Abstract
Calorie restriction without malnutrition (CR) is considered as the most effective nongenetic nor pharmacological intervention that promotes healthy aging phenotypes and can extend lifespan in most model organisms. Lifelong CR leads to an increase of cytochrome b5 reductase-3 (CYB5R3) expression and activity. Overexpression of CYB5R3 confers some of the salutary effects of CR, although the mechanisms involved might be independent because key aspects of energy metabolism and lipid profiles of tissues go in opposite ways. It is thus important to study if some of the metabolic adaptations induced by CR are affected by CYB5R3 overexpression. CYB5R3 overexpression greatly preserved body and liver weight in mice under CR conditions. In liver, CR did not modify mitochondrial abundance, but lead to increased expression of mitofusin Mfn2 and TFAM, a transcription factor involved in mitochondrial biogenesis. These changes were prevented by CYB5R3 overexpression but resulted in a decreased expression of a different mitochondrial biogenesis-related transcription factor, Nrf1. In skeletal muscle, CR strongly increased mitochondrial mass, mitofusin Mfn1, and Nrf1. However, CYB5R3 mice on CR did not show increase in muscle mitochondrial mass, regardless of a clear increase in expression of TFAM and mitochondrial complexes in this tissue. Our results support that CYB5R3 overexpression significantly modifies the metabolic adaptations of mice to CR.
Keywords: Calorie restriction, Cytochrome b5 reductase, Liver, Mitochondria, Skeletal muscle
Introduction
Aging can be defined as the time-dependent progressive functional decline with decreased fertility and increased susceptibility of the organism to endogenous and external threats. Aging is the greater risk factor for all chronic diseases including metabolic, degenerative and neoplastic disorders (Harman 1956). The free radical theory of aging, despite some draw backs and criticisms (Liochev 2015), is still one of the most accepted theories enunciated to explain the causes of aging (Barja 2013; Barja 2014). This theory is based on the overproduction of several oxidant species—mainly of mitochondrial origin—with advancing age as the main causative factor of aging, which may be also concomitant with a decrease of antioxidant protective mechanisms (Harman 1956). Indeed, mitochondrial dysfunction has been increasingly identified as a causative factor in many aging-related diseases (Sure et al. 2018). Since the preservation of mitochondrial function is a viable strategy to counteract some of the deleterious manifestations of aging (Csiszar et al. 2019), understanding the alterations of mitochondrial metabolism with aging is of utmost importance (Sakamuri et al. 2018).
Several interventions have been reported to delay aging and to provide protection against aging-related diseases. Among them, calorie restriction without malnutrition (CR) has revealed as the most effective nongenetic intervention that preserves health and extends lifespan in many model organisms (Sohal and Weindruch 1996). There is a strong interest in elucidating how CR produces its antiaging effects, not only to get basic knowledge about the molecular mechanisms involved, but also because this research could give us novel clues on the identification of targets susceptible of intervention. As a general agreement, it has been established that optimization of mitochondrial function plays a key mechanistic role in the prolongevity action of CR (Lopez-Lluch et al. 2006). In accordance, our recent comparative study aimed to elucidate the role of sex, strain and energy intake on hallmarks of aging in mice has shown that the maintenance of mitochondrial function is indeed one of the major predictors of longevity extension in mice fed a CR diet age. Interestingly, the two other main predictors of longevity extension highlighted in our previous study were the maintenance of NAD+ levels and the preservation of an optimal amount of fat mass with age (Mitchell et al. 2016).
NADH-cytochrome b5 reductases (EC 1.6.2.2) constitute a family of flavoproteins encoded by 4 different genes in mammals, designed as CYB5R1–4, which catalyse one electron transfer from NADH (which is thus oxidized to NAD+) not only to cytochrome b5 but also to a variety of alternative acceptors including coenzyme Q and other redox cyclers (de Cabo et al. 2009). Recently, a great interest has been paid to CYB5R3 in the fields of metabolism and aging research (de Cabo et al. 2009; Diaz-Ruiz et al. 2018; Martin-Montalvo et al. 2016). Two CYB5R3 isoforms can be generated by alternative splicing. A soluble cytosolic isoform, known as methaemoglobin reductase, is expressed exclusively in the erythroid lineage, whereas a membrane-bound isoform expressed in many cell types is attached to the cytosolic side of the mitochondrial outer membrane, the endoplasmic reticulum and the plasma membrane and participates in many physiological processes including elongation and desaturation of fatty acids (Oshino et al. 1971), cholesterol biosynthesis (Reddy et al. 1977) and drug metabolism (Jansson and Schenkman 1973; Sacco and Trepanier 2010). A transplasma membrane redox system which relies on membrane-bound CYB5R3 and coenzyme Q protects cells against oxidants (Navas et al. 2007) and is upregulated by CR, thus potentiating resilience of cells against oxidative damage (De Cabo et al. 2004; Lopez-Lluch et al. 2005; Navas et al. 2007).
To gain new insights into the physiological role this enzyme plays in metabolism and aging, we generated transgenic Drosophila melanogaster flies overexpressing CYB5R and transgenic mice overexpressing CYB5R3 (Tg mice) and, interestingly, both experimental models were found to live longer than their wild-type (WT) counterparts (Martin-Montalvo et al. 2016). Tg mice exhibited increased insulin sensitivity and improved regulation of glucose homeostasis both when fed standard and high-fat diets. Conversely, mice with a β-cell-specific deletion of CYB5R3 had impaired insulin secretion, resulting in glucose intolerance and diet-induced hyperglycaemia. Moreover, respiratory response to glucose was blunted in CYB5R3-deficient cells, which also displayed impaired NAD+ homeostasis and extensive mitochondrial abnormalities (Fan et al. 2020). On the other hand, Tg mice overexpressing CYB5R3 showed less inflammation and decreased oxidative damage, and were protected against induced cancer, which resembled the healthy effect of CR. However, Tg mice were fatter but not heavier than WT controls when fed a standard diet ad libitum and preferentially used carbohydrate to meet their energy needs, which differs from other anti-aging interventions as CR or metformin supplementation, where increased longevity required the use of fat as a source of fuel (Guarente 2008; Martin-Montalvo et al. 2013). Thus, CYB5R3 overexpression could contribute to extend lifespan in mice by mechanisms that may be distinct from those described for CR. Further studies aimed at gain knowledge on mitochondrial modifications in tissues from mice overexpressing CYB5R3 are lacked. Furthermore, the existence of a putative crosstalk between CR and CYB5R3 overexpression has not been explored.
The aim of this research was to study the impact of CR on key markers related to mitochondrial function in Tg mice in comparison with their WT littermates. We extended our studies to both liver and skeletal muscle because these tissues are major contributors to whole animal energy expenditure, and they represent mitotic (liver) (Spindler and Dhahbi 2007) and postmitotic (skeletal muscle) tissue models (Ramsey et al. 2000). Our results support that metabolic adaptations of mice fed under CR can be altered by CYB5R3 overexpression.
Materials and methods
Animals and diets
Tg mice were generated as previously reported (Martin-Montalvo et al. 2016). Briefly, the rat CYB5R3 gene was cloned into the pRC/CMV-rDTD plasmid (Belcourt et al. 1998). The transgene insert was cleaved from the DNA cloning vector by digestion with SwaI and NruI restriction enzymes and the purified transgene (under the control of the human cytomegalovirus immediate-early promoter and the SV40 poly-adenylation sequences) was then microinjected into fertilized C57BL/6J eggs at the University of Michigan Transgenic Animal Model Core Facility (http://www.med.umich.edu/tamc/). Surviving eggs were transferred to pseudopregnant B6D2F1 female mice. Stable incorporation of the construct into the genome was validated as described in our previous report (Martin-Montalvo et al. 2016). Tg males were crossed with WT females of the C57BL/6J background obtained from Charles River (Barcelona, Spain) to establish a colony that was maintained under barrier conditions at the Service of Experimentation Animals (SAEX) of the University of Córdoba. Tg and WT mice were distinguished by PCR genotyping with DNA obtained from tail tissue using the primers CACCAAAATCAACGGGACTT (forward) and AGACCGGGGAGAGTACCACT (reverse) to reveal the presence of the transgene. As internal control, we used amplification of the IL2 gene with the primers CTAGGCCACAGAATTGAAAGATCT (forward) and GTAGGTGGAAATTCTAGCATCATCC (reverse).
