Abstract
IMPORTANCE
Surgeons make complex, high-stakes decisions under time constraints and uncertainty, with significant effect on patient outcomes. This review describes the weaknesses of traditional clinical decision-support systems and proposes that artificial intelligence should be used to augment surgical decision-making.
OBSERVATIONS
Surgical decision-making is dominated by hypothetical-deductive reasoning, individual judgment, and heuristics. These factors can lead to bias, error, and preventable harm. Traditional predictive analytics and clinical decision-support systems are intended to augment surgical decision-making, but their clinical utility is compromised by time-consuming manual data management and suboptimal accuracy. These challenges can be overcome by automated artificial intelligence models fed by livestreaming electronic health record data with mobile device outputs. This approach would require data standardization, advances in model interpretability, careful implementation and monitoring, attention to ethical challenges involving algorithm bias and accountability for errors, and preservation of bedside assessment and human intuition in the decision-making process.
CONCLUSIONS AND RELEVANCE
Integration of artificial intelligence with surgical decision-making has the potential to transform care by augmenting the decision to operate, informed consent process, identification and mitigation of modifiable risk factors, decisions regarding postoperative management, and shared decisions regarding resource use.
Surgeons make complex, high-stakes decisions when offering an operation, addressing modifiable risk factors, managing complications and optimizing resource use, and conducting an operation. Diagnostic and judgment errors are the second most common cause of preventable harm incurred by surgical patients.1 Surgeons report that lapses in judgment are the most common cause of their major errors.2 Surgical decision-making is dominated by hypothetical deductive reasoning and individual judgment, which are highly variable and ill-suited to remedy these errors. Traditional clinical decision support tools, such as the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator, can reduce variability and mitigate risks, but their clinical adoption is hindered by suboptimal accuracy and time-consuming manual data acquisition and entry requirements.3–8
Although decision-making is one of the most difficult and important tasks that surgeons perform, there is a relative paucity of research investigating surgical decision-making and strategies to improve it. The objectives of this review are to describe challenges in surgical decision-making, review traditional clinical decision-support systems and their weaknesses, and propose that artificial intelligence models fed with live-streaming electronic health record data (EHR) would obviate these weaknesses and should be integrated with bedside assessment and human intuition to augment surgical decision-making.
Methods
PubMed and Cochrane Library databases were searched from their inception to February 2019 (eFigure in the Supplement). Articles were screened by reviewing their abstracts for the following criteria: (1) published in English, (2) published in a peer-reviewed journal, and (3) primary literature or a review article. Articles were selected for inclusion by manually reviewing abstracts and full texts for these criteria: (1) topical relevance, (2) methodologic strength, and (3) novel or meritorious contribution to existing literature. Articles of interest cited by articles identified in the initial search were reviewed using the same criteria. Forty-nine articles were included and assimilated into relevant categories (Table 1).1–49
Table 1.
Summary of Included Studies
| Source | Study Design | Population | Sample Size | Major Findings Pertinent to This Scoping Review | Sources of Funding; Conflicts of Interest |
|---|---|---|---|---|---|
| Adhikari et al9 | Retrospective | Patients undergoing inpatient surgery | 2911 | A machine learning algorithm accurately predicted postoperative acute kidney injury using preoperative and intraoperative data | NIGMS, University of Florida CTSI; NCATS; I Heerman Anesthesia Foundation; SCCM Vision Grant |
| Artis et al10 | Observational | Trainee ICU presentations | 157 | Potentially important data were omitted from 157 of157 presentations; missing an average 42% of all data elements | AHRQ |
| Bagnall et al11 | Retrospective | Patients who had colorectal surgery | 1380 | Six traditional risk models used to predict postoperative morbidity and mortality had weak accuracy with AUC, 0.46–0.61 | St Mark’s Hospital Foundation |
| Bechara et al12 | Observational | Healthy volunteers and participants with prefrontal cortex damage | 16 | Participants began to decide advantageously before they could consciously explain what they were doing or whythey were doing it | National Institute of Neurological Diseases and Stroke |
| Bertrand et al13 | Prospective | ICU patients and attending surgeons | 419 | Clinicians thought 45% of all patients had decision-making capacity; a minimental status examination found that 17% had decision-making capacity | Pfizer, Fisher & Paykel; Pfizer; Alexion; Gilead; Jazz Pharma; Baxter; Astellas |
| Bertsimas et al14 | Retrospective | Emergency surgery patients | 382 960 | A app-based machine learning model accurately predicted mortality and 18 postoperative complications (AUC, 0.92) | None reported |
| Bihorac et al15 | Retrospective | Patients undergoing major surgery | 51 457 | A machine learning algorithm using automated EHR data predicted 8 postoperative complications (AUC, 0.82–0.94)and predicted mortality at 1,3, 6,12, and 24 mo (AUC, 0.77–0.83) | NIGMS; NCATS |
| Blumenthal-Barby et al16 | Review | Articles about heuristics in medical decision-making | 213 | Among studies investigating bias and heuristics among medical personnel, 80% identified evidence of bias and heuristics | Greenwall Foundation; Pfizer |
| Brennan et al17 | Prospective | Physicians | 20 | A machine learning algorithm was significantly more accurate than physicians in predicting postoperative complications (AUC, 0.73–0.85 vsAUC, 0.47–0.69) | NIGMS, University of Florida CTSI; NCATS |
| Che et al18 | Retrospective | Pediatric ICU patients | 398 | A gradient boosting trees method allowed for quantification of the relative importance of deep model inputs in determining model outputs | NSF; Coulter Translational Research Program |
| Chen-Ying et al19 | Retrospective | Clinic patients | 840 487 | A deep model predicted 5-y stroke occurrence with greater sensitivity (0.85 vs 0.82) and specificity (0.87 vs 0.86) than logistic regression | None reported |
| Christie et al20 | Retrospective | Trauma patients | 28212 | A machine learning ensemble accurately predicted mortality among trauma patients in the United States, South Africa, and Cameroon with AUC ≥0.90 inall settings | NIH |
| Clark et al4 | Retrospective | Surgical patients | 885 502 | The ACS Surgical Risk Calculator accurately predicted mortality (AUC, 0.94) and morbidity (AUC, 0.83) | None reported |
| Cohen et al6 | Retrospective | Studies assessing the ACS Surgical Risk Calculator | 3 | Externalvalidation studies assessing ACS Surgical Risk Calculator performance may have been compromised by small sample size, case-mix heterogeneity, and use of data from a small number of institutions | None reported |
| Delahanty et al21 | Retrospective | ICU patients | 237 173 | A machine learning algorithm accurately predicted inpatient death (AUC, 0.94) | Alesky Belcher; Intensix, Advanced ICU |
| Dybowski et al22 | Retrospective | ICU patients | 258 | An artificial neural network predicted in-hospital mortality more accurately than logistic regression (AUC 0.86 vs 0.75) | Special Trustees for St Thomas’ Hospital |
| Ellis et al23 | Prospective | Volunteers | 1948 | Induction of fear and anger had unique and significant influences on decisions to take hypothetical medications | NCI |
| Gage et al24 | Prospective | Patients with atrial fibrillation | 2580 | Models commonly used to predict risk of stroke were moderately accurate with AUC ranging from 0.58–0.70 | AHA, NIH, Danish and Netherlands Heart Foundations, Zorg Onderzoek Nederland Prevention Fund, Bayer, UK StrokeAssociation |
| Gijsberts et al25 | Retrospective | Patients with no baseline cardiovascular disease | 60 211 | Associations between risk factors and development of atherosclerotic cardiovascular disease were different across racial and ethnic groups | Netherlands Organization for Health Research and Development, NIH |
