AUD and CUD symptom severity and neural dysfunction during decision-making. Adolescents and adults often engage in co-morbid alcohol use and cannabis use (35, 36). Although much of the literature to date examines AUD and CUD individually (20, 23, 27, 37), there is emerging data indicating that increasing AUD and CUD symptom severity have differential associations with neural dysfunctions in adolescents (14, 38-40). One paper to date has shown that adolescents with co-morbid alcohol and cannabis use show increased striatal modulation during the balloon analogue risk task (BART) compared to adolescents who use only alcohol, only cannabis, or no substances (11). However, work from our group has indicated that AUD severity, but not CUD severity, are associated with reduced striatal modulation by reward value during the MID task (14).
Third, much of the literature to date on reward processing in SUDs has focused exclusively on adult populations (e.g., 23, 24, 26, 27, 29). Very little work has been conducted with adolescents. Yet, the striatum is undergoing considerable development during adolescence with suggestions, and data from animal and human neuro-imaging work, indicating that striatal reward responsiveness peaks in mid-adolescence (12, 40, 41; for a meta-analytic review, see 42). Adolescents are thought to be particularly susceptible to the adverse effects of substance use partly because of this increased striatal reward responsiveness (5, 6). One study, utilizing a group-based approach, reported that adolescents with co-morbid alcohol and cannabis use histories show reduced striatal modulation during the BART (11). In contrast a second study reported that adolescent tobacco use, but not alcohol or cannabis use, is associated with reduced striatal response to rewards during a MID task (43). Our own work utilizing a dimensional approach to AUD/CUD severity, has indicated that AUD symptom severity, but not CUD symptom severity, is associated with reduced striatal reward signaling in adolescents during the MID task (14).
The current study aimed to address these gaps in the literature. It examines differential associations between AUD and CUD symptomatology and reward versus punishment responsiveness in the context of an instrumental learning task in adolescent participants. On the basis of our previous work, we predicted that AUD symptom severity would be inversely associated with differential responses to reward versus punishment feedback within striatum and other reward-sensitive structures involved in instrumental learning-based decision-making (e.g., vmPFC and PCC).
Methods
Participants
Study participants included 154 youths aged 14-18 from both a residential treatment program (Boys Town) and the surrounding Omaha community. These participants were recruited as part of a broader study determining neural correlates of youth with behavioral and emotional problems, specifically substance use disorders (at least 40% of the population) and mental health concerns (Attention Deficit/Hyperactivity Disorder [ADHD], Conduct Disorder [CD], Major Depressive Disorder [MDD] and Generalized Anxiety Disorder [GAD]); (see 14, 38-40); specifically, of the 141 participants in the current sample, 60 participated in Aloi et al., 2018; 87 in Aloi et al., 2019; 58 in Blair et al., 2019; 66 in Leiker et al., 2019. Thirteen youths were excluded due to excessive movement (>5mm maximum displacement) during fMRI scanning (details below). This resulted in a final sample of 141 youths (101 from the residential treatment program and 40 from the community); average age=16.6 (SD=1.12), average IQ=100.1 (SD=10.13), 90 males. See Supplemental Methods for information on recruitment, consent/assent, and exclusion criteria.
Measures
Passive Avoidance Task.
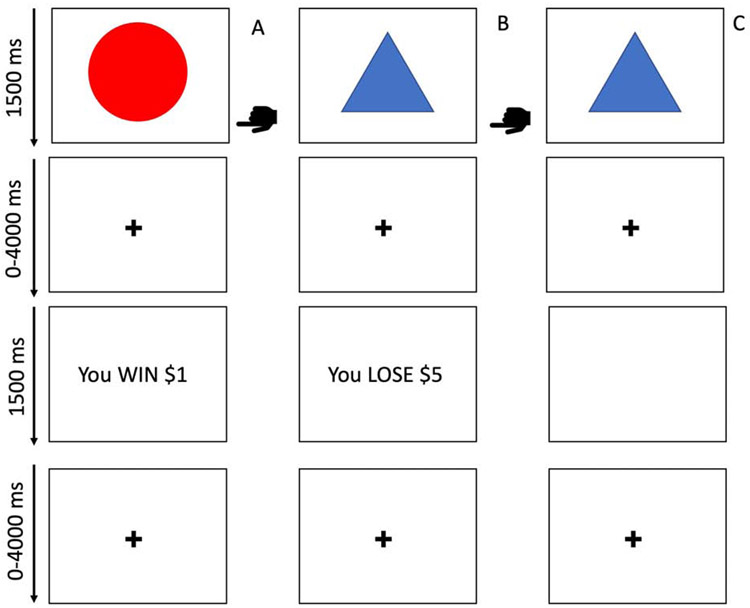
The Passive Avoidance (PA) task (44-46) is a paradigm where participants are presented with one of four shapes on each trial. Each trial requires participants to decide whether or not to respond to a shape. If responded to, two of the shapes yield a virtual reward (80% probability of winning $1 or $5 and 20% probability of losing $1 or $5) while the other two yield a virtual punishment (80% probability of losing $1 or $5 and 20% probability of winning $1 or $5). No reinforcement is received if the stimulus is not responded to. Each trial involves: i) the presentation of a shape (1500ms), a jittered fixation cross interval (1000-4000ms), reward/punishment feedback (1500ms) and a second jittered fixation cross interval (1000-4000ms). Shapes were presented in random order and participants responded via button press. There were 27 trials for each shape, totaling 108 trials. See Figure 1 for more details.
