Abstract
Objectives
Most treatment outcome studies for people with chronic low back pain (CLBP) have based analyses on and reported only the mean levels of these factors. However, high levels of pain, mood, function, and sleep volatility may represent unique factors contributing to diminished quality of life. Our goal was to determine whether bright light treatment affected both mean levels of pain, mood, function, and sleep and reduced volatility in these outcomes.
Methods
US military veterans with CLBP (N = 22) underwent an open trial with a seven-day baseline, followed by 13 days of a one-hour morning bright light treatment self-administered at their home and a 30-day follow-up. Participants completed daily diary measures at 12 Pm and 6 Pm every day during the three study epochs.
Results
Using location scale modeling, results suggested that, in addition to being associated with changes in mean levels of pain intensity, pain interference, negative affect, and sleep quality, bright light treatment was also related to reductions in the volatility of pain intensity and negative affect, reductions that were largely maintained during follow-up.
Conclusions
Changes in mean levels and volatility were independent factors, suggesting that bright light treatment was related to participants experiencing fewer “pain flares.” These findings underscore the potential importance of volatility as a future treatment target.
Keywords: Bright Light, Chronic Low Back Pain, Function, Mood, Volatility
Introduction
Most studies of pain intensity, mood, function, and sleep among people with chronic low back pain (CLBP) have based analyses on and reported only the mean levels of these factors [1]. This is almost exclusively the case for treatment outcome studies in which pre- to post-treatment changes in mean levels of pain, mood, function, and sleep are reported. Indexes that may reflect pain flares or frequent fluctuations in pain, mood, function, and sleep have typically not been analyzed, with only occasional exceptions [2,3]. We echo the arguments of others who claim that high levels of pain volatility may exacerbate the unpredictability and uncertainty of chronic pain and contribute to diminished quality of life [4]. If so, then it is critical to study the effects of chronic pain interventions on the volatility of pain, mood, function, and sleep, in addition to effects on mean levels.
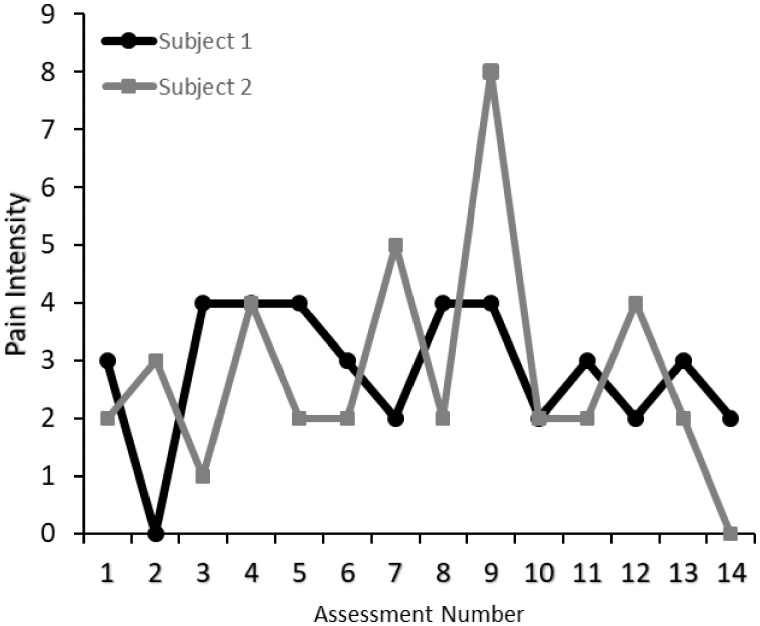
Electronic daily diary methods employing repeated assessments over the course of hours or days can be used to capture measures of central tendency (e.g., mean scores for outcomes collapsed over observations) and indexes of volatility (e.g., variance of outcomes across many observations [5]). The implementation of intensive longitudinal data collection has contributed to increased research on within-subject variation in behavioral processes including pain and pain-related impairments [3,6]. Figure 1 illustrates the phenomenon of pain volatility with the raw baseline data from two participants in the study described below. They have similar mean levels of pain intensity (2.8 vs 2.5) but markedly different standard deviations (1.97 vs 0.82). Thus, Subject 1’s scores range from 0 to 8, whereas Subject 2’s scores range from 0 to 4. Moreover, as we have reported [6], pain intensity may be related not only to mean levels of negative emotions, but uniquely to variability in negative emotions. As these results and those of others suggest [3], volatility may describe an independent factor affecting the well-being of people with chronic pain.
Figure 1.
