Abstract
BACKGROUND
Abnormalities in cortical excitation and inhibition (E/I) balance are thought to underlie sensory and information processing deficits in schizophrenia (SZ). Deficits in early auditory information processing (EAIP) mediate both neurocognitive and functional impairment, and appear to be normalized by acute treatment with the NMDA antagonist, memantine (MEM).
METHODS
Thirty-six subjects with a diagnosis of schizophrenia and 31 control subjects (NCS) underwent EEG recordings. Subjects ingested either placebo or MEM (10 or 20 mg) in a double-blind, within-subject cross-over randomized design. The aperiodic, 1/f-like scaling property of the neural power spectra, which is thought to index relative E/I balance, was estimated using a robust linear regression algorithm.
RESULTS
SZ patients had greater aperiodic components compared to NCS (p<.01, d=.64), which was normalized after 20 mg MEM. Analysis revealed a significant dose x diagnosis interaction (p<.0001, d=.82). Further, the “MEM effect” (change in aperiodic component in MEM vs. placebo conditions) was associated with baseline attention and vigilance (r=.54, p<.05) and MEM-induced enhancements in gamma power (r=−.60, p<.01).
CONCLUSIONS
Findings confirmed E/I balance abnormalities in SZ that were normalized with acute MEM administration and suggest that neurocognitive profiles may predict treatment response based on E/I sensitivity. These data provide “proof-of-concept” evidence for the utility of E/I balance indices as metrics of acute pharmacologic sensitivity for central nervous system therapeutics.
Clinical Trials Registration Name
Memantine Effects on Sensorimotor Gating and Neurocognition in Schizophrenia
URL: https://clinicaltrials.gov/ct2/show/NCT03860597
Registration #: NCT03860597
Keywords: excitation/inhibition balance, electroencephalography, early auditory information processing, memantine, cognition, schizophrenia
Introduction
Neurocognitive deficits are core pathophysiologic dimensions of chronic psychotic disorders (1,2). While psychotic symptoms fluctuate over the disease course, cognitive symptoms are pervasive and are refractory to conventional treatment modalities. Moreover, cognitive symptoms mediate the degree of functional impairment, disability, and clinical course in chronic psychotic disorders (3–5). Despite the pervasiveness of cognitive impairments, the biology underlying these cognitive deficits remains unclear and there are no reliable biomarkers that can be used to predict response to novel therapeutics.
Converging evidence from preclinical and translational studies suggest that disruptions in cortical NMDA receptor signaling in schizophrenia (SZ) affect the large scale spatial and temporal organization of excitation and inhibition (E/I) in neural networks that mediate cognition and behavior (6,7). In addition to the distributed effects of cortical NMDA hypofunctioning, GABA-ergic interneuron signaling also appears to be profoundly affected in SZ. Postmortem studies in SZ have consistently demonstrated an abnormal pattern of expression of GABA signaling molecules in interneuron populations across cortical structures (8–10). These disruptions in both excitatory and inhibitory signaling are thought to underlie the pathological cognitive and perceptual states associated with SZ and other neuropsychiatric disorders (11–14).
The merging of computational models of E/I balance with experimental approaches has provided novel insights into the hierarchical organization of neural networks (15–18). Yet, the study of E/I balance as it relates to human cortical physiology and neural network dynamics is in its relative infancy. Traditional measures of E/I balance often require invasive methods, such as single-unit or voltage-clamp recordings in rodents and/or non-human primates (19–21), which have precluded their application to in vivo human studies. Recent findings suggest that the aperiodic, 1/f-like component of the neural power spectra may index tonic E/I balance and can be studied non-invasively using electroencephalographic (EEG) recordings. Interestingly, this aperiodic component is dynamically modulated by conscious and perceptual states (22–25), and preliminary findings suggest that it may be also be altered in SZ (26).
One way to explicate the biology underlying the E/I balance and its potential abnormalities in SZ is to assess changes in E/I balance in response to pharmacologic probes. Previous studies from our group demonstrated that the NMDA receptor antagonist, memantine (MEM), “normalized” EEG measures of early auditory information processing (EAIP) (27,28), which have been shown to mediate neurocognition in schizophrenia (3). Preclinical studies suggest that MEM can also “normalize” pathological E/I tone (29,30). We hypothesized: 1) that the aperiodic, 1/f-like scaling properties of the EEG power spectrum would be disrupted in patients with SZ, consistent with an aberrant E/I balance; and 2) that aberrant E/I balance in SZ would also be “normalized” by acute exposure to MEM. The present study was designed to test this hypothesis, by applying a novel signal processing algorithm to EEG recordings acquired during EAIP testing described in previous reports (27).