Figure 1. Schematic representation of the Passive Avoidance (PA) task.
In the PA task, participants are presented with one of four objects to which they decide to respond or not to respond. Reinforcement was provided in a probabilistic manner and the selection of two of the four objects would result in a net profit over the course of the task while selection of the other two objects would result in a net loss. Column A depicts a participant choosing to respond and receiving rewarding feedback. Column B depicts a participant choosing to respond and receiving punishing feedback. Column C depicts a participant choosing not to respond and receiving no feedback.  indicates the participant has chosen to respond.
indicates the participant has chosen to respond.
Substance Use Disorder Assessments.
Participants completed both the AUD Identification Test (AUDIT) and CUD Identification Test (CUDIT; 47-49). These scales assess overall symptom severity of AUD and CUD, respectively, including overall quantity/frequency of use, abuse symptoms, and dependence symptoms. They show high validity, as higher scores on these scales are associated with a high likelihood of an AUD and/or CUD diagnosis, respectively (47, 49). Cigarette smoking status was determined via the Monitoring the Future Survey (50). Although participants were subject to random drug testing as part of the treatment program, they were not drug tested on the day of scanning.
Functional MRI Parameters and Analysis
Whole-brain BOLD functional MRI data were acquired via a 3T MAGNETOM Skyra magnetic resonance imaging scanner (Siemens Medical Solutions; see supplemental materials for further details on MRI parameters and fMRI preprocessing). Data were analyzed with a random-effects general linear model using Analysis of Functional NeuroImages (AFNI; 51). Four indicator regressors were generated: one for approached stimuli, one for avoided stimuli, one for reward feedback, and one for punishment feedback. Conditions were modeled with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. GLM fitting was performed with the four regressors listed, six motion regressors, and a regressor modeling baseline drift (-polort 4). This produced a β-coefficient and an associated t-statistic for each voxel and regressor.
Statistical Analyses
To reduce skewness and kurtosis, a Rankit Transformation was applied to participants' AUDIT scores (52). The CUDIT score distribution did not show significant skewness or kurtosis, so a Rankit-Transformation was not applied to the CUDIT scores. The Rankit-Transformed AUDIT scores and the raw CUDIT scores were then z-scored, and these values were used as continuous covariates in all analyses.
To examine relationships between AUDIT/CUDIT scores and psychiatric diagnoses, correlations were performed between AUDIT/CUDIT scores and (i) psychiatric diagnosis status for the four main co-morbid conditions in our sample: ADHD, CD, MDD, and GAD; (ii) prescribed use of stimulant, antidepressant, or antipsychotic medication; and (iii) sex. For these analysis, presence of diagnosis/prescribed use of a drug class was coded as 1, absence of diagnosis/prescribed use coded as 0. The association between AUDIT/CUDIT scores and smoking status was also determined (smoking scores ranged from 0-4 ; for this analysis, the range of options was coded from 0 (“Never”) to 4 (“Regularly now”) based on the Monitoring the Future survey (50). Steiger z-tests were performed to compare the relative strength of the correlations between AUDIT scores and psychiatric diagnoses, prescribed medication use, and smoking versus correlations between CUDIT scores and these variables. Two-sample t-tests were conducted to test for differences between AUDIT/CUDIT scores and sex.
To examine relationships between AUDIT/CUDIT scores and behavioral data on the PA task, a one-way (Error Type: Commission Error, Omission Error) repeated measures ANCOVA was conducted; rankit-transformed, z-scored AUDIT scores and z-scored CUDIT scores were used as continuous covariates. Commission Errors occurred when participants responded to stimuli that were probabilistically associated with punishment. Omission Errors occurred when participants did not respond to stimuli that were probabilistically associated with reward.