| Hao et al26 | Retrospective | ICU patients | 15 647 | Deep learning models predicted 28-d mortalitywith 84%−86%accuracy | None reported |
| Healey et al1 | Retrospective | Surgical inpatients | 4658 | Behind technical errors, diagnostic and judgment errors were the second most common cause of preventable harm | None reported |
| Henry et al27 | Retrospective | ICU patients | 16234 | A machine learning early warning score accurately predicted the onset of septic shock (AUC 0.83), identifying approximately two-thirds of all cases prior to the onset of organ dysfunction | NSF; Google Research, Gordon and Betty Moore Foundation |
| Hubbard et al28 | Prospective | Trauma patients | 980 | A machine learning ensemble predicted mortalitymore accurately than logistic regression (5% gain) | US Army Medical Research and Materiel Command, NIH |
| Hyde et al7 | Prospective | Patients undergoing colorectal resections | 288 | The likelihood of a serious complication was underestimated bythe ACS Surgical Risk Calculator (AUC, 0.69), but the calculator accurately predicted postoperative mortality (AUC, 0.97) | None reported |
| Kim et al29 | Retrospective | ICU admissions | 38 474 | A decision tree model predicted in-hospital mortalitymore accurately than APACHE III (AUC, 0.89 vs 0.87) | NCRR |
| Knops et al30 | Systematic review | Studies about decision aids in surgery | 17 | Decision aid use was associated with more knowledge regarding treatment options and preference for less invasive treatment options with no observable differences in anxiety, quality of life, or complications | None reported |
| Komorowski et al31 | Retrospective | Septic ICU patients | 96 156 | A reinforcement learning model recommending intravenous fluid and vasopressor strategies outperformed human clinicians; mortality was lowest when decisions made by clinicians matched recommendations from the reinforcement learning model | Orion Pharma, Amomed Pharma, Ferring Pharma, Tenax Therapeutics; Baxter Healthcare; Bristol-Myers Squibb; GSK; HCA International |
| Koyner et al32 | Retrospective | Hospital admissions | 121 158 | A machine learning algorithm accurately predicted development of acute kidney injury within 24 h (AUC, 0.90) and 48 h (AUC, 0.87) | Satellite Healthcare; Philips Healthcare; EarlySense; Quant HC |
| Leeds et al8 | Observational | Surgery residents | 124 | Residents reported that lack of electronic and clinical workflow integration were major barriers to routine use of risk communication frameworks | NCI; ASCRS; AHRQ |
| Legare et al33 | Systematic review | Studies about shared decision-making | 38 | Time constraints impair the shared decision-making process among providers, patients, and caregivers | Tier 2 Canada Research Chair |
| Loftus et al34 | Retrospective | Patients with lower intestinal bleeding | 147 | An artificial neural network predicted severe lower intestinal bleeding more accurately than a traditional clinical prediction rule (AUC, 0.98 vs 0.66) | NIGMS, NCATS |
| Lubitz et al5 | Retrospective | Patients undergoing colorectal surgery | 150 | The ACS Surgical Risk Calculator accurately predicted morbidityand mortalityfor elective surgery but underestimated risk for emergent surgery | None reported |
| Ludolph et al35 | Systematic review | Articles about debiasing in health care | 68 | Many debiasing strategies targeting health care clinicians effectively decrease the effect of bias on decision-making | University of Lugano Institute of Communication and Health |
| Lundgren-Laine et al36 | Observational | Academic intensivists | 8 | Academic intensivists made approximately 56 ad hoc patient care and resource use decisions per day | Finnish Funding Agency for Technology and Innovation; Tekes; Finnish Cultural Foundation |
| Morris et al37 | Interviews | Academic surgeons | 20 | Younger surgeons felt uncomfortable defining futility and felt pressured to perform operations that were likely futile | AHRQ |
| Pirracchio et al38 | Retrospective | ICU patients | 24508 | A machine learning ensemble predicted in-hospital mortality (AUC, 0.85) more accurately than SAPS-II (AUC, 0.78) and SOFA (AUC, 0.71) | Fulbright Foundation; Doris Duke Clinical Scientist Development Award; NIH |
| Pirracchio et al39 | Observational | Simulated data sets | 1000 | A machine learning ensemble predicted propensity scores more accurately than logistic regression and individual machine learning algorithms | Fulbright Foundation; Assistance Publique-Hopitaux de Paris; NIH |
| Raymond et al3 | Prospective | Preoperative clinic patients | 150 | After reviewing ACS Surgical Risk Calculator results, 70% would participate in prehabilitation and 40% would delay surgery for prehabilitation | GE Foundation; Edwards Lifesciences; Cheetah Medical |
| Sacks et al40 | Observational | Surgeons | 767 | Facing clinical vignettes for urgent and emergent surgical diseases; surgeons exhibited wide variability in the decision to operate (49%−85%) | Robert Wood Johnson/Veterans Affairs Clinical Scholars program |
| Schuetz et al41 | Retrospective | Clinical encounters in an EHR | 32 787 | A deep model predicted the onset of heart failure more accurately than logistic regression (AUC, 0.78vsAUC, 0.75) | NSF; NHLBI |
| Shanafelt et al2 | Observational | Members of the ACS | 7905 | Nine percent of all surgeons reported making a major medical error in the last 3 mo, and lapses in judgment were the most common cause (32%) | None reported |
| Shickel et al42 | Retrospective | ICU admissions | 36216 | A deep model using SOFA variables predicted in-hospital mortality with greater accuracy than the traditional SOFAscore (AUC 0.90 vs 0.85) | NIGMS; NSF, University of Florida CTSI; NCATS; J Crayton Pruitt Family Department of Biomedical Engineering; Nvidia |
| Singh et al43 | Systematic review | Articles about CRP to predict leak after colorectal surgery | 7 | The positive predictive value of serum C-reactive protein 3–5 d after surgerywas 21%−23% | Auckland Medical Research Foundation, New Zealand Health Research Council |
| Stacey et al44 | Systematic review | Randomized trials about decision aids | 105 | Participants exposed to decision aids felt that they were more knowledgeable, informed, and clear about their values and played a more active role in the shared decision-making process | Foundation for Informed Medical Decision Making, Healthwise |
| Strate et al45 | Prospective | Patients with acute lower intestinal bleeding | 275 | A bedside clinical prediction rule using simple cutoff values predicted severe lower intestinal bleeding (AUC, 0.75) | American College of Gastroenterology, National Research Service Award, American Society for Gastrointestinal Endoscopy |
| Sun et al46 | Observational | Simulated type 1 diabetics | 100 | A reinforcement learning model performed as well as standard intermittent self-monitoring and continuous glucose monitoring methods, but with fewer episodes of hypoglycemia | Swiss Commission of Technology and Innovation |
| Van den Bruel et al47 | Retrospective | Primary care patients | 3890 | Clinician intuition identified patients with illness severity that was underrepresented bytraditional clinical parameters | Research Foundation Flanders, Eurogenerics, NIHR |
| Van den Bruel et al48 | Systematic review | Articles about clinical parameters for serious infections | 30 | Traditional clinical parameters associated with serious infection were often absent among patients with serious infections | Health Technology Assessment, NIHR |
| Vohs et al49 | Observational | Undergraduate students |
34 | Higher decision-making volume was associated with decreased physical stamina, persistence, qualityand quantity of mathematic calculations, and more procrastination | NIH, Social Sciences and Humanities Research Council, Canada Research Chair Council, McKnight Land-Grant |
Abbreviations: ACS, American College of Surgeons; AHA, American Heart Association; AHRQ, Agency for Healthcare Research and Quality; ASCRS, American Society of Colon and Rectal Surgeons; AUC, area under the curve; CRP, C-reactive protein; CTSI, Clinical and Translational Sciences Institute; EHR, electronic health record; GSK, Glaxo Smith Kline; ICU, intensive care unit; NCATS, National Center for Advancing Translational Sciences; NCI, National Cancer Institute; NCRR, National Center for Research Resources, Acute Physiology, and Chronic Health Evaluation; NHLBI, National Heart, Lung, and Blood Institute; NIGMS, National Institute of General Medical Sciences; NIH, National Institutes of Health; NIHR, National Institute for Health Research; NSF, National Science Foundation; SAPS, Simplified Acute Physiology Score; SCCM, Society of Critical Care Medicine; SOFA, Sequential Organ Failure Assessment.
