Abstract
As average human lifespans increase across the globe, companion animals, specifically dogs and cats, are also living longer with more age-related morbidities. However, a similar trend is not seen in mammalian livestock species. Cows, pigs, goats, and sheep, as well as more niche mammalian species raised across the world, have been primarily raised for their economic benefit to humans and are culled from the population once their production declines. To this end, we lack clear knowledge about the age-related morbidities and causes of death that afflict livestock animals due to natural aging, as well as detailed age-specific survival rates. Here, we review the current state of the field of agricultural mammal aging, as well as provide specific questions and directions that may provide novel resources for veterinarians and aging biologists. By raising awareness of the overall quality of life and ongoing health of individual livestock animals, we can potentially increase production into older life stages, leading to decreased costs to farmers and improved welfare for the animals themselves.
Keywords: Lifespan, Healthspan, Companion animals, Agricultural animals, Longevity, Livestock
Introduction
Understanding the drivers and consequences of the aging process has come to the forefront of the medical field due to the rapidly increasing elderly population (Kennedy et al. 2014). While many individuals remain active and in good health throughout their lifespan, a large percentage of older adults will be faced with reduced mobility, multimorbidity, cognitive decline, and/or a decrease in overall quality of life in later years. This is placing huge economic burdens on individuals and governments across the globe. In parallel, aged companion animals, specifically cats and dogs in high-income countries, are living longer with more sophisticated medical treatments, such that many of their age-related issues mimic the human condition. However, how or when livestock and domesticated, economically relevant animals age is poorly defined in the veterinary sciences. The study of aging in mammalian agricultural species (e.g., pigs, goats, cows, and sheep) provides an untapped resource for developing novel aging hypotheses biologists, as well as providing essential information to veterinarians and veterinary scientists. Strikingly, when looking at the total numbers of livestock animals, the sample sizes available to study aging would far exceed both laboratory and companion animals. According to the Food and Agricultural Organization (FAO), the global animal population of pigs comes closer to a billion individuals, half of which are raised in China. Similarly, there are 500 million goats, over one billion sheep, as well as 1.4 billion cattle being currently raised across the globe.
Historically, horses pulling wagons and dogs watching animal herds were medically attended to solely for their benefits to the human population, not the animals themselves. In addition to “working animals,” those animals kept for their meat, fur, milk, and other products needed to stay free from major diseases, especially zoonoses, such that their economic value remained high. Generally, in agriculture, we do not understand the natural aging process, because once an animal’s “productive” life is complete, the animal is removed from the population. Taking advantage of this, farmers and breeders have spent decades selecting for traits that improve the economic benefits of their livestock. This often entails selection on those genes that lead to faster and increased growth (e.g., Biscarini et al. 2015). Larger muscles are desirable in meat animals, and similarly, reaching adulthood quickly allows milk-producing animals to more readily become an economic benefit, as well as for individuals to start reproducing quickly for the next generation of livestock. Consequently, those genetic variants that often lead to increased growth and reproduction, and thus are selected by agriculturists, have a negative effect on overall late-life health and longevity of the animals. In other words, aging is an incidental trade-off; genes that help animals survive to adulthood and reproduce can cause detrimental physiological effects in the post-reproductive period, commonly referred to as antagonistic pleiotropy. While it is assumed that these trade-offs exist in all animal species, the majority of these trade-offs, as well as the individual genes that cause these trade-offs, have been discovered in laboratory animal models, due to their ease of genetic manipulations in highly controlled environmental conditions (Austad and Hoffman 2018).
Conversely to agricultural animals, companion animal veterinarians and veterinary scientists have been treating age-related diseases broadly over the past several decades, especially as companion animals have made the shift from “property” to “family.” While veterinary science has developed large resources for treating animal aging in cats, it has been the companion dog that has become the focus of the biology of aging field (e.g., Fleming et al. 2011; O’Neill et al. 2013). Established medical procedures, sophisticated pathology, and natural aging resources are second only to humans in “man’s best friend” (Hoffman et al. 2018a). While veterinarians have been treating age-related morbidities in the dog for years, it is only recently that understanding the underlying molecular mechanisms that influence aging in the dog have come to the forefront. This is most exemplified by the Dog Aging Project, which was recently started (Kaeberlein et al. 2016) to longitudinally follow dogs as they age, in addition to testing a health- and lifespan extending pharmacological intervention (Urfer et al. 2017). Thus, the push to understand companion animal aging is growing rapidly. In addition to the companion dog, companion cats have also been studied fairly extensively with regard to aging age-related diseases (Bellows et al. 2016a; Bellows et al. 2016b), though minimally with regard to actual longevity and causes of death (O'Neill et al. 2015). A similar trend is not seen with regard to domesticated agricultural animals, with an exception of horses, as explained in more detail later.
One big advantage of studying aging in companion animals, and potentially agricultural animals, is that their lifespan reflects largely their healthspan as they are often euthanized if they are in ill-health and suffering (Hoffman et al. 2018a; Ahlman et al. 2011). By identifying age-related and characteristic symptoms arising during an animal’s life and by giving attention to measure healthspan, we can potentially provide a significant gain to agricultural production, animal welfare, and veterinary science. As the economic aspect is particularly important for agricultural animals, we hypothesize that a slowing of the rate of aging in these animals, leading to an improved health, may expectedly lead to an extension of an animal’s overall productivity, though gains may take longer in an animal’s life to reach. We propose that observing aging in agricultural animals that are the outcome of long-standing selection for productivity traits, often counter to improved lifespan, may lead to testable hypotheses about the underlying mechanisms that influence healthspan as opposed to lifespan. We feel that due to the enormous recent progress in aging research, it is a very timely and relevant task to bridge the fields between aging research and veterinary medicine by taking advantage from what has been learned from laboratory-derived research in well- established model organisms. We believe that implementation of this knowledge can potentially extend the phase of productivity of livestock to human benefit and, notwithstanding, optimize maintenance and welfare of agricultural animals.
Here, we review what is known about aging in individual agricultural species, with all maximum lifespans taken from AnAge unless otherwise cited (https://genomics.senescence.info/species/, Tacutu et al. 2018), and then describe how using the longevity dividend from aging biology may ultimately improve health and longevity in these agricultural animals. We then propose three pressing questions on livestock aging, for which the answers may lead to slowed aging and improved agricultural animals’ health, as well as provide critical information on animal aging for both veterinarians and biologists. Finally, we summarize the, so far identified, most promising interventions to slow aging in model organisms (fruit flies, nematodes, and mice) that we believe may be applicable to agricultural animals in the future. Overall, we want to raise the awareness for the necessity of understanding and cataloging livestock aging for the benefit of both veterinary scientists and aging biologists.