Correlational analyses were conducted to determine potential associations of AUDIT/CUDIT scores and movement variables (number of censored TR’s, average motion per TR, or maximum displacement during the task). These analyses are reported in the supplemental material.
To examine associations between AUDIT/CUDIT scores and dysfunctions within brain regions involved in reward and punishment feedback processing, we ran a one-way (Feedback: Reward, Punishment) ANCOVA on the BOLD response data with AUDIT scores, CUDIT scores, and the AUDIT-by-CUDIT interaction as continuous covariates in the same model. Follow-up partial correlations and Steiger-Z tests were performed within SPSS 22.0 (IBM Corp., Armonk, NY) and using freely available online tools (53). In order to facilitate future meta-analytic work, effect sizes for all clusters are reported. All clusters reported exceeded the cluster-wise threshold of k=16 voxels at an initial threshold of p=.001 [corrected cluster-wise p<.05]. Details regarding multiple comparison correction procedures may be found in the supplemental material.
There were a number of potential confounds that could have contributed to the current results; e.g., medication usage, co-morbid psychiatric conditions, placement and sex differences. Briefly, we identified nine potential confounds and for each potential confound we conducted an additional analysis that repeated the main analysis controlling for that specific confound. We chose to run separate models for each confounding variable because of both power concerns with an 11 regressor model, inter-confound suppressor effects and our interest in determining the extent of influence of each individual confound.
Results
Clinical Data
Of the final sample of 141 adolescents, 100 youths endorsed past-year use of either alcohol and/or cannabis. All adolescents had been abstinent from any substance use for at least 4 weeks prior to scanning. AUDIT scores ranged from 0-34 [M=3.56, SD=5.82] and CUDIT scores ranged from 0-32 [M=8.47, SD=9.33]. Seventy youths met the clinical cutoffs on the AUDIT and/or CUDIT suggestive of adolescent AUD (AUDIT≥4) or CUD (CUDIT≥8; 47-49). Forty-three participants had an AUDIT score≥4 and 62 participants had a CUDIT score≥8. In line with prior work indicating high rates of poly-substance use in adolescents (35), 35 participants had both an AUDIT score≥4 and CUDIT score≥8. For details, see supplemental materials.
Correlation analyses revealed a significant positive relationship between AUDIT and CUDIT scores [r=0.59, p<.001]; see Table 1. Both AUDIT and CUDIT scores were significantly positively associated with presence of MDD, GAD, and CD diagnoses; use of antidepressant medications; and level of smoking [r’s=0.17-0.63, all p’s<.05]; see Table 1. Only CUDIT scores were significantly positively associated with ADHD diagnosis status. In all cases, except ADHD status and sex, follow up Steiger’s z tests showed no significantly stronger associations between AUDIT/CUDIT scores and any of these indices (Steiger z’s=−0.57-1.50, p’s>.05). There was a stronger relationship between CUDIT scores and ADHD status than AUDIT scores and ADHD status (Steiger’s z=2.16, p<.05). There were no significant relationships between age, IQ, stimulant use, or antipsychotic use and AUDIT scores or CUDIT scores (see Table 1).
Table 1.
Demographic and Clinical Variables
| Average (SD) | ADHD (N=74) |
CD (N=66) |
MDD (N=21) |
GAD (N=39) |
Stimulants (N=16) |
Antidepressants (N=20) |
Antipsychotics (N=9) |
Age | IQ | AUDIT | CUDIT | Smoking | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 16.1 (1.12) | ||||||||||||
| IQ | 100.1 (10.13) | 0.00 | |||||||||||
| AUDIT | 3.6 (5.82) | 0.15 | 0.35* | 0.17† | 0.34* | −0.03 | 0.18† | −0.05 | 0.15 | 0.03 | |||
| CUDIT | 8.5 (9.33) | 0.31* | 0.39* | 0.18† | 0.23* | 0.02 | 0.20† | −0.00 | 0.07 | −0.01 | 0.59* | ||
| Smoking | 1.5 (1.48) | 0.08 | 0.06 | 0.63* | 0.62* | ||||||||
| Sex | 90 males | −0.01a | −0.06a | −0.16a | 0.04a | 0.01a |
significant at p<0.05
significant at p<0.01
correlations coded as male=1, female=0; ADHD=Attention Deficit/Hyperactivity Disorder, CD=Conduct Disorder, MDD=Major Depressive Disorder, GAD=Generalized Anxiety Disorder, AUDIT=Alcohol Use Disorder Identification Test, CUDIT=Cannabis Use Disorder Identification Test
A two-sample t-test revealed that females had significantly greater AUDIT scores than males [t(139)=2.40, p<.05]. The average AUDIT score for females was 5.10 (SD=7.57) and the average AUDIT score for males was 2.69 (SD=4.36). There was no significant difference between females and males on CUDIT scores [t(139)=0.26, p>.05]. The average CUDIT score for females was 8.20 (SD=9.32) and the average CUDIT score for males was 8.62 (SD=9.38).