Observations
The quality of surgical decision-making is influenced by patient values and emotions, patient-surgeon interactions, decision-making volume and complexity, time constraints, uncertainty, hypothetical deductive reasoning, and individual judgment. There are effective and ineffective methods for dealing with each of these factors, which lead to positive and negative outcomes, respectively (Figure 1).
Figure 1. Surgical Decision-Making Paradigm.
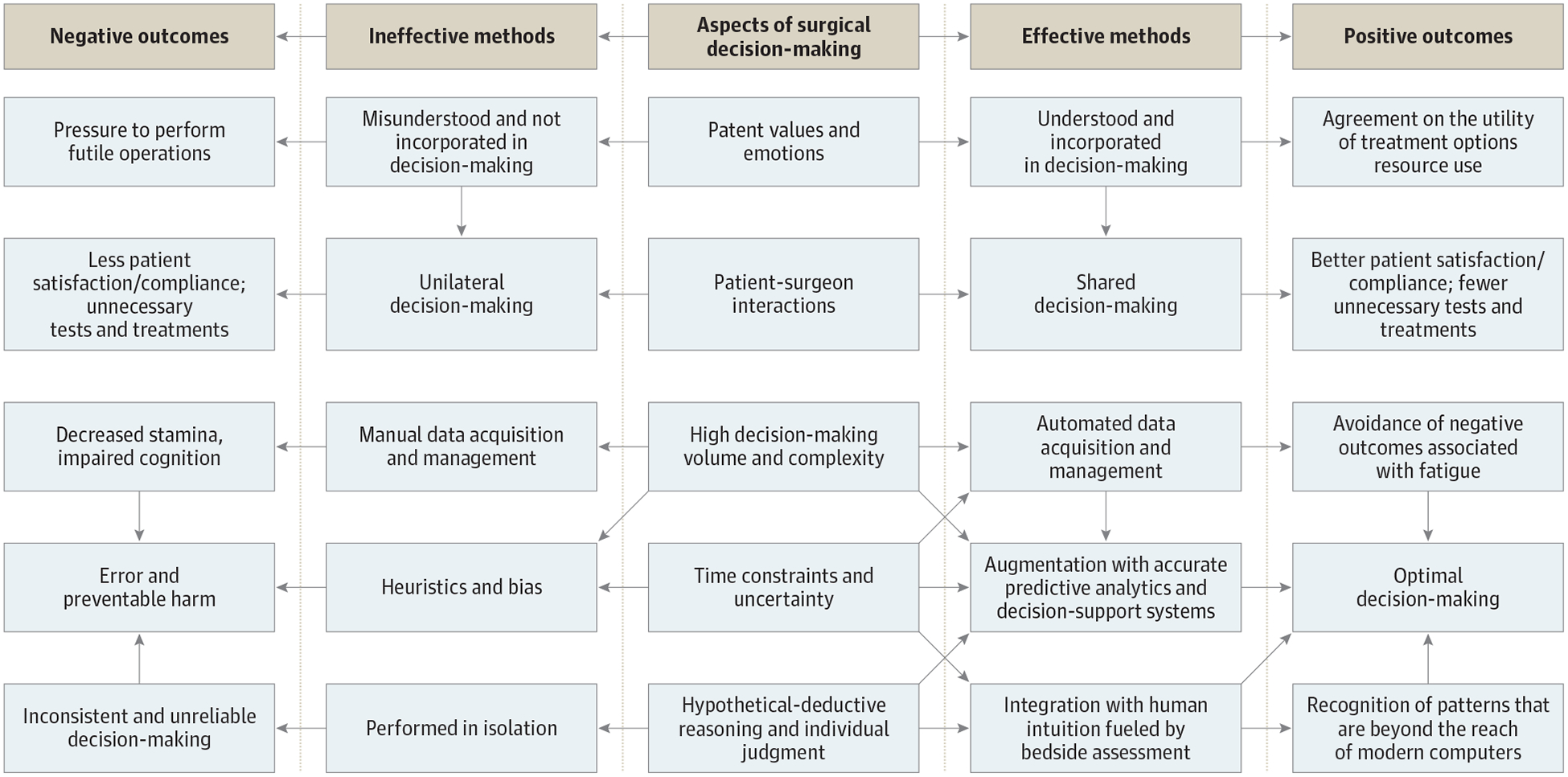
Challenges in Surgical Decision-Making
Complexity
In the hypothetical-deductive decision-making model that dominates surgical decision-making, initial patient presentations are assessed to develop a list of possible diagnoses that are differentiated by diagnostic testing or response to empirical therapy. This depends on the surgeon’s ability to form a complete list of all likely diagnoses, all life-threatening diagnoses, and all unlikely diagnoses that may be considered if the initial workup excludes other causes. It also requires recognition of strengths and limitations of available tests. Once the diagnosis is established, the surgeon must recommend a plan using sound judgment. Each step introduces variability and opportunities for error.40
Values and Emotions
Patient values are individualized by nature, precluding the creation of a criterion standard of optimal decision-making. Understanding and incorporating these values is essential to an effective shared decision-making process.50 This may be accomplished by simply asking patients and caregivers about their goals of care and what they value most in life. Shared decision-making improves patient satisfaction and compliance and may reduce costs associated with undesired tests and treatments. However, patients, caregivers, and clinicians often misunderstand one another, their goals may differ, and patients and caregivers are often expected to make decisions with limited background knowledge and no medical training.13,33,50 Surgical diseases may evoke fear and anger, which influence perceptions of risks and benefits.23,51 Emotions surrounding an acute surgical condition may also create a sense of urgency and pressure on surgeons to perform futile operations.37
Time Constraints and Uncertainty
Surgical decision-making is often hindered by uncertainty owing to missing or incomplete data. This occurs when decisions regarding an urgent or emergent condition must be made before all relevant data can be gathered and analyzed. Nonurgent decisions may be hindered by time constraints and uncertainty owing to sheer decision-making volume, the time-consuming nature of manual data acquisition, and team dynamics. Academic intensivists make approximately 56 patient care and resource use decisions per day.36 In an assessment of medical student and resident intensive care unit (ICU) patient presentations, potentially important data were omitted from 157 of 157 presentations.10 Even when data collection and analysis are complete, high decision-making volume begets decision fatigue, manifesting as procrastination, less persistence when facing adversity, decreased physical stamina, and lower quality and quantity of mathematic calculations.49 These impairments are exacerbated by acute and chronic sleep deprivation, which occurs in as many as two-thirds of all acute care surgeons taking in-house call.52,53 For a surgical oncologist with a busy outpatient clinic, automated production of prognostic data from artificial intelligence models could improve efficiency and preserve face-to-face patient-surgeon interactions by obviating manual data acquisition and entry into prognostic models.