Equines (horses and donkeys)
While horses, Equus caballus, and donkeys, Equus asinus, are still considered “livestock” due to their traditional role as “working” animals, they are currently more often reared for pleasure riding and companionship as their role has been largely replaced by machinery. In addition, at least in high-income countries, they are rarely culled from the population due to a lack of productivity. Many horses are retired to pastures and only euthanized when their quality of life drops below a certain threshold, more similar to companion cats and dogs than other livestock species. And as such, more is known about aging in horses than other larger bodied livestock animals. Horses have an average lifespan of 20–30 years, with a maximum lifespan over 40 years (Cozzi et al. 2017). Elderly horses present with high rates of joint problems, dental and ophthalmologic issues, and neoplasias (Brosnahan and Paradis 2003; Ireland et al. 2012), in addition to general “aging” phenotypes (Ireland et al. 2012). And similar to studies in humans, horses with multimorbidity have decreased survival compared to those with no morbidities (Welsh et al. 2016). In parallel, donkeys can also easily reach an age of 30 years, and they show similar age-related disorders to horses including arthritis/joint problems, dental issues, and vascular disorders (Morrow et al. 2011). Combined, aging in horses and donkeys show similar trends and patterns to those seen in other companion animals and even humans. To this end, we are not going to discuss these species further, as their aging resources are more in line with companion animals than with other agricultural species. However, we do wish to note that due to the similar pathologies seen in equines compared to humans, equines may provide ideal large-bodied models of aging for both veterinary and translational sciences.
Cows
Perhaps the best described agricultural animal in regard to productive aging is the cow, Bos taurus. Depending on the breed, cattle are generally raised for either meat or dairy production with a maximum lifespan recorded at around 20 years, though anecdotal evidence suggests Texas Longhorn cattle can live over 30 years, but to the authors’ knowledge, this has never been officially published. However, the average lifespan of cattle is only 25% of the recorded maximum lifespan due to culling when productivity is reduced (Fig. 1). As meat cows are slaughtered once they meet a minimum age/size requirement, longevity has mostly been assigned in the species from dairy cow, yet little information exists on natural population longevity, nor age-related diseases in the species.
Fig. 1.
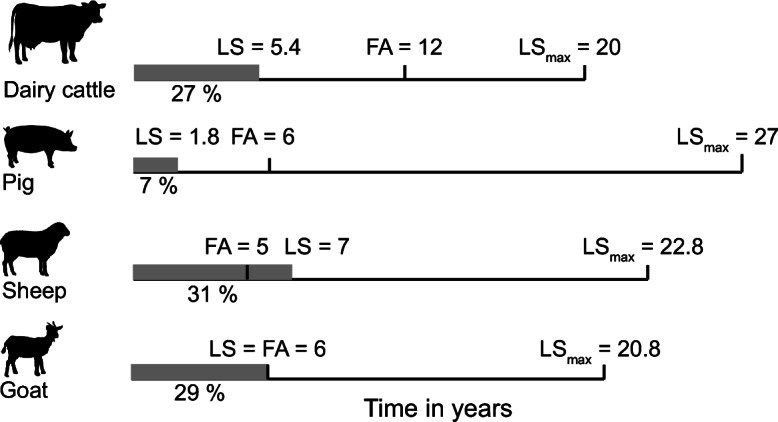
Effective lifespan (LS) in years and in % over time and the species-specific maximum expected lifespan (LSmax) from AnAge database for the economically most relevant agricultural species. Fertile age (FA) is distinguished with a mark and was estimated according to the literature (Ahlman et al. 2011; Klein 2019)
Multiple genome wide association studies have been completed on longevity in dairy cows, indicating novel loci for potential selection on for improved lactational longevity (Steri et al. 2019; Zhang et al. 2016). Similarly, genetics of longevity in beef cattle suggest the heritability is quite low (0.18) such that selection on longevity itself will not have strong improvements on beef cattle production (Hamidi Hay and Roberts 2017). While milk productivity and reproductive factors are the largest contributors to culling in dairy cattle, age itself can be a large reason for culling, independent of other diseases (Heise et al. 2016). Interestingly, telomere length has been associated with culling in dairy cows where individual animals with shorter telomeres are more likely to culled from 1 year to the next, independent of age (Brown et al. 2012), and telomere length appears to remain stable after 1 year of age (Seeker et al. 2018). Therefore, specific molecular markers might signify future age-related physiological changes in the cow.
The limited information we do know about natural cow aging largely comes from India, as cows are considered sacred, and the slaughter of cows is illegal in many Indian states. The average age of sheltered cows, those cows that are no longer productive, is 11 years (Sharma and Phillips 2019); much higher than the averages seen in commercial farming. Unlike previous work in dairy cows (Haskell et al. 2006), age was not found to be associated with increased risk of lameness in Indian sheltered cows (Sharma and Phillips 2019). While these sheltered cows are allowed to age naturally, they are not necessarily maintained in a high quality environment, and they often develop diseases and morbidities that would be more easily treated in high-income countries (Kennedy et al. 2018). However, they do offer a glimpse into natural aging in bovines. Unfortunately, there are not many peer-reviewed studies to date assessing these animals, but they may provide an ideal untapped resource for understanding cow aging.
Pigs
Sus scrofa domestica, the domesticated pig, has been used as a major source of protein for 7000 years (Caliebe et al. 2017). Unlike the majority of other mammalian agricultural animals, there is only one product that pigs have been selected for, meat. As such, growth is heavily favored in the species, as the faster an animal grows, the faster it can be slaughtered to provide an economic benefit. Thus, pigs are commonly euthanized before reaching 10% of their possible lifespan (Fig. 1). Similar to cows and other large-bodied agricultural animals, lameness and joint conditions are some of the most prevalent disorders in the pig (Kilbride et al. 2009; Noppibool et al. 2016), at least partially due to the rapid weight gain seen in the species. Even in relatively young pigs (1–2 years old), osteoarthritis and lameness are major factors influencing pig production and culling (Table 1), and the pig has recently been proposed as a strong translational model of naturally occurring osteoarthritis (Macfadyen et al. 2019). Additionally, pigs do develop spontaneous cancers including tumors in their digestive tract (Albuquerque et al. 2018) as well as melanomas (Millikan et al. 1974), and genetic models of cancer in the pig are currently being developed (Flisikowska et al. 2013).
Table 1.