Experimental groups were established with males of the two genotypes (WT and Tg in C57BL/6J background). The animals were maintained from weaning on 12-h light/dark cycle at 22 °C with a free access to water and a standard chow until they reached an age of 3 months. Then, they were switched to a purified AIN93M diet and separated into the two dietary groups: ad libitum (AL) and calorie restriction (CR) with a 40% reduction of the ad libitum intake. Mice were fed with experimental diets for 4 months and then sacrificed by cervical dislocation. Muscle from hind limb and liver were rapidly excised and frozen by immersion in liquid nitrogen in a buffered medium containing 10% DMSO as cryoprotectant and then stored at − 80 °C. Procedures with experimentation animals were approved by the bioethics committee of the University of Córdoba and authorized by the Consejería de Agricultura, Pesca y Desarrollo Rural, Junta de Andalucía (authorization code: 20/04/2016/053).
Preparation of tissue extracts
Muscle and liver tissues were trimmed and homogenized in radioimmunoprecipitation assay (RIPA) buffer, which contains 50 mM Tris-HCl pH 8, 150 mM NaCl, 0.5% deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mM DTT, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 μg/mL each of chymostatin, leupeptin, antipain, and pepstatin A (CLAP) and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich) diluted at 1:100. Tissues were homogenized using a mechanical tissue disrupter (Ultra-Turrax T25, IKA, Staufen, Germany) for 30 s. Homogenates were centrifuged at 10,000g for 15 min at 4 °C to separate supernatants containing the protein extracts, which were transferred to new tubes and stored frozen at − 80 °C until use. Total amount of protein in the extracts was estimated by using the Stoscheck modification (Stoscheck 1990) of the dye-binding method of Bradford (Bradford 1976).
Electrophoresis and Western blot immunodetection
Polyacrylamide gel electrophoresis was performed as described by Lopez-Dominguez et al. (2013). Membranes were incubated with primary antibodies (obtained from Santa Cruz Biotech unless otherwise stated) raised against the following mitochondrial components: CYB5R3 (Proteintech, 10894-1-AP), VDAC1/2/3 (sc-98708), TOTAL OxPhos Complex Kit (Life technologies, 458099), NRF1 (sc-33771), TFAM (sc-2358), Mfn1 (sc-50330), Mfn2 (sc-50331) and Fis1 (sc-98900). Antibody against CYB5R3 was used at 1:10,000 dilution, antibodies against Mfn2 and Fis 1 were used at 1:500 dilution, antibodies against TFAM and VDAC were used at 1:1000 dilution, antibody against NRF1 was used at 1:2000 dilution and TOTAL OxPhos Complex antibody Kit was used at 1:4000 dilution. Appropriate species-specific secondary antibodies coupled to horseradish peroxidase were used to reveal binding sites by enhanced chemiluminescence (ECL-Plus, GE Healthcare Life Sciences). The signal was recorded using a ChemiDoc Imaging System (Bio-Rad) and staining intensity of positive bands was quantified with Image LabTM Software (Bio-Rad). To correct for possible differences in protein load between samples, data obtained from the quantification of the immunostained bands (in arbitrary units) were normalized to density data of the corresponding lanes stained with Ponceau S.
Statistics
Data values were analysed using the GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). All the data shown in this paper are means ± SEM. Normality of data was verified using the Kolmogorov-Smirnov normality test. The means were compared using the two-way ANOVA. We assessed overall differences due to “diet” (independently of genotype), “genotype” (independently of diet) and the interaction “diet × genotype”, as well as individual differences between experimental groups. Significant differences were expressed as follows: * (p < 0.05), ** (p < 0.01), *** (p < 0.001) and **** (p < 0.0001).
Results
Body and liver weight
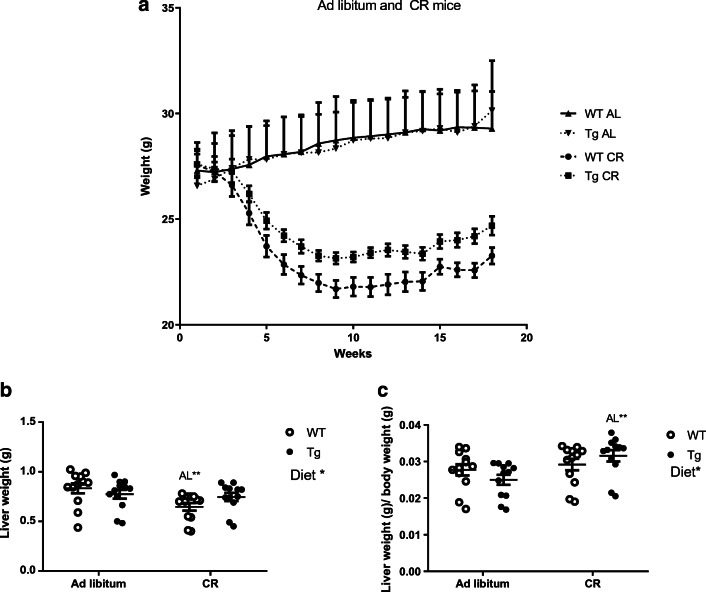
We first determined body and liver weight in WT and Tg mice that had been fed an AIN93M diet for 4 months, either ad libitum or under conditions of 40% restriction. No differences in body weight between genotypes were observed when mice were fed ad libitum and, as expected, CR produced a significant decrease of body weight in both genotypes (p < 0.0001). However, weight loss due to CR intervention was significantly more pronounced in WT than in Tg mice. As a result, Tg mice weighed significantly more than WT littermates when fed under CR conditions (p < 0.0001) (Fig. 1a).
Fig. 1.
Changes in body (a) and liver (b) weight in WT and Tg mice overexpressing CYB5R3, fed ad libitum or under CR. The body weight (a) was monitored weekly during 19 weeks of intervention. b displays liver weights after 19 weeks of intervention and c shows the relative liver-to-body weight at the end of the intervention. Data are represented as mean ± SEM of at least 12 mice
Liver weight was also reduced in WT mice fed under CR conditions in comparison with mice of the same genotype fed ad libitum (Fig. 1b) although no difference between the two dietary conditions was observed when liver weight was normalized to body weight (Fig. 1c). No differences in liver weight or in liver weight normalized to body weight were found when comparing mice of the two genotypes when fed ad libitum. Of note, in contrast to what was found for WT mice, CR did not result in a significant decrease of liver weight in Tg mice (Fig. 1b). Moreover, liver weight normalized to body weight was significantly increased in Tg mice fed under CR in comparison with mice of the same genotype fed ad libitum (Fig. 1c).
CYB5R3 polypeptide in liver and skeletal muscle from WT and Tg mice
Next, we studied the levels of CYB5R3 polypeptide in tissue extracts obtained from liver and hind limb skeletal muscle from WT and Tg mice fed the two experimental diets. As shown in Fig. 1a, b, the effect of CR and CYB5R3 overexpression on this parameter was strongly tissue-dependent. Overexpression of the CYB5R3 gene led to a modest albeit significant increase of the CYB5R3 polypeptide in liver from Tg mice fed ad libitum, but this increase was blunted by CR in such a way that no differences between genotypes were noted in mice submitted to this dietary intervention. Interestingly, levels of hepatic CYB5R3 polypeptide were significantly decreased by CR in mice of both genotypes, with this effect being much more striking in Tg mice (Fig. 2a). In skeletal muscle, overexpression of the CYB5R3 gene led to a dramatic increase in the levels of the CYB5R3 polypeptide regardless the intake of calories. In contrast with the results obtained in liver, the levels of CYB5R3 polypeptide in skeletal muscle was not affected by CR regardless of genotype (Fig. 2b).
