Abstract
Purpose
To report a case of conjunctivitis due to Mycobacterium abscessus in the setting of keratoconjunctivitis sicca due to Sjögren's syndrome in the absence of other known risk factors such as surgery, trauma or immunosuppressive therapy.
Observations
A 61-year-old woman with a history of keratoconjunctivitis sicca secondary to Sjögren's syndrome presented with dryness, irritation, redness, and discharge in her left eye for 2 months. She was diagnosed with chronic conjunctivitis and began a regimen of moxifloxacin and an ocular ointment of dexamethasone, neomycin, and polymyxin with no improvement of symptoms. Concurrent cultures grew Mycobacterium abscessus and the patient began treatment with amikacin drops, oral clarithromycin and intravenous imipenem, followed by amikacin drops, oral clarithromycin, and oral clofazimine, but her course was complicated by a perforated corneal ulcer that required a corneal patch graft. The patient eventually recovered despite persistent colonization.
Conclusions/importance
We present a case of Mycobacterium abscessus conjunctivitis in a patient with keratoconjunctivitis sicca secondary to Sjögren's syndrome without previous history of surgery, trauma, or other known risk factors. Because of low suspicion and clinician awareness, ocular nontuberculous mycobacteria (NTM) infection may have a delayed diagnosis and treatment. Clinicians should consider NTM in the differential diagnosis in patients with autoimmune disease such as Sjögren's syndrome. Treatment may be lengthy, requiring topical and systemic antibiotics and is often complicated due to resistance.
Keywords: Mycobacterium, Conjunctivitis, Sjögren's syndrome
1. Introduction
Nontuberculous mycobacteria (NTM) are a set of over 30 atypical mycobacterial species commonly acquired from water and soil. Risk factors for ocular NTM infection include topical corticosteroid use, surgery, or an immunocompromised state.1,2 NTM as a source of ocular infection has been reported since 1965, and has been implicated in ocular surface infections (keratitis, scleritis, and conjunctivitis).1,3 NTM conjunctivitis in absence of typical risk factors has only occasionally been reported, including one case associated with soft contact lenses and contact with parrots.4
M. abscessus complex is a group of rapid-growing, multidrug-resistant NTM species.1 Due to a modified erm(41) gene, subspecies may have induced resistance to macrolides and have varying susceptibilities to other antimicrobials, though some studies report successful therapy with amikacin and clarithromycin for ocular infections3.5.
We report a case of M. abscessus conjunctivitis and keratitis in a patient with Sjögren's syndrome and no other additional risk factors. The condition was diagnosed by culture, and treatment was completed with a combination of topical and systemic antibiotics.
2. Case report
A 61-year-old woman presented with a 2-month history of left eye dryness, redness, irritation, and mucopurulent discharge for 2 months. She had an ocular history of myopia and also had keratoconjunctivitis sicca secondary to Sjögren's syndrome. Her primary ophthalmologist had started a regimen of topical moxifloxacin drops and combination neomycin, polymyxin B, dexamethasone ointment for presumed bacterial conjunctivitis. The patient had poor response with this regimen and the patient stopped antibiotics and started fluoromethalone drops three times per day. She was referred to our practice for further evaluation.
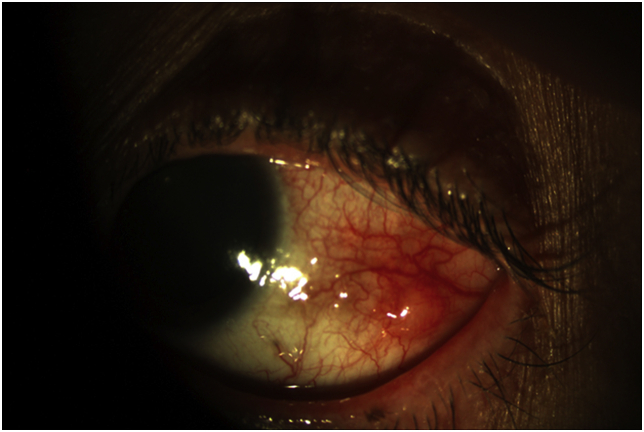
The patient's ophthalmic exam showed a best corrected visual acuity of 20/20 in the right eye and 20/50 + 2 in the left. The left eye showed 2–3+ conjunctival injection, with follicular reaction in the tarsal and bulbar conjunctiva and a 2 × 2mm nodule on the bulbar conjunctiva temporally (see Fig. 1). All other exam features were normal. Cultures of the left eye were obtained, and the patient was restarted on hourly topical moxifloxacin regimen and fluoromethalone was increased to four times daily.