Illustration of individual subject means and within-subject variation in pain intensity. This figure depicts raw baseline data from two participants who have similar mean levels of pain intensity. Both subjects have similar mean levels of pain intensity (Subject 1 mean = 2.86, Subject 2 mean = 2.50), but Subject 2’s standard deviation (1.97) is nearly twice as large as Subject 1’s standard deviation (0.82).
Results of meta-analyses indicate that exposure to bright light in the morning can improve sleep [7] and mood [8]. In our program of research, which examines the possible beneficial effects of bright light for people with chronic pain, we extrapolate from the observation that chronic pain is a multidimensional phenomenon, interrelated with many factors, including negative mood and poor sleep. We recently reported results from a small sample of women with fibromyalgia who underwent bright light treatment. The women reported clinically meaningful improvements in pain and function in response to morning bright light [9]. The present study is based on data collected during a home-based morning bright light treatment for US military veterans with CLBP [10]. The purpose of the original study was to examine the feasibility, acceptability, and effects of a bright light treatment on pain, mood, sleep, and circadian timing in US military veterans with chronic low back pain. To our knowledge, bright light treatment had not theretofore been tested as a potential treatment for chronic low back pain. Participants (N = 37) underwent an open trial with a seven-day baseline, followed by 13 days of a one-hour morning bright light treatment self-administered at their home and a 30-day follow-up. Using standardized questionnaires at approximately weekly intervals, we found that pain intensity and pain behavior decreased significantly, and physical function and sleep quality improved significantly [10].
In the present study, we analyzed electronic daily diary data that were not reported in the original article. Participants completed the diary measures at 12 Pm and 6 Pm every day during the seven-day baseline period, 13-day treatment period, and 30-day follow-up. Electronic daily diary items assessed pain intensity, pain interference, negative affect, and sleep quality. Put simply, frequently assessed measures of pain, mood, function, and sleep—as in a daily diary format—are critical for revealing patterns of volatility and change over time. We expected that changes in mean levels of the diary factors would reveal improvement during treatment, consistent with changes found with the questionnaires reported previously [10]. Our focus, however, was less on revealing changes wrought by bright light treatment per se, and more on demonstrating the existence of substantial volatility and illustrating whether volatility could be affected by a complementary/integrative treatment. Thus, we also examined whether volatility in outcomes would decrease from baseline to treatment and whether these gains would be maintained into follow-up.
Methods
Design Overview
This was a single-arm trial, in which all subjects received morning bright light treatment. The study consisted of a seven-day baseline during which subjects slept at home on their usual sleep schedule (ad lib). This baseline was followed by a daily morning bright light treatment. The morning light treatment was for one hour per day for a total of 13 days and started each morning at the subject’s average wake time (derived from the baseline week of wrist actigraphy) or up to one hour earlier to accommodate morning social responsibilities (e.g., work, child care) [11].
Participants
Thirty-seven US military veterans who reported CLBP were enrolled in the study. Participants were required to provide proof of veteran status (e.g., DD Form 214, a certificate of release or discharge from active military duty). The presence of significant CLBP was determined from a self-report of CLBP for at least the previous six months, with an average pain intensity of at least 4/10 (1 = no pain to 10 = worst pain possible). Participants also signed an authorization form to obtain their medical records regarding their back pain, which was used to verify a preexisting complaint of chronic low back pain to a medical provider.
Exclusion criteria were: a) other significant chronic disease (apart from medication-controlled diabetes and hypertension); b) other condition associated with chronic pain (including chronic headaches, fibromyalgia, complex regional pain syndrome, rheumatoid arthritis); c) past or present psychosis or bipolar disorder; d) present alcohol or substance abuse problems; e) suicidal ideation; f) high risk for obstructive sleep apnea or restless leg syndrome or seasonal affective disorder [12–14]; g) taking daily nonsteroidal anti-inflammatory medications (NSAIDs) and/or beta-blockers [15,16]; h) high risk for seasonal affective disorder [14]. Accepted participants reported no retinal pathology or eye surgery, and none were taking photosensitizing medications. No participants were color blind, as determined from the Ishihara test, and none had any prior experience with bright light treatment. Prescribed or over-the-counter sleep aids (apart from exogenous melatonin) and antidepressants were permitted, provided medication use remained stable 30 days before and during the study. We did exclude based on seasonal affective disorder, recent jet travel, and shift work. No participants had worked any night shifts or traveled outside the Central Time Zone in the month preceding the study. All participants had normal baseline wrist actigraphy not suggestive of any other circadian rhythm sleep disorder (advanced sleep phase, delayed sleep phase disorder, non-24-hour disorder, and irregular sleep wake disorder). The study was approved by the Rush University Medical Center Institutional Review Board, and all participants gave written informed consent before participation. This clinical trial was registered as NCT02373189 on clinicaltrials.gov.