Behavioral Data
The one-way ANCOVA conducted on the error data revealed a main effect of error type [F(1,137)=34.95, p<.001]; participants made more commission [Mcomission=19.28, SD=12.41] than omission errors [Momission=11.72, SD=9.27]. However, participants’ AUDIT and CUDIT scores were not associated with error rates.
fMRI Results
We hypothesized that increasing scores on the AUDIT would be associated with reduced responsiveness to reward relative to punishment feedback. Main effects of feedback are reported in the Supplemental Material. Our main analysis revealed the following interaction effects:
AUDIT-by-Feedback Interaction:
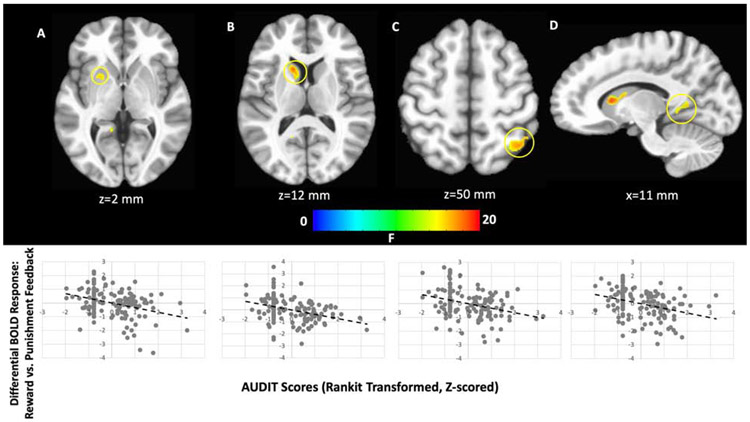
There was a significant AUDIT-by-Feedback interaction within regions including caudate, putamen, PCC, and superior parietal lobule (Figure 2, Table 2). In all brain regions there was a significant negative relationship between AUDIT scores and differential BOLD responsiveness to reward relative to punishment feedback. Within the caudate/putamen and sPL clusters this manifested as significant negative relationships between AUDIT scores and reward responsiveness [rp=−0.19 & =−0.18, p<.05 respectively]. However, within the more posterior putamen, PCC and occipital cortex clusters this manifested as significant positive relationships between AUDIT scores and punishment responsiveness [rp’s=0.19-0.29, p’s<.05].
Figure 2. AUDIT-by-Feedback interaction.
within the (A) putamen, (B) caudate, (C) superior parietal lobule, and (D) posterior cingulate cortex. In all cases, increasing AUDIT scores were associated with decreasing differential responses to rewarding feedback relative to punishing feedback.
Table 2.
Brain regions demonstrating significant AUDIT-by-Feedback Interactions
| Coordinates of Peak Activationb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regiona | Hemisphere | BA | x | y | z | F | Partial η2 | Voxels |
| AUDIT-by-Feedbackc | ||||||||
| Caudate/Putamen | L | - | −13 | 14 | 8 | 20.50 | 0.130 | 28 |
| Putamen | L | - | −19 | 8 | 2 | 17.84 | 0.115 | 20 |
| Posterior Cingulate Cortex | L | 29/30 | −10 | −49 | 5 | 17.76 | 0.115 | 19 |
| Superior Parietal Lobule | R | 7 | 38 | −55 | 57 | 16.85 | 0.110 | 21 |
| Occipital Cortex | R | 19/31 | 32 | −73 | 17 | 16.79 | 0.109 | 20 |
Note: According to the Talairach Daemon Atlas (http://www.nitrc.org/proiects/tal-daemon/)
Based on the Tournoux & Talairach standard brain template
Note that all interactions reflect a negative relationship between AUDIT scores and the reward > punishment contrast, BA= Brodmann’s Area
No regions showed either a CUDIT-by-Feedback interaction or AUDIT-by-CUDIT-by-Feedback Interaction that survived correction for multiple comparisons.