Fig. 1.
Slit lamp photo of the left eye at presentation, demonstrating a nodular lesion on the temporal conjunctiva.
The patient did not improve after a week with this regimen, and 2 weeks after her initial presentation cultures returned positive for a rapidly growing mycobacterium. The patient was diagnosed with nontuberculous mycobacteria (NTM) conjunctivitis. She was given a regimen of ciprofloxacin ointment, and hourly ofloxacin drops (substituted for moxifloxacin due to cost). The patient was also given oral clarithromycin and ciprofloxacin but did not start the regimen immediately. Three weeks after initial presentation to our clinic, the patient returned and noted that her symptoms had worsened, with a new complaint of photophobia. Several small epithelial defects were noted on the left inferotemporal cornea. Speciation later showed M. abscessus susceptible to amikacin, imipenem, clofazimine, azithromycin, and clarithromycin and resistant to moxifloxacin and ciprofloxacin. At this point topical amikacin was started and the patient was referred to infectious disease specialists for guidance on antibiotic treatment.
The patient's symptoms continued to worsen and the patient was started on an 8 week course of intravenous imipenem 500 mg every 6 hours, oral clarithromycin, and topical amikacin, after consultation with an infectious disease specialist.
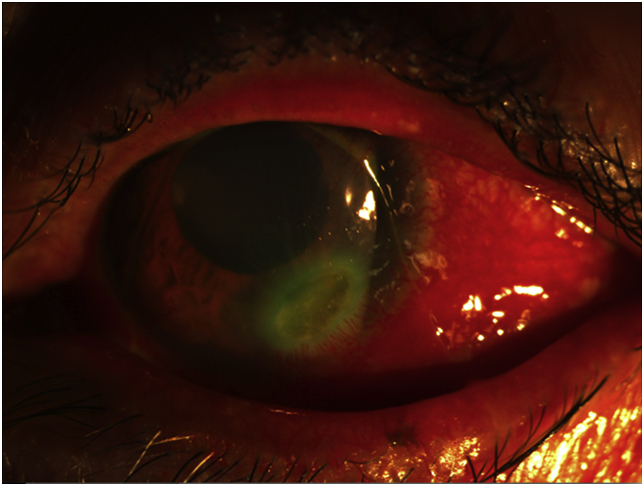
The patient returned with severe, constant throbbing pain to the left eye, and the epithelial defect developed significant thinning inferotemporally (Fig. 2) without associated infiltrate. Azithromycin eye drops were added and prednisolone eye drops were also started to control inflammation, due to concern that the ulcer represented a complication of Sjögren's syndrome, rather than an infectious cause. Several days later, after 3 weeks of topical amikacin and oral clarithromycin and after 2 weeks of imipenem therapy, the corneal ulcer perforated spontaneously. Cyanoacrylate glue was used to stabilize the globe, however the patient required a corneal patch graft 3 days later. Due to ongoing concern for Sjögren's related inflammation, she was placed on methotrexate after the graft was repaired. The patient was transitioned to oral clofazimine after 2 months and the imipenem was stopped. Oral clarithromycin was continued.
Fig. 2.
Slit lamp photo of the left eye demonstrating corneal ulcer inferiorly.
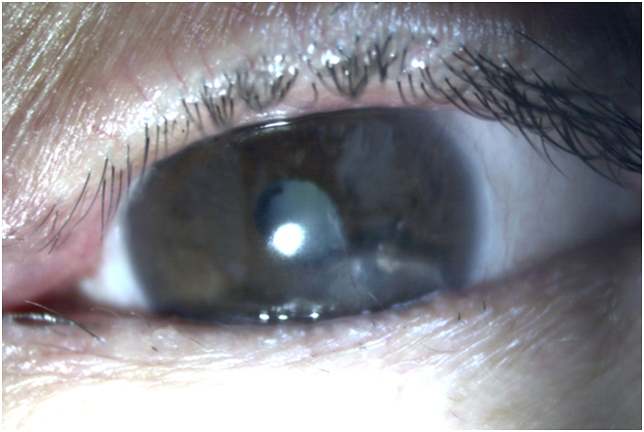
Ultimately, at 10.5 months after initial presentation after receiving over 4 months of systemic antibiotics with imipenem, clofazimine, and clarithromycin, and over 6 months of clarithromycin and amikacin drops, the patient had no signs of active ocular infection, but cultures continued to show colonization (Fig. 3). The patient's final visual acuity was 20/80 with correction and 20/50 with pinhole.