Detailed reasons for culling farm animals
| Species | Reason for culling | Potential age-related cause | Reference |
|---|---|---|---|
| Cattle | Udder health | Immune system | Ahlman et al. 2011 |
| Low fertility | Loss of reproductive function | ||
| Low production | |||
| Pig | Low fertility | Reproductive tract pathology | Vaarst et al. 2003 |
| Leg weakness | Osteochondrosis | ||
| Sheep | Low fertility | Loss of reproductive function | Byun 2010 |
| Disease susceptibility | Immune system | ||
| Udder health | Wear and tear | ||
| Tooth wear and loss | |||
| Goat | Udder disorder | Immune system | Malher et al. 2001 |
| Locomotor disorder | Wear and tear | ||
| Digestive disorder | Loss of reproductive function | ||
| Reproductive disorder | |||
| emaciation |
While little is known about aging in agricultural pigs, pigs have been used for years in the laboratory as a translational model for human medical advances (Lunney 2007). However, the use of pigs in aging research is limited, as even in a translational setting, most pigs are euthanized at a relatively young age. Researchers have found age-related transcriptional changes in pig brain (Chen et al. 2019b), liver (Chen et al. 2019a), and skeletal muscle (Chen et al. 2018), but samples sizes were very low. While not the standard farm-raised pig, studies in the micropig, very small pigs arising from crosses of commercial and native pigs that are often kept as companion pets, suggest that age-related changes in the microbiome are similar to those in humans (Lim et al. 2019), and we hypothesize that similar patterns would be seen in agriculturally raised pigs, due to the close relationship between the two species.
Sheep
Domesticated sheep were traditionally raised for their wool; however, Ovis aries, are also used for meat and milk production, making today’s commercial dairy sheep industry a largely profitable business in Europe, Mideastern countries, and even China. Among the rarely specified health considerations that lead to culling in sheep are lameness, skin lesions, and setbacks in reproduction (Munoz et al. 2019), all similar to other agricultural animals. While sheep are culled at an average age of 5 years, some farms in developed countries allow ewes to survive into their teens (Byun 2010; Kern et al. 2010), with a maximum reported age of just under 23 years (Weigl 2005). Interestingly, in sheep, several “aging” genes have been found to be associated with longevity including the insulin-like growth factor 1 receptor gene (Byun et al. 2012) and the forkhead box class O3 class gene (Byun et al. 2011). However, variation in the toll-like receptor 4 (TLR4) gene (Byun 2010) and calpastatin gene (Byun et al. 2010) failed to be to associated with longevity. Like other mammals, sheep do develop spontaneous cancers including adenocarcinomas (Youssef et al. 2015) and squamous cell carcinomas (Albuquerque et al. 2018); however, similar to other livestock animals, these are rarely thoroughly investigated for their age-associated effects. In addition, the domesticated sheep has been proposed as a model of human genetic disorders, yet, the majority of these diseases are early-onset, not age-related (Pinnapureddy et al. 2015).
Goats
Similar to cows and sheep, goats, Capra hircus, are raised both for meat and milk. Due to its nutritional content, goat milk is considered the closest alternative to human breast milk (Clark and Mora Garcia 2017), and thus, the infant milk powder market has an increase in goat milk demand in the Asia-Pacific area. Fifty-two percent of the world’s 202 million dairy goats are found in China, India, Bangladesh, Pakistan, and Indonesia (Liang and Paengkoum 2019). As goats are tolerant towards many plant secondary metabolites, they can consume a wider variety of byproducts and non-conventional feed compared to other livestock, making them easier to rear in varied environments. Similar to other livestock species, their expected lifespan is much longer than their productive age as they are removed from the population relatively early in life (Fig. 1). An average age of just of 4 years has been suggested for one population of dairy goats in France with the majority of mortalities and cullings occurring due to nervous system, digestive system, reproductive system, and lameness issues (Malher et al. 2001); however, some goats are allowed to remain in the population past the age of 8. Similarly, results in New Zealand goats suggest a mean culling age of 4.5 years (Gautam et al. 2017) in one study and 4.5–5.7 years in another (Scholtens et al. 2018).
Other livestock mammals
While cows, sheep, goats, and pigs make up the majority of all agriculturally raised mammals in high-income countries, there are perhaps a dozen or so other mammalian species that are raised for human benefits across the globe. These include yaks, water buffaloes, bison, oxen, camels, alpacas, and llamas. As these species are produced on a smaller scale, they often are kept around longer than on commercial farms. As such, there is often more known about aging in these rarer species than the four most commonly raised species discussed above.
Water buffalo and bison
The domestic water buffalo, Bubalus bubalis, contributes a significant share of global milk production and is a major milk-producing animal in countries such as China, India, and Pakistan, but also Egypt and other African countries. As water buffalo are often kept on a smaller scale, it has anecdotally been reported that they have a longer productive life than cattle with reproduction and milk production lasting until they are 20 years of age (Borghese 2005) with a maximum lifespan of approximately 35 years. However, mean longevity is estimated to be 7–11 years across populations due to culling (El Debaky et al. 2019). Similarly, the bison (Bos bison synonymous Bison bison) can live up to 33.5 years in captivity while the median age in the European bison (Bison bonasus) is reported to be around 4 years (Korec et al. 2019), combining both captive and wild populations. However, little is known about the specific morbidities and mortalities that lead to culling and death in the species.
Llamas and alpacas
Llamas (Lama glama) and alpacas (Vicugna pacos) are South American ruminants from the Andean mountains raised for wool and transportation of goods; however, recently, especially in high-income countries, these two species are raised as companion animals, and can anecdotally reach ages over 30 years (AnAge suggested maximum lifespan of just under 29 years). As such, there are guidelines for care and management of elderly individuals (Fowler 1994). Llamas show significant musculoskeletal deterioration with age, as well as increases in dental issues with potential declines in vision and hearing (Fowler 1994) and neoplasias (Smith 1989), though, interestingly, neoplasias may be more age-related in llamas as compared to alpacas (Valentine and Martin 2007). However, many of the organ-specific changes that occur with age are unknown in these animals, and no work has attempted to understand the molecular mechanisms that lead to aging-related declines in llamas and alpacas.
Camels
Related to llamas and alpacas, camels are subdivided into two species, the dromedaries (Camelus dromedarius) of north Africa and the middle east and the Bactrian camel (C. bactrianus) of Asia. Camels were originally domesticated for milk but and are broadly used as a means of transport, as well as meat production (Raziq et al. 2008). While dromedaries are considered the main herbivores in the Sahelian countries, knowledge about their lifespan is restricted to their maximum lifespan in captivity which is 28.4 years for C. dromedaries. For Bactrian camels, maximum lifespan is reported at over 35 years. Given their extraordinary tolerance towards heat and drought, camels as a multipurpose livestock species have an enormous future potential as climate change continues to lead to increased worldwide temperatures. However, to really expand on the use of camels in agriculture, we must understand more about their age-related morbidities and mortalities.
