Abstract
An increase in the burden of senescent cells in tissues with age contributes to driving aging and the onset of age-related diseases. Genetic and pharmacologic elimination of senescent cells extends both health span and life span in mouse models. Heterochronic parabiosis in mice has been used to identify bloodborne, circulating pro- and anti-geronic factors able to drive or slow aging, respectively. However, whether factors in the circulation also regulate senescence is unknown. Here, we measured the expression of senescence and senescence-associated secretory phenotype (SASP) markers in multiple tissues from 4- to 18-month-old male mice that were either isochronically or heterochronically paired for 2 months. In heterochronic parabionts, the age-dependent increase in senescence and SASP marker expression was reduced in old mice exposed to a young environment, while senescence markers were concurrently increased in young heterochronic parabionts. These findings were supported by geropathology analysis using the Geropathology Grading Platform that showed a trend toward reduced hepatic lesions in old heterochronic parabionts. In summary, these results demonstrate that senescence is regulated in part by circulating geronic factors and suggest that one of the possible mediators of the rejuvenating effects with heterochronic parabiosis is through the reduction of the senescent cell burden.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00185-1) contains supplementary material, which is available to authorized users.
Keywords: Parabiosis, Cellular senescence, SASP, Geropathology, Aging
Introduction
A number of common aging mechanisms, termed pillars of aging, that influence life span and health span have been identified in multiple models of aging. Of these pillars, cellular senescence has received considerable attention recently as a key driver of aging as well as a potentially druggable target to prevent or treat multiple aging comorbidities (Kirkland et al. 2017; LeBrasseur et al. 2015; Tchkonia et al. 2013). Cellular senescence is an essentially irreversible growth arrest that occurs when cells experience persistent DNA damage or potential oncogenic insults (Gorgoulis et al. 2019), consistent with its role as an anticancer mechanism. Senescent cells also can secrete soluble factors, termed the senescence-associated secretory phenotype (SASP), that can induce secondary senescence both in a proximal and distal fashion. SASP factors are made up of metalloproteinases, growth factors, chemokines, and pro-inflammatory molecules that can induce cellular stress and recruit immune cell populations to sites of senescence (Coppe et al. 2010). However, with advancing age, senescent cells accumulate (Herbig et al. 2006; Waaijer et al. 2012), presumably due to inefficient removal by the immune system and their resistance to apoptosis (Campisi and d’Adda di Fagagna 2007; Zhu et al. 2015). By depleting the mitotically active progenitor pool and secreting factors that directly affect regenerative, inflammatory, and tissue remodeling processes, senescent cells contribute to age-related tissue deterioration, inflammation, fibrosis, and even hyperproliferation (Campisi and d’Adda di Fagagna 2007; Munoz-Espin and Serrano 2014). Correspondingly, genetic and/or pharmacologic clearance of senescent cells and/or suppression of the SASP is coupled with significant therapeutic benefits in murine models of natural aging (Baker et al. 2016), accelerated aging (Baker et al. 2011; Zhu et al. 2015), vascular dysfunction (Roos et al. 2016), wound healing (Demaria et al. 2014), atherosclerosis (Childs et al. 2016), lung disease (Schafer et al. 2017), diabetes (Schafer et al. 2016; Xu et al. 2015), osteoarthritis (Jeon et al. 2017), intervertebral disc degeneration (Patil et al. 2019), and osteoporosis (Farr et al. 2017).
The surgical pairing of animals, termed parabiosis, to establish a shared circulatory system has been used extensively to study the effects of circulating factors in driving disease. In addition, heterochronic parabiosis, involving the pairing of mice of young and old mice, has been used extensively in recent years to investigate whether exposure to a young circulatory system could act as a source of rejuvenation for an older animal as well as whether circulating pro-geronic factors from old mice can drive aging in young mice. These studies have shown the ability of circulating factors in young mice to improve myogenesis, hepatogenesis, regrowth of bone, and neurogenesis in old mice (Brack et al. 2007; Carlson et al. 2009a, b; Conboy et al. 2015; Conboy et al. 2005; Rebo et al. 2016; Villeda et al. 2011; Villeda et al. 2014; Yousef et al. 2015). Conversely, circulating factors in old mice, including β2-microglobulin (β2M), C-C Motif Chemokine Ligand 11 (CCL11), and transforming growth factor-β (TGF-β) (Carlson et al. 2009a; Smith et al. 2015; Villeda et al. 2011; Yousef et al. 2015), can drive aging in young mice.
While rejuvenating or aging effects of heterochronic parabiosis in mice have been well documented, very little is known about the impact of circulating factors on cellular senescence. To determine whether circulating factors can increase or decrease the senescent cell burden with aging in a cell non-autonomous manner, here we measured senescence (Sharpless and Sherr 2015; Yousefzadeh et al. 2019) in multiple tissues of isochronically and heterochronically paired male mice. We demonstrate that the age-related increase in markers of senescence found in old isochronic pairings was reduced in old heterochronic parabionts, whereas conversely, the markers of senescence were enhanced in young heterochronic mice. These findings were correlated with histopathologic analysis for common age-specific lesions in the mice. Taken together, these results demonstrate that senescence can be modified by circulating factors in a murine model of heterochronic parabiosis and suggest the pro- and anti-geronic effects observed in heterochronic parabiosis could be mediated, in part, via the regulation of cellular senescence.