Fig. 3.
Slit lamp photo of the left eye after 10.5 months of treatment.
3. Discussion
Ocular NTM infections are difficult to identify, have an indolent course, and may be misdiagnosed, causing delays in treatment. Additionally, the infection may be refractory to multiple medical and surgical treatments. A recent review found that the majority of ocular NTM infections are keratitis (69% of cases), while conjunctivitis comprises only 0.7% of cases.6
Few other cases of NTM conjunctivitis have been reported, including the aforementioned case associated with soft contact lens use and contact with parrots,4 case of M. chelonae conjunctivitis following vitrectomy,7 a case of M. abscessus nodular conjunctivitis following cataract surgery,8 and a case of an AIDS patient who presented with NTM conjunctivitis with CMV coinfection in the setting of bacillary angiomatosis.2 NTM infection is usually environmentally derived, as these are ubiquitious organisms that are commonly found in water and soil. The known risk factors for acquiring NTM infection include corticosteroid use, surgery, or an immunocompromised state, with the main risk factor for NTM keratitis being trauma with penetration of the corneal epithelium.1
Our patient had none of the typically reported risk factors for NTM conjunctivitis such as ocular trauma, ocular surgery, contact lens use or antecedent topical steroid use. To our knowledge, this case represents the first known case of M. abscessus conjunctivitis without these typical risk factors. However, this patient may have been predisposed to infection due to keratoconjunctivitis sicca and possible relative immune suppression in the setting of Sjögren's syndrome. It is known that patients with keratoconjuctivitis sicca have decreased quantities of tear film proteins such as lactoferrin9 which aid in ocular surface immune function, leading to potential susceptibility to infection.
Patients with autoimmune diseases such as Sjögren's syndrome and rheumatoid arthritis may have increased risk for NTM infection. Temporal association between Sjögren's syndrome and NTM infection have been described. Chao et al. described a series of over 5000 newly diagnosed Sjögren's syndrome patients and noted a significant association of newly diagnosed Sjögren's syndrome in patients with a history of previous NTM infection.10 While NTM infection preceded diagnosis of Sjögren's syndrome in this study, the temporal association between these two conditions remains to be evaluated. Another study also by Chao et al. reported an increased risk of developing NTM infection in patients with Sjögren's syndrome who also had immunosuppressive therapy within the first year of diagnosis.11 In the present case, our patient did not report using immunosuppressive medications at the time of her diagnosis, but had methotrexate instituted shortly after her corneal ulcer and graft repair. The patient's history of keratoconjunctivitis sicca and Sjögren's syndrome may have been a risk factor in increasing her susceptibility for developing ocular infection with M. abscessus.
When our patient developed a corneal ulcer during her course, this was determined to be an immune mediated phenomenon due to Sjögren's syndrome rather than an infectious cause, because of the ulcer's lack of underlying infiltrate, and previous medical treatment with both topical and systemic antibiotics. Immune-mediated corneal melts have been reported in a few instances.12,13 While there have been no reported cases of NTM infection with Sjögren's syndrome alone as a risk factor, Van Der Beek et al. reported a case of M. chelonae keratitis in a patient with keratoconjunctivitis sicca and rheumatoid arthritis without other risk factors.14
Diagnosis of NTM conjunctivitis is confirmed by staining and culture. Acid-fast staining using fluorescein conjugated stains such as auramine-rhodamine can be helpful in making the diagnosis, as some Ziehl-Neelsen stains may not detect slender NTM bacilli.1 This stain should be considered in the differential diagnosis for patients who have conjunctivitis that does not respond with typical antibiotics.
Treatment for NTM conjunctivitis often requires surgical excision, as well as systemic antibiotics. Surgical excision can be employed on portions of infected conjunctiva, with repeat excision, as necessary.2,4,8 Resistance to antibiotics is common. M. chelonae and M. abscessus have a single copy of ribosomal RNA, making single point mutations a potential cause for drug resistance to ribosomally active drugs such as amikacin and clarithromycin.5 In the present case, the patient did have the erm(41) gene modification, which has been shown to be associated with inducible macrolide resistance in M. abscessus.5 Treatment of NTM infections can be lengthy, and systemic therapy with IV antibiotics may be necessary.