Heuristics and Bias
When facing time constraints and uncertainty, decision-making may be influenced by heuristics or cognitive shortcuts.54,55 Heuristics may lead to bias or predictable and systematic cognitive errors, as described in Table 2.16,35
Table 2.
Sources of Bias in Surgical Decision-Making
| Source of Bias | Examples |
|---|---|
| Framing effect | Aclinician presents a clinical scenario to a surgeon in different context than the surgeon would have perceived during an independent assessment |
| Overconfidence bias | Asurgeon falsely perceives that weaknesses and failures disproportionatelyaffect their peers |
| Commission bias | Asurgeon tends toward action when inaction may be preferable, especially in the context of overconfidence bias |
| Anchoring bias | Patients are informed of expected outcomes using data from aggregate patient populations without adjusting for their personalized risk profile |
| Recall bias | Recent experiences with a certain patient population or operation disproportionately affect surgical decision-making relative to remote experiences |
| Confirmation bias | Outcomes are predicted using personal beliefs rather than evidence-based guidelines |
Traditional Predictive Analytics and Clinical Decision Support
Decision Aids
Decision aids provide specific patient populations with background information, options for diagnosis and treatment, risks and benefits for each option, and outcome probabilities. In a systematic review44 including 31 043 patients facing screening or treatment decisions, patients exposed to decision aids felt more knowledgeable and played a more active role in the decision-making process. In a systematic review of 17 studies investigating decisions made by surgical patients, decision aids were associated with more knowledge regarding treatment options, preference for less invasive treatments, and no observable differences in anxiety, quality of life, morbidity, or mortality.30 However, because decision aids apply to heterogeneous patient populations with 1 common clinical presentation or choice, they do not consider individual patient physiology and risk factors.
Prognostic Scoring Systems
Traditional prognostic scoring systems use regression modeling on aggregate patient populations to identify static variable risk factor thresholds, which are applied to individual patients. For example, elevated serum levels of C-reactive protein (CRP) are associated with anastomotic leak after colorectal surgery. A meta-analysis43 found that the optimal postoperative day 3 CRP cutoff value was 172 mg/L (to convert to nanomoles per liter, multiply by 9.524). This is easy to apply at the bedside but does not accurately reflect pathophysiology. Serum CRP has a relatively constant half-life, and its production is directly associated with with inflammation along a continuum.56 If 4 different patients have CRP levels of 10 mg/L, 171 mg/L, 173 mg/L, and 1000 mg/L 3 days after a colectomy, few clinicians would group these patients according to the 172 mg/L cutoff. The negative predictive value was 97%, such that a low value usually indicates no leak, but the positive predictive value was 21%.
Most diseases are not driven by a single physiologic parameter; therefore, prognostic scoring systems often incorporate multiple parameters for tasks such as measuring illness severity and predicting stroke and severe gastrointestinal bleeding.24,45,57 Parametric regression prognostic scoring systems assume that relationships among input variables are linear.22,29 When the relationships are nonlinear, the scoring system is similar to a coin toss.11
To facilitate clinical adoption, prognostic scoring systems have been implemented as online risk calculators. The NSQIP Surgical Risk Calculator is a prominent example. Calculator use may increase the likelihood that patients will participate in risk-reduction strategies such as prehabilitation.3 However, input variables must be entered manually, and its predictive accuracy is suboptimal, especially for nonelective operations, representing opportunities for improvement.4–7
Artificial Intelligence Predictive Analytics and Augmented Decision-Making
In 1970, William B. Schwartz published a Special Article in the New England Journal of Medicine stating, “Computing science will probably exert its major effects by augmenting and, insomecases, largely replacing the intellectual functions of the physician.”58 Despite extraordinary advances in computer technology, this vision has not been realized. Several factors may contribute. Traditional clinical decision-support systems require time-consuming manual data acquisition and entry, which impairs their adoption.8,33 Even the most successful and widely used static variable cutoff values do not accurately represent individual patient pathophysiology, as reflected by their suboptimal accuracy.34,43,56 Parametric regression equations also fail to represent the complex, nonlinear associations among input variables, further limiting the accuracy of traditional multivariable regression models.22,29 The weaknesses of traditional approaches may be overcome by artificial intelligence models fed with livestreaming intraoperative and EHR data to augment surgical decision-making through hpreoperative, intraoperative, and postoperative phases of care (Figure 2).
Figure 2. Optimal and Suboptimal Approaches to Surgical Decision-Making.

Artificial intelligence refers to computer systems that mimic human cognitive functions such as learning and problem-solving. In the broadest sense, a computer program using simple decision tree functions can mimic human intelligence. However, artificial intelligence usually refers to computer systems that learn from raw data with some degree of autonomy, as occurs with machine learning, deep learning, and reinforcement learning (Figure 3). Whereas traditional clinical decision-support systems use rules to generate codes and algorithms, artificial intelligence models learn from examples. Herein lies the strength of artificial intelligence for predictive analytics in medicine: human disease is simply too broad and complex to be explained and interpreted by rules.59,60
Figure 3. Summary of Artificial Intelligence Techniques.