Methods and Materials
Participants, assessments, and experimental design
All procedures were approved by the UCSD Human Subject Institutional Review Board. Detailed descriptions of the recruitment and ascertainment methods were reported previously (27). Briefly, sixty-seven adults (with a diagnosis of a chronic psychotic disorder (SZ, n=36) or normal comparison subject (NCS, n=31) participated in the study. All subjects underwent baseline screening with demographic and clinical questionnaires, hearing tests, and urine toxicology. Neurocognitive functioning was assessed with the MATRICS Consensus Cognitive Battery. Diagnosis of SZ was confirmed with the MINI International Neuropsychiatric Interview and symptom severity was assessed with the positive and negative syndrome scale. Participants who met inclusion criteria were tested on two days separated a week apart in a double-blind, placebo-controlled, pseudo-randomized balanced drug order design where MEM HCl (10 or 20 mg) or placebo (PBO) was administered (p.o.). Testing of experimental measures was timed to coincide with peak blood levels of MEM in healthy subjects (31); sensorimotor gating (prepulse inhibition, “PPI”) was tested 210 min after pill administration and EEG measures were recorded about 345 min post-pill. Detailed methods of these measures were previously described (27,28).
EEG data acquisition and processing
A passive auditory oddball paradigm comprised of standard (50-ms, 1000-Hz) and deviant stimuli that differed from the standard in duration (125-ms, 1000-Hz), pitch (50-ms, 1100-Hz), or both pitch and duration (125-ms, 1100-Hz). Stimuli were presented in a pseudo-randomized sequence as per previous reports (27). EEG data were continuously recorded from 64 channels using a BioSemi ActiveTwo system at a sampling rate of 2048-Hz, which was downsampled offline to 512-Hz. Data processing was performed offline using MATLAB, EEGLAB, and BrainVision Analyzer and as per established protocols (27,28,32,33). Briefly, vertical and horizontal eye movement artifacts were corrected using independent component analysis (34). Continuous data were segmented at 500 ms epochs relative to the stimulus onset and each epoch was baseline-corrected relative to the 100 ms pre-stimulus interval. Epochs containing ± 70 μV were automatically rejected. All artefact-free epochs from all stimulus types were included in our analyses of aperiodic slopes and spectral parameters. Mismatch negativity (MMN) was calculated as the mean amplitude from the 135–205 ms range on the difference waveform between deviant and standard tones at electrode FZ and was averaged across all deviant types as previously reported (27).
Analysis of aperiodic and periodic spectral features
EEG signals were decomposed into their frequency-domain components via power spectral density (PSD) estimation using Welch’s method. PSDs from the 4–50 Hz range were used to characterize the aperiodic “background” or 1/f-like signal and oscillatory components using a robust linear regression algorithm (35). Additional measures of oscillatory power were derived to better characterize the drivers of the aperiodic signal and putative shifts in E/I balance. The algorithm treated PSDs as the linear sum of the aperiodic component in log-log space and modeled the superimposed oscillatory peaks (i.e., regions of the power spectrum rising above the aperiodic background signal) as Gaussian functions. The peak oscillatory amplitudes (the distance between the peak of the Gaussian and the aperiodic fit) represent the oscillatory power of the EEG signal at that given frequency band. The oscillatory peaks were then iteratively modeled and removed from the signal, providing a robust approximation of the aperiodic, 1/f-like slope. The aperiodic, 1/f-like slope, and oscillatory components of the EEG power spectra were calculated from trial-by-trial PSDs, generating spectral estimates for all stimulus types (standard and deviants), which were then averaged across all electrodes.