Deer
Deer (family Cervidae) are very diverse hoofed ruminant mammals consisting of two main groups, the Cervinae to which the red deer (Cervus elaphus), sika deer (Cervus nippon), American elk (Cervus elaphus canadensis), and fallow deer (Cervus dama) belong. The other family, the Capreolinae, comprise reindeer (Rangifer tarandus), roe deer (Capreolus capreolus), moose (Alces alces), and white-tailed deer (Odocoileus virginianus). Domesticated deer are kept in situations varying from small enclosures to fauna parks to extensive grazing at low rates on pastures. Their economic importance rests upon use of their meat, skins and antlers, velvet, leather, hides, and even milk. Reindeer and red deer live 10–11 years in captivity on average, similar to that seen in wild populations (Muller et al. 2010); however, roe deer have shorter lifespans in captivity compared to free range (5.15 vs 6.85 years on average), suggesting husbandry is not ideal yet in many deer species. In sum, despite being considered a wild mammal, deer can be domesticated and maintained in captivity, and their lifespan would remain comparable to their free-ranging conspecifics. It is also worth noting that comparative biogerontology including natural populations of larger bodied mammals such as deer are considered very informative sources for senescence and inter-individual rates of aging (Austad 1993; Clutton-Brock and Isvaran 2007; Nussey et al. 2007).
Yaks
Yaks, Bos grunniens, and Bos mutus, are the domestic and wild forms, respectively, of the herding ungulate bovids inhabiting remote high-elevation alpine meadows and steppes in the Tibetan Plateau, China, and other parts of Central Asia. They are herbivorous ruminants traditionally used for milk, meat, and transporting goods. They are reported to reach a maximum lifespan of 25 years; however, nothing is reported on their effective lifespan. We speculate that, as they are utilized on a smaller scale than cattle, combined with a very distinct environment, their value to individual farmers may elicit the need for an extended effective lifespan compared to their commercial relatives. Thus, similar to others of these more niche species, studying natural aging in yaks may be more easily accomplished than in more commercially raised species.
Longevity dividend and importance of livestock animals for the field
The “Longevity Dividend” (LD) posits that delaying the age of onset of senescence-related symptoms of aging may extend life- and healthspan significantly more than curing individual age-related diseases (Olshansky et al. 2007). To derive testable hypotheses from this concept, broad knowledge of age-specific survivorship and mortality rates are required, which is lacking in livestock animals. Due to the economic choices of agriculturists, we lack reliable data on age-specific natural mortality in most of the common livestock animals. Obtaining such data collaboratively and broadly has so far proven successful in small companion animals within the framework of the “VetCompass” Programme (www.rvc.ac.uk) in the UK as well as the Veterinary Medical Database (VMDB) in the USA (Creevy et al. 2016). Below are three testable questions whose answers may provide novel insights into aging and longevity in livestock animals.
What are the main symptoms of worsening health and declining quality of life in agricultural animals? In general, livestock animals are culled when they are no longer “productive.” This is often manifested in slowed growth (or reaching a minimum threshold), declining reproduction, or reduced product production (i.e., milk). However, we do not thoroughly understand what age-related morbidities would affect these animals later in life. For example, similar to most mammals, livestock animals develop multiple types of cancer, yet studies on these are often small and fail to look at the age-related trajectories of cancers (e.g., Albuquerque et al. 2018; Millikan et al. 1974). In the light of the LD, we suggest that by better understanding the age-related morbidities and mortalities of livestock animals, we can best begin to understand how slowing the aging rate may improve health, longevity, and productivity in these animal species.
-
2.
How do hormonal manipulations affect healthspan and lifespan in livestock animals? Previous work in companion animals has shown that surgical sterilization improves lifespan in dogs (reviewed in Urfer and Kaeberlein 2019) and cats (O'Neill et al. 2015) with what appears to be a stronger effect in females. In addition, small studies in Korean eunuchs (Min et al. 2012) and males institutionalized in the USA (Hamilton and Mestler 1969), suggest castrated men live longer than those allowed to remain intact. Sterilization leads to a strong shift in hormone profiles that is presumably driving this observed lifespan extension; however, if this relationship holds in agricultural animals is still unknown. Livestock animals may provide a rich source of information on surgical sterilization due to the long experience with sterilization and the well described phenotypic changes caused by neutering. In pigs, males are usually castrated to prevent an offensive odor and flavor in pork, often referred to as “boar taint” (Bonneau and Weiler 2019), and a similar procedure is performed to a lesser extent on male sheep and cows raised for meat (Prescott and Lamming 1964). However, how castration affects adult lifespan in agricultural animals has not yet been answered. We therefore suggest that by understanding the effects of sterilization on healthspan and longevity, we may be able understand ways to slow aging in these animals and improve their LD. One study in a population of horses found no difference in lifespan between stallions and castrated geldings (Tapprest et al. 2017); however, anecdotal evidence suggests geldings maybe longer lived. We do wish to caveat that determining the effects of sterilization on longevity and health may only be feasible on a large scale in males of most agricultural species, due to logistics of female sterilization in large-bodied animals.
-
3.
How does reproduction influence aging in agricultural animals? In a similar vein to no. 2 above, one poorly understood relationship influencing time of death in agricultural animals is the correlation between onset of reproduction or more generally reproduction and aging. Trade-offs exist between reproduction and survival, such that most laboratory experimental studies have shown that virgin animals are longer lived than their mated counterparts. Despite extensive work from evolutionary biologists to answer this question in laboratory environments, larger-bodied, agricultural animals may provide a rich and meaningful source as to examine the question what specifically in reproducing animals makes them age faster than non-reproductive individuals. In addition, agricultural animals can help us determine how changes in reproductive timing, including timing of first reproduction, influence lifespan. For example, it is well established that the timing of reproduction influences the optimal onset of lactation for dairy cows, but reproductive timing effects on overall health and longevity are unknown (Bieber et al. 2019; Horn et al. 2012).
Combined, we hypothesize that timely veterinary research can benefit from answering the above three questions in livestock animals. With all the technological progress and the improved monitoring in farming aided by computing, it may be possible, in line with the above stated hypotheses, to extend the productivity and lifespan of the animals. Not only would these investigations allow veterinarians to better treat livestock aging, but aging science would benefit from insights from abundant, large-bodied mammalian species.
How can aging research help veterinarians and vice versa, with insights from larger-bodied, economically relevant livestock animals?
The following genetic, pharmacological, and environmental interventions, which affect aging in model organisms, have been presented over the past at least 100 years and may provide robust methods to improve healthspan, lifespan, and productivity in agricultural animals.
Genes. Over 30 genes have been individually manipulated to extend lifespan in mice by up to 50% (Pedro de Magalhaes et al. 2018). Selection on longevity in livestock species can improve economic benefits to farmers, but it is rarely addressed, as farmers often are interested in the quickest economic benefits. Genome wide association studies have been used to identify genes that are associated with “productive” or “functional” lifespan in livestock animals, including cows, sheep, and pigs. However, it is unknown, if these genes are also associated with overall animal lifespan. We believe that focusing on understanding the genes and genetic variants that influence longevity in agricultural animals will provide a wealth of knowledge for farmers, veterinarians, and aging biologists.