4. Conclusions
Diagnosis of non-tuberculous mycobacterium can be difficult due to lack of awareness, low clinical suspicion and the need for special cultures. Risk factors include trauma and surgery but NTM infection should be considered in patients with significant keratoconjuntivitis sicca especially in the setting of Sjögren's syndrome or other autoimmune conditions such as rheumatoid arthritis. Treatment with systemic antibiotics is often necessary and resistance to multiple antibiotics is not uncommon. Early involvement of infectious disease specialists may be valuable.
Patient consent
Verbal consent from the patient was obtained to publish the present work.
Funding
Both The Moran Eye Center at the University of Utah and The Department of Ophthalmology at University of California, Irvine, are recipients of an institutional Research to Prevent Blindness Unrestricted Grant.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None of the authors have conflicts of interest related to the present work.
Acknowledgements
None.
References
- 1.Moorthy R.S., Valluri S., Rao N.A. Nontuberculous mycobacterial ocular and adnexal infections. Surv Ophthalmol. 2012;57(3):202–235. doi: 10.1016/j.survophthal.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Edmonson B.C., Morris W.R., Osborn F.D. Bacillary angiomatosis with cytomegaloviral and mycobacterial infections of the palpebral conjunctiva in a patient with AIDS. Ophthalmic Plast Reconstr Surg. 2004:168–170. doi: 10.1097/01.iop.0000116379.78461.57. [DOI] [PubMed] [Google Scholar]
- 3.Lee M., Sheng W., Hung C., Yu C., Lee L., Hsueh P. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21(9):1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng C., Hsiao C., Hu F. Nontuberculous mycobacterial conjunctival granuloma detected by nested polymerase chain reaction. J Formos Med Assoc. 2014;113(10):760–761. doi: 10.1016/j.jfma.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Nash K.A., Zhang Y., Brown-elliott B.A., Wallace R.J. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J Antimicrob Chemother. 2005;55:170–177. doi: 10.1093/jac/dkh523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheir W.J., Sheheitli H., Abdul Fattah M., Hamam R.N. Nontuberculous mycobacterial ocular infections: a systematic review of the literature. BioMed Res Int. 2015;2015 doi: 10.1155/2015/164989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margo, Curtis E., Pavan P.R. Mycobacterium chelonae conjunctivitis and scleritis following vitrectomy. Arch Ophthalmol. 2000;118:1125–1128. doi: 10.1001/archopht.118.8.1125. [DOI] [PubMed] [Google Scholar]
- 8.Merani R., Mmed M., Franzco S.O. Postoperative Mycobacterium abscessus nodular conjunctivitis. Clin Exp Ophthalmol. 2008;36:371–373. doi: 10.1111/j.1442-9071.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 9.Jonssen P.T., Dijsrerveld OP Van. Origin and biosynthesis of human tear fluid proteins. Invest Ophthalmol Vis Sci. 1983;24(5):623–630. [PubMed] [Google Scholar]
- 10.Chao W., Lin C., Liao T., Chen Y. 2017. Association between a History of Mycobacterial Infection and the Risk of Newly Diagnosed Sjögren’s Syndrome : A Nationwide , Population-Based Case-Control Study; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao W., Lin C., Liao T., Chen Y., Hsu C., Chen J. The risk of nontuberculous mycobacterial infection in patients with Sjögren’s syndrome : a nationwide , population-based cohort study. BMC Infect Dis. 2017;17(1):1–8. doi: 10.1186/s12879-017-2930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfister Roswell, Murphy G. Corneal ulceration and perforation associated with Sjögren’s syndrome. Arch Ophthalmol. 1980;98(1):89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- 13.Gudas P.P., Altman B., Nicholson D.H., Green W.R. Corneal perforations in sjögren syndrome. Arch Ophthalmol. 1973;90(6):470–472. doi: 10.1001/archopht.1973.01000050470014. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Beek M.T., Bernards A.T., Lapid-Gortzak R. Mycobacterium chelonae keratitis in a patient with Sjögren’s syndrome. Eur J Ophthalmol. 2008;18(2):294–296. doi: 10.1177/112067210801800221. [DOI] [PubMed] [Google Scholar]