Five participants failed drug and alcohol screening on the first day of the study and did not participate further. An additional seven participants dropped out before the start of the light treatment, due to a variety of reasons including jail time, job offers, and family crises. Therefore, a total of 25 participants started the bright light treatment. One veteran dropped out after six days of light treatment in order to go on a vacation, and the electronic diary failed for two participants. Thus, electronic diary data from 22 participants were analyzed. See Table 1 for demographic information.
Table 1.
Demographic characteristics
| Characteristic | Total Sample (N = 22) |
|---|---|
| Sex, No. (%) | |
| Female | 5 (23) |
| Male | 17 (77) |
| Age, y | |
| Mean (SD) | 48.4 (13.9) |
| Race, No. (%) | |
| African American | 11 (50) |
| White | 10 (45.5) |
| Other | 1 (4.5) |
| Partner status, No. (%) | |
| Single | 8 (36) |
| Domestic partner | 14 (64) |
| Educational status, No. (%) | |
| Postgraduate degree | 1 (5) |
| College graduate | 11 (50) |
| Some college | 10 (45) |
| Body mass index, kg/m² | |
| Mean (SD) | 29.9 (4.6) |
Electronic Diary
The personal digital assistant (PDA) program signaled participants to complete two assessments each day at 12:00 Pm and at 6:00 Pm. These times were chosen to ensure the alarms would not disturb the participants’ sleep. Frequent assessments helped minimize retrospective bias in ratings [3]. Daily diary data obtained in this manner also appear to suffer little from reactivity effects that are sometimes caused by monitoring [17,18]. Variability in ratings within the day is also captured well by this method [18]. Previous studies support the reliability, validity, and compliance with electronic diary strategies when used to assess pain, affect, and behavior [17–20]. Electronic diaries with time-stamped entries also allowed us to accurately assess when ratings were made, something that cannot be done with paper diary methods [18].
Participants completed electronic diary measures throughout baseline, treatment, and follow-up for a total of 49 consecutive days. We used the Experience Sampling Program (ESP) [21] on handheld Palm Zire 22 PDAs running the Palm OS platform. The PDA program blocked participants from altering the items or alarm times.
The 22 participants were prompted twice daily across 49 days, resulting in 2,156 possible diary prompts. Data were completed for 76.6–82.1% of the 2,156 total prompts across the various items in the diary. This amount of missing records was in the range typically observed in other electronic diary studies involving people with chronic pain [22].
Measures
Pain-Related Variables
At each assessment, participants rated the questions “How intense was your pain?” and “To what degree did your pain interfere with you being physically active?” during the past three hours. These responses were rated on nine-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely).
State Negative Affect
At each assessment, participants rated the extent to which they felt on edge, tense, sad, discouraged, irritated, and angry during the past three hours. These items were summed and averaged to create a composite State Negative Affect rating. Responses were made on the same nine-point scales described above.
Sleep
At the 12 Pm assessment, participants were asked, “Last night, your sleep quality was…?” Responses were made using a five-point scale with anchors 1 (very poor), 2 (poor), 3 (fair), 4 (good), and 5 (very good).
Bright Light Treatment at Home
After the seven-day baseline, research staff visited participants in their homes to set up the light boxes. Two broad-spectrum white light boxes (33×18×55 cm, EnergyLight HF3318/60, Philips, Inc. Andover, MA, USA) were set up to the left and right at a distance that allowed the subject to view a TV or computer in front of them. Each participant’s comfort was first maximized using comfortable chairs and/or cushions, as appropriate, and then the light boxes were positioned to maximize light intensity (>3,000 lux; Extech EA33 light meter, Nashua, NH, USA). A 60-cm string was taped to the base of each light box to remind the participants how close they needed to sit near the light boxes, and painter’s tape was placed around the base of each light box to show where the boxes should remain. The morning light treatment was for one hour per day for a total of 13 days and started each morning at the participant’s average wake time or up to one hour earlier to accommodate morning social responsibilities (e.g., work, child care [11]). A photosensor (Actiwatch Spectrum, Philips, Inc.) was taped facing inwards to the outside of each light box to confirm adherence. An alarm clock was set to the start of the bright light treatment and was placed near the light boxes (subjects also set their home alarm clock). Participants were given a list of written reminders, including a) not to permit anyone to touch the light boxes, b) only to turn on the light boxes during the scheduled time, and c) to turn on all ambient lighting during light treatment time.