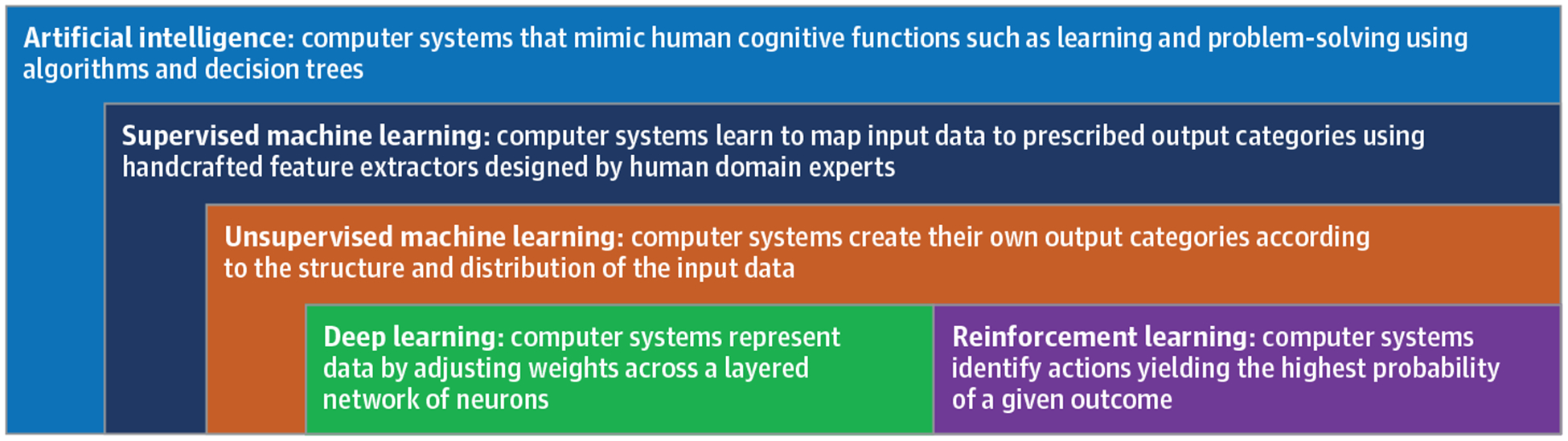
AI indicates artificial intelligence; EHR, electronic health record.
Machine Learning
Machine learning is a subfield of artificial intelligence in which a computer system performs a task without explicit instructions. Supervised machine learning models require human domain expertise and computer engineering to design handcrafted feature extractors capable of transforming raw data into desired representations. The algorithm learns associations between input data and prescribed output categories. Once trained, a supervised model is capable of classifying new unseen input data. With unsupervised techniques, input data have no corresponding annotated output categories; the algorithm creates its own output categories according to the structure and distribution of the input data. This approach allows discovery of patterns and phenotypes that were unrecognized prior to model development.
Machine learning has been used to accurately predict sepsis, in-hospital mortality, and acute kidney injury using intraoperative time-series data.9,21,27,32 Each machine learning algorithm has distinct advantages and disadvantages for different tasks such that performance depends on fit between algorithm and task. To capitalize on this phenomenon, Super Learner ranks a set of candidate algorithms20,28,38,39 by their performance and applies an optimal weight to each, creating ensemble algorithms that can accurately predict transfusion requirements and mortality among trauma patients.20,28,38,39 Supervised and unsupervised machine learning input features must be handcrafted using domain knowledge. In deep learning, features are extracted by the model itself.
Deep Learning
Deep learning is a subfield of machine learning in which computer systems learn and represent highly dimensional data by adjusting weighted associations among input variables across a layered hierarchy of neurons or artificial neural network. Early warning systems that alert clinicians to unstable vital signs illustrate data dimension ality. As the number of vital sign data sources increases linearly, the combinations of alarm parameters that trigger early warning system alarms increase exponentially, resulting in frequent false alarms. Even without a corresponding exponential increase in observations, data are highly dimensional when many variables are used to represent a single patient or event, especially when the number of patients or events in the data set is relatively low, producing unique and rare mixtures of data. Prediction models are less effective when classifying mixtures of data that are rare or absent in the development or training data set. The ability of deep models to represent highly dimensional data is important to their application to surgical decision-making.
In deep models, the initial input and final output layers are connected by hidden layers containing hidden nodes. Each hidden node is assigned a weight that is influenced by previous layers, affects the output from that neuron, and has the potential to affect the outcome classification of the entire network. An algorithm optimizes and updates weights as the model is trained to achieve the strongest possible association between input and output layers. This structure allows accurate representation of chaotic and nonlinear yet meaningful relationships among input features. Deep models automatically learn optimal feature representations from raw data without handcrafted feature engineering, providing a logistical advantage over machine learning models that require time-intensive feature engineering.61 Automatic feature extraction also promotes discovery of novel patterns and phenotypes that may have been overlooked by handcrafted feature selection techniques.
Clinical applications of deep learning benefit from the ability to include multiple different types and sources of data as inputs for a single model, including wearable sensors and cameras capturing patient movements and facial expressions with computer vision, an artificial intelligence subfield in which deep models use pixels from images and videos as inputs.60,62,63 Deep models have successfully performed patient phenotyping, disease prediction, and mortality prediction tasks.19,26,41,64 When applied to the same variable set used to calculate SOFA scores, deep models outperform traditional SOFA modeling in predicting in-hospital mortality for ICU patients.42 Preliminary data suggest that deep models are theoretically capable of accurately predicting risk for perioperative and postoperative complications and augmenting recommendations for operative management and the informed consent process. Despite their utility for predictive analytics, deep learning only provides outcome probabilities that loosely correspond to specific decisions and actions. In contrast, reinforcement learning is well suited to support specific decisions made by patients, caregivers, and surgeons.
Reinforcement Learning
Reinforcement learning is an artificial intelligence subfield in which computer systems identify actions yielding the highest probability of an outcome. Reinforcement models can be trained by series of trial and error scenarios, exposing the model to expert demonstrations, or a combination of these strategies. This occurs in a Markov decision process framework, consisting of a set of states, a set of actions, the probability that a certain action in a certain state will lead to a new state, and the reward that results from the new state. Using this framework, the system creates a policy that identifies the choice or action with the highest probability of a desired outcome, assessing total rewards attributable to multiple actions performed over time and the relative importance of present and future rewards, facilitating application of reinforcement learning to clinical scenarios that evolve over time.
Reinforcement learning has been used to recommend optimal fluid resuscitation and vasopressor administration strategies for patients with sepsis.31 Ninety-day mortality was lowest when care provided by clinicians was concordant with model recommendations. Reinforcement learning has also been used to recommend basal and bolus insulin administration for virtual type 1 diabetics.46 The algorithm performed as well as standard intermittent self-monitoring and continuous glucose monitoring methods, but with fewer episodes of hypoglycemia. Similar methods could be applied to augment the decision to operate.
Implementation
Automated Electronic Health Record Data
The Health Information Technology for Economic and Clinical Health Act of 2009 incentivized adoption of HER systems.65 Within 6 years, more than 4 of 5 US hospitals adopted EHRs.66 The volume of data generated by EHRs is staggering and will likely increase over time. Approximately 153 billion GB of data were generated in 2013, with projected growth of 48% per year.67 This data volume is ideal for artificial intelligence models, which thrive on large data sets.
