Abstract
Alzheimer’s disease (AD) is the most common cause of dementia worldwide. AD is a multifactorial disease with simultaneous occurrence of several connected pathological processes including mitochondrial dysfunction and impaired proteostasis. Most of these are also implicated in organismal aging per se. The presence of separable pathological conditions poses the opportunity to try combination treatments that target these different processes separately. This approach may provide an effective strategy to target AD; therefore, we investigated whether a combination of metformin (targeting mitochondria and energy metabolism) and lithium (targeting proteostasis) could result in synergistic benefits. In this perspective paper, we looked for benefits in lifespan and healthspan using a transgenic nematode strain, GRU102, which expresses pan-neuronal human amyloid-beta (Aβ). Individually, metformin and lithium extended the lifespan of both non-transgenic GRU101 controls and GRU102. Combination treatment using metformin and lithium did not result in any synergistic increase in GRU102 lifespan, but this treatment did result in a significant compression of morbidity when compared with each individual drug, resulting in relative and absolute extension of healthspan. Despite over-expressing pathogenic human Aβ in their neurons, GRU102 worms treated with the combination treatment enjoyed longer lifespans and significantly compressed morbidity, even compared with untreated non-transgenic animals. These findings suggest combination treatment as a strategy to compress morbidity, and highlight the distinction between healthspan and lifespan.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00169-1) contains supplementary material, which is available to authorized users.
Keywords: C. elegans, Alzheimer’s, Combination therapy, Synergy
Alzheimer’s disease: a multifactorial disease
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder characterized by cognitive decline and memory loss. As the most common form of dementia, the global socioeconomic cost of AD and dementia-related illnesses is currently estimated to be USD 818 billion (Wimo et al. 2017). As populations around the world are rapidly aging, this cost is set to increase rapidly. Yet, and despite extensive efforts, no disease-modifying therapy has been approved for treatment of AD.
The key pathological hallmarks of AD are amyloid plaques and neurofibrillary tangles, which form the basis of the amyloid cascade and Tau hypotheses, respectively. While some promising results have been obtained in pre-clinical and early phase clinical trials targeting modulation of Aβ or Tau, unfortunately, none of these interventions have been effective in phase III trials thus far (Cummings et al. 2018; Hara et al. 2019). These findings question whether targeting Aβ or Tau alone is sufficient to modulate AD, and highlight the need to explore alternative or complementary therapies.
The pathogenesis of AD appears to be complex and multifactorial, involving more than one pathological factor (Gong et al. 2018). In addition to Aβ and Tau, mitochondrial dysfunction, oxidative stress, increased inflammation, and impairment in proteostasis have been identified as contributing factors in the pathophysiology of AD (Sery et al. 2013). Due to this multifactorial nature, combination therapy simultaneously targeting separate pathological events may be more likely to succeed as a preventative or disease-modifying therapy. Notably, combination therapy is already a common research theme in the cancer field (Bayat Mokhtari et al. 2017; Lu et al. 2017). In Drosophila neurodegeneration and AD models, several studies have reported protective effects of combination treatment (Kerr et al. 2017; Sarkar et al. 2008). We have also recently demonstrated the feasibility of using combination drug therapy to target multiple pathways of aging, demonstrating a synergistic increase in nematode and Drosophila lifespans (Admasu et al. 2018).
Geroscience approach to target AD
Aging is the leading risk factor for AD (Austad 2016; Sierra and Kohanski 2017), and many of the pathological processes central to AD are also altered during biological aging itself (Hara et al. 2019). In fact, age-dependent changes are always easy to delineate from those underlying age-dependent diseases. For example, brain autopsies of subjects above the age of 85 showed that AD-like pathology, characterized by presence of Aβ plaques and Tau tangles, was observable even though no clinical dementia was present (Polvikoski et al. 2006). Such observations question the distinction between aging and age-dependent diseases, including AD, and suggest an alternative approach of targeting AD by directly targeting key biological processes of aging, an approach known as “geroscience.”