Potential Confounds:
There were a number of potential confounds in our sample, including medication usage, co-morbid psychiatric conditions, placement, and sex differences. Briefly, we conducted an additional analysis for each of nine potential confounds that repeated the main analysis controlling for that specific confound. The striatal and parietal cortex findings remained significant in each of these analyses (for fuller descriptions of the results, see the supplemental material).
Discussion
The goal of the current study was to investigate whether severity of AUD (and/or CUD) was related to dysfunction in differential reward versus punishment feedback responsiveness during an instrumental learning decision-making task. In line with our hypotheses, increasing AUDIT scores were inversely associated with responsiveness to reward relative to punishment processing within regions of striatum, PCC, and parietal cortex.
Previous work has indicated that long-term substance use leads to hypo-responsiveness to non-drug reward related cues (16, 54). Consistent with this, the current study indicates that, in adolescents, increasing AUD symptom severity is associated with decreasing differential responsiveness to reward relative to punishment within striatum, PCC, parietal and occipital cortices. Most previous studies reporting either reduced striatal or PCC responsivity to monetary rewards in individuals with AUD has used the MID task (10, 14, 19, 20). In this task, participants receive reward but they do not need to learn which actions engender reward. As such, the current study extends this previous work indicating dysfunctional reinforcement processing is seen as a function of AUD symptom severity during reward-related instrumental learning. These findings may have important clinical implications. Alcohol substance abuse may compromise differential reward-punishment responsiveness to non-drug rewards, increasing the probability that instrumental behavior in non-drug reward contexts will be compromised and perhaps extinguished. This may increase the individual’s focus on behaviors associated with substance use when instrumentally choosing actions to gain reward.
The striatum and PCC are implicated in the representation of subjective value (34) while the PCC and parietal cortex are hypothesized to orchestrate attention to reinforcement-related external stimuli that is then additionally represented in occipital cortex (55). As such, these findings could suggest that increasing AUD symptom severity is associated with both a reduction in the value of received rewards via striatal/PCC dysfunction and a relative failure to attend to stimulus features associated with reward receipt via PCC/parietal cortex dysfunction. However, the interpretation is complicated by two features of the results. First, while the changes in differential reward vs. punishment responsiveness within the caudate/putamen and sPL clusters manifested as significant negative relationships between AUDIT scores and reward responsiveness, those within the more posterior putamen, PCC and occipital cortex clusters manifested as significant positive relationships between AUDIT scores and punishment responsiveness. Second, participants with the highest AUDIT scores not only failed to show appropriately increased responding to reward relative to punishment within these regions, many also showed greater responses to punishment than reward (i.e., they were not just showing a deficient response to reward but an elevated response to punishment). In short, the results indicate that during instrumental learning, increased AUD symptom severity is positively associated with compromised reward responsiveness within caudate/putamen and sPL but heightened punishment responsiveness within more posterior putamen, PCC and occipital cortex. This latter component of the results was not predicted a priori. As such, we are cautious about interpreting further. However, the issue is worthy of future investigation – particularly as heightened punishment processing might have further implications regarding the extinction of behaviors associated with non-drug cues.
CUD severity, unlike AUD severity, was not associated with differential responsiveness to reward relative to punishment. This is consistent with our earlier work with the MID task on a partially overlapping sample (14). It is also in line with previous work that has not reported reduced reward responsiveness in individuals with CUD (25, 43)– though two studies have reported reduced reward responsiveness in individuals with CUD histories, see (22, 23). Potential reasons for the differential associations between CUD and AUD severity and reward responsiveness are interesting to consider.
It is argued that individuals with SUDs show reduced reward responsiveness to non-drug cues because long-term substance use is associated with repeated stimulation of dopamine receptors in the ventral striatum (following drug usage) resulting in receptor downregulation and thus reduced dopaminergic neurotransmission within this region (18). Interestingly, molecular data indicates different mechanisms of striatal impact for alcohol and cannabis. Short-term alcohol use induces striatal activity through the inhibition of GABAergic neurons in the ventral tegmental area while short-term cannabis use induces striatal activity through activation of striatal cannabinoid receptors (56, 57). Moreover, human positron emission tomography studies suggest differences in level of synaptic dopamine following alcohol versus cannabis ingestion. While both short-term alcohol use and short-term cannabis use induce increases in synaptic dopamine in the ventral striatum, the increase following alcohol appears larger (58, 59). As such, the differential associations between CUD and AUD severity and reward versus punishment responsiveness may reflect differences between cannabis and alcohol in molecular mechanism of impact and/or the strength of the induced increase in synaptic dopamine in the ventral striatum (though note this molecular hypothesis does not account for indications of heightened punishment responsiveness in some regions as a function of AUD severity; see above).