Because EHRs are continuously updated as patient data become available, artificial intelligence models can provide real-time predictions and recommendations. Works published within the last year demonstrate the feasibility of this approach. The My Surgery Risk platform uses EHR data for 285 variables to predict 8 postoperative complications with an area under the curve (AUC) of 0.82–0.94 and to predict mortality at 1, 3, 6, 12, and 24 months with an AUC of 0.77–0.83.15 Electronic health record data feed the algorithm automatically, obviating manual data search and entry and overcoming a major obstacle to clinical adoption. In a prospective study, the algorithm predicted postoperative complications with greater accuracy than physicians.17
Mobile Device Outputs
To optimize clinical utility and facilitate adoption, automated model outputs could be provided to mobile devices. This would require several elements that communicate with one another reliably and efficiently, including robust quality filters, a public key infrastructure, and encryption that can only be deciphered by the intended receiver.68 Model outputs could be provided to mobile devices equipped with the appropriate Rest API client-server relationship and security clearance or through Google Cloud Messaging. To our knowledge, automated surgical risk predictions with mobile device out puts have not yet been reported. However, efforts to use manual data entry to feed machine learning models for surgical risk prediction on mobile devices have been successful.14
Human Intuition
Human intuition seems to arise from dopaminergic limbic system neurons that modify their connections with one another when a certain pattern or situation leads to a reward or penalty such as pleasure or pain.69,70 Subsequently, similar patterns or situations evoke positive and negative emotions, or gut feelings, which are powerful and effective decision-making tools. In a sentinel investigation12 of intuitive decision-making, participants drew cards from 1 of 4 decks for a cash reward. Two decks were rigged to be advantageous and 2 were rigged to be disadvantageous. Participants could explain differences between decks after drawing 80 cards, but demonstrated measurable anxiety and perspiration when reaching for a disadvantageous deck after drawing 10 cards and began to favor the advantageous deck after 50 cards before they could consciously explain what they were doing or why they were doing it. Similar phenomena occur in fight-or-flight survival responses, naval warfare, and financial decision-making.71,72 Intuition can also identify patients with life-threatening conditions that would be underappreciated by traditional clinical parameters alone.47,48
Challenges to Adoption
Data Standardization and Technology Infrastructure
To produce models that may be integrated with any EHR in any setting, data must be standardized. The Fast Healthcare Interoperability Resources framework establishes standards for health information exchange using a set of universal components assembled into systems that facilitate data sharing across EHRs and cloud-based communications. In addition, the Epic EHR that dominates the market has exclusive rights to develop new functions. To avoid legal conflicts, virtual models can live outside the EHR.15 However, this requires technology infrastructure that is not currently available in all clinical settings.
Interpretability
Diligent clinicians and informed patients will want to know why a computer program made a certain prediction or recommendation. Several techniques address this challenge, including attention mechanisms that reveal periods during which model inputs contributed disproportionately to the output, plotting pairwise similarities between data points to display phenotypic clusters, and training models on labeled patient data and then a linear gradient-boosting tree so that the model will assign relative importance to patient data input features.18,42,73
Safety and Monitoring
If model inputs are flawed or model outputs are not carefully monitored by data scientists and interpreted by astute clinicians, many patients could be harmed in a short time frame. Artificial intelligence models trained on erroneous or misrepresentative data are likely to obscure the truth. Because studies with positive results are more likely to be submitted and published, artificial intelligence literature may be overly optimistic. Prior to clinical implementation, machine and deep learning models must be rigorously analyzed in a retrospective fashion and externally validated to ensure generalizability. Performing a stress test of artificial intelligence models by simulating erroneous and rare model inputs and assessing how the model responds may allow clinicians to better understand how and why failures occur. Initial prospective implementation should occur on a small scale under close monitoring, similar to phase 1 and 2 clinical trials for experimental medications, with analysis of how decision-support tools affect decisions across populations and among individual patients.74 In cooperation with the International Medical Device Regulators Forum, the US Food and Drug Administration created the Software as Medical Device category and developed a voluntary Software Precertification Program to aid health care software developers in creating, testing, and implementing Software as Medical Device. Medicolegal regulation of Software as Medical Device is not rigidly defined.
Ethical Challenges
When algorithms are trained on data sets that are influenced by bias, algorithm outputs will likely reflect similar bias. In 1 prominent example, a model designed to augment judicial decision-making by predicting the likelihood of crime recidivism demonstrated predilection for racial/ethnic discrimination.75 When data used to train an algorithm are predominantly derived from patient populations with different demographics than the patient for whom the algorithm is applied, accuracy may suffer. For example, the Framingham heart study primarily included white participants. A model trained on this data may reflect racial and ethnic bias because associations between cardiovascular risk factors and events differ by race and ethnicity.25 Accountability for errors poses another challenge. Our justice system is well-equipped to address scenarios in which an individual clinician is responsible for making an errant decision, but it may prove difficult to assign blame to a computer program and its developers.
Conclusions
Surgical decision-making is impaired by time constraints, uncertainty, complexity, decision fatigue, hypothetical-deductive reasoning, and bias, leading to preventable harm. Traditional decision-support systems are compromised by time-consuming manual data entry and suboptimal accuracy. Automated artificial intelligence models fed with livestreaming EHR data can address these weaknesses. Successful integration of artificial intelligence with surgical decision-making would require data standardization, advances in model interpretability, careful implementation and monitoring, attention to ethical challenges, and preservation of bedside assessment and human intuition in the decision-making process. Artificial intelligence models must be rigorously analyzed in a retrospective fashion with robust external validation prior to prospective clinical application under the close scrutiny of astute clinicians and data scientists. Properly applied, artificial intelligence has the potential to transform surgical care by augmenting the decision to operate, the informed consent process, identification and mitigation of modifiable risk factors, recognition and management of complications, and shared decisions regarding resource use.
Supplementary Material
Funding/Support:
Dr Efron was supported by R01 GM113945-01 from the the National Institute of General Medical Sciences (NIGMS). Drs Bihorac and Rashidi were supported by R01 GM110240 from the NIGMS. Drs Bihorac and Efron were supported by P50 GM-111152 from the NIGMS. Dr Rashidi was supported by CAREER award NSF-IIS 1750192 from the National Science Foundation, Division of Information and Intelligent Systems, and by the National Institute of Biomedical Imaging and Bioengineering (grant R21EB027344-01). Dr Tighe was supported by R01GM114290 from the NIGMS. Dr Loftus was supported by a postgraduate training grant (T32 GM-008721) in burns, trauma, and perioperative injury from the NIGMS.
Role of the Funder/Sponsor: The National Institute of General Medical Sciences, National Science Foundation, and the National Institute of Biomedical Imaging and Bioengineering had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Tighe reported grants from the National Institutes of Health during the conduct of the study. Dr Rashidi reported patents to Method and Apparatus for Pervasive Patient Monitoring pending and Systems and Methods for Providing an Acuity Score for Critically Ill or Injured Patients pending. Dr Bihorac reported grants from the National Institutes of Health and the National Science Foundation during the conduct of the study; in addition, Dr Bihorac has a patent to Systems and Methods for Providing an Acuity Score for Critically Ill or Injured Patients pending. No other disclosures were reported.