Research staff phoned each participant daily, shortly after the start of the light treatment to confirm correct use of the light boxes, ensure that the photosensor on their wrist monitor was uncovered, and assess potential side effects. Research staff also visited participants at home at midtreatment (after six days of light treatment) to confirm that the light box setup was unchanged and at post-treatment (after 13 days of light treatment) to collect the light boxes.
Statistical Analysis
Descriptive statistics were computed with SPSS, version 22. We computed person-level means and standard deviations for each of the study variables during baseline and examined their bivariate correlations. However, this approach masks the underlying structure of longitudinal data, including nesting and autocorrelation, and does not account for the possibility of compounding correlated measurement error. Therefore, the primary study hypotheses were tested using the Mixregls Package in R. The Mixregls package was used to estimate location scale models in which mean levels and within-subject variation—our index of volatility—were regressed across the three study epochs (i.e., baseline, bright light treatment, and follow-up). In brief, location scale models can model several characteristics of intensive longitudinal data including mean levels and variation in a data series. These variables can then be regressed on predictors. For this study, the predictors of interest were study epochs (baseline, bright light treatment, and follow-up). Epochs were entered as dummy-coded variables in order to compare treatment and follow-up epochs with baseline. Random location can be computed to determine whether the mean level of a data series is associated with the variance in the same series. For instance, participants with higher levels of pain intensity might also experience greater variation. Random scale estimates determine whether there is significant variation between subjects with regard to their within-subject variation after adjustment for covariates. In this analysis, the covariates were the three study epochs.
Mixregls computes the location scale models in three stages. In the first model, mean levels of the dependent variable are regressed on predictors. In this stage of the analysis, significant effects would indicate that mean levels of outcomes differed across treatment epochs. In the second model, within-subject variation is regressed on predictors. In this second stage of analysis, significant effects would indicate that volatility in outcomes differed across treatment epochs. In the third and final model, the random location and random scale effects are added. In this stage of analysis, significant random location effects would indicate whether mean levels and volatility would be significantly correlated, and significant random scale effects would indicate whether significant volatility or within-subject variation remained to be accounted for. Likelihood ratio tests can be used to compare fit across the three models.
Results
Means and standard deviations for the study variables are presented in Table 2. At baseline, the participants reported pain and pain-related impairments that were of mild to moderate intensity. In general, there were patterns of decreased pain and pain-related impairment over the course of the study. Correlations between subject-level means and standard deviations aggregated across baseline are presented in Table 3. Higher average pain intensity, pain interference, and negative affect were accompanied by greater volatility in these outcomes. Higher average sleep quality was not associated with volatility.
Table 2.
Means and standard deviations collapsed over observations in each epoch
| Baseline |
Treatment |
Follow-up |
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Pain intensity | 2.50 | 1.28 | 1.94 | 1.10 | 1.98 | 1.63 |
| Pain interference | 1.99 | 1.20 | 1.75 | 1.18 | 1.65 | 1.49 |
| Negative affect | 8.30 | 7.93 | 8.20 | 7.60 | 6.51 | 6.75 |
| Sleep quality | 3.03 | 0.55 | 3.05 | 0.69 | 3.28 | 0.41 |
Table 3.
Association of person-level means and standard deviations aggregated across baseline
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 Pain intensity mean | – | 0.79** | 0.57** | –0.30 | 0.57 ** | 0.69** | 0.25 | –0.04 | |
| 2 Pain interference mean | – | 0.32 | –0.15 | 0.52* | 0.68 ** | 0.07 | –0.07 | ||
| 3 Negative affect mean | – | –0.43* | 0.00 | 0.43* | 0.49 * | –0.02 | |||
| 4 Sleep quality mean | – | –0.19 | –0.47* | –0.45* | –0.19 | ||||
| 5 Pain intensity SD | – | 0.64** | 0.45* | 0.34 | |||||
| 6 Pain interference SD | – | 0.53* | 0.28 | ||||||
| 7 Negative affect SD | – | 0.07 | |||||||
| 8 Sleep quality SD | – |
Correlations of a variable’s person-level mean and standard deviation are bolded for ease of interpretation.
P < 0.05; **P < .01.