Fig. 2.
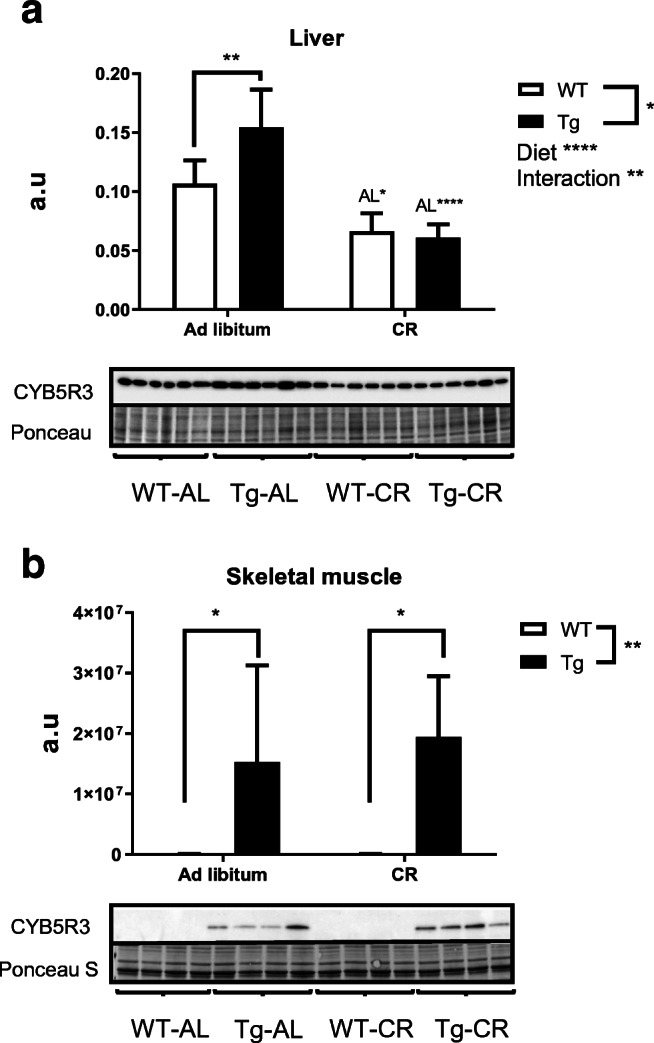
Expression levels of CYB5R3 measured by Western blots in protein extracts obtained from liver (a) and hind limb skeletal muscle (b) of wild-type and Tg mice. Depicted data are mean ± SEM of 4 replicates. In all graphs, asterisks without a letter refer to statistically significant differences between genotypes, whereas “AL” followed by asterisks indicates statistically significant differences between ad libitum and CR for a given genotype. Significant interactions between diet and genotype are also indicated when appropriated. In Figs. 2, 3, 4, 5, 6, and 7, representative Western blots and Ponceau S-stained lanes as loading control are included below each graph. a.u. arbitrary units
Mitochondrial dynamics and biogenesis markers
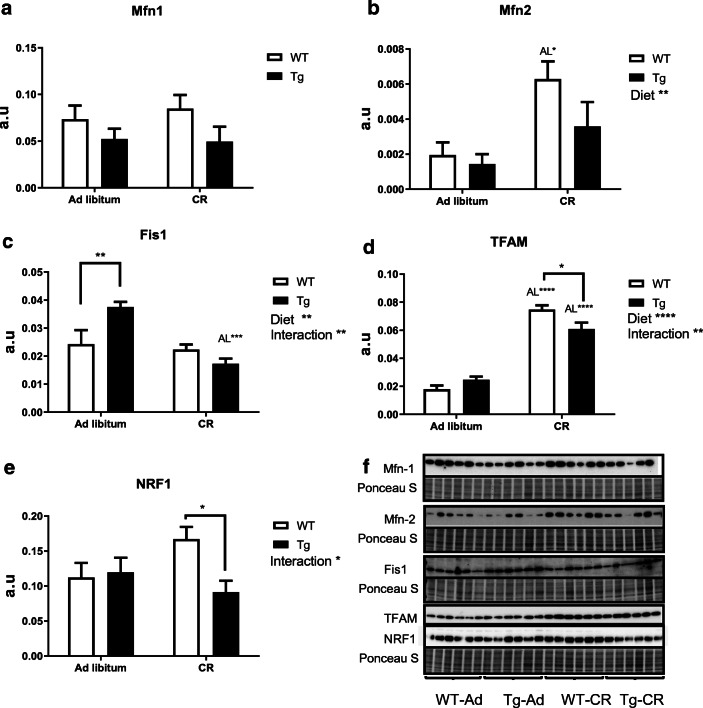
We next investigated the impact that CR and/or overexpression of CYB5R3 gene imposes on mitochondrial dynamics by studying the levels of key marker proteins related to mitochondrial fusion and fission phenomena. As depicted in Fig. 3a, no significant differences were detected for the levels of hepatic Mfn1 in both ad libitum and CR-fed mice, but Mfn2 was significantly increased in WT mice fed under CR in comparison with WT mice fed ad libitum. Interestingly, the extent of this increase by CR was attenuated in Tg mice in such a way that no significant differences were observed when comparing Tg mice fed ad libitum or under CR (Fig. 3b). The fission marker Fis1 was not altered by CR in WT mice, but it was significantly increased in Tg mice fed ad libitum in comparison with WT mice fed the same diet. As observed for CYB5R3 polypeptide, this effect was also vanished in mice fed under CR conditions. Consequently, Fis1 levels were significantly lower in Tg mice fed under CR in comparison with animals of the same genotype fed ad libitum (Fig. 3c). We also measured the levels of TFAM and NRF1, two key transcription factors regulating mitochondrial biogenesis. TFAM polypeptide exhibited a dramatic increase by CR both in WT and in Tg mice although, as found for another markers, a decrease was also observed in Tg mice fed under CR in comparison with WT mice fed the same diet (Fig. 3d). NRF1 levels also tended to increase with CR in WT mice in comparison with their ad libitum controls, although in this case the differences did not reach statistical significance (p = 0.1). Interestingly, a significant decrease of this mitochondrial biogenesis marker was again observed in Tg mice fed under CR in comparison with WT mice fed the same diet (Fig. 3e). Figure 3f depicts Western blots used for quantification of protein levels in liver with their corresponding Ponceau S-stained lanes used for normalization of protein loading.
Fig. 3.