Removal of old, dysfunctional cells. Senescent cells that are not cleared by autophagy can produce harmful metabolites and proteins that negatively affect surrounding cells and may ultimately lead to tissue and organ malfunction. Previous work in mouse models suggests that senescent cell burden increases with age and removal of these cells (either genetically or pharmacologically) leads to improved health and lifespan (Baker et al. 2011; Xu et al. 2018). However, outside of rodent models and small pilot studies in diseases in humans (Hickson et al. 2019; Justice et al. 2019), the effects of accumulation of senescent cells with age on health and longevity in other mammalian species is unknown. Potentially, removal of these cells starting in early to mid-life could potentially lead to economic benefits of increased production leading to increased lifespan in livestock animals.
Nutrition. Reducing overall calorie intake has been the most replicated method to increase lifespan in laboratory organisms (Fontana et al. 2010), and the longevity and health benefits of calorie restriction have been extended to rhesus macaques (Mattison et al. 2017), with preliminary promising results on health in younger humans (Kraus et al. 2019). In addition, lowering dietary protein, and even individual amino acids, most reproducibly methionine, has shown both health and lifespan benefits in rodent models (Mirzaei et al. 2014). Low protein diets have previously been shown to provide adequate nutrition for livestock pigs and goats for a lower cost (Wang et al. 2018). However, the effects of lower protein on pig growth are conflicting, with some studies showing slowed growth under low protein, and others not, especially when diets were supplemented with branched chain amino acids (Wang et al. 2018). However, the long-term effects of caloric, protein, or amino acid restriction in livestock animals are unknown.
Growth hormone (GH)/insulin-like growth factor I (IGF-I). IGF-1, insulin, and their receptors are conserved from invertebrates to vertebrates, and they are heavily involved in multiple signaling pathways (reviewed in Bartke et al. 2013). It is widely accepted in the field that a combination of reduced GH, IGF-1, and insulin signaling contributes to extended longevity in line with increased cellular and in vivo stress resistance (Bartke et al. 2013). IGF-1 and GH are intrinsically connected because the key action of GH is to stimulate hepatic expression of the IGF-1 gene. Small dogs are longer lived than large dogs, and it is hypothesized this is due to decreases in GH and IGF-I in small individuals (Favier et al. 2001; Greer et al. 2011). A similar pattern is observed in horses where ponies are clearly longer-lived than larger horses, and presumably, GH and IGF-I at least partially regulate this relationship as well, though data is conflicting (Thomas et al. 1998). In addition, genetic knockdown of GH is arguably the most robust genetic manipulation to increase lifespan in rodent models (Bartke et al. 2013). However, how changes in these endocrine factors influence health and longevity in livestock animals is unknown. As most agricultural animals are selected for increased rates of growth and would thus be expected to have high levels of both GH and IGF-I, this might be inadvertently reducing overall health and longevity in the animals. As mentioned above, variation in the IGF-I receptor is associated with longevity in sheep (Byun et al. 2012), but overall effects of GH and its downstream targets on aging and longevity are largely unknown in livestock animals. Dairy cattle in the USA are commonly given recombinant bovine somatotropin hormone, synthetic GH, to promote growth but also to increase milk production, and this treatment may affect the risk of culling (Dohoo et al. 2003a; Dohoo et al. 2003b). Large livestock species may provide an ideal model to identify the roles GH and IGF-1 in animals due to their selection history as well as their size variation seen within a species. Experimentally testing this hypothesis may contribute to a better understanding of the underlying effects of GH, as well as assess its importance in an agricultural context. In this context, interesting novel experimental evidence has shown that disruption of the GH receptor in pigs leads to decreased body size, as well as other physiological changes similar to lowered GH in mice and humans (Hinrichs et al. 2018); however, if these pigs are significantly longer lived has not yet been determined.
Drugs. In recent years, multiple pharmacological interventions have been administered to model organisms to extend health and longevity; the most studied of which has been rapamycin. Preliminary work in companion dogs suggests rapamycin given short term in middle to old age is safe (Urfer et al. 2017), with phase II clinical trials currently ongoing. However, the effects of rapamycin, as well as other pharmacological interventions that extend lifespan in laboratory models, on both companion and agricultural animal species are unknown. The use of drugs to improve health and lifespan in livestock animals has the potential to provide the most direct economic benefits to farmers. However, many of these interventions lead to a decrease in body weight, such that any economic improvement in animals with these supplements may take longer to manifest. In addition, as many agricultural animals are used for human consumption only, the effects of these interventions on human health would need to be vigorously considered. Due to the growing market for organic farming worldwide, a renascence of the use of naturally occurring plant secondary compounds with proven medical (i.e. antimicrobial) effects is increasing such that use of more natural pharmacological interventions may be more promising avenues of pharmacological interventions to improve livestock animal health.
Future directions
The common aim of all biomedical aging research is to improve healthspan in the older population, and as stated by the Longevity Dividend, alleviating “aging” would improve lifespan and health more than curing individual diseases. This premise has the potential to extend to all mammalian species, including agricultural animals, yet as described above, our knowledge in livestock aging is lacking. We believe there are several avenues of research that can be addressed over the next decade to improve our understanding of agricultural animal aging.
As large agriculture animals require large amounts of resources (money and space), they are rarely raised in the laboratory, with the exception of some pigs; thus, completing “experiments” to uncover the molecular mechanisms associated with aging in livestock can be difficult. However, over the last decade, advances in large “omics” technologies have provided more avenues for researchers to use easily attainable biological samples. This has probably been most exemplified by recent work in the companion dog. Using blood plasma samples, tryptophan metabolism was found to vary in large, long-lived compared to small, short-lived dogs (Hoffman et al. 2019), and using fairly easily collected fibroblast cells, mitochondrial metabolism was also found to be associated with size (Jimenez et al. 2018; Nicholatos et al. 2019). As collection of these types of samples would also be possible on a large scale for large agricultural animals, this adds to the potential of using livestock animals as aging models.
Recently, understanding sex differences in aging has become one of the most studied topics in the biology of aging field. Women live significantly longer than males in all countries, yet they will spend more years with multimorbidity and low quality of life. In addition, causes of death differ between males and females, with males dying more of cardiovascular and cancer morbidities, while females are more likely to be diagnosed with Alzheimer’s and other dementias. To this end, models of sex differences are necessary to understand the physiological changes that differ between the sexes with age. In general, it is assumed that females are longer lived in most mammalian species (Austad and Fischer 2016). However, sex differences in mouse models differ based on genetic background and study conditions, and work in companion dogs suggests no real difference in longevity between males and females, with no difference in overall causes of death (Hoffman et al. 2018b). In livestock animals, differences in lifespan between males and females is rarely reported. Interestingly, one of the few species groups for which we do have information is the genus Bison, where it was suggested that females live approximately 2 years longer on average than males (Korec et al. 2019). However, the low median lifespan in the populations studied, make it hard to determine if these results are applicable to all bison. Overall, we believe agricultural animals provide a large, untapped resource to develop novel models and hypotheses about sex differences in aging.