Location Scale Models
Pain Intensity
Fit statistics indicated that the third model that included epochs as predictors of both means and within-subject variation, and also included random location and scale effects, provided the best fit to the data (Table 4). Mean levels of pain intensity were significantly lower during treatment (B = –0.20, SE = 0.04, P < 0.001) and follow-up (B = –0.21 SE = 0.04, P < 0.001) than during baseline. In addition, within-subject variation in pain intensity was significantly lower during treatment (B = –0.80, SE = 0.13, P < 0.001, exp(B) = 0.45) and follow-up (B = –0.83, SE = 0.13, P < 0.001, exp(B) = 0.44) compared with baseline. The random location effect indicated that higher mean levels of pain intensity were accompanied by greater within-subject variation in pain intensity. That is, both epochs and mean levels of pain intensity were significant predictors of within-subject variability in pain intensity. On average, patients with higher levels of pain tended to have greater variation in pain variability. Even accounting for this association, both average levels of pain and within-subject variation of pain were lower during treatment and follow-up compared with baseline.
Table 4.
Location scale model coefficients for pain intensity
| Pain Intensity | ||||
|---|---|---|---|---|
| Estimate | SE | Z | P | |
| Mean intercept | 2.11 | 0.24 | 8.89 | <0.001 |
| Mean treatment | –0.20 | 0.04 | –4.52 | <0.001 |
| Mean follow-up | –0.21 | 0.04 | –5.08 | <0.001 |
| Between-subject intercept | 0.12 | 0.32 | 0.39 | 0.370 |
| Within-subject intercept | 0.93 | 0.30 | 3.08 | 0.003 |
| Within-subject treatment | –0.80 | 0.13 | –5.93 | <0.001 |
| Within-subject follow-up | –0.83 | 0.13 | –6.33 | <0.001 |
| Random location | 0.93 | 0.24 | 3.80 | <0.001 |
| Random scale | 0.90 | 0.14 | 6.28 | <0.001 |
Group mean levels of pain are estimated across conditions (mean intercept, mean treatment, mean follow-up). An intercept for between-subject variation is estimated. Within-subject variation or volatility is estimated across conditions (within-subject intercept, within-subject treatment, within-subject follow-up).
Estimate = unstandardized estimate; SE = asymmetric standard error; Z = Z-test statistic.
Pain Interference
Fit statistics indicated that the first model that included only epochs for the mean level of pain interference provided the best fit. Results of this model are reported in Table 5. Within-subject variation did not differ significantly across baseline, treatment, or follow-up. Mean levels of pain interference were significantly lower during treatment (B = –0.24, SE = 0.12, P = 0.047) and follow-up compared with baseline (B = –0.34, SE = 0.10, P = 0.002).
Table 5.
Location scale model coefficients for pain interference
| Pain Interference | ||||
|---|---|---|---|---|
| Estimate | SE | Z | P | |
| Mean intercept | 1.99 | 0.25 | 7.88 | <0.001 |
| Mean treatment | –0.24 | 0.12 | –2.07 | 0.047 |
| Mean follow-up | –0.34 | 0.10 | –3.23 | 0.002 |
| Between-subject intercept | 0.20 | 0.31 | 0.63 | 0.328 |
| Within-subject intercept | 0.76 | 0.04 | 21.63 | <0.001 |
Estimate = unstandardized estimate; SE = asymmetric standard error; Z = Z-test statistic.
Negative Affect
Fit statistics indicated that the third model that included epochs as predictors for both means and within-subject variation, along with random location and scale effects, provided the best fit to the data. Results of this model are reported in Table 6. Mean levels of negative affect were significantly lower during treatment (B = –1.41, SE = 0.21, P < 0.001) and follow-up (B = –1.68, SE = 0.20, P < 0.001) compared with baseline. In addition, within-subject variation in negative affect was significantly lower during treatment (B = –0.76, SE = 0.12, P < 0.001, exp(B) = 0.47) and follow-up (B = –1.33, SE = 0.15, P < 0.001, exp(B) = 0.26) compared with baseline. The random location effect indicated that higher mean levels of negative affect were accompanied by greater within-subject variation in negative affect. That is, both epochs and mean levels of negative affect were significant predictors of within-subject variability in negative affect. On average, subjects with higher levels of negative affect tended to have greater variation in negative affect. Even accounting for this association, both mean levels of negative affect and within-subject variation of negative affect were lower during treatment and follow-up compared with baseline.
Table 6.
Location scale model coefficients for negative affect
| Negative Affect | ||||
|---|---|---|---|---|
| Estimate | SE | Z | P | |
| Mean intercept | 8.24 | 1.21 | 6.80 | <0.001 |
| Mean treatment | –1.41 | 0.21 | –6.63 | <0.001 |
| Mean follow-up | –1.68 | 0.20 | –8.45 | <0.001 |
| Between-subject intercept | 3.52 | 0.25 | 14.26 | <0.001 |
| Within-subject intercept | 3.66 | 0.35 | 10.43 | <0.001 |
| Within-subject treatment | –0.76 | 0.12 | –6.12 | <0.001 |
| Within-subject follow-up | –1.33 | 0.12 | –10.95 | <0.001 |
| Random location | 1.33 | 0.15 | 9.14 | <0.001 |
| Random scale | 1.01 | 0.15 | 6.53 | <0.001 |
Estimate = unstandardized estimate; SE = asymmetric standard error; Z = Z-test statistic.