The results of this study must be viewed in light of several limitations. First, we did not conduct urine or breathalyzer testing for alcohol or cannabis use at the time of scanning. However, all but one participant with a significant alcohol and/or cannabis use history were residents of a highly supervised residential treatment facility and were subject to random drug testing as part of treatment for at least four weeks prior to scanning. Exclusion of this participant elicited highly similar results. Second, this study was cross-sectional. As such, the relationships reported in the present study might reflect the effects of alcohol use on the developing brain or pre-existing risk factors for alcohol use disorder. Notably, though, the dominant view is that increased reward responsiveness increases the risk for use of substances generally (15). Notwithstanding any concerns regarding this dominant view (13) - no case has been made that reduced reward responsiveness is a selective risk for AUD but not CUD severity. Third, there was a high degree of psychiatric co-morbidity in the psychiatric sample. It could be argued that the current findings are reflective of psychiatric co-morbidities of AUD rather than AUD/CUD itself. Prior work has often excluded participants with psychiatric conditions (20, 37, 60). However, this approach is problematic as these samples are clinically atypical. AUD and CUD are associated with a number of co-morbid psychiatric conditions (16, 35, 61). Approximately half of individuals with a SUD present with one or more co-morbid psychiatric conditions (62) and the majority of adolescents whose SUD is significant enough to warrant treatment have at least one psychiatric co-morbidity (63). Notably, though, our supplemental analyses showed that including MDD, GAD, CD, or ADHD diagnosis as covariates did not significantly alter the main results. As such, the current findings likely reflect severity of AUD rather than any psychiatric co-morbidity. On a related note, AUD and CUD were highly co-morbid in our sample, of the 43 adolescents with an AUDIT≥4 only 8 did not had a CUDIT<8. This reflects adolescent substance use where epidemiological data indicate that co-use is extremely common (64). Importantly, this makes interpretation of group-based studies complex (unless a clinically atypical adolescent single drug use using group is identified). However, our dimensional analysis approach enables differentiation. Fourth, there was no association between AUD symptom severity and behavioral impairment in instrumental learning on the task. An absence of group differences in behavior but differences in neural reward responsiveness has been previous reported (11, 14). Given the clinical relevance of instrumental learning to AUD, it will be important to determine if/under what circumstances dysfunctional differential reinforcement processing at the neural level is accompanied by behavioral impairments in learning. Relatedly, the PA task, as currently analyzed, does not differentiate between reward responsivity and the ability to learn from rewarding (or punishing) experiences. Future computational modelling work will be necessary to disentangle these possibilities.
In summary, we found that AUDIT scores were negatively related to reward versus punishment responsiveness within regions of striatum, parietal cortex, occipital cortex, and PCC during a passive avoidance learning task. However, we did not find evidence of a relationship between CUDIT scores and striatal reward responsiveness. These data replicate prior work from our group indicating that AUD symptom severity is associated with striatal dysfunction during reward receipt and extend this finding to indicate that similar striatal dysfunction also exists during reward receipt in an instrumental learning task.