Statistical analysis
Linear mixed effects models were used to analyze the relationships between aperiodic slope, oscillatory power, and MEM in our sample. Dependent variables were regressed onto contrast-coded diagnosis, dose (0, 10, 20), and stimulus types, as well as interaction terms modeled as fixed effects. All models included centered fixed effects and random intercepts for subjects. Additionally, we provide complementary estimates of effects size (Cohen’s d) to help clarify effects of diagnosis and interactions (calculated using MEM minus PBO difference scores, henceforward referred to as the “MEM effect”). Note: aperiodic slope and oscillatory power responses to standard vs. deviant stimuli did not interact significantly with diagnosis or dose in linear mixed models; hence, spectral features were averaged across stimulus type and electrodes in “MEM effect” analyses. To further characterize the clinical and physiologic implications of the “MEM effect” on EEG spectral features, we assessed their relationships to: 1) MEM effects on traditional measures of EAIP (MMN and PPI); and 2) demographic, clinical, and cognitive measures in SZ patients. Statistical analysis were implemented using the “lme4” package in R (36) and the “statsmodels” package in Python (37).
Results
Demographic, clinical and neurocognitive assessments
Demographic and clinical characteristics of the sample were previously reported (27) and are summarized in Table 1. NCS participants were significantly younger compared to SZ patients (t=4.28, p<.001), although age was not significantly different between SZ dose groups (10 vs. 20 mg: t=.50, p>.5). Age did not significantly contribute to any statistical model in post hoc analyses; therefore, it was not included in the linear mixed effects analyses reported below.
Table 1.
Demographics and Clinical Characteristics (Mean (SEM))
| NCS (n=31) | SZ (n=36) | |
|---|---|---|
| Age (years)a | 28.0 (0.5) | 36.5 (0.4) |
| Sex (M:F) | 24:7 | 24:12 |
| Smoker (%) | 0 | 53.3 |
| WRAT | 103.0 (1.5) | 93.4 (0.8) |
| SZ dose group | 10 mg | 20 mg |
| Age (years) | 35.3 (1.9) | 37.0 (1.7) |
| Age of onset (years) | 19.9 (0.4) | 16.0 (0.6) |
| GAF | 58.7 (0.7) | 56.7 (0.4) |
| PANSS scores | ||
| Positive symptomsb | 12.4 (0.5) | 17.6 (0.4) |
| Negative symptoms | 16.2 (0.4) | 17.5 (0.6) |
| General psychopathologya | 24.1 (0.6) | 31.9 (0.9) |
| Totala | 52.8 (1.1) | 67.1 (1.5) |
| Medications | ||
| Aypical: Typical: Both: None | 9:1:3:4 | 13:1:4:1 |
| CPZ equivalents | 418.3 (46.7) | 501.6 (40.1) |
p<.05
p<.01
Despite careful randomization for key demographic variables, there was a modest cohort effect on baseline measures of PANSS Positive (t=2.9, p<.01), PANSS General Psychopathology (t=2.6, p<.05), and PANSS Total Symptoms (t=2.7, p<.05) in the 10 vs. 20 mg dosage groups (Table 1). The effects of these phenomenological differences were analyzed post hoc; none contributed significantly to the analyses of the primary dependent measures when treated as covariates in linear mixed effects models. No other demographic or clinical variables were found to be significantly different between patients, control, or dosage groups.
Relationships between diagnosis, MEM, and the aperiodic signal
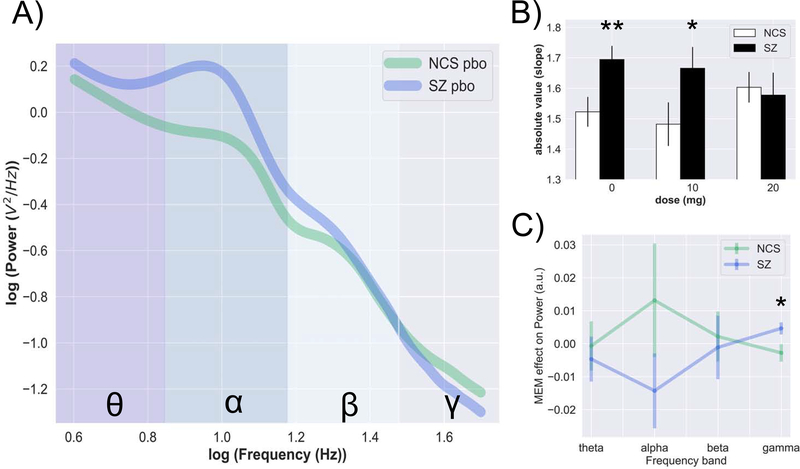
SZ patients had greater aperiodic slopes compared to HCS during the PBO condition (Figure 1b; t=2.60, p=.011, d=.64). Figure 1a shows how diagnostic differences in aperiodic slopes manifest on PSDs during the PBO condition. MEM 20 mg “normalized” aperiodic slope differences in SZ relative to NCS and abolished the effect of diagnosis (Figure 1b; t=.25, p=.801, d=.09). Analysis revealed a significant dose x diagnosis interaction (b=0.12, SE=.02, t=−4.92, p<.0001, d=.82) among individuals receiving 20 mg MEM vs PBO. Consistent with previous EAIP studies (26, 27), MEM 10 mg appeared to be inert as contrasts that included MEM 10 mg did not yield significant main effects or interactions. Exploratory analyses did not detect significant associations between aperiodic slope and clinical variables in these SZ patients (Supplemental Table 1). See Supplemental Material for PSD plots during drug conditions and scalp topographies.