The geroscience approach is supported by evidence from model organisms, ranging from yeast to mice, suggesting that aging can be delayed and aging trajectories modified (Kennedy and Pennypacker 2014; Mitchell et al. 2015; Zainabadi 2018). Importantly, interventions that delay aging are typically accompanied not by increased survival in a diseased state but by a delay in the onset age-related diseases and conditions (Kennedy et al. 2014; Sierra 2016). Given these observations, a combination therapy targeting the fundamental processes of aging may be a promising approach to prevent age-related disease such as AD (Finkel 2005; Goldberg et al. 2018; Hara et al. 2019; Kennedy et al. 2014).
Combination therapy for AD
Here, we explore this concept by testing a combination therapy for AD consisting of metformin (targeting mitochondria and energy metabolism) and lithium (targeting proteostasis). These two compounds were chosen because both drugs are currently approved for other conditions in humans and have a well-documented safety profile, making them good candidates for drug-repurposing. Worldwide, metformin is the recommended first-line drug for treatment of type 2 diabetes (Piskovatska et al. 2019). Interestingly, type 2 diabetic patients with metformin treatment were found to have longer survival than age-matched non-diabetic controls despite having more metabolic syndromes and comorbidities at baseline (Bannister et al. 2014), suggesting that metformin reduces mortality in humans.
Experimentally, metformin has been shown to extend lifespan and healthspan in nematodes, mice, and rats (reviewed in (Anisimov 2013)). Studies from nematodes have suggested that metformin improves lifespan by promoting stress resistance (Onken and Driscoll 2010), inducing mitohormesis (De Haes et al. 2014), and altering microbial folate and methionine metabolism (Cabreiro et al. 2013). Metformin has also been reported to improve AD-like pathology in transgenic nematode models that express human Aβ peptides (Ahmad and Ebert 2017). In transgenic mouse models of AD, metformin has been shown to reduce Aβ levels and prevent cognitive impairment (Matthes et al. 2015), although its effect on cognition in these mice may be gender-specific (DiTacchio et al. 2015). In a small randomized placebo-controlled crossover trial of 20 non-diabetic human subjects suffering from mild cognitive impairment or mild dementia due to AD, metformin treatment significantly improved executive function, and trends suggested improvement in learning/memory and attention (Koenig et al. 2017). Larger placebo-controlled randomized controlled trials are needed to verify the effects of metformin on slowing, or even potentially reversing, the cognitive decline of AD. One such trial, targeting aging with metformin (TAME), is currently underway to investigate the potential of metformin in preventing the occurrence of age-related conditions, including dementia, in humans (Barzilai et al. 2016).
Lithium is commonly used for treatment of bipolar disease and mood disorder (Licht 2012). Lithium concentration in drinking water was found to have an inverse correlation with all-cause mortality in 18 Japanese municipalities (Zarse et al. 2011), suggesting that exposure to lithium decreases mortality in humans too. Similar to metformin, treatment with lithium increased the lifespan of nematodes and Drosophila (Castillo-Quan et al. 2016; McColl et al. 2008; Tam et al. 2014; Zarse et al. 2011). While the main molecular targets of lithium are thought to be inhibition of inositol monophosphatase (IMPA) and glycogen synthase kinase-3 (GSK-3) (Kerr et al. 2018), animal studies have revealed that lithium reduces oxidative damage and increases the expression of antioxidant enzymes in Drosophila and rats (Kasuya et al. 2009; Kerr et al. 2017; Khan et al. 2015), and also promotes longevity via a hormetic mechanism that is dependent on nuclear factor erythroid 2-related factor 2 (NRF-2), a key regulator of cellular resistance to oxidants (Castillo-Quan et al. 2016). Lithium has also been reported to enhance autophagy and reduce protein translation in nematodes and Drosophila (Sarkar et al. 2005; Sarkar and Rubinsztein 2006; Sofola et al. 2010; Sofola-Adesakin et al. 2014; Tam et al. 2014). A recent systematic review by Heard et al. (2018) found that lithium produced statistically significant benefits on cognition in animal models of AD (5 mice, 2 rats, 1 Drosophila, and 1 zebrafish; P < 0.05). The results in humans with AD were mixed however with three randomized controlled studies showing a statistically significant benefit of lithium on cognition, while two other open-label and one randomized controlled study did not (Heard et al. 2018).