While we do not foresee commercial farming making large changes to their culling practices, such that we can learn more about aging in livestock animals, there is a strong potential for study of smaller farms in developed countries. Similar to equines as mentioned above, many small hobby farms raise cows, goats, and sheep for companionship, not necessarily production (though they are often used productively, just not culled once their productivity ends). To this end, large animal veterinarians are already treating aging livestock animals in private practice; however, any data from this is anecdotal, not scientifically rigorous. We propose that aging biologists, working in conjunction with practicing veterinarians, may be able to design, execute, and publish highly needed literature on aging in specific livestock species.
Conclusions
Overall, the study of aging, healthspan, and longevity in livestock agricultural animals has the potential to increase agricultural production, improve animal welfare and husbandry, and provide novel models in the biology of aging field for comparative studies. We have focused here on large-bodied mammalian livestock species; however, our ideas and thoughts are very applicable to smaller niche mammalian species (e.g., rabbits and capybaras), as well as the many avian species raised for both meat and eggs. Veterinary scientists and biologists are at a crossroads where both fields have the potential to forge strong collaborations to push both areas of research in promising directions. We are excited with the promise these collaborations hold and look forward to their fruition over the next decade.
Acknowledgments
We would like to thank all the organizers and attendants of the 14th International Symposium on Neurobiology and Neuroendocrinology of Aging, especially Holly Brown-Borg and Kurt Borg which allowed us to meet and begin our collaboration. Special thanks to Steven Austad for critical reading of the manuscript. We would also like to thank the two reviewers for their insightful comments.
Author contributions
Both authors contributed equally to the design and writing of this review.
Funding information
JMH is supported by the National Institutes of Health K99AG059920.
Availability of data and material
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica M. Hoffman, Email: jmhoffm@uab.edu
Teresa G. Valencak, Email: teresa.valencak@vetmeduni.ac.at
References
- Ahlman T, Berglund B, Rydhmer L, Strandberg E. Culling reasons in organic and conventional dairy herds and genotype by environment interaction for longevity. J Dairy Sci. 2011;94:1568–1575. doi: 10.3168/jds.2010-3483. [DOI] [PubMed] [Google Scholar]
- Albuquerque TAF, Drummond do Val L, Doherty A, de Magalhaes JP. From humans to hydra: patterns of cancer across the tree of life. Biol Rev Camb Philos Soc. 2018;93:1715–1734. doi: 10.1111/brv.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Retarded senescence in an insular population of Virginia opossums. J Zool. 1993;229:695–708. doi: 10.1111/j.1469-7998.1993.tb02665.x. [DOI] [Google Scholar]
- Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN, Hoffman JM. Is antagonistic pleiotropy ubiquitous in aging biology? Evol Med Public Health. 2018;2018:287–294. doi: 10.1093/emph/eoy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows J, et al. Aging in cats: common physical and functional changes. J Feline Med Surg. 2016;18:533–550. doi: 10.1177/1098612X16649523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows J, et al. Evaluating aging in cats: how to determine what is healthy and what is disease. J Feline Med Surg. 2016;18:551–570. doi: 10.1177/1098612X16649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber A, et al. Production level, fertility, health traits, and longevity in local and commercial dairy breeds under organic production conditions in Austria, Switzerland, Poland, and Sweden. J Dairy Sci. 2019;102:5330–5341. doi: 10.3168/jds.2018-16147. [DOI] [PubMed] [Google Scholar]
- Biscarini F, Nicolazzi EL, Stella A, Boettcher PJ, Gandini G. Challenges and opportunities in genetic improvement of local livestock breeds. Front Genet. 2015;6:33. doi: 10.3389/fgene.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau M, Weiler U. Pros and cons of alternatives to piglet castration: welfare, boar taint, and other meat quality traits. Animals (Basel). 2019;9. 10.3390/ani9110884. [DOI] [PMC free article] [PubMed]
- Borghese A (2005) Buffalo production and research. REU Technical Series 67. FAO Regional Office for Europe
- Brosnahan MM, Paradis MR. Assessment of clinical characteristics, management practices, and activities of geriatric horses. J Am Vet Med Assoc. 2003;223:99–103. doi: 10.2460/javma.2003.223.99. [DOI] [PubMed] [Google Scholar]
- Brown DE, Dechow CD, Liu WS, Harvatine KJ, Ott TL. Hot topic: association of telomere length with age, herd, and culling in lactating Holsteins. J Dairy Sci. 2012;95:6384–6387. doi: 10.3168/jds.2012-5593. [DOI] [PubMed] [Google Scholar]
- Byun S (2010) Genes assoicated with variation in longevity and fecundity in sheep. Lincoln University
- Byun SO, Zhou H, Frampton CM, Hickford JG. No association between variation in the ovine calpastatin gene and either longevity or fertility in sheep. Animal Genetics. 2010;41:223–224. doi: 10.1111/j.1365-2052.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Byun SO, Zhou H, Hickford JG. Characterization of genetic variation in the Forkhead box class O3 gene (FOXO3) in sheep. DNA Cell Biol. 2011;30:449–452. doi: 10.1089/dna.2010.1193. [DOI] [PubMed] [Google Scholar]
- Byun SO, Forrest RH, Frampton CM, Zhou H, Hickford JG. An association between lifespan and variation in insulin-like growth factor I receptor in sheep. J Anim Sci. 2012;90:2484–2487. doi: 10.2527/jas.2011-4148. [DOI] [PubMed] [Google Scholar]