Figure 1.
Aperiodic and periodic features – effects of acute MEM. SZ is characterized by steeper aperiodic slopes relative to NCS, indicative of greater power at lower frequencies. PSD are presented in log-log space; color-coded region represent canonical frequency bands as follows: theta (dark grey, 4–8 Hz), alpha (blue, 8–12 Hz), beta (light blue, 12–30 Hz), gamma (light grey, 30–50 Hz) (a). Acute dose of MEM 20 mg had a “normalizing” effect on aperiodic slopes (b). The “memantine effect” was more pronounced at gamma frequencies (c). Data are presented as mean ± SEM. Note: **p < .01, *p < .05.
Relationships between diagnosis, MEM, and oscillatory power
Since differences in oscillatory power may contribute to E/I balance abnormalities in SZ, we assessed the effects of MEM in canonical frequency bands. Analyses of power bands during the PBO condition revealed diagnostic differences; SZ patients had significantly greater power in theta (t=2.81, p=.006, d=.69) and alpha (t=2.99, p=.004, d=.73) frequency bands and lower gamma power (t=−2.42, p=.018, d=.59) relative to NCS. Among individuals receiving PBO vs. 20 mg MEM, there was a significant dose x diagnosis interaction (Figure 1c) for alpha (b=−.09, SE=.02, t=−3.45, p<.001, d=.51) and gamma (b=.18, SE=.04, t=3.87, p<.001, d=.88) frequency bands.
MEM effects, EAIP measures, and cognition
The parameterization of EEG spectral features is a novel method for extracting meaningful signals from neural power spectra (35). Given our prior reports of the relationships between MEM effects on MMN and PPI (27), we sought to assess the relationships between these EAIP measures and MEM effects on EEG spectral features during the 20 mg condition (which had a significant effect on E/I balance) (Table 2). Interestingly, there was no significant relationship between MEM effect on aperiodic slopes vs. MEM effects on either MMN or PPI. MEM effects on aperiodic slope did correlate significantly with the magnitude of MEM-enhanced spectral gamma power (r=−.60, p=.008), but not with MEM-induced changes in any other frequency band. Further, MEM effects on gamma power were associated with both MMN (r=−.65, p=.005) and PPI (r=.55, p=.043). It is important to note that these correlations do not survive Bonferroni correction for multiple comparisons (α<.002).
Table 2.