Methods
Heterochronic parabiosis
Parabiotic mouse surgeries were performed by the Einstein Health Span Core in young (4-month-old) and old (18-month-old) male inbred C57BL/6 mice that were obtained from the National Institutes of Aging aged rodent colony. Surgical unions were performed between isochronic young, isochronic old, and heterochronic pairing of young and old mice (n = 8 mice per group). Following surgery, animals were kept on a partial heating pad to recover overnight. Paired mice were rigorously monitored and received subcutaneous injections of Banamine (2 mg/kg each) immediately postoperative and b.i.d. for 3 days and once daily for 4 days. Animals also received Ringers lactate subcutaneously immediately post-op and daily for 3 additional days to prevent dehydration. Animals remained conjoined for approximately 2 months before sacrifice. Samples isolated from young isochronic pairings (YY), old isochronic pairings (OO), young mice heterochronically paired to old mice (YO), and an old mice heterochronically paired to young mice (OY) are respectively denoted in Fig. 1A. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
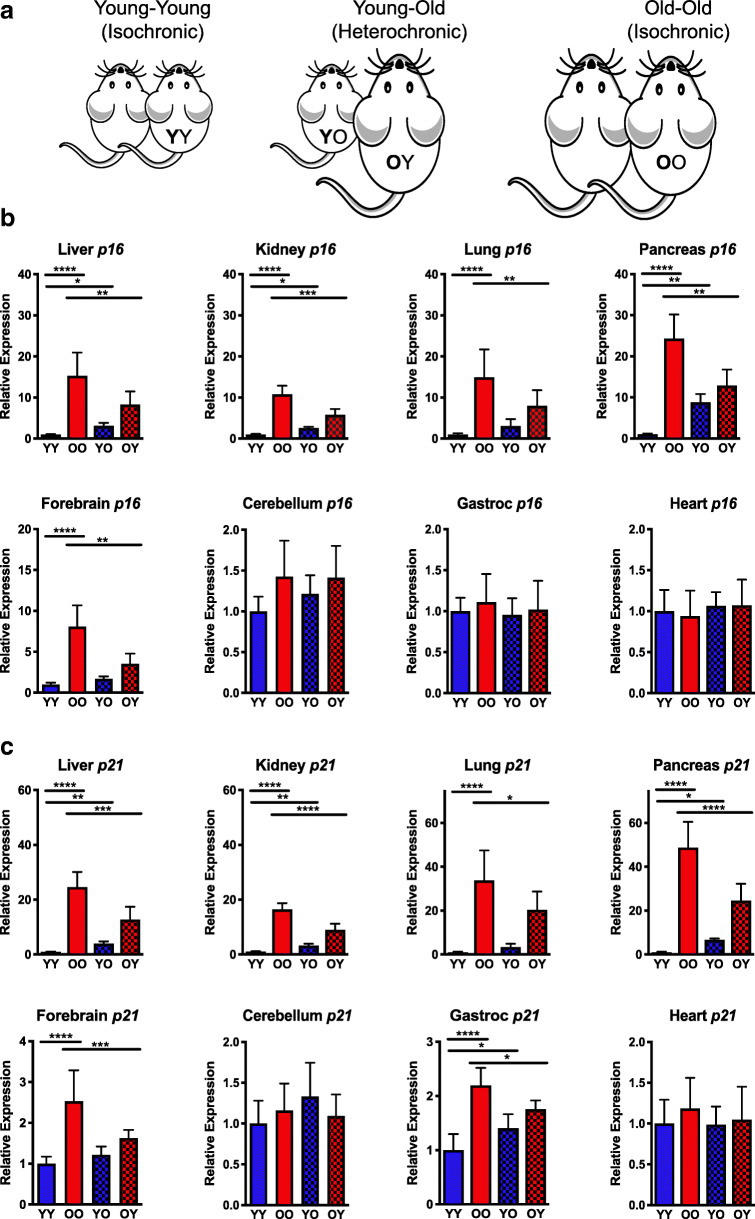
Fig. 1.
Expression of p16Ink4a and p21Cip1 in various tissues of parabiotic mice. A Schematic of isochronic and heterochronic parabiotic pairings of 4- and 18-month-old mice. B Total RNA was isolated from snap-frozen tissues collected from parabiotic mice (n = 5–8 mice per group). Expression of senescence markers Bp16Ink4a and Cp21Cip1 was measured by qPCR using the ∆∆Ct method and normalized to Gapdh expression. Values represent the mean ± SD and one-way ANOVA with