Preliminary evidence from Caenorhabditis elegans
Given that metformin and lithium target distinct biological pathways related to aging, we reasoned that combination treatment might lead to an effective AD intervention. We investigated whether the combination of metformin and lithium could result in a synergistic increase in lifespan and healthspan of a transgenic nematode strain, GRU102, which expresses pan-neuronal human Aβ. The GRU102 animals express low levels of pan-neuronal Aβ and display mild age-dependent deterioration in neuromuscular behaviour, such as abnormal head shake and reduced pumping behaviour (Fong et al. 2016). The GRU102 animals do, however, exhibit clear healthspan and lifespan detriments in comparison with the GRU101 non-transgenic vector controls (Fong et al. 2016), providing two measurements to test as the phenotypic readouts for our combination therapy.
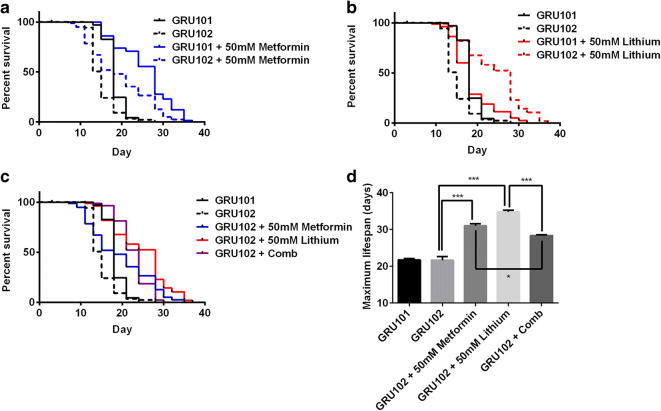
We found that metformin and lithium both individually extended the lifespan of GRU102 animals (Fig. 1 a and b). At the dose investigated, metformin, but not lithium, treatment increased the median lifespan of the GRU101 animals (vector controls for GRU102 with wild-type behaviour). Comparing the effects of drug treatment between the GRU101 and GRU102 animals, the increase in median lifespan due to metformin treatment was greater in the GRU101 controls than in GRU102 animals, with approximately 55% increase in that of GRU101 controls (median lifespan of treated vs untreated controls: 28 vs 18 days) and 20% increase in that of the GRU102 animals (median lifespan of treated vs untreated GRU102: 18 vs 15 days). These observations suggest that metformin treatment cannot completely compensate for the Aβ-induced detriments and therefore benefits the controls more than the Aβ-expressing GRU102 animals. These data also suggest that the lifespan benefits of metformin in the GRU102 animals result from an effect in modulating the aging process in general, rather than specifically modulating Aβ.
Fig. 1.
Effects of metformin, lithium, and combination treatment on the lifespan of GRU102 animals. a Metformin treatment significantly increased the lifespan of GRU101 and GRU102 animals (log-rank test P < 0.001; n = 70–110 animals per group). b Lithium treatment significantly increased the lifespan of GRU102 animals (log-rank test P < 0.001; n = 60–110 animals per group) but not the GRU101 controls. c Combination treatment using metformin and lithium (Comb) in the GRU102 animals. d Maximum lifespan, defined as the last 10% surviving animals of the cohort. One-way ANOVA Sidak’s multiple comparison test, *P < 0.05, ***P < 0.001. Lifespan experiments in a–d have been repeated in at least one other independent trial with similar results obtained (Supplementary Table S1)
Lithium treatment, on the other hand, resulted in a ~ 90% increase in median lifespan in the GRU102 animals (median lifespan of treated vs untreated GRU102: 28 vs 15 days), although no difference in the median lifespan was observed between the lithium-treated and untreated GRU101 controls. From our initial dose optimization experiments (Fig. S1), we established that a higher concentration of lithium (50 mM) was required for benefits in GRU102 animals, compared with the concentration (10 mM) previously found to extend lifespan in wild-type nematodes (McColl et al. 2008; Tam et al. 2014). The higher lithium dose in our study may therefore have induced toxicity in the GRU101 controls, thereby resulting in a lack of lifespan benefits.