Sleep Quality
Fit statistics indicated that the third model that included covariates for both means and within-subject variation, along with random location and scale effects, provided the best fit to the sleep quality data. Results of this model are reported in Table 7. Mean levels of sleep quality during treatment were not significantly different from baseline. Sleep quality was significantly higher during follow-up compared with baseline (B = 0.26, SE = 0.07, P < 0.001). Levels of within-subject variability in sleep quality did not differ across conditions. The random location effect indicated that regardless of treatment epoch, there was a trend for higher mean levels of sleep quality to be associated with less within-subject variation in sleep quality (B = –0.2, B = 0.10, P = 0.052). The random scale effect was also significant, indicating that significant within-subject variation in sleep quality existed. That is, regardless of treatment epoch, participants experienced significant levels of sleep volatility.
Table 7.
Location scale model coefficients for sleep
| Sleep Quality | Estimate | SE | Z | P |
|---|---|---|---|---|
| Mean intercept | 3.03 | 0.11 | 28.62 | <0.001 |
| Mean treatment | 0.08 | 0.08 | 1.01 | 0.240 |
| Mean follow-up | 0.26 | 0.07 | 3.73 | <0.001 |
| Between-subject intercept | –1.81 | 0.33 | –5.46 | <0.001 |
| WS intercept | –0.67 | 0.16 | –4.27 | <0.001 |
| WS treatment | 0.14 | 0.17 | 0.84 | 0.280 |
| WS follow-up | 0.12 | 0.15 | 0.84 | 0.280 |
| Random location | –0.21 | 0.10 | –2.02 | 0.052 |
| Random scale | 0.38 | 0.08 | 4.58 | <0.001 |
Estimate = unstandardized estimate; SE = asymmetric standard error; Z = Z-test statistic.
Discussion
Because most studies of treatments for CLBP have focused on changes in mean levels of pain intensity, mood, function, and sleep, a critical aspect of chronic pain may have been ignored, namely day-to-day variability in levels of these outcomes. Our analysis of bivariate associations between pain-related impairment and volatility revealed that the intensity of pain and pain-related impairment is strongly associated with greater volatility. As others have argued, these high levels of pain volatility may worsen the unpredictability and uncertainty of chronic pain and contribute to diminished quality of life [4]. Thus, reducing volatility through chronic pain interventions may be as important as improving mean levels of pain, mood, function, and sleep. Widely used complementary/integrative interventions (e.g., CBT) may indeed reduce volatility in pain, mood, function, and sleep; the problem is that we do know whether this is in fact the case. Using daily diary data collected during an open trial of bright light therapy to treat CLBP among US military veterans, we examined whether this complementary/integrative intervention may have an impact on within-subject variability in pain, mood, function, and sleep. Results suggested that, in addition to being associated with changes in mean levels of pain intensity, pain interference, negative affect, and sleep quality, bright light treatment was also related to reductions in the volatility of pain intensity and negative affect in particular.
It is critical to interpret these results in the context of an open trial of bright light treatment. Although mean levels and volatility of outcomes changed from baseline to treatment, without comparison with effects of, for instance, a placebo intervention, we cannot conclude that bright light treatment per se exerted any effects. Instead, we focus here on the potential importance of reducing fluctuations in pain intensity and negative affect via some kind of complementary/integrative intervention.
Consistent with questionnaire results reported previously [10], participants reported via daily diaries significant baseline-to-treatment decreases in mean levels of pain intensity. These gains were maintained during the follow-up epoch. In addition, findings indicate that within-subject variability in pain intensity also decreased significantly during bright light treatment, and these decreases were also maintained during follow-up. Thus, participants reported not only that their levels of pain intensity were lower on average during and after treatment, but also that their day-to-day levels of pain intensity had become less volatile. Put another way, the intervention may have been associated with participants experiencing fewer “pain flares.” Recall that mean levels of pain intensity, within-subject variation in pain intensity, and their association were estimated simultaneously in the model. Assessment of bivariate correlations and review of random location effects revealed that mean levels and within-subject variability in pain intensity were related significantly, such that participants with higher mean levels of pain had higher variability in pain. It is not clear, therefore, whether the bright light treatment reduced mean levels of pain, which in turn affected variability, or vice versa. What is clear is that the intervention was associated with independent changes in mean levels and volatility, underscoring the potential importance of the latter as a future treatment target.