Expression levels of proteins related to mitochondrial fusion: Mfn-1 (a) and Mfn-2 (b); fission: Fis1 (c); and biogenesis: TFAM (d) and NRF1 (e) in liver of wild-type and Tg mice fed ad libitum or under CR. When detected, general effects of diets and interaction between genotype and diet are represented on the corresponding panels. Data are represented as mean ± SEM of 6 replicates. Western blots used for quantification of protein levels with their corresponding Ponceau S-stained lanes used for normalization of protein loading (f)
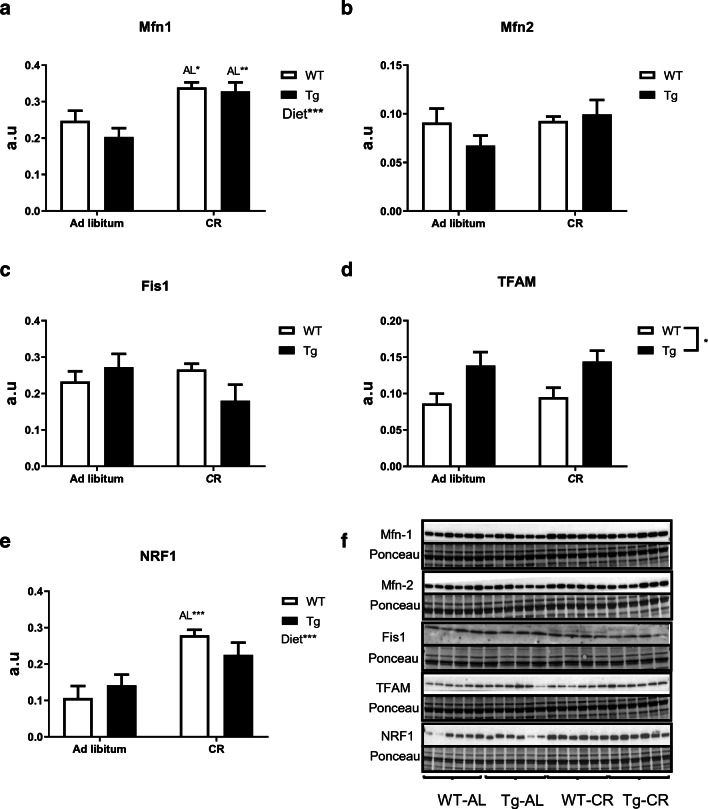
Changes elicited by CR and/or CYB5R3 overexpression in skeletal muscle were different to those observed in liver. In skeletal muscle, CR produced a significant increase in the levels of Mfn1 in mice of both genotypes without any change attributable to CYB5R3 overexpression (Fig. 4a), while the levels of Mfn2 (Fig. 4b) and Fis1 (Fig. 4c) were unaffected by either CR or CYB5R3 overexpression. We found an increase of TFAM levels by CYB5R3 overexpression regardless of the diet (Fig. 4d), while NRF1 was substantially increased by CR in WT mice but no significant change was observed in Tg mice (Fig. 4e). Figure 4f depicts Western blots used for quantification of protein levels in skeletal muscle with their corresponding Ponceau S-stained lanes used for protein loading normalization.
Fig. 4.
Expression levels of protein related to mitochondrial fusion Mfn-1 (a) and Mfn-2 (b), fission Fis1 (c) and biogenesis TFAM (d) and NRF1 (e) in skeletal muscle of wild-type and transgenic animals fed ad libitum or under CR. General effects of diet and interaction between genotype and diet are represented, when detected, on the corresponding panel. Data are shown as mean ± SEM of 6 replicates except in c (Fis1) with 4 replicates. Western blots used for quantification of protein levels with their corresponding Ponceau S-stained lanes used for normalization of protein loading (f)
Mitochondrial mass and electron transport chain complexes
To further study the impact CR and/or CYB5R3 overexpression exert on mitochondria, we measured the levels of VDAC (porin), an outer membrane protein which is considered as a biochemical marker of mitochondrial abundance (Grünewald et al. 2014), and several marker subunits of the electron transport chain (ETC) complexes in the inner membrane. Neither CR nor CYB5R3 overexpression altered VDAC levels in liver (Fig. 5a), but CR strongly increased VDAC in skeletal muscle from WT mice. It is noteworthy that the effect of CR was again abated in mice overexpressing CYB5R3, so that VDAC levels in Tg mice fed under CR were not only much lower than those of WT mice fed the same diet, but even lower than those of Tg mice fed ad libitum (Fig. 5b).
Fig. 5.
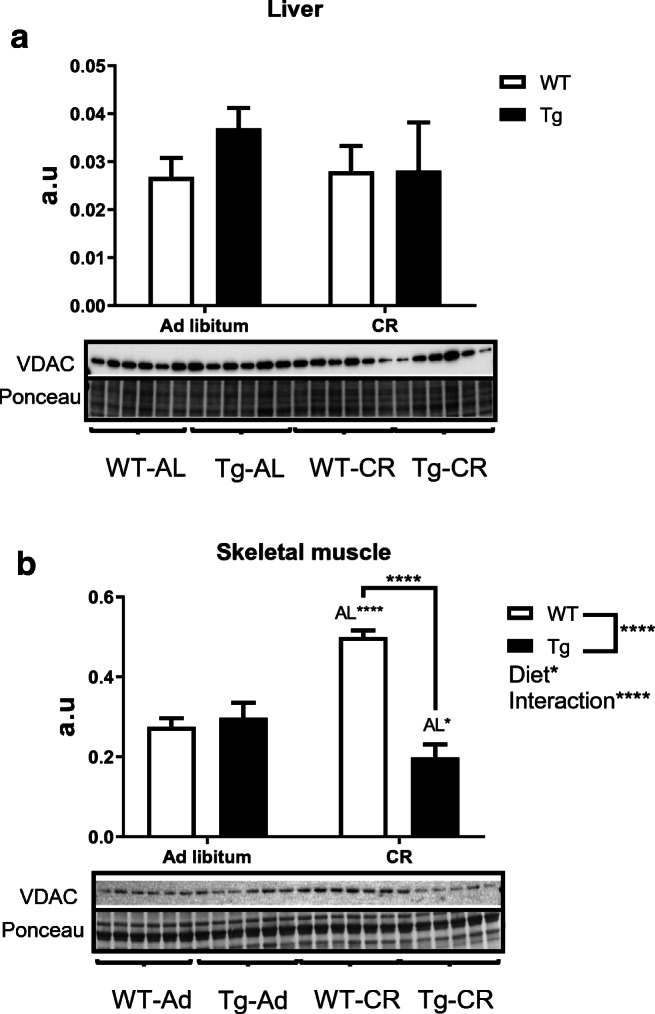
Expression levels of VDAC as mitochondrial mass marker in protein extracts obtained from liver (a) and hind limb skeletal muscle (b) of WT and Tg mice. Data are depicted as mean ± SEM of 6 replicates
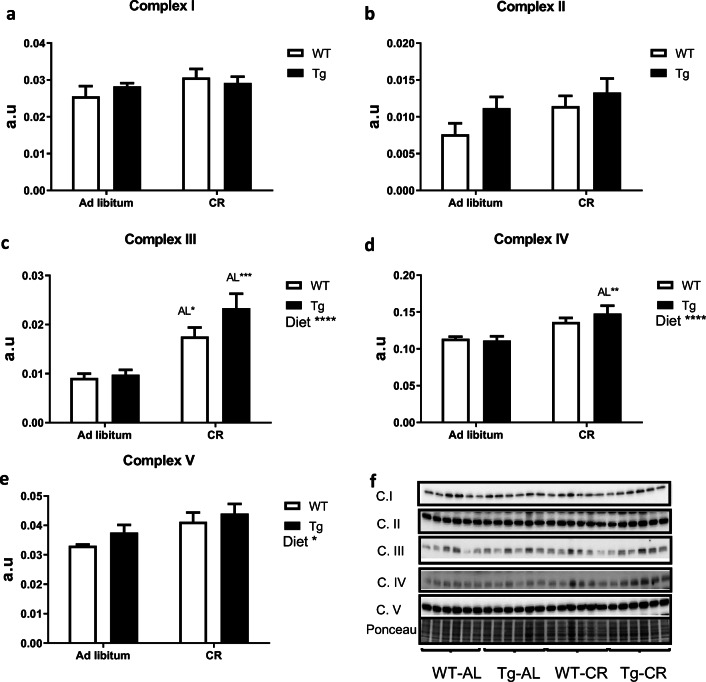
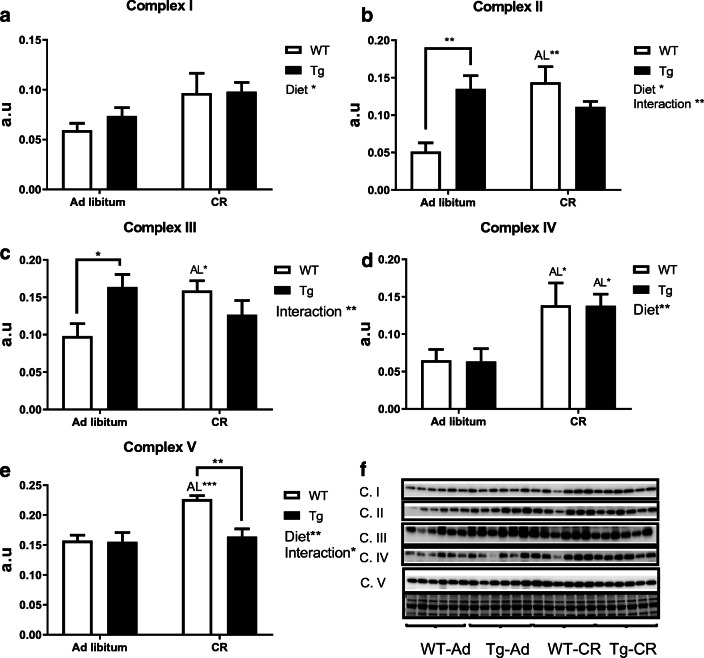
Regarding the abundance of marker subunits of the electron transport chain complexes, we found no changes attributable to diet or genotype in hepatic levels of complexes I and II (Fig. 6a, b), but CR produced substantial increases of hepatic complex III (with statistical significance in both genotypes, see Fig. 6c), complex IV (with statistical significance in Tg mice, see Fig. 6d) and complex V (where statistical significance for the effect of diet was obtained when mice of both genotypes were combined, see Fig. 6e). Figure 6f depicts the Western blots used for quantification of protein levels, with their corresponding Ponceau S-stained lanes used for protein loading normalization.