Drug synergies
Given the individual benefits of metformin and lithium in the GRU102 animals, we tested the combination treatment in our transgenic animals, looking for synergistic lifespan benefits. Here, it may be beneficial to clarify our terminology related to the concept of synergy (summarized in Fig. S2A). Synergy can be defined in terms of the numerical size of any measure quantifying a given phenotype. For instance, benefits in terms of lifespan and healthspan are not restricted to lifespan alone but may, for example, include measures of healthy lifespan or relative period of morbidity. A drug combination is considered synergistic relative to a parameter if the combined effect on that parameter is numerically greater than expected from combining the individual benefits of each drug, that is, the benefits of the combination is statistically significantly larger than the sum of the individual effects. Central to the concept of synergy is the idea of additive effect; a combination is considered fully additive, if the combined effect is statistically indistinguishable from the sum of the individual effects. However, biological effect sizes, especially lifespan effects, are intrinsically highly variable, and it is often difficult to quantify the expected additive effects of a drug combination precisely. In practice, any effect that is significantly larger than expected for the better (more efficacious) of the two drugs individually is often considered additive. Moreover, the mathematical definition of additivity remains controversial (Roell et al. 2017). When the interaction between two drugs results in a reduction of effect size compared with the better of the individual drugs, this can be considered an antagonistic interaction. Finally, if combination treatment results in effects that are statistically indistinguishable from the effect of either of the single drugs alone, then the two drugs can be considered redundant.
It should be noted that the above definitions are exclusively based on comparison of the numerical size of single, specific effects. An alternative definition of synergy, based on that used to explain non-linear interactions in dynamical systems (Anderson 1972; Strogatz 2014), may also be useful. Synergy in this context can be defined based on identifying mechanism or benefit where the effect of whole (drug combination) is qualitatively different from the sum of its parts (effects of each drug individually). We previously applied this definition of synergy to transcriptional changes, observing that some drug combinations result in recruitment of significant additional/different genes and pathways, from those affected by either drug individually (Admasu et al. 2018).
Qualitatively, the combination treatment comprising metformin and lithium appears to have a redundant effect on lifespan (Fig. S2B), as the increase in median lifespan from the combination treatment was similar to lithium treatment alone. The combination treatment even resulted in an antagonistic effect on the maximum lifespan, as the maximum lifespan of GRU102 with combination treatment was statistically lower than both the individual drug treatment (Fig. 1d, Fig. S2C).
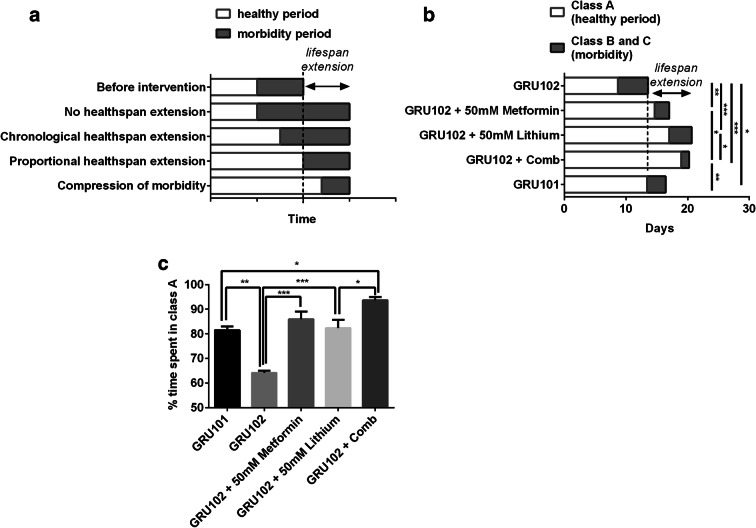
Yet, lifespan alone as an endpoint measurement is of limited value to determine the effectiveness of an intervention. Long lifespan alone does not necessarily translate to a longer healthspan, defined as the period of healthy and functional, morbidity-free time (Hansen and Kennedy 2016). Interventions that increase lifespan can have complex effects on healthspan (Hansen and Kennedy 2016) (Fig. 2a). At one extreme, an intervention targeting disease-specific causes of death may improve survival but extend the morbidity period without extending healthspan at all. Alternatively, an intervention may lead to a chronological healthspan extension with both the healthspan and the period of morbidity being extended proportionately. In such a case, absolute healthspan and morbidity are both increased but relative morbidity remains unchanged. Clearly, the most desirable case would be to increase lifespan by adding only healthy lifespan, while maintaining unchanged or even compressing morbidity. We, therefore, looked at the effects of individual and combination treatments on the healthspan of our animals.
Fig. 2.