Supplementary Material
Acknowledgements:
We would like to thank Ron Copsey, Kim VanHorn, Michael Wright, Mark Timm, and Rhonda Tuel for their contributions to data collection. We would like to thank all participants and their families for their participation. This work was supported by Boys Town National Research Hospital. JA was supported by the AACAP Jeanne Spurlock Medical Student Research Fellowship in Substance Abuse and Addiction and a Program of Excellence Fellowship from the University of Nebraska Medical Center. RJB was supported by K22-MH109558 and R34-DA050286, SFW was supported by K01-MH110643, KSB was supported by P20-GM109023, and KIC was supported by T32-MH018869 and U54-DA016511. The funders had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Conflicts of Interest: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. (2015): Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry. 72: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. (2016): Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry. 173: 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winters KC, Lee CYS (2008): Likelihood of developing an alcohol and cannabis use disorder during youth: Association with recent use and age. Drug Alcohol Depend. 92: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B (1992): Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 49: 599–608. [DOI] [PubMed] [Google Scholar]
- 5.Filbey FM, McQueeny T, DeWitt SJ, Mishra V (2015): Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 16: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squeglia LM, Tapert SF, Sullivan E V., Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A (2015): Brain Development in Heavy-Drinking Adolescents. Am J Psychiatry. 172: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DJLG Schutter, Van Bokhoven I, Vanderschuren LJMJ, Lochman JE, Matthys W (2011): Risky decision making in substance dependent adolescents with a disruptive behavior disorder. J Abnorm Child Psychol. 39: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenbaum G, Roesch MR, Stalnaker TA (2006): Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 29: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White SF, Tyler P, Botkin ML, Erway AK, Thornton LC, Kolli V, et al. (2016): Youth with substance abuse histories exhibit dysfunctional representation of expected value during a passive avoidance task. Psychiatry Res Neuroimaging. 257: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT (2010): Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 5: e12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claus ED, Feldstein Ewing SW, Magnan RE, Montanaro E, Hutchison KE, Bryan AD (2017): Neural mechanisms of risky decision making in adolescents reporting frequent alcohol and/or marijuana use. Brain Imaging Behav. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvan A (2010): Adolescent development of the reward system. Front Hum Neurosci. 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair RJR (2019): Modelling the comorbidity of Cannabis Abuse and Conduct Disorder/Conduct Problems from a Cognitive Neuroscience Perspective. J Dual Diagn. 0: 1–19. [DOI] [PubMed] [Google Scholar]
- 14.Aloi J, Meffert H, White SF, Blair KS, Hwang S, Tyler PM, et al. (2019): Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev Cogn Neurosci. 36. doi: 10.1016/j.dcn.2019.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heitzeg MM, Villafuerte S, Weiland BJ, Enoch M-A, Burmeister M, Zubieta J-K, Zucker R a (2014): Effect of GABRA2 Genotype on Development of Incentive-Motivation Circuitry in a Sample Enriched for Alcoholism Risk. Neuropsychopharmacology. 39: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Koob GF, McLellan AT (2016): Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 374: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW (2019): Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology. 44: 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koob GF, Volkow ND (2016): Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. (2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 35: 787–794. [DOI] [PubMed] [Google Scholar]
- 20.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. (2009): Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiatry. 66: 734–742. [DOI] [PubMed] [Google Scholar]
- 21.Cousijn J, Goudriaan AE, Ridderinkhof KR, Van Den Brink W, Veltman DJ, Wiers RW (2013): Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol. 18: 570–580. [DOI] [PubMed] [Google Scholar]
- 22.van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF (2010): Long-term effects of cannabis use on the human reward system: An fMRI study. Eur Neuropsychopharmacol. 20: 153–163. [DOI] [PubMed] [Google Scholar]
- 23.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM (2016): Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA Psychiatry. 73: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper R, Huettel SA (2013): Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 133: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filbey FM, Dunlop J (2014): Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 140: 5421–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN (2014): Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: An exploratory study of relationships with abstinence during behavioral treatment1. Drug Alcohol Depend. 140: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jager G, Block RI, Luijten M, Ramsey NF (2013): Tentative Evidence for Striatal Hyperactivity in Adolescent Cannabis-Using Boys: A Cross-Sectional Multicenter fMRI Study. J Psychoactive Drugs. 45: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestor L, Hester R, Garavan H (2010): Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 49: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson B, Westdorp A, Kaiser E, Hommer D (2000): FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. Neuroimage. 12: 20–27. [DOI] [PubMed] [Google Scholar]
- 30.Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM (2003): Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 26: 475–479. [DOI] [PubMed] [Google Scholar]