- Caliebe A, Nebel A, Makarewicz C, Krawczak M, Krause-Kyora B. Insights into early pig domestication provided by ancient DNA analysis. Sci Rep. 2017;7:44550. doi: 10.1038/srep44550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Comprehensive transcriptional landscape of porcine cardiac and skeletal muscles reveals differences of aging. Oncotarget. 2018;9:1524–1541. doi: 10.18632/oncotarget.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Comprehensive transcriptional profiling of aging porcine liver. Peer J. 2019;7:e6949. doi: 10.7717/peerj.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Comprehensive transcriptional profiling of porcine brain aging. Gene. 2019;693:1–9. doi: 10.1016/j.gene.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Clark S, Mora Garcia MB. A 100-year review: advances in goat milk research. J Dairy Sci. 2017;100:10026–10044. doi: 10.3168/jds.2017-13287. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proc Biol Sci. 2007;274:3097–3104. doi: 10.1098/rspb.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi B, Ballarin C, Mantovani R, Rota A. Aging and veterinary care of cats, dogs, and horses through the records of three university veterinary hospitals. Front Vet Sci. 2017;4:14. doi: 10.3389/fvets.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creevy KE, Austad SN, Hoffman JM, O'Neill DG, Promislow DE. The companion dog as a model for the longevity dividend cold spring. Harb Perspect Med. 2016;6:a026633. doi: 10.1101/cshperspect.a026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP, et al. A reassessment of genes modulating aging in mice using demographic measurements of the rate of aging. Genetics. 2018;208:1617–1630. doi: 10.1534/genetics.118.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo IR, DesCoteaux L, Leslie K, Fredeen A, Shewfelt W, Preston A, Dowling P. A meta-analysis review of the effects of recombinant bovine somatotropin. 2. Effects on animal health, reproductive performance, and culling. Can J Vet Res. 2003;67:252–264. [PMC free article] [PubMed] [Google Scholar]
- Dohoo IR, Leslie K, DesCoteaux L, Fredeen A, Dowling P, Preston A, Shewfelt W. A meta-analysis review of the effects of recombinant bovine somatotropin. 1. Methodology and effects on production. Can J Vet Res. 2003;67:241–251. [PMC free article] [PubMed] [Google Scholar]
- El Debaky HA, Kutchy NA, Ul-Husna A, Indriastuti R, Akhter S, Purwantara B, Memili E. Potential of water buffalo in world agriculture: challenges and opportunities. Appl Anim Sci. 2019;35:255–268. doi: 10.15232/aas.2018-01810. [DOI] [Google Scholar]
- Favier RP, Mol JA, Kooistra HS, Rijnberk A. Large body size in the dog is associated with transient GH excess at a young age. J Endocrinol. 2001;170:479–484. doi: 10.1677/joe.0.1700479. [DOI] [PubMed] [Google Scholar]
- Fleming JM, Creevy KE, Promislow DEL. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25:187–198. doi: 10.1111/J.1939-1676.2011.0695.X. [DOI] [PubMed] [Google Scholar]
- Flisikowska T, Kind A, Schnieke A. The new pig on the block: modelling cancer in pigs. Transgenic Res. 2013;22:673–680. doi: 10.1007/s11248-013-9720-9. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler ME. Health care of the geriatric llama and alpaca. Vet Clin North Am Food Anim Pract. 1994;10:391–399. doi: 10.1016/s0749-0720(15)30571-5. [DOI] [PubMed] [Google Scholar]
- Gautam M, Stevenson MA, Lopez-Villalobos N, McLean V. Risk factors for culling, sales and deaths in New Zealand dairy goat herds, 2000–2009. Front Vet Sci. 2017;4:191. doi: 10.3389/fvets.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer KA, Hughes LM, Masternak MM. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr) 2011;33:475–483. doi: 10.1007/s11357-010-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi Hay E, Roberts A. Genomic prediction and genome-wide association analysis of female longevity in a composite beef cattle breed. J Anim Sci. 2017;95:1467–1471. doi: 10.2527/jas.2016.1355. [DOI] [PubMed] [Google Scholar]
- Hamilton JB, Mestler GE. Mortality and survival: comparison of eunuchs with intact men and women in a mentally retarded population. J Gerontol. 1969;24:395–411. doi: 10.1093/geronj/24.4.395. [DOI] [PubMed] [Google Scholar]
- Haskell MJ, Rennie LJ, Bowell VA, Bell MJ, Lawrence AB. Housing system, milk production, and zero-grazing effects on lameness and leg injury in dairy cows. J Dairy Sci. 2006;89:4259–4266. doi: 10.3168/jds.S0022-0302(06)72472-9. [DOI] [PubMed] [Google Scholar]
- Heise J, Liu Z, Stock KF, Rensing S, Reinhardt F, Simianer H. The genetic structure of longevity in dairy cows. J Dairy Sci. 2016;99:1253–1265. doi: 10.3168/jds.2015-10163. [DOI] [PubMed] [Google Scholar]
- Hickson LJ, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs A, et al. Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab. 2018;11:113–128. doi: 10.1016/j.molmet.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Creevy KE, Franks A, O'Neill DG, Promislow DEL. The companion dog as a model for human aging and mortality. Aging Cell. 2018;17:e12737. doi: 10.1111/acel.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, O’Neill DG, Creevy KE, Austad SN. Do female dogs age differently than male dogs? J Gerontol A Biol Sci Med Sci. 2018;73:150–156. doi: 10.1093/gerona/glx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, et al. Tryptophan metabolism is differently regulated between large and small dogs. Geroscience. 2019. 10.1007/s11357-019-00114-x. [DOI] [PMC free article] [PubMed]
- Horn M, Knaus W, Kirner L, Steinwidder A. Economic evaluation of longevity in organic dairy cows. Organic Agric. 2012;2:127–143. doi: 10.1007/s13165-012-0027-6. [DOI] [Google Scholar]
- Ireland JL, McGowan CM, Clegg PD, Chandler KJ, Pinchbeck GL. A survey of health care and disease in geriatric horses aged 30 years or older. Vet J. 2012;192:57–64. doi: 10.1016/j.tvjl.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Jimenez AG, Winward J, Beattie U, Cipolli W. Cellular metabolism and oxidative stress as a possible determinant for longevity in small breed and large breed dogs. Plos One. 2018;13:e0195832. doi: 10.1371/journal.pone.0195832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome. 2016;27:279–288. doi: 10.1007/s00335-016-9638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy U, Sharma A, Phillips CJC. The sheltering of unwanted cattle, experiences in India and implications for cattle industries elsewhere. Animals (Basel). 2018;8. 10.3390/ani8050064. [DOI] [PMC free article] [PubMed]
- Kern G, Kemper N, Traulsen I, Henze C, Stamer E, Krieter J. Analysis of different effects on longevity in four sheep breeds of northern Germany. Small Ruminant Res. 2010;90:71–74. doi: 10.1016/j.smallrumres.2010.01.005. [DOI] [Google Scholar]
- Kilbride AL, Gillman CE, Green LE. A cross-sectional study of the prevalence of lameness in finishing pigs, gilts and pregnant sows and associations with limb lesions and floor types on commercial farms in England. Anim Welf. 2009;18:215–224. [Google Scholar]
- Klein B. Cunningham’s textbook of veterinary physiology. 6th ed: Saunders; 2019.