Fig. 6.
Expression levels of mitochondrial electron transport chain complexes in liver from WT and Tg mice fed ad libitum or under CR. a to e represent the levels of mitochondrial complexes I to V, respectively. Statistically significant differences between groups are indicated where detected. Data are mean ± SEM of 6 replicates. Western blots used for quantification of protein levels with their corresponding Ponceau S-stained lanes used for normalization of protein loading (f)
In the case of skeletal muscle, we evidenced a global and genotype-independent effect of CR to increase the abundance of marker subunits of complexes I (where statistical significance was obtained when mice of both genotypes were combined, see Fig. 7a) and IV (with statistical significance in mice of both genotypes, see Fig. 7d). On the other hand, complexes II and III shared another pattern of changes as a function of diet and/or genotype, i.e. the abundance of these complexes was increased in WT mice fed a CR diet and in Tg mice fed ad libitum, in comparison with their WT controls fed ad libitum. No differences were however found when comparing the two dietary groups of Tg mice, and CR did not affect significantly the levels of complexes II and III in these mice (see Fig. 7b, c). Finally, the pattern of complex V changes with diet and/or genotype was similar to that exhibited by VDAC (see above), i.e. CR produced a significant increase in WT mice, but this increase was abated by CYB5R3 overexpression. Complex V abundance was thus significantly lower in Tg mice fed under CR in comparison with WT mice fed the same diet, and no change as a function of genotype was observed in mice fed ad libitum (Fig. 7e). Figure 7f depicts Western blots used for quantification of protein levels, and their corresponding Ponceau S-stained lanes used for protein loading normalization.
Fig. 7.
Expression levels of mitochondrial electron transport chain complexes quantified in skeletal muscle of WT and Tg mice fed either ad libitum or under CR. a to e represent complexes I to V, respectively. Statistical differences between groups as well as general effect of diet and interactions genotype-diet are indicated on the corresponding panel. Data were represented as mean ± SEM of 6 replicates. Western blots used for quantification of protein levels with their corresponding Ponceau S-stained lanes used for normalization of protein loading (f)
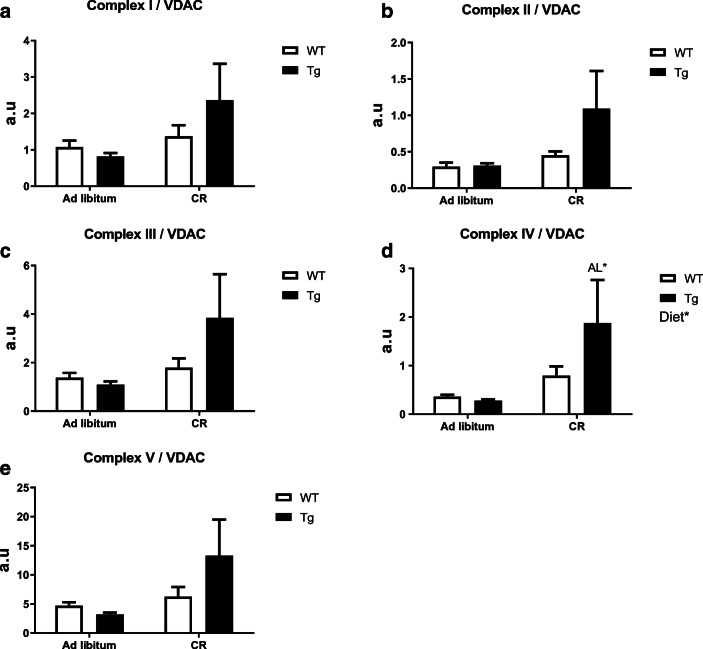
The alterations in the levels of several mitochondrial complexes with diet and/or genotype we document here could be accounted by changes in mitochondrial abundance and/or by modifications in the intrinsic composition of mitochondrial membranes. To distinguish between these two possibilities, we calculated the levels of mitochondrial complexes relative to VDAC, as previously published (Grünewald et al. 2014), and the results are depicted in Figs. 8 (for liver) and 9 (for skeletal muscle). Interestingly, a consistent pattern of changes was highlighted for both tissues when the abundance of each mitochondrial complex was referred to the corresponding value of VDAC. In liver, CYB5R3 overexpression did not alter the abundance of ETC complexes relative to VDAC in mice fed ad libitum, but a general trend towards the increase of all complexes was found in Tg mice fed under CR (see Fig. 8a–e), with statistically significant differences being obtained for complex IV:VDAC in comparison with Tg mice fed ad libitum (Fig. 8d).
Fig. 8.
Levels of mitochondrial complexes relative to VDAC in liver. a to e as in Fig. 5. Statistical differences were found only for complex IV/VDAC (d)
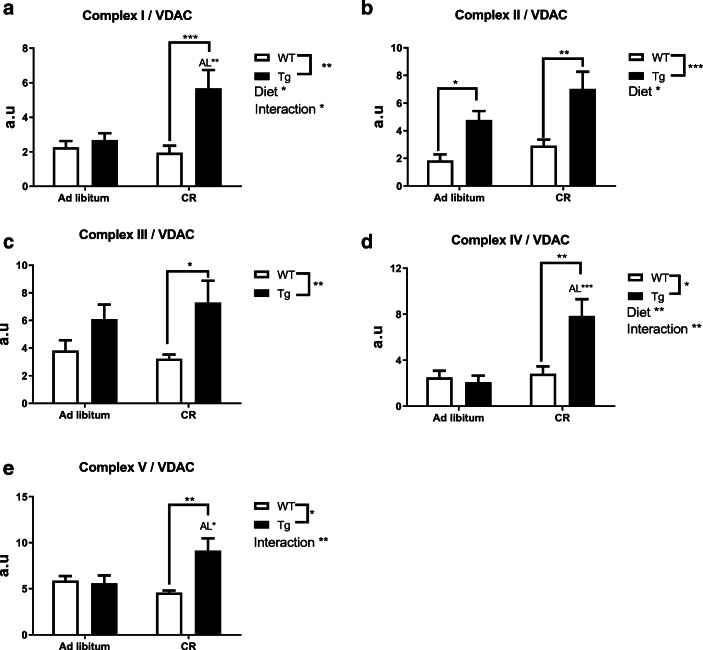
Fig. 9.