MEM effect and relationship to other measures of EAIP
| Aperiodic slope | Theta Power | Alpha Power | Beta Power | Gamma Power | MMN | |
|---|---|---|---|---|---|---|
| Theta power | 0.07 | |||||
| Alpha power | 0.08 | 0.67c | ||||
| Beta power | −0.25 | 0.63c | 0.47 | |||
| Gamma power | −0.60c | 0.04 | 0.04 | 0.14 | ||
| MMN | 0.30 | −0.51a | −0.47 | −0.36 | −0.65c | |
| PPI | 0.09 | 0.28 | 0.41 | 0.23 | 0.55a | −0.70b |
p < .05
p < .01
p < .005
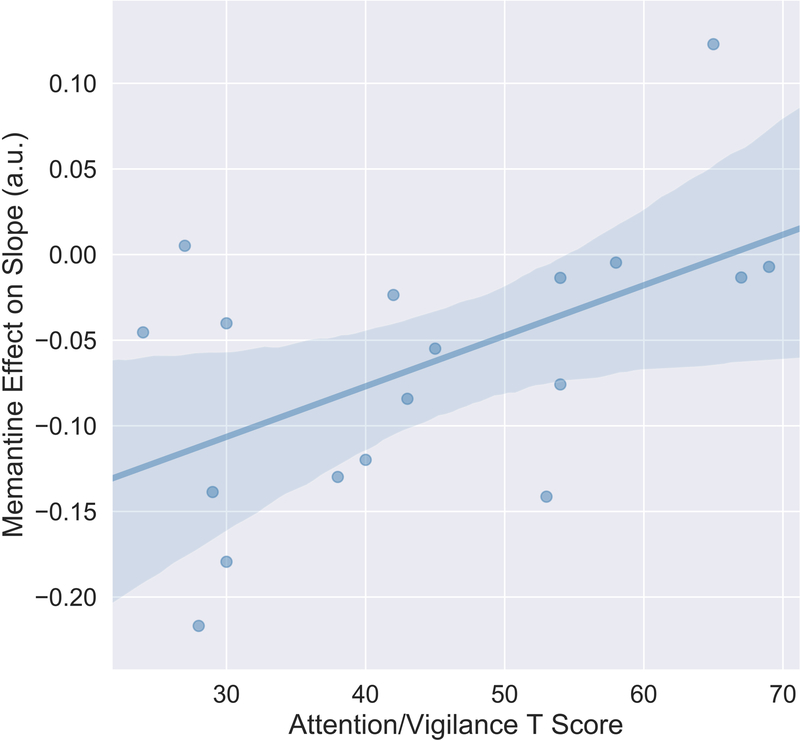
Recent findings from our laboratory suggest that MEM-induced gains in specific neurocognitive and sensory measures may be greatest among individuals with low attentional functioning (38). Therefore, we assessed the relationship between attention and MEM effects on aperiodic slopes in this 20 mg patient dose group. MEM effects on aperiodic slope were positively associated with attention and vigilance (Figure 2; r=.54, p=.021); this reflected the fact that MEM had greater “normalizing” effects (slope reduction) in individuals with lower vs. higher baseline attention. The effect of MEM on the spectral parameter did not correlate significantly with other clinical measures, including age of illness onset, symptom severity, chlorpromazine equivalents, or cognitive measures.
Figure 2.
Baseline Attention and Vigilance predicts MEM effect of aperiodic slopes. MEM had greater “normalizing” effects (slope reduction) in individuals with lower vs. higher baseline attention (r= .54; p< .05).
Discussion
Despite major advances in psychiatric neuroscience, the pathophysiology of SZ and its associated cognitive impairment remain elusive. This lack of progress is underscored by the dearth of effective therapies for the disabling cognitive symptoms of SZ. While several neuromodulatory systems have been implicated in this disorder, converging lines of evidence point to a fundamental role of cortical glutamatergic dysfunction and a disruption in the dynamic balance of excitation and inhibition in the pathogenesis of SZ (39–41). Yet, the investigations of E/I balance in SZ and its applications to CNS therapeutics have been limited by the invasiveness of electrophysiological measures of E/I and the lack of reliable biomarkers. To this end, the 1/f-like, aperiodic scaling property of the EEG power spectrum has emerged as a candidate biomarker of cortical E/I balance.
The present findings demonstrate E/I balance abnormalities in SZ patients during auditory information processing as measured by non-invasive electrophysiologic recordings. Specifically, “steeper” aperiodic slopes were detected in patients with SZ relative to NCS during PBO conditions, similar to findings in an independent sample of chronic SZ patients (26). Further, SZ patients had greater power in theta and alpha frequency bands and less power in gamma frequencies relative to NCS, suggesting a possible framework for understanding how deficits in oscillatory dynamics may contribute to E/I balance disturbances.
As hypothesized, MEM had “normalizing” effects on aperiodic slopes in SZ patients. MEM’s neurochemical properties are distinct from other NMDA receptor antagonists: it appears to spare physiologic NMDA receptor functioning and its therapeutic effects may involve both glutamatergic and non-glutamatergic targets (42–47). Interestingly, scalp topographies reveal that the ‘MEM effect’ extends beyond the fronto-central electrodes in both groups, reflecting a bi-frontal aperiodic slope enhancement in NCS and a more distributed slope reduction in SZ (Supplemental Figure 2). These differences in drug effects may be partially explained by MEM’s actions on pathological vs. normal functioning brain states. While it is highly speculative, the MEM-induced shift towards higher frequency spectra in SZ patients - particularly those with low baseline attention - may result in greater neurophysiological resources to extract signal from noise, and thereby to more effectively process attentionally-demanding information.