Effects of drug treatments on the healthspan of the GRU102 animals. a An illustration showing the range of effects on healthspan of a lifespan-extending intervention (adapted from Hansen and Kennedy (2016)). b Healthy vs morbidity period of the GRU102 animals. Data plotted were the average values from 3 independent lifespan trials, with 60–120 animals per condition for each trial. One-way ANOVA Sidak’s multiple comparison test for time spent in morbidity period, *P < 0.05, **P < 0.05, ***P < 0.001. c Percentage of time spent in healthy period (class A). Data plotted were the average values from 3 independent lifespan trials, with 60–120 animals per condition for each trial. One-way ANOVA Sidak’s multiple comparison test for time spent in morbidity period, *P < 0.05, **P < 0.05, ***P < 0.001
To determine the healthspan of GRU102 animals, we used the health classification scheme (ABC scoring) described by Herndon et al. (2002). On solid media, C. elegans display sinusoidal movement, and as they age, this sinusoidal movement becomes increasingly disorganized. Based on their locomotor movements, nematodes that display spontaneous sinusoidal movement are classified as class A animals; nematodes that only move in response to prodding and in a non-sinusoidal manner are classified as class B animals; while class C animals are those that only exhibit head or tail movement upon prodding. We examined the time spent in class A or healthy state and B and C or unhealthy state in the treated GRU102 animals. Metformin or lithium treatment alone increased the healthy period of the GRU102 animals (time spent in healthy period (mean ± SEM) for GRU102 vs GRU102 + Met vs GRU102 + Li, 8.7 ± 0.3 vs 14.6 ± 0.3 vs 17.0 ± 1.4 days) and decreased the morbidity period (time spent in morbidity period (mean ± SEM) for GRU102 vs GRU102 + Met vs GRU102 + Li, 4.8 ± 0.1 vs 2.4 ± 0.5 vs 3.6 ± 0.6 days), suggesting that individual drug treatment resulted in a compression of morbidity (Fig. 2b). Combination treatment even resulted in a significantly shorter period of morbidity than the untreated non-transgenic GRU101 controls (Fig. 2b). Although we did not observe any synergistic increase in median lifespan and even observed a small reduction in maximum lifespan of the combination treatment compared with the individual drug treatment, the combination treatment resulted in the greatest compression of morbidity compared with all other conditions was tested (time spent in morbidity period (mean ± SEM) for GRU102 + Comb, 1.3 ± 0.2 days; one-way ANOVA post-test against all other condition P < 0.05, Fig. 2b). Qualitatively, the compression of morbidity appears to be additive, since the reduction in morbidity period of the combination treatment was similar to the sum of the individual treatment (Fig. S2E).
Interestingly, comparing the effects of single/combinatorial drug on healthspan and morbidity, the combination treatment has the greatest effects in both healthspan extension and morbidity compression. In other words, the combination treatment resulted in the longest healthspan and shortest morbidity period. We next examined the healthspan vs morbidity index of the different treatments, by taking the ratio between number of days spent in healthy period vs that of morbidity period. The combination treatment appears to have a synergistic effect on this index, as the effect size of the combination treatment was qualitatively greater than the sum of the individual treatment (Fig. S2F). We further found that the percentage of time spent in the healthy period was also the greatest in the combination treatment group (Fig. 2c), again supporting that the combination treatment has a significantly beneficial effect on both extending healthspan and reducing morbidity period.
In summary, our findings that metformin and lithium improve the lifespan of transgenic AD nematodes are in support of the geroscience approach to tackle age-related diseases. Although, when compared with individual drug treatment, combination treatment using both metformin and lithium did not lead to any further increase in median lifespan of the GRU102 animals, it did however lead to a synergistic increase in healthspan and a compression of morbidity. GRU102 animals with combination treatment not only lived longer than untreated GRU101 controls but also spent a significantly shorter period and fraction of time in morbidity, suggesting that combination treatment is able to normalize the Aβ-induced detriments. Finally, our observations highlight important differences between lifespan and healthspan, and suggest the importance of examining the healthspan effects of any lifespan-extending intervention, before concluding its anti-aging effects.