Levels of mitochondrial complexes relative to VDAC in hind limb skeletal muscle. a to e, as in Fig. 6. Significant differences between groups or diets and genotype-diet interaction were found where noted
In skeletal muscle, it was highlighted again that abundance of most complexes relative to VDAC was not altered by CYB5R3 overexpression in mice fed ad libitum (see Fig. 9a, c–e), resembling the results obtained in liver, with the sole exception of complex II which was increased significantly in Tg compared with WT mice (Fig. 9b). Of note, the abundance of all complexes relative to VDAC was consistently increased in Tg mice fed under CR in comparison with their WT littermates fed the same diet (see Fig. 9a–e). Moreover, statistically significant increases with respect to Tg mice fed ad libitum were also observed for complexes I, IV, and V (see Fig. 9a, d, e).
Discussion
The NADH dehydrogenase CYB5R3 has direct implications in cell protection against oxidative stress and in the regulation of NAD+ levels, and thus, a fundamental role for CYB5R3 in metabolism and aging has been proposed (de Cabo et al. 2009; Siendones et al. 2014). Moreover, it has been considered that some of the beneficial effects CR exerts on health could be related, at least in part, to CYB5R3 induction (De Cabo et al. 2004; Lopez-Lluch et al. 2005; Navas et al. 2007). However, whereas some of the salutary effects of CR are indeed mimicked in transgenic mice overexpressing CYB5R3 (Tg mice) (Martin-Montalvo et al. 2016), the mechanisms involved in metabolic adaptation of Tg mice might be independent of those described for CR. In this sense, Tg mice fed a standard diet ad libitum were fatter but not heavier than the corresponding WT controls, and preferentially used carbohydrate to meet their energy needs. These two traits clearly depart from a typical CR phenotype, which is characterized by the decrease of body weight, adiposity and the use of fat as a fuel source (Guarente 2008; Martin-Montalvo et al. 2013). Since saturated fatty acids are required to allow mitochondrial β-oxidation to proceed (Kunau et al. 1995), metabolic adaptations elicited by CYB5R3 overexpression might be related to the action of this enzyme in enhancing elongation and desaturation of fatty acids, which may lead to the inhibition of fatty acid β-oxidation and, thus, to the preferential use of carbohydrate metabolism (Martin-Montalvo et al. 2016). Lipid composition of membranes is also modified by CR, but the changes again differ from those produced by CYB5R3 overexpression. In this sense, CR decreases the levels of long-chain PUFA and increases monounsaturated fatty acids without any change in saturated fatty acids, as result of a metabolic reprogramming leading to lower levels of oxidative damage which could contribute to the extension of lifespan (Jove et al. 2014; Laganiere and Yu 1989; Laganiere and Yu 1993).
Because CR and CYB5R3 overexpression modify key aspects of energy metabolism and the lipid profile of tissues in opposite ways, it is important to study if some of the metabolic adaptations induced by CYB5R3 overexpression are affected by CR. In accordance with this idea, our previous research has shown that increasing n-3 PUFA in tissues can be beneficial to mice fed ad libitum (Hagopian et al. 2010), while shortening lifespan in mice fed a CR diet (Lopez-Dominguez et al. 2015).
After being fed a standard diet for 4 months ad libitum, CYB5R3 mice showed no significant differences in body weight with respect to their WT controls, which is in agreement with our previous research in these mice fed closely related standard or high-fat diets (Martin-Montalvo et al. 2016). However, although CR produced a significant decrease of body weight in both genotypes, CYB5R3 overexpression led to a greater preservation of body weight—and to a greater extent of liver weight. Thus, CYB5R3 emerges for the first time as a key determinant in the control of organ and body weight in mice under CR.
Quantification of CYB5R3 polypeptide levels in liver and skeletal muscle homogenates from mice fed ad libitum also confirmed our previous observations, with a modest although statistically significant increase of CYB5R3 polypeptide in liver and a dramatic augmentation in skeletal muscle from Tg in comparison with WT mice (Martin-Montalvo et al. 2016). Skeletal muscle is thus a suitable model to study the direct effects of CYB5R3 overexpression in the cellular physiology.
While CR did not affect CYB5R3 levels in skeletal muscle, this intervention produced a decrease in liver, particularly in Tg mice. The decrease of hepatic CYB5R3 in young mice submitted to CR is in accordance with our previous research carried out in Fischer-344 rats (De Cabo et al. 2004) and C57BL/6J mice (Lopez-Lluch et al. 2005), which allowed us to document that CR has a dual effect on CYB5R3 levels in liver plasma membrane. Of note, while a decrease in a CYB5R3-dependent activity was observed in young animals fed a CR diet, a remarkable increase was observed in old animals fed the CR diet lifelong. Furthermore, hepatic expression and polypeptide levels of CYB5R were also decreased in C57BL/6J mice after 18-h fasting, which was confirmed in HepG2 and Hepa1.6 cell lines cultured under glucose deprivation (Jakobs et al. 2014). In brain, CYB5R3 levels were unchanged by CR in young, but markedly increased in old Ficher-344 rats fed a CR diet. A substantial increase of CYB5R3 levels was also observed in SH-SY5Y neuroblast cells cultured with serum obtained from rats that had been fed under CR, in comparison with cells cultured with serum obtained from rats fed ad libitum (Hyun et al. 2006). Taken together, these observations demonstrate the existence of age and tissue-specific mechanisms regulating CYB5R3 levels by CR.
Of note, whereas the expression of ectopic CYB5R3 in Tg mice is controlled by a viral promoter (CMV), expression of the endogenous CYB5R3 gene is controlled by SP1 transcription factor (Toyoda et al. 1995), which can be further upregulated through FOXO3a and Nrf2 under conditions of nutritional and oxidative stress (Siendones et al. 2014). Since the levels of CYB5R3 polypeptide were similarly affected by CR in both WT and Tg mice, it is very likely that this regulation is post-transcriptional.
Previous research has firmly established that CR results in increased mitochondrial number and mass, and it may reduce ROS production without decreasing cellular respiration (Civitarese et al. 2007; Lopez-Lluch et al. 2006). However, how CR targets mitochondrial metabolism in mice overexpressing CYB5R3 is completely unknown. To elucidate the putative crosstalk between CR and CYB5R3 overexpression in the regulation of mitochondrial mass and function, we used here a battery of antisera raised against key mitochondrial proteins, including VDAC, different subunits of mitochondrial complexes, the mitofusins Mfn1 and Mfn2, the fission factor Fis1 and two transcription factors involved in mitochondrial biogenesis: Nrf1 and TFAM.
VDAC is an abundant protein of the outer mitochondrial membrane whose levels serve as a biochemical estimate of mitochondrial mass (Grünewald et al. 2014). No significant differences among experimental groups were found for hepatic VDAC, but a substantial increase was observed in skeletal muscle from WT mice on CR in comparison with their ad libitum controls, supporting a higher mitochondrial abundance in this tissue under CR conditions. In accordance with previous investigations (Villalba et al. 2015), our results support that mitochondrial metabolism is rapidly adapted to CR in skeletal muscle compared with other tissues as liver, although another pathways which also affects the rate of aging, as mTOR signalling and the ubiquitin-proteasome pathway, were not affected by CR in skeletal muscle from young rats but a substantial inhibition was achieved in middle-age animals (Chen et al. 2019). The observations reported here are however in contrast with our previous studies based on a quantitative approach using electron microscopy, which allowed us to document that a 6-month CR intervention based on AIN93G diet was sufficient to increase mitochondrial abundance in mouse liver (Khraiwesh et al. 2014; Khraiwesh et al. 2013) whereas these changes were not detected in skeletal muscle (Gutierrez-Casado et al. 2019). Since the amount of fat constitutes the main difference between AIN93G (7% fat) and AIN93M (4% fat) diets, the possibility exists that this factor plays a key role in determining the rate of mitochondrial adaptations of different tissues to CR. However, controversial results regarding changes of mitochondrial mass with CR and aging may also arise from the heterogeneity in the methods used to assess mitochondrial content (Cartee et al. 2016), as discussed in our previous publication (Gutierrez-Casado et al. 2019).