REFERENCES
- 1.Healey MA, Shackford SR, Osler TM, Rogers FB, Burns E. Complications in surgical patients. Arch Surg. 2002;137(5):611–617. doi: 10.1001/archsurg.137.5.611 [DOI] [PubMed] [Google Scholar]
- 2.Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995–1000. doi: 10.1097/SLA.0b013e3181bfdab3 [DOI] [PubMed] [Google Scholar]
- 3.Raymond BL, Wanderer JP, Hawkins AT, et al. Use of the American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator during preoperative risk discussion: the patient perspective. Anesth Analg. 2019;128(4): 643–650. doi: 10.1213/ANE.0000000000003718 [DOI] [PubMed] [Google Scholar]
- 4.Clark DE, Fitzgerald TL, Dibbins AW. Procedure-based postoperative risk prediction using NSQIP data. J Surg Res. 2018;221:322–327. doi: 10.1016/j.jss.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Lubitz AL, Chan E, Zarif D, et al. American College of Surgeons NSQIP risk calculator accuracy for emergent and elective colorectal operations. J Am Coll Surg. 2017;225(5):601–611. doi: 10.1016/j.jamcollsurg.2017.07.1069 [DOI] [PubMed] [Google Scholar]
- 6.Cohen ME, Liu Y, Ko CY, Hall BL. An examination of American College of Surgeons NSQIP surgical risk calculator accuracy. J Am Coll Surg. 2017;224(5): 787–795.e1. [DOI] [PubMed] [Google Scholar]
- 7.Hyde LZ, Valizadeh N, Al-Mazrou AM, Kiran RP. ACS-NSQIP risk calculator predicts cohort but not individual risk of complication following colorectal resection. Am J Surg. 2019;218(1):131–135. doi: 10.1016/j.amjsurg.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 8.Leeds IL, Rosenblum AJ, Wise PE, et al. Eye of the beholder: risk calculators and barriers to adoption in surgical trainees. Surgery. 2018;164(5): 1117–1123. doi: 10.1016/j.surg.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhikari L, Ozrazgat-Baslanti T, Ruppert M, et al. Improved predictive models for acute kidney injury with IDEA: Intraoperative Data Embedded Analytics. PLoS One. 2019;14(4):e0214904. doi: 10.1371/journal.pone.0214904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artis KA, Bordley J, Mohan V, Gold JA. Data omission by physician trainees on ICU rounds. Crit Care Med. 2019;47(3):403–409. doi: 10.1097/CCM.0000000000003557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnall NM, Pring ET, Malietzis G, et al. Perioperative risk prediction in the era of enhanced recovery: a comparison of POSSUM, ACPGBI, and E-PASS scoring systems in major surgical procedures of the colorectal surgeon. Int J Colorectal Dis. 2018;33(11):1627–1634. doi: 10.1007/s00384-018-3141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304): 1293–1295. doi: 10.1126/science.275.5304.1293 [DOI] [PubMed] [Google Scholar]
- 13.Bertrand PM, Pereira B, Adda M, et al. Disagreement between clinicians and score in decision-making capacity of critically ill patients. Crit Care Med. 2019;47(3):337–344. doi: 10.1097/CCM.0000000000003550 [DOI] [PubMed] [Google Scholar]
- 14.Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA. Surgical risk is not linear: derivation and validation of a novel, user-friendly, and machine-learning-based Predictive Optimal Trees in Emergency Surgery Risk (POTTER) calculator. Ann Surg. 2018;268(4):574–583. doi: 10.1097/SLA.0000000000002956 [DOI] [PubMed] [Google Scholar]
- 15.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, et al. MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg. 2019;269(4):652–662. doi: 10.1097/SLA.0000000000002706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Making. 2015;35(4):539–557. doi: 10.1177/0272989X14547740 [DOI] [PubMed] [Google Scholar]
- 17.Brennan M, Puri S, Ozrazgat-Baslanti T, et al. Comparing clinical judgment with the MySurgeryRisk algorithm for preoperative risk assessment: a pilot usability study. Surgery. 2019; 165(5):1035–1045. doi: 10.1016/j.surg.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che Z, Purushotham S, Khemani R, Liu Y. Interpretable deep models for ICU outcome prediction. AMIA Annu Symp Proc. 2017;2016: 371–380. [PMC free article] [PubMed] [Google Scholar]
- 19.Hung Chen-Ying, Chen Wei-Chen, Lai Po-Tsun, Lin Ching-Heng, Lee Chi-Chun. Comparing deep neural network and other machine learning algorithms for stroke prediction in a large-scale population-based electronic medical claims database. Conf Proc IEEE Eng Med Biol Soc. 2017; 2017:3110–3113. [DOI] [PubMed] [Google Scholar]
- 20.Christie SA, Hubbard AE, Callcut RA, et al. Machine learning without borders? an adaptable tool to optimize mortality prediction in diverse clinical settings. J Trauma Acute Care Surg. 2018;85 (5):921–927. doi: 10.1097/TA.0000000000002044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delahanty RJ, Kaufman D, Jones SS. Development and evaluation of an automated machine learning algorithm for in-hospital mortality risk adjustment among critical care patients. Crit Care Med. 2018;46(6):e481–e488. doi: 10.1097/CCM.0000000000003011 [DOI] [PubMed] [Google Scholar]
- 22.Dybowski R, Weller P, Chang R, Gant V. Prediction of outcome in critically ill patients using artificial neural network synthesised by genetic algorithm. Lancet. 1996;347(9009):1146–1150. doi: 10.1016/S0140-6736(96)90609-1 [DOI] [PubMed] [Google Scholar]
- 23.Ellis EM, Klein WMP, Orehek E, Ferrer RA. Effects of emotion on medical decisions involving tradeoffs. Med Decis Making. 2018;38(8):1027–1039. doi: 10.1177/0272989X18806493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93 [DOI] [PubMed] [Google Scholar]
- 25.Gijsberts CM, Groenewegen KA, Hoefer IE, et al. Race/ethnic differences in the associations of the framingham risk factors with carotid IMT and cardiovascular events. PLoS One. 2015;10(7):e0132321. doi: 10.1371/journal.pone.0132321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Hao, Ghassemi MM Mengling Feng. The effects of deep network topology on mortality prediction. Conf Proc IEEE Eng Med Biol Soc. 2016; 2016:2602–2605. [DOI] [PubMed] [Google Scholar]
- 27.Henry KE, Hager DN, Pronovost PJ, Saria S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. 2015; 7(299):299ra122. doi: 10.1126/scitranslmed.aab3719 [DOI] [PubMed] [Google Scholar]
- 28.Hubbard A, Munoz ID, Decker A, et al. ; PROMMTT Study Group. Time-dependent prediction and evaluation of variable importance using superlearning in high-dimensional clinical data. J Trauma Acute Care Surg. 2013;75(1)(suppl 1): S53–S60. doi: 10.1097/TA.0b013e3182914553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Kim W, Park RW. A comparison of intensive care unit mortality prediction models through the use of data mining techniques. Healthc Inform Res. 2011;17(4):232–243. doi: 10.4258/hir.2011.17.4.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knops AM, Legemate DA, Goossens A, Bossuyt PM, Ubbink DT. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Ann Surg. 2013;257(5):860–866. doi: 10.1097/SLA.0b013e3182864fd6 [DOI] [PubMed] [Google Scholar]
- 31.Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24(11):1716–1720. doi: 10.1038/s41591-018-0213-5 [DOI] [PubMed] [Google Scholar]
- 32.Koyner JL, Carey KA, Edelson DP, Churpek MM. The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med. 2018;46(7):1070–1077. doi: 10.1097/CCM.0000000000003123 [DOI] [PubMed] [Google Scholar]
- 33.Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–535. doi: 10.1016/j.pec.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 34.Loftus TJ, Brakenridge SC, Croft CA, et al. Neural network prediction of severe lower intestinal bleeding and the need for surgical intervention. J Surg Res. 2017;212:42–47. doi: 10.1016/j.jss.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludolph R, Schulz PJ. Debiasing health-related judgments and decision making: a systematic review. Med Decis Making. 2018;38(1):3–13. [DOI] [PubMed] [Google Scholar]