Electronic supplementary material
(DOCX 94 kb)
Dose optimization of lithium. 50 mM lithium treatment resulted in the greatest lifespan extension in GRU102 (n = 80–150 nematodes per condition). (PNG 65 kb)
Drug synergies in GRU102. A) An illustration of the different outcomes of a drug combination. Qualitative illustration of the effects of drug combination on different phenotype: B) Lifespan, C) Maximum lifespan, D) Healthspan, E) Morbidity and F) Healthspan vs morbidity index. Met: Metformin treatment, Li: Lithium treatment, Comb: Combination treatment, E(Met + Li): Expected additive effect of metformin and lithium. (PNG 1562 kb)
Author contributions
E.T. and J.G. conceived and designed the study. E.T. performed the experimental works. All authors analyzed the experimental data and wrote the manuscript.
Funding information
The study was funded by the Ministry of Education Singapore (Grant R-184-000-230-112, 2014-T2-2-120), Yale-NUS grant IG17-LR006 and IG18-LR001. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Admasu TD, Chaithanya Batchu K, Barardo D, Ng LF, Lam VYM, Xiao L, et al. Drug synergy slows aging and improves healthspan through IGF and SREBP lipid signaling. Dev Cell. 2018;47(1):67–79.e65. doi: 10.1016/j.devcel.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Ebert PR. Metformin attenuates abeta pathology mediated through levamisole sensitive nicotinic acetylcholine receptors in a C. elegans model of Alzheimer’s disease. Mol Neurobiol. 2017;54(7):5427–5439. doi: 10.1007/s12035-016-0085-y. [DOI] [PubMed] [Google Scholar]
- Anderson PW. More is different. Science. 1972;177(4047):393–396. doi: 10.1126/science.177.4047.393. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483–3489. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. The geroscience hypothesis: is it possible to change the rate of aging? In: Sierra F, Kohanski R, editors. Advances in Geroscience. Cham: Springer International Publishing; 2016. pp. 1–36. [Google Scholar]
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16(11):1165–1173. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Quan JI, Li L, Kinghorn KJ, Ivanov DK, Tain LS, Slack C, Kerr F, Nespital T, Thornton J, Hardy J, Bjedov I, Partridge L. Lithium promotes longevity through GSK3/NRF2-dependent hormesis. Cell Rep. 2016;15(3):638–650. doi: 10.1016/j.celrep.2016.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A. 2014;111(24):E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTacchio KA, Heinemann SF, Dziewczapolski G. Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2015;44(1):43–48. doi: 10.3233/jad-141332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6(12):971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- Fong S, Teo E, Ng LF, Chen CB, Lakshmanan LN, Tsoi SY, Moore PK, Inoue T, Halliwell B, Gruber J. Energy crisis precedes global metabolic failure in a novel caenorhabditis elegans Alzheimer disease model. Sci Rep. 2016;6:33781. doi: 10.1038/srep33781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Moltó PB, Cuezva JM, Maher P, Petrascheck M, Schubert D (2018) The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell 17(2). 10.1111/acel.12715 [DOI] [PMC free article] [PubMed]
- Gong CX, Liu F, Iqbal K. Multifactorial hypothesis and multi-targets for Alzheimer’s disease. J Alzheimers Dis. 2018;64(s1):S107–s117. doi: 10.3233/jad-179921. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kennedy BK. Does longer lifespan mean longer healthspan? Trends Cell Biol. 2016;26(8):565–568. doi: 10.1016/j.tcb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019;92(2):84–93. doi: 10.1212/wnl.0000000000006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard DS, Tuttle CSL, Lautenschlager NT, Maier AB. Repurposing proteostasis-modifying drugs to prevent or treat age-related dementia: a systematic review. Front Physiol. 2018;9:1520. doi: 10.3389/fphys.2018.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Kasuya J, Kaas G, Kitamoto T. Effects of lithium chloride on the gene expression profiles in drosophila heads. Neurosci Res. 2009;64(4):413–420. doi: 10.1016/j.neures.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Pennypacker JK. Drugs that modulate aging: the promising yet difficult path ahead. Transl Res. 2014;163(5):456–465. doi: 10.1016/j.trsl.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr F, Bjedov I, Sofola-Adesakin O. Molecular mechanisms of lithium action: switching the light on multiple targets for dementia using animal models. Front Mol Neurosci. 2018;11:297. doi: 10.3389/fnmol.2018.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, F., Sofola-Adesakin, O., Ivanov, D. K., Gatliff, J., Gomez Perez-Nievas, B., Bertrand, H. C., . . . Partridge, L. (2017). Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. 13(3), e1006593. doi:10.1371/journal.pgen.1006593 [DOI] [PMC free article] [PubMed]