Findings for negative affect closely parallel those for pain intensity. In short, participants reported not only that their levels of negative affect were lower on average during and after treatment, but also that their day-to-day levels of negative affect had become less volatile. The intervention may have been associated with participants experiencing fewer “mood swings.” Similar to pain intensity, mean levels and within-subject variability in negative affect were related significantly, such that participants with higher mean levels of negative affect had higher variability in negative affect. Again, it is not clear whether the bright light treatment reduced mean levels of negative affect, which in turn affected variability, or vice versa. Still, results hint at how a complementary/alternative intervention can affect potentially problematic fluctuations in two important aspects of the adjustment to chronic pain.
Although mean levels of pain interference changed over the course of treatment and follow-up, contrary to expectations, within-subject variability in this factor did not. People with CLBP may simply not be faced with day-to-day vacillations in how much their pain impedes activity. Therefore, a complementary/alternative intervention would face a floor effect in affecting volatility and be less capable of reducing something already rather low. On another level, findings could signal that volatility in pain interference is relatively independent from volatility in pain intensity and negative affect, whereas fluctuations in the latter two factors may accompany one another.
Similarly, mean levels of sleep quality changed over the course of treatment and follow-up, but within-subject variability in this factor did not. Results did indicate that participants experienced significant fluctuations in sleep quality across the baseline, treatment, or follow-up epochs. So, on one level, the findings imply that, on average, people with CLBP experience wide day-to-day shifts in the quality of their sleep. Unpredictability and uncertainty about how well someone will sleep on a given night could detrimentally affect quality of life, as well as the actual frequent day-to-day shifts from good to not-so-good sleep. On another level, results hint that bright light therapy may not adequately target and impact this aspect of sleep quality, although with this small sample, it is too early to draw firm conclusions.
The frequent assessments and dense longitudinal data collection we used in this study may have revealed a potentially critical and heretofore largely hidden aspect of CLBP adjustment: day-to-day variability in pain, negative affect, and sleep quality. Following Schneider and colleagues [1] and our previous findings regarding variability [6], day-to-day and even hour-by-hour variability may need to be assessed to gain a full understanding of a patient’s experience of and adjustment to chronic pain. Beyond assessment, and as others have argued [1], these phenomena may need to be deliberately addressed in CLBP treatment to enhance the well-being of patients and overall treatment potency. Before such efforts, research will need to document the importance and uniqueness of the effects of volatility on patient quality of life. Our findings offer a promising glimpse into the potential effectiveness of complementary/integrative interventions in reducing pain flares and mood swings among people with CLBP. Clearly, more research is needed to uncover whether the wide array of existing complementary/integrative interventions can reduce volatility, and by what therapeutic mechanisms. To illustrate, in the present study, bright light treatment favorably impacted volatility in two of four outcome domains. Bright light treatment may not include the therapeutic mechanisms that would drive improvements in volatility in, say, sleep quality. Another complementary/integrative intervention may include relaxation mechanisms and techniques that may reduce sleep volatility. Another possibility is to investigate whether components of treatments that focus on dysregulation of emotions, such as dialectical behavior therapy [23], may aid patients in reducing volatility in negative affect.
Caveats must be issued. First, data were collected in an open trial. Without comparisons to some kind of control group, we cannot infer that changes in volatility were wrought by bright light treatment. Outcome changes during treatment may have been due to patient expectations of improvement, or contact with study staff, or simply due to patients self-monitoring their pain, mood, function, and sleep over the course of the study. A randomized controlled trial—particularly using a group receiving a placebo light treatment—is needed to increase confidence that exposure to morning bright light as such exerted effects on mean levels and volatility of outcomes. Second, the parent study was a treatment development project, and thus the sample was small. Confidence in results is buoyed by the large number of diary observations made over the course of 49 days, but having such a small sample increases the possibility that the results are unreliable. Third, the project was funded to study US military veterans, and high levels of acute and chronic pain may deter some individuals from enrolling or fully participating in the study protocol. These factors confer the risk that the results may not be generalizable to all adults. Fourth, we relied exclusively on self-reported sleep quality. To fully examine volatility in sleep parameters and its impact on patient quality of life, objective measures—such as those offered by actigraphy—will need to be collected.