Theoretical and experimental models of SZ posit that NMDA hypofunction at parvalbumin-expressing interneurons leads to disinhibited pyramidal firing and excess cortical excitatory tone or “increased” E/I ratio within local microcircuits. These microcircuit abnormalities exist in the setting of widespread deficits to both “E” and “I”, which likely contribute to variations in E/I balance across different circuits and contexts. In contrast, the present findings of increased aperiodic slopes relative to NCS suggest a “decreased” E/I ratio in SZ patients (48) during passive auditory information processing. These findings should be interpreted from the perspective that aperiodic slopes are a reflection of the autocorrelation structure of PSDs (49) and do not preclude the possibility of regional E/I variations during other task-related paradigms. A “steeper” slope in SZ patients vs. NCS implies tighter temporal correlations, particularly within lower frequencies. Greater aperiodic slopes are also seen in other states where slow waves and greater inhibitory tone (i.e. reduced E/I ratio) predominate, such as sleep and altered states of consciousness where GABAergic tone is explicitly manipulated (50,51). These findings are largely consistent with neural circuit models of cortical dysfunction in SZ (52–55), where disruptions in cortical E/I balance may lead to a state of pathological “overcoupling” (15,56,57). This rigidity in the temporal structure of ongoing neural activity may represent an inability to properly tune responses and discern signal from noise in environmental stimuli (53,54,57,58).
Results of the present study should be considered in light of potential limitations. First, most of our current understanding of E/I balance reflects studies conducted at the level of cellular electrophysiology. By contrast, the current study applies novel signal processing methods to estimate E/I balance based on non-invasive EEG recordings. Scalp electrodes detect the aggregate activity of both excitatory and inhibitory populations from local and distant circuitries; the aperiodic slope does not dissociate the individual contributions of “E” or “I”, rather it represents a theoretical measurement of presumed E/I tone. Future computational and experimental studies are needed to clarify the precise contributions of “E” and “I” to aperiodic slopes.
Second, data were collected while subjects were exposed to passive auditory stimuli. Therefore, the parameterization of aperiodic (background slope) and periodic (oscillatory) spectral features likely reflects both task-evoked and non-task or “resting” features. While linear mixed effect analysis did not detect any differences as a function of stimulus type, future studies are needed to clarify the extent to which task (EAIP-evoked) and non-task (resting state) responses affect the neural power spectrum.
Third, it is possible that group (NCS vs. SZ) differences in E/I balance reflect the impact of antipsychotic medications. All SZ patients in this study were treated with antipsychotics, but no significant relationships were detected between E/I measures and antipsychotic load (chlorpromazine equivalents). Fourth, the present study included clinically stable outpatients with longstanding illness, and thus its findings may not generalize to other patient populations. Fifth, MEM effects on the key dependent measures of E/I balance were evident in the 20 mg dose group, but not the 10 mg dose group, similar to previous findings with MMN, PPI or gamma power and coherence (27,28); conceivably this apparent dose difference may have reflected a cohort effect (i.e. the use of clinically or demographically distinct patients in the 10 vs 20 mg groups). Importantly, none of the group differences in clinical or demographic variables (Table 1) accounted for the observed differences on MEM effects on E/I balance.
In summary, this study confirms the presence of cortical E/I balance deficits in antipsychotic-treated SZ patients and highlights a novel biomarker of an important construct in psychiatric neuroscience (i.e., “E/I balance”). The findings demonstrate that E/I balance can be transiently normalized in SZ patients with a single dose of MEM. These data provide “proof-of-concept” evidence for the utility of indices of E/I balance as metrics of pharmacologic sensitivity in drug discovery.