- 31.O’Doherty JP, Cockburn J, Pauli WM (2017): Learning, Reward, and Decision Making. Annu Rev Psychol. 68: 73–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M (2008): Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl). 196: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinz A, Beck A, Halil MG, Pilhatsch M, Smolka MN, Liu S (2019): Addiction as Learned Behavior Patterns. J Clin Med. 8: 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clithero JA, Rangel A (2013): Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 9: 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss HB, Chen CM, Yi H ye (2014): Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 136: 51–62. [DOI] [PubMed] [Google Scholar]
- 36.Duncan SC, Gau JM, Farmer RF, Seeley JR, Kosty DB, Lewinsohn PM (2015): Comorbidity and Temporal Relations of Alcohol and Cannabis Use Disorders from Youth through Adulthood. Drug Alcohol Depend. 149: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nees F, Witt SH, Dinu-Biringer R, Lourdusamy A, Tzschoppe J, Vollstadt-Klein S, et al. (2015): BDNF Val66Met and reward-related brain function in adolescents: Role for early alcohol consumption. Alcohol. 49: 103–110. [DOI] [PubMed] [Google Scholar]
- 38.Aloi J, Blair KS, Crum KI, Meffert H, White SF, Tyler PM, et al. (2018): Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries. NeuroImage Clin. 19: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair RJ., White SF, Tyler PM, Johnson K, Lukoff J, Thornton LC, et al. (2019): Threat Responsiveness as a Function of Cannabis and Alcohol Use Disorder Severity. J Child Adolesc Psychopharmacol. XX: cap.2019.0004. [DOI] [PubMed] [Google Scholar]
- 40.Leiker EK, Meffert H, Thornton LC, Taylor BK, Aloi J, Ba HA, et al. (2019): Alcohol Use Disorder and Cannabis Use Disorder symptomatology in adolescents are differentially related to dysfunction in brain regions supporting face processing. Psychiatry Res Neuroimaging. doi: 10.1016/j.pscychresns.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laviola G, Pascucci T, Pieretti S (2001): Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 68: 115–124. [DOI] [PubMed] [Google Scholar]
- 42.Silverman MH, Jedd K, Luciana M (2015): Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage. doi: 10.1111/obr.12065.Variation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW (2015): Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White SF, Tyler PM, Erway AK, Botkin ML, Kolli V, Meffert H, et al. (2016): Dysfunctional representation of expected value is associated with reinforcement-based decision-making deficits in adolescents with conduct problems. J Child Psychol Psychiatry. doi: 10.1111/jcpp.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finger EC, Marsh A a, Blair KS, Reid ME, Ng P, Pine DS, Blair RJR (2011): Disrupted Reinforcement Signaling in Orbital Frontal Cortex and Caudate in Youths with Conduct Disorder/Oppositional Defiant Disorder and High Psychopathic Traits. Am J Psychiatry. 168: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman JP, Kosson DS (1986): Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol. 95: 252–256. [PubMed] [Google Scholar]
- 47.Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, Sellman JD (2010): An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 110: 137–143. [DOI] [PubMed] [Google Scholar]
- 48.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993): Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 88: 791–804. [DOI] [PubMed] [Google Scholar]
- 49.Fairlie AM, Sindelar HA, Eaton CA, Spirito A (2006): Utility of the AUDIT for screening adolescents for problematic alcohol use in the emergency department. Int J Adolesc Med Heal. 18: 115–122. [DOI] [PubMed] [Google Scholar]
- 50.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2016): Monitoring the Future national survey results on drug use, 1975-2015: Volume I, Secondary school students. [Google Scholar]
- 51.Cox RW (1996): AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 52.Bliss CI, Greenwood ML, White ES (1956): A Rankit Analysis of Paired Comparisons for Measuring the Effect of Sprays on Flavor. Biometrics. 12: 381–403. [Google Scholar]
- 53.Lee IA, Preacher KJ (2013): Calculation for the test of the difference between two dependent correlations with one variable in common. Retrieved from http://quantpsy.org/corrtest/corrtest2.htm. [Google Scholar]
- 54.DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM (2015): The hyper-sentient addict: an exteroception model of addiction. Am J Drug Alcohol Abuse. 41: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gusnard D, Raichle M (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2: 685–694. [DOI] [PubMed] [Google Scholar]
- 56.Lupica CR, Riegel AC, Hoffman AF (2004): Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 143: 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nestler EJ (2005): Is there a common molecular pathway for addiction? Nat Neurosci. 8: 1445–1449. [DOI] [PubMed] [Google Scholar]
- 58.Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. (2003): Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 49: 226–231. [DOI] [PubMed] [Google Scholar]
- 59.Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. (2009): Δ9-Tetrahydrocannabinol Induces Dopamine Release in the Human Striatum. Neuropsychopharmacology. 34: 759–766. [DOI] [PubMed] [Google Scholar]
- 60.Enzi B, Lissek S, Edel MA, Tegenthoff M, Nicolas V, Scherbaum N, et al. (2015): Alterations of monetary reward and punishment processing in long-term cannabis users: An fMRI study. PLoS One. 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moss HB, Lynch KG (2001): Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug Alcohol Depend. 64: 75–83. [DOI] [PubMed] [Google Scholar]
- 62.SAMHSA (2017): Key Substance Use and Mental Health Indicators in the US: Results from the 2016 National Survey on Drug Use and Health. 1–101. [Google Scholar]
- 63.Chan YF, Dennis ML, Funk RR (2008): Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat. 34: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mason WA, Chmelka MB, Howard BK, Thompson RW (2013): Comorbid alcohol and cannabis use disorders among high-risk youth at intake into residential care. J Adolesc Heal. 53: 350–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.