- Khan A, Jamwal S, Bijjem KR, Prakash A, Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3beta modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287:66–77. doi: 10.1016/j.neuroscience.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, Detre JA, Wolk DA, Arnold SE. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord. 2017;31(2):107–113. doi: 10.1097/wad.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht RW. Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Ther. 2012;18(3):219–226. doi: 10.1111/j.1755-5949.2011.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Lu TR, Yarla NS, Wu HY, Xu B, Ding J, Zhu H. Drug combination in clinical cancer treatments. Rev Recent Clin Trials. 2017;12(3):202–211. doi: 10.2174/1574887112666170803145955. [DOI] [PubMed] [Google Scholar]
- Matthes F, Hettich MM, Ryan DP, Ehninger D, Krauss S. The anti-diabetic drug metformin improves cognitive impairment and reduces amyloid-beta in a mouse model of Alzheimer’s disease. Alzheimer Dementia. 2015;11(7):P845. doi: 10.1016/j.jalz.2015.06.1880. [DOI] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ. Pharmacogenetic analysis of lithium-induced delayed aging in caenorhabditis elegans. J Biol Chem. 2008;283(1):350–357. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskovatska, V., Stefanyshyn, N., Storey, K. B., Vaiserman, A. M., & Lushchak, O. (2019). Metformin as a geroprotector: experimental and clinical evidence. 20(1), 33-48. doi:10.1007/s10522-018-9773-5 [DOI] [PubMed]
- Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinisto L, Notkola IL, et al. Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology. 2006;26(2):76–82. doi: 10.1159/000090252. [DOI] [PubMed] [Google Scholar]
- Roell KR, Reif DM, Motsinger-Reif AA. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol. 2017;8:158. doi: 10.3389/fphar.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet. 2008;17(2):170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy. 2006;2(2):132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- Sery O, Povova J, Misek I, Pesak L, Janout V. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: a review. Folia Neuropathol. 2013;51(1):1–9. doi: 10.5114/fn.2013.34190. [DOI] [PubMed] [Google Scholar]
- Sierra F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med. 2016;6(4):a025163. doi: 10.1101/cshperspect.a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. Geroscience and the trans-NIH geroscience interest group, GSIG. Geroscience. 2017;39(1):1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Inhibition of GSK-3 ameliorates abeta pathology in an adult-onset drosophila model of Alzheimer’s disease. PLoS Genet. 2010;6(9):e1001087. doi: 10.1371/journal.pgen.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola-Adesakin O, Castillo-Quan JI, Rallis C, Tain LS, Bjedov I, Rogers I, Li L, Martinez P, Khericha M, Cabecinha M, Bähler J, Partridge L. Lithium suppresses abeta pathology by inhibiting translation in an adult drosophila model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:190. doi: 10.3389/fnagi.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. Nonlinear dynamics and chaos: with applications to physics, biology, chemistry, and engineering CRC press. 2014. [Google Scholar]
- Tam ZY, Gruber J, Ng LF, Halliwell B, Gunawan R. Effects of lithium on age-related decline in mitochondrial turnover and function in caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2014;69(7):810–820. doi: 10.1093/gerona/glt210. [DOI] [PubMed] [Google Scholar]
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainabadi K. A brief history of modern aging research. Exp Gerontol. 2018;104:35–42. doi: 10.1016/j.exger.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur J Nutr. 2011;50(5):387–389. doi: 10.1007/s00394-011-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 94 kb)
Dose optimization of lithium. 50 mM lithium treatment resulted in the greatest lifespan extension in GRU102 (n = 80–150 nematodes per condition). (PNG 65 kb)
Drug synergies in GRU102. A) An illustration of the different outcomes of a drug combination. Qualitative illustration of the effects of drug combination on different phenotype: B) Lifespan, C) Maximum lifespan, D) Healthspan, E) Morbidity and F) Healthspan vs morbidity index. Met: Metformin treatment, Li: Lithium treatment, Comb: Combination treatment, E(Met + Li): Expected additive effect of metformin and lithium. (PNG 1562 kb)