Supplementary Material
Acknowledgements and Disclosures
This work was supported by the National Institute of Mental Health (MH059803, MH094320, and MH101072), the Brain and Behavior Research Foundation, the VISN-22 Mental Illness Research, Education, and Clinical Center (MIRECC), and the Sidney R Baer, Jr Research Foundation. GAL has served as a consultant for Astellas, Boehringer Ingelheim, Merck, Lundbeck, Neuroverse, NeuroSig, and Takeda. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barch DM, Ceaser A (2012): Cognition in Schizophrenia: Core Psychological and Neural Mechanisms. Trends Cogn Sci 16 10.1016/j.tics.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MF, Harvey PD (2014): Cognition in schizophrenia: Past, present, and future. Schizophr Res Cogn 1: e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, et al. (2017): Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry 74: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuesta MJ, Peralta V (1995): Cognitive disorders in the positive, negative, and disorganization syndromes of schizophrenia. Psychiatry Res 58: 227–235. [DOI] [PubMed] [Google Scholar]
- 5.Green MF, Kern RS, Braff DL, Mintz J (2000): Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26: 119–136. [DOI] [PubMed] [Google Scholar]
- 6.Braun U, Schäfer A, Bassett DS, Rausch F, Schweiger JI, Bilek E, et al. (2016): Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proc Natl Acad Sci USA 113: 12568–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, et al. (2012): NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA 109: 16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. (2011): Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 31: 11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA (2011): Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 168: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB (2005): Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 57: 252–260. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AR, Bygrave AM, Sanderson DJ, Boyden ES, Bannerman DM, Kullmann DM, Kätzel D (2018): Optogenetic induction of the schizophrenia-related endophenotype of ventral hippocampal hyperactivity causes rodent correlates of positive and cognitive symptoms. Sci Rep 8: 12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. (2011): Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starc M, Murray JD, Santamauro N, Savic A, Diehl C, Cho YT, et al. (2017): Schizophrenia is associated with a pattern of spatial working memory deficits consistent with cortical disinhibition. Schizophr Res 181: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, et al. (2015): N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77: 569–580. [DOI] [PubMed] [Google Scholar]
- 15.Yang GJ, Murray JD, Wang X-J, Glahn DC, Pearlson GD, Repovs G, et al. (2016): Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA 113: E219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang X-J (2014): Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex 24: 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirtaş M, Burt JB, Helmer M, Ji JL, Adkinson BD, Glasser MF, et al. (2019): Hierarchical Heterogeneity across Human Cortex Shapes Large-Scale Neural Dynamics. Neuron 101: 1181–1194.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolls ET, Loh M, Deco G, Winterer G (2008): Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci 9: 696–709. [DOI] [PubMed] [Google Scholar]
- 19.Haider B, Duque A, Hasenstaub AR, McCormick DA (2006): Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwin-Kumar A, Doiron B (2012): Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat Neurosci 15: 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS (2014): Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 81: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, et al. (2008): Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He BJ, Zempel JM, Snyder AZ, Raichle ME (2010): The temporal structures and functional significance of scale-free brain activity. Neuron 66: 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podvalny E, Noy N, Harel M, Bickel S, Chechik G, Schroeder CE, et al. (2015): A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114: 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, Gazzaley A (2015): Age-Related Changes in 1/f Neural Electrophysiological Noise. J Neurosci 35: 13257–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson EJ, Rosen BQ, Campbell AM, Belger A, Voytek B (2018): 1/f neural noise is a better predictor of schizophrenia than neural oscillations. bioRxiv 113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow NR, Bhakta S, Chou H-H, Talledo JA, Balvaneda B, Light GA (2016): Memantine Effects On Sensorimotor Gating and Mismatch Negativity in Patients with Chronic Psychosis. Neuropsychopharmacology 41: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Light GA, Zhang W, Joshi YB, Bhakta S, Talledo JA, Swerdlow NR (2017): Single-Dose Memantine Improves Cortical Oscillatory Response Dynamics in Patients with Schizophrenia. Neuropsychopharmacology. 10.1038/npp.2017.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Povysheva NV, Johnson JW (2016): Effects of memantine on the excitation-inhibition balance in prefrontal cortex. Neurobiol Dis 96: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipton SA (2007): Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci 8: 803–808. [DOI] [PubMed] [Google Scholar]