Interestingly, CYB5R3 overexpression abated the increase of VDAC levels in skeletal muscle from mice fed under CR, an effect that might be related to the inhibition of mitochondrial β-oxidation and the preferential use of carbohydrate metabolism in Tg mice (Martin-Montalvo et al. 2016). Mfn2 (but not Mfn1) and TFAM were dramatically increased and NRF1 tended to increase by CR in liver of WT mice, which is partially in accordance with a previous research that documented the upregulation of hepatic Mfn2 and TFAM mRNA in mice fed under CR for 3 or 12 months starting at 2 months of age (Nisoli et al. 2005). However, this research also evidenced an increase of Mfn1 mRNA that we have not confirmed here at the level of polypeptide. In accordance with our results, NRF1 mRNA levels increase in HeLa cells cultured with CR serum and this effect is inhibited by insulin (Lopez-Lluch et al. 2006), and TFAM and NRF1 protein levels decrease with aging in rat liver, and this decrease can be prevented by CR (Picca et al. 2013). According to our results, CR not only prevents the aging-related decrease of hepatic TFAM in old animals, but it even increases the level of this factor in young mice. The lack of Fis1 changes in WT mice fed under CR is in contrast with the increase reported for this fission factor in mice fed an AIN-93G diet for 6 months under CR conditions (Khraiwesh et al. 2013). This highlights again the importance of dietary fat content (AIN-93G vs. AIN-93M) and/or duration of intervention (6 months vs. 4 months) in determining the outcome of CR on mitochondrial dynamics.
Of note, CYB5R3 overexpression abated (for Mfn-2 and Fis1) or at least attenuated (for TFAM) the effect of CR in lever. CYB5R3 overexpression led to an increase of hepatic Fis1 in mice fed ad libitum, but this effect was abated by CR. Moreover, CYB5R3 overexpression also led to decreased hepatic NRF1, indicating a global effect on mitochondrial metabolism, dynamics and biogenesis in mice fed under CR. Mfn2 is a key protein of facilitating of contacts between endoplasmic reticulum and mitochondria which might result in an improvement in the processing of fat deposits (de Brito and Scorrano 2008; Rieusset 2018). The absence of CR effect of hepatic levels of Mfn2 in Tg mice and the decrease of TFAM and NRF1 in Tg mice fed under CR in comparison with WT mice fed the same diet might be related to the enhancement of carbohydrate metabolism in mice overexpressing CYB5R3 (Martin-Montalvo et al. 2016).
Changes elicited by CR and/or CYB5R3 overexpression in skeletal muscle were different to those observed in liver, indicating the existence of tissue specificity for mitochondrial adaptations to the interventions. In skeletal muscle, we evidenced an increase in the level of Mfn1 protein by CR in both genotypes, although Mfn2 was unaffected. It has been demonstrated elsewhere that CR increases the protein levels of Mfn2 in skeletal muscle of female swiss mice fed for 6 months with the same diet used here (AIN93M) starting at 1 month of age (Cerqueira et al. 2011). This discrepancy may be caused by differences in sex and strain (Mitchell et al. 2016). Previous results of our own group have also demonstrated an increase of Mfn2 levels in skeletal muscle of C57BL/6 mice fed the AIN93G diet under CR conditions for 6 months starting at 3 months of age (Gutierrez-Casado et al. 2019). This fact revels again the importance of the amount of fat in the diet. The increase of Mfn1 in skeletal muscle of mice of both genotypes fed under CR could indicate an increase in mitochondrial fusion needed to protect against autophagic degradation of these organelles (Tilokani et al. 2018). Additional studies will be necessary to elucidate putative changes in autophagy by CYB5R3 overexpression in mice fed under CR.
Unlike the results obtained in liver, levels of TFAM were not affected by CR but, interestingly, we evidenced a general effect of CYB5R3 overexpression increasing this factor independently of diet. While Civitarese et al. (2007) have demonstrated that TFAM mRNA is increased in muscle of healthy humans under CR, the intervention was not evaluated for TFAM at the levels of polypeptide. The dramatic increase of NRF1 polypeptide in skeletal muscle from WT mice fed under CR is in accordance with the similar increase observed for the mitochondrial abundance marker VDAC (see above). Of note, as also found for VDAC, the increase of NRF1 levels by CR was not observed in Tg mice, reinforcing the interference of both interventions.
While we found no major changes in the marker of hepatic mitochondrial abundance (VDAC) in liver tissue, an increase of fatty acid β-oxidation in response to CR could influence the activity and abundance of mitochondrial complex subunits (Chen et al. 2012a). Levels of complex III were increased by CR in both WT and Tg mice, which could facilitate electron flow through the mitochondrial chain. In skeletal muscle, CYB5R3 overexpression by itself increased the levels of complexes II and III in mice fed ad libitum, and CR apparently produced a generalized increase in the levels of mitochondrial complexes, although this effect seemed to be blunted when the dietary intervention was combined with CYB5R3 overexpression. Nevertheless, when the levels of mitochondrial complexes were normalized to those of VDAC to analyse those intrinsic changes taking place in the mitochondrial membranes independently of changes accounted by alterations in mitochondrial abundance, we were able to evidence a consistent effect of CYB5R3 overexpression to increase the levels of ETC complexes in both tissues, being the effect particularly striking in skeletal muscle. It has been shown that the increase in the levels of complex I by CR does not produce an increase in the levels of ROS in skeletal muscle (Chen et al. 2012b). The increase of ETC complexes could indicate a higher production of ATP without higher ROS production in Tg mice, as we have already demonstrated in liver (Martin-Montalvo et al. 2016).
In summary, our data support that many alterations elicited by CR in mitochondrial abundance and dynamics can be hindered by CYB5R3 overexpression, but these effects are compensated by a greater abundance of mitochondrial ETC complexes in Tg mice submitted to this dietary intervention.
Authors’ contributions
JMVM and RdC conceived and designed the project; SRL was responsible of raising and maintaining the colony of mice; SRL and SLB performed the experimental determinations and conducted the data analysis; JAGR and MIB provided valuable advice; SRL, SLB and JMVM wrote the manuscript with approval from all authors.
Funding information
This study was supported by Spanish Ministerio de Economía y Competitividad (MINECO) grant BFU2015-64630-R, cofinanced with EU FEDER funds, and Ministerio de Ciencia, Innovación y Universidades (MICIU) grant RTI2018-100695-B-I00, Spanish Junta de Andalucía (BIO-276) and Universidad de Córdoba (to JMVM). SRL held a FPI predoctoral contract funded by MINECO. SLB held a FPU predoctoral contract awarded by the Spanish Ministerio de Educación, Cultura y Deporte (MECD). RdC is supported by the Intramural Research Program of the National Institute on Aging.
Compliance with ethical standards
All animals were cared for in accordance with the University of Córdoba policy for animal welfare, which complies current European, Spanish and Andalusian regulations and is in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. This study was approved by the bioethics committee of the University of Córdoba and authorized by the Consejería de Agricultura, Pesca y Desarrollo Rural, Junta de Andalucía (authorization code: 20/04/2016/053).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sandra Rodríguez-López and Sara López-Bellón contributed equally to this work.