- 36.Lundgrén-Laine H, Kontio E, Perttilä J, Korvenranta H, Forsström J, Salanterä S. Managing daily intensive care activities: an observational study concerning ad hoc decision making of charge nurses and intensivists. Crit Care. 2011;15(4):R188. doi: 10.1186/cc10341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris RS, Ruck JM, Conca-Cheng AM, Smith TJ, Carver TW, Johnston FM. Shared decision-making in acute surgical illness: the surgeon’s perspective. J Am Coll Surg. 2018;226(5): 784–795. doi: 10.1016/j.jamcollsurg.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 38.Pirracchio R, Petersen ML, Carone M, Rigon MR, Chevret S, van der Laan MJ. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): a population-based study. Lancet Respir Med. 2015;3(1):42–52. doi: 10.1016/S2213-2600(14)70239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirracchio R, Petersen ML, van der Laan M. Improving propensity score estimators’ robustness to model misspecification using super learner. Am J Epidemiol. 2015;181(2):108–119. doi: 10.1093/aje/kwu253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacks GD, Dawes AJ, Ettner SL, et al. Surgeon perception of risk and benefit in the decision to operate. Ann Surg. 2016;264(6):896–903. doi: 10.1097/SLA.0000000000001784 [DOI] [PubMed] [Google Scholar]
- 41.Choi E, Schuetz A, Stewart WF, Sun J. Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc. 2017;24(2):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shickel B, Loftus TJ, Adhikari L, Ozrazgat-Baslanti T, Bihorac A, Rashidi P. DeepSOFA: a continuous acuity score for critically ill patients using clinically interpretable deep learning. Sci Rep. 2019;9(1):1879. doi: 10.1038/s41598-019-38491-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(4):339–346. doi: 10.1002/bjs.9354 [DOI] [PubMed] [Google Scholar]
- 44.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strate LL, Saltzman JR, Ookubo R, Mutinga ML, Syngal S. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(8):1821–1827. doi: 10.1111/j.1572-0241.2005.41755.x [DOI] [PubMed] [Google Scholar]
- 46.Sun Q, Jankovic M, Budzinski J, et al. A dual mode adaptive basal-bolus advisor based on reinforcement learning [published online Dec 17, 2018]. IEEE J Biomed Health Inform. doi: 10.1109/JBHI.2018.2887067 [DOI] [PubMed] [Google Scholar]
- 47.Van den Bruel A, Thompson M, Buntinx F, Mant D. Clinicians’ gut feeling about serious infections in children: observational study. BMJ. 2012;345:e6144. doi: 10.1136/bmj.e6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D; European Research Network on Recognising Serious Infection investigators. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet. 2010;375 (9717):834–845. doi: 10.1016/S0140-6736(09)62000-6 [DOI] [PubMed] [Google Scholar]
- 49.Vohs KD, Baumeister RF, Schmeichel BJ, Twenge JM, Nelson NM, Tice DM. Making choices impairs subsequent self-control: a limited-resource account of decision making, self-regulation, and active initiative. J Pers Soc Psychol. 2008;94(5): 883–898. doi: 10.1037/0022-3514.94.5.883 [DOI] [PubMed] [Google Scholar]
- 50.Kopecky KE, Urbach D, Schwarze ML. Risk calculators and decision aids are not enough for shared decision making. JAMA Surg. 2019;154(1): 3–4. doi: 10.1001/jamasurg.2018.2446 [DOI] [PubMed] [Google Scholar]
- 51.Ferrer RA, Green PA, Barrett LF. Affective science perspectives on cancer control: strategically crafting a mutually beneficial research agenda. Perspect Psychol Sci. 2015;10(3):328–345. doi: 10.1177/1745691615576755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman JJ, Robinson CK, Zarzaur BL, Timsina L, Rozycki GS, Feliciano DV. To sleep, perchance to dream: acute and chronic sleep deprivation in acute care surgeons. J Am Coll Surg. 2019;229(2):166–174. doi: 10.1016/j.jamcollsurg.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 53.Stickgold R Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. doi: 10.1038/nature04286 [DOI] [PubMed] [Google Scholar]
- 54.Goldenson RM. The Encyclopedia of Human Behavior; Psychology, Psychiatry, and Mental Health. Garden City, NY: Doubleday; 1970. [Google Scholar]
- 55.Groopman JE. How Doctors Think. Boston: Houghton Mifflin; 2007. [Google Scholar]
- 56.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440(7088): 1217–1221. doi: 10.1038/nature04672 [DOI] [PubMed] [Google Scholar]
- 57.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz WB. Medicine and the computer: the promise and problems of change. N Engl J Med. 1970;283(23):1257–1264. doi: 10.1056/NEJM197012032832305 [DOI] [PubMed] [Google Scholar]
- 59.Schwartz WB, Patil RS, Szolovits P. Artificial intelligence in medicine: where do we stand? N Engl J Med. 1987;316(11):685–688. doi: 10.1056/NEJM198703123161109 [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Ann Surg. 2018;268(1):70–76. doi: 10.1097/SLA.0000000000002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J Biomed Health Inform. 2018;22(5): 1589–1604. doi: 10.1109/JBHI.2017.2767063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–29. doi: 10.1038/s41591-018-0316-z [DOI] [PubMed] [Google Scholar]
- 63.Davoudi A, Malhotra KR, Shickel B, et al. Intelligent ICU for autonomous patient monitoring using pervasive sensing and deep learning. Sci Rep. 2019;9(1):8020. doi: 10.1038/s41598-019-44004-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson PN. Deep phenotyping for precision medicine. Hum Mutat. 2012;33(5):777–780. doi: 10.1002/humu.22080 [DOI] [PubMed] [Google Scholar]
- 65.Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345–359. doi: 10.1146/annurev-publhealth-031914-122747 [DOI] [PubMed] [Google Scholar]
- 66.Adler-Milstein J, Holmgren AJ, Kralovec P, Worzala C, Searcy T, Patel V. Electronic health record adoption in US hospitals: the emergence of a digital “advanced use” divide. J Am Med Inform Assoc. 2017;24(6):1142–1148. doi: 10.1093/jamia/ocx080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanford Medicine. Stanford medicine 2017 health trends report: harnessing the power of data in health. http://med.stanford.edu/content/dam/sm/sm-news/documents/StanfordMedicineHealthTrendsWhitePaper2017.pdf. Accessed February 23, 2019.
- 68.Feng Z, Bhat RR, Yuan X, et al. Intelligent perioperative system: towards real-time big data analytics in surgery risk assessment. DASC PICom DataCom CyberSciTech 2017 (2017). 2017;2017: 1254–1259. doi: 10.1109/DASC-PICom-DataCom-CyberSciTec.2017.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976 [DOI] [PubMed] [Google Scholar]
- 70.Kahneman D. Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2013. [Google Scholar]
- 71.LeDoux J Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58(5):662–671. doi: 10.1016/j.neuron.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 73.van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–2605. [Google Scholar]
- 74.Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA. 2018;320(21):2199–2200. doi: 10.1001/jama.2018.17163 [DOI] [PubMed] [Google Scholar]
- 75.Angwin J, Larson J, Mattu S, Kirchner L. Machine bias. https://www.propublica.org/article/machine-bias-risk-assessments-in-criminal-sentencing. Published May 23, 2016. Accessed January 24, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