In sum, we focused on variability in pain, mood, function, and sleep because this veiled aspect of chronic pain could contribute to decreased quality of life. First, our results indicate that significant day-to-day fluctuations in pain, mood, and sleep may indeed characterize the experience of people with CLBP. Second, we found that, in addition to changes in mean levels of pain-related factors, changes in volatility may be produced during a complementary/integrative intervention. Specifically, day-to-day fluctuations in pain and negative affect may be reduced through such an intervention. More work is needed to study both the efficacy of bright light therapy and the therapeutic mechanisms by which bright light may reduce volatility. In addition, the impact on volatility of psychosocial treatments with demonstrated efficacy in improving mean levels of pain-related factors must also be considered.
Acknowledgments
We thank Morgan Corich, Joshua Dein, Aahad Kahn, Catherine Keefner, Mary Kennedy, Fumitaka Kikyo, Athanasios Kondilis, Othon Nunez-Montelongo, Daria Orlowska, Philip Sanchez, Monica Thomas, Marie Vallido, and Amanda Vatinno for their assistance with data collection. We thank Mark Aloia, PhD, at Philips, Inc., who donated the light boxes for this pilot study.
Funding sources: Research reported in this manuscript was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number R34AT008347.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: Dr. Burgess is a consultant for Natrol, LLC.
References
- 1. Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE.. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: Associations with psychological variables. Pain 2012;153(4):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S.. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain 2007;131(1):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rost S, Van Ryckeghem DML, Koval P, Sütterlin S, Vögele C, Crombez G.. Affective instability in patients with chronic pain: A diary approach. Pain 2016;157(8):1783–90. [DOI] [PubMed] [Google Scholar]
- 4. Rahman QA, Janmohamed T, Pirbaglou M, et al. Defining and predicting pain volatility in users of the Manage My Pain app: Analysis using data mining and machine learning methods. J Med Internet Res 2018;20(11):e12001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone AA, Broderick JE, Schneider S, Schwartz JE.. Expanding options for developing outcome measures from momentary assessment data. Psychosom Med 2012;74(4):387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerhart JI, Burns JW, Bruehl S, et al. Variability in negative emotions among individuals with chronic low back pain: Relationships with pain and function. Pain 2018;159(2):342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Maanen A, Meijer AM, van der Heijden KB, Oort FJ.. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev 2016;29:52–62. [DOI] [PubMed] [Google Scholar]
- 8. Al-Karawi D, Jubair L.. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J Affect Disord 2016;198:64–71. [DOI] [PubMed] [Google Scholar]
- 9. Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J.. Morning versus evening bright light treatment at home to improve function and pain sensitivity for women with fibromyalgia: A pilot study. Pain Med 2017;18(1):116–23. [DOI] [PubMed] [Google Scholar]
- 10. Burgess HJ, Rizvydeen M, Kimura M, et al. An open trial of morning bright light treatment among US military veterans with chronic low back pain: A pilot study. Pain Med 2019;20(4):770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM.. Bright light treatment of winter depression: A placebo-controlled trial. Arch Gen Psychiatry 1998;55(10):883–9. [DOI] [PubMed] [Google Scholar]
- 12. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP.. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131(7):485–91. [DOI] [PubMed] [Google Scholar]
- 13. Hening WA, Allen RP.. Restless legs syndrome (RLS): The continuing development of diagnostic standards and severity measures. Sleep Med 2003;4(2):95–7. [DOI] [PubMed] [Google Scholar]
- 14. Terman M, Williams J.. Personal Inventory for Depression and SAD (PIDS). J Prac Psychiatry Behav Health 1998;5:301–3. [Google Scholar]
- 15. Murphy PJ, Myers BL, Badia P.. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav 1996;59(1):133–9. [DOI] [PubMed] [Google Scholar]
- 16. Cowen PJ, Bevan JS, Gosden B, Elliott SA.. Treatment with B-adrenoceptor blockers reduces plasma melatonin concentration. Br J Clin Pharmacol 1985;19(2):258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP.. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain 2001;91(3):277–85. [DOI] [PubMed] [Google Scholar]
- 18. Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PF, Bensing JM.. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain 2000;84(2):181–92. [DOI] [PubMed] [Google Scholar]
- 19. Cruise CE, Broderick J, Porter L, Kaell A, Stone AA.. Reactive effects of diary self-assessment in chronic pain patients. Pain 1996;67(2–3):253–8. [DOI] [PubMed] [Google Scholar]
- 20. Stone AA, Shiffman S.. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med 1994;16(3):199–202. [Google Scholar]
- 21. Barrett LF, Barrett DJ.. An introduction to computerized experience sampling in psychology. Soc Sci Comp Review 2001;19(2):175–85. [Google Scholar]
- 22. Shiffman S, Stone AA, Hufford MR.. Ecological momentary assessment. Annu Rev Clin Psychol 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 23. Linehan M. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York: Guilford Press; 1993. [Google Scholar]