References
- Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013;19:1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci. 2014;127:1–27. doi: 10.1016/B978-0-12-394625-6.00001-5. [DOI] [PubMed] [Google Scholar]
- Belcourt MF, Hodnick WF, Rockwell S, Sartorelli AC. The intracellular location of NADH:cytochrome b5 reductase modulates the cytotoxicity of the mitomycins to Chinese hamster ovary cells. J Biol Chem. 1998;273:8875–8881. doi: 10.1074/jbc.273.15.8875. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Laurindo FR, Kowaltowski AJ. Mild mitochondrial uncoupling and calorie restriction increase fasting eNOS, akt and mitochondrial biogenesis. PLoS One. 2011;6:e18433. doi: 10.1371/journal.pone.0018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, CALERIE Pennington Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–617. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CN, Liao YH, Tsai SC, Thompson LV. Age-dependent effects of caloric restriction on mTOR and ubiquitin-proteasome pathways in skeletal muscles. Geroscience. 2019;41:871–880. doi: 10.1007/s11357-019-00109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. The influence of dietary lipid composition on liver mitochondria from mice following 1 month of calorie restriction. Biosci Rep. 2012;33:83–95. doi: 10.1042/BSR20120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction. J Gerontol A Biol Sci Med Sci. 2012;67:1121–1131. doi: 10.1093/gerona/gls113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Siendones E, Minor R, Navas P. CYB5R3: a key player in aerobic metabolism and aging? Aging (Albany NY) 2009;2:63–68. doi: 10.18632/aging.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz A, et al. Overexpression of CYB5R3 and NQO1, two NAD(+)-producing enzymes, mimics aspects of caloric restriction. Aging Cell. 2018;17:e12767. doi: 10.1111/acel.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, et al. Cyb5r3 links FoxO1-dependent mitochondrial dysfunction with β-cell failure. Mol Metab. 2020;34:97–111. doi: 10.1016/j.molmet.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald A, Lax NZ, Rocha MC, Reeve AK, Hepplewhite PD, Rygiel KA, Taylor RW, Turnbull DM. Quantitative quadruple-label immunofluorescence of mitochondrial and cytoplasmic proteins in single neurons from human midbrain tissue. J Neurosci Methods. 2014;232:143–149. doi: 10.1016/j.jneumeth.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Casado E, et al. The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2019;74:760–769. doi: 10.1093/gerona/gly161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian K, et al. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS One. 2010;5:e12696. doi: 10.1371/journal.pone.0012696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs HH, et al. The N-reductive system composed of mitochondrial amidoxime reducing component (mARC), cytochrome b5 (CYB5B) and cytochrome b5 reductase (CYB5R) is regulated by fasting and high fat diet in mice. PLoS One. 2014;9:e105371. doi: 10.1371/journal.pone.0105371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson I, Schenkman JB. Evidence against participation of cytochrome b5 in the hepatic microsomal mixed-function oxidase reaction. Mol Pharmacol. 1973;9:840–845. [PubMed] [Google Scholar]
- Jove M, Naudi A, Ramirez-Nunez O, Portero-Otin M, Selman C, Withers DJ, Pamplona R. Caloric restriction reveals a metabolomic and lipidomic signature in liver of male mice. Aging Cell. 2014;13:828–837. doi: 10.1111/acel.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh H, et al. Mitochondrial ultrastructure and markers of dynamics in hepatocytes from aged, calorie restricted mice fed with different dietary fats. Exp Gerontol. 2014;56:77–88. doi: 10.1016/j.exger.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh H, et al. Alterations of ultrastructural and fission/fusion markers in hepatocyte mitochondria from mice following calorie restriction with different dietary fats. J Gerontol A Biol Sci Med Sci. 2013;68:1023–1034. doi: 10.1093/gerona/glt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau WH, Dommes V, Schulz H. Beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Laganiere S, Yu BP. Effect of chronic food restriction in aging rats. II. Liver cytosolic antioxidants and related enzymes. Mech Ageing Dev. 1989;48:221–230. doi: 10.1016/0047-6374(89)90084-5. [DOI] [PubMed] [Google Scholar]
- Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology. 1993;39:7–18. doi: 10.1159/000213509. [DOI] [PubMed] [Google Scholar]
- Liochev SI. Reflections on the theories of aging, of oxidative stress, and of science in general. Is it time to abandon the free radical (oxidative stress) theory of aging? Antioxid Redox Signal. 2015;23:187–207. doi: 10.1089/ars.2014.5928. [DOI] [PubMed] [Google Scholar]
- Lopez-Dominguez JA, et al. Dietary fat modifies mitochondrial and plasma membrane apoptotic signaling in skeletal muscle of calorie-restricted mice. Age (Dordr) 2013;35:2027–2044. doi: 10.1007/s11357-012-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Dominguez JA, et al. The influence of dietary fat source on life span in calorie restricted mice. J Gerontol A Biol Sci Med Sci. 2015;70:1181–1188. doi: 10.1093/gerona/glu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Rios M, Lane MA, Navas P, de Cabo R. Mouse liver plasma membrane redox system activity is altered by aging and modulated by calorie restriction. Age (Dordr) 2005;27:153–160. doi: 10.1007/s11357-005-2726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, et al. Cytochrome b5 reductase and the control of lipid metabolism and healthspan. NPJ Aging Mech Dis. 2016;2:16006. doi: 10.1038/npjamd.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7(Suppl):S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Oshino N, Imai Y, Sato R. A function of cytochrome b5 in fatty acid desaturation by rat liver microsomes. J Biochem. 1971;69:155–167. doi: 10.1093/oxfordjournals.jbchem.a129444. [DOI] [PubMed] [Google Scholar]
- Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C, Lezza AM. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS One. 2013;8:e74644. doi: 10.1371/journal.pone.0074644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radical Bio Med. 2000;29:946–968. doi: 10.1016/S0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Reddy VV, Kupfer D, Caspi E. Mechanism of C-5 double bond introduction in the biosynthesis of cholesterol by rat liver microsomes. J Biol Chem. 1977;252:2797–2801. [PubMed] [Google Scholar]
- Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 2018;9:388. doi: 10.1038/s41419-018-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco JC, Trepanier LA. Cytochrome b5 and NADH cytochrome b5 reductase: genotype-phenotype correlations for hydroxylamine reduction. Pharmacogenet Genomics. 2010;20:26–37. doi: 10.1097/FPC.0b013e3283343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamuri S, et al. Measurement of respiratory function in isolated cardiac mitochondria using Seahorse XFe24 Analyzer: applications for aging research. Geroscience. 2018;40:347–356. doi: 10.1007/s11357-018-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siendones E, et al. Membrane-bound CYB5R3 is a common effector of nutritional and oxidative stress response through FOXO3a and Nrf2. Antioxid Redox Signal. 2014;21:1708–1725. doi: 10.1089/ars.2013.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR, Dhahbi JM. Conserved and tissue-specific genic and physiologic responses to caloric restriction and altered IGFI signaling in mitotic and postmitotic tissues. Annu Rev Nutr. 2007;27:193–217. doi: 10.1146/annurev.nutr.27.061406.093743. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-P. [DOI] [PubMed] [Google Scholar]
- Sure VN, et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience. 2018;40:365–375. doi: 10.1007/s11357-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda A, Fukumaki Y, Hattori M, Sakaki Y. Mode of activation of the GC box/Sp1-dependent promoter of the human NADH-cytochrome b5 reductase-encoding gene. Gene. 1995;164:351–355. doi: 10.1016/0378-1119(95)00443-a. [DOI] [PubMed] [Google Scholar]
- Villalba JM, et al. The influence of dietary fat source on liver and skeletal muscle mitochondrial modifications and lifespan changes in calorie-restricted mice. Biogerontology. 2015;16:655–670. doi: 10.1007/s10522-015-9572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]