- 31.Sonkusare SK, Kaul CL, Ramarao P (2005): Dementia of Alzheimer’s disease and other neurodegenerative disorders--memantine, a new hope. Pharmacol Res 51: 1–17. [DOI] [PubMed] [Google Scholar]
- 32.Light GA, Swerdlow NR, Braff DL (2007): Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci 19: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez VB, Tarasenko M, Miyakoshi M, Pianka ST, Makeig SD, Braff DL, et al. (2017): Mismatch Negativity is a Sensitive and Predictive Biomarker of Perceptual Learning During Auditory Cognitive Training in Schizophrenia. Neuropsychopharmacology 42: 2206–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell AJ, Sejnowski TJ (1995): An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7: 1129–1159. [DOI] [PubMed] [Google Scholar]
- 35.Haller M, Donoghue T, Peterson E, Varma P, Sebastian P, Gao R, et al. (2018): Parameterizing neural power spectra. bioRxiv 299859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates D, Mächler M, Bolker B, Walker S (2015): Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- 37.Seabold S, Perktold J (2010): Statsmodels: Econometric and Statistical Modeling with Python. Proceedings of the 9th Python in Science Conference. [Google Scholar]
- 38.Swerdlow NR, Bhakta SG, Clifford R, Talledo J, Kotz JE, Benster L, et al. (2019): Memantine Enhances Measures of Auditory Fidelity and Learning in Schizophrenia. Proceedings Am Col of Neuropsychopharmacology: Orlando, Fl: M159. [Google Scholar]
- 39.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. (2012): Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 169: 1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT, et al. (2013): Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry 18: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK (2016): Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry 73: 665–674. [DOI] [PubMed] [Google Scholar]
- 42.Xia P, Chen HV, Zhang D, Lipton SA (2010): Memantine Preferentially Blocks Extrasynaptic over Synaptic NMDA Receptor Currents in Hippocampal Autapses. J Neurosci 30: 11246–11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeman P, Caruso C, Lasaga M (2008): Memantine agonist action at dopamine D2High receptors. Synapse 62: 149–153. [DOI] [PubMed] [Google Scholar]
- 44.Glasgow NG, Povysheva NV, Azofeifa AM, Johnson JW (2017): Memantine and Ketamine Differentially Alter NMDA Receptor Desensitization. J Neurosci 37: 9686–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onogi H, Ishigaki S, Nakagawasai O, Arai-Kato Y, Arai Y, Watanabe H, et al. (2009): Influence of memantine on brain monoaminergic neurotransmission parameters in mice: neurochemical and behavioral study. Biol Pharm Bull 32: 850–855. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow NR, Eastvold A, Karban B, Ploum Y, Stephany N, Geyer MA, et al. (2002): Dopamine agonist effects on startle and sensorimotor gating in normal male subjects: time course studies. Psychopharmacology (Berl) 161: 189–201. [DOI] [PubMed] [Google Scholar]
- 47.Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, et al. (2015): Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 40: 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao R, Peterson EJ, Voytek B (2017): Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158: 70–78. [DOI] [PubMed] [Google Scholar]
- 49.He BJ (2014): Scale-free brain activity: past, present, and future. Trends Cogn Sci (Regul Ed) 18: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthukumaraswamy SD, Liley DT (2018): 1/f electrophysiological spectra in resting and drug-induced states can be explained by the dynamics of multiple oscillatory relaxation processes. Neuroimage 179: 582–595. [DOI] [PubMed] [Google Scholar]
- 51.Colombo MA, Napolitani M, Boly M, Gosseries O, Casarotto S, Rosanova M, et al. (2019): The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. Neuroimage 189: 631–644. [DOI] [PubMed] [Google Scholar]
- 52.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. (2000): Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10: 1078–1092. [DOI] [PubMed] [Google Scholar]
- 53.Winterer G, Musso F, Beckmann C, Mattay V, Egan MF, Jones DW, et al. (2006): Instability of prefrontal signal processing in schizophrenia. Am J Psychiatry 163: 1960–1968. [DOI] [PubMed] [Google Scholar]
- 54.Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR (2004): Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry 161: 490–500. [DOI] [PubMed] [Google Scholar]
- 55.Berman KF, Torrey EF, Daniel DG, Weinberger DR (1992): Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Arch Gen Psychiatry 49: 927–934. [DOI] [PubMed] [Google Scholar]
- 56.Voytek B, Knight RT (2015): Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry 77: 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, et al. (2000): Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol 111: 837–849. [DOI] [PubMed] [Google Scholar]
- 58.Joshi YB, Breitenstein B, Tarasenko M, Thomas ML, Chang W-L, Sprock J, et al. (2018): Mismatch negativity impairment is associated with deficits in identifying real-world environmental sounds in schizophrenia. Schizophr Res 191: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.