- Korec E, et al. Genus Bison has the biggest sex-related difference in longevity among mammals. Approaches Poult Dairy Vet Sci. 2019;5:1–4. doi: 10.31031/APDV.2019.05.000620. [DOI] [Google Scholar]
- Kraus WE, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. doi: 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JB, Paengkoum P. Current status, challenges and the way forward for dairy goat production in Asia - conference summary of dairy goats in Asia Asian-Australas. J Anim Sci. 2019;32:1233–1243. doi: 10.5713/ajas.19.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MY, Song EJ, Kang KS, Nam YD. Age-related compositional and functional changes in micro-pig gut microbiome. Geroscience. 2019;41:935–944. doi: 10.1007/s11357-019-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JK. Advances in swine biomedical model genomics. Int J Biol Sci. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfadyen MA, Daniel Z, Kelly S, Parr T, Brameld JM, Murton AJ, Jones SW. The commercial pig as a model of spontaneously-occurring osteoarthritis. BMC Musculoskelet Disord. 2019;20:70. doi: 10.1186/s12891-019-2452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malher X, Seegers H, Beaudeau F. Culling and mortality in large dairy goat herds managed under intensive conditions in western France. Livest Prod Sci. 2001;71:75–86. doi: 10.1016/S0301-6226(01)00242-1. [DOI] [Google Scholar]
- Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millikan LE, Boylon JL, Hook RR, Manning PJ. Melanoma in Sinclair swine: a new animal model. J Invest Dermatol. 1974;62:20–30. doi: 10.1111/1523-1747.ep12676714. [DOI] [PubMed] [Google Scholar]
- Min KJ, Lee CK, Park HN. The lifespan of Korean eunuchs. Curr Biol. 2012;22:R792–R793. doi: 10.1016/j.cub.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow LD, et al. Retrospective analysis of post-mortem findings in 1,444 aged donkeys. J Comp Pathol. 2011;144:145–156. doi: 10.1016/j.jcpa.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Muller DWH, Gaillard JM, Lackey LB, Hatt JM, Clauss M. Comparing life expectancy of three deer species between captive and wild populations. Eur J Wildl Res. 2010;56:205–208. doi: 10.1007/s10344-009-0342-8. [DOI] [Google Scholar]
- Munoz CA, Campbell AJD, Hemsworth PH, Doyle RE. Evaluating the welfare of extensively managed sheep. PLoS One. 2019;14:e0218603. doi: 10.1371/journal.pone.0218603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholatos JW, et al. Cellular energetics and mitochondrial uncoupling in canine aging. Geroscience. 2019;41:229–242. doi: 10.1007/s11357-019-00062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppibool U, Koonawootrittriron S, Elzo MA, Suwanasopee T. Factors affecting length of productive life and lifetime production traits in a commercial swine herd in Northern Thailand. Agric Nat Resour. 2016;50:71–74. [Google Scholar]
- Nussey DH, Wilson AJ, Brommer JE. The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol. 2007;20:831–844. doi: 10.1111/j.1420-9101.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. 2013;198:638–643. doi: 10.1016/j.tvjl.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg. 2015;17:125–133. doi: 10.1177/1098612X14536176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnapureddy AR, Stayner C, McEwan J, Baddeley O, Forman J, Eccles MR. Large animal models of rare genetic disorders: sheep as phenotypically relevant models of human genetic disease. Orphanet J Rare Dis. 2015;10:107. doi: 10.1186/s13023-015-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott JHD, Lamming GE. The effects of castration on meat production in cattle, sheep and pigs. J Agric Sci. 1964;63:341–357. doi: 10.1017/S0021859600016026. [DOI] [Google Scholar]
- Raziq A, Younas M, Kakar MA. Camel- a potential dairy animal in difficult environment. Pakistan J Agric Sci. 2008;45:263–267. [Google Scholar]
- Scholtens MR, Lopez-Villalobos N, Garrick DJ, Blair HT. Heritability of longevity in New Zealand dairy goats. N Z J Anim Sci Prod. 2018;78:11–15. [Google Scholar]
- Seeker LA, et al. Bovine telomere dynamics and the association between telomere length and productive lifespan. Sci Rep. 2018;8:12748. doi: 10.1038/s41598-018-31185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Phillips CJC. Lameness in sheltered cows and its association with cow and shelter attributes. Animals (Basel). 2019;9. 10.3390/ani9060360. [DOI] [PMC free article] [PubMed]
- Smith JA. Noninfectious diseases, metabolic diseases, toxicities, and neoplastic diseases of South American camelids. Vet Clin North Am Food Anim Pract. 1989;5:101–143. doi: 10.1016/s0749-0720(15)31006-9. [DOI] [PubMed] [Google Scholar]
- Steri R, Moioli B, Catillo G, Galli A, Buttazzoni L. Genome-wide association study for longevity in the Holstein cattle population. Animal. 2019;13:1350–1357. doi: 10.1017/S1751731118003191. [DOI] [PubMed] [Google Scholar]
- Tacutu R, et al. Human ageing genomic resources: new and updated databases. Nucleic Acids Res. 2018;46:D1083-D1090. doi: 10.1093/nar/gkx1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapprest J, et al. Fallen stock data: an essential source of information for quantitative knowledge of equine mortality in France. Equine Vet J. 2017;49:596–602. doi: 10.1111/evj.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MG, Bennett-Wimbush K, Keisler DH, Loch WE. Plasma concentrations of growth hormone and insulin-like growth factor-I in prepuberal quarter horses and ponies. J Equine Vet Sci. 1998;18:52–55. doi: 10.1016/S0737-0806(98)80187-1. [DOI] [Google Scholar]
- Urfer SR, Kaeberlein M. Desexing dogs: a review of the current literature. Animals (Basel). 2019:9. 10.3390/ani9121086. [DOI] [PMC free article] [PubMed]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarst M, Roderick S, Lund V, Lockeretz W (2003) Animal health and welfare in organic agriculture. CABI
- Valentine BA, Martin JM. Prevalence of neoplasia in llamas and alpacas (Oregon State University, 2001–2006) J Vet Diagn Invest. 2007;19:202–204. doi: 10.1177/104063870701900213. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou J, Wang G, Cai S, Zeng X, Qiao S. Advances in low-protein diets for swine. J Anim Sci Biotechnol. 2018;9:60. doi: 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigl R (2005) Longevity of mammals in captivity: from the living collections of the world. A list of mammalian longevity in captivity. Schweizerbart'sche, E.
- Welsh CE, Duz M, Parkin TDH, Marshall JF. Prevalence, survival analysis and multimorbidity of chronic diseases in the general veterinarian-attended horse population of the UK. Prev Vet Med. 2016;131:137–145. doi: 10.1016/j.prevetmed.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Xu M, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef G, Wallace WA, Dagleish MP, Cousens C, Griffiths DJ. Ovine pulmonary adenocarcinoma: a large animal model for human lung cancer. Ilar J. 2015;56:99–115. doi: 10.1093/ilar/ilv014. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Guldbrandtsen B, Thomasen JR, Lund MS, Sahana G. Genome-wide association study for longevity with whole-genome sequencing in 3 cattle breeds. J Dairy Sci. 2016;99:7289–7298. doi: 10.3168/jds.2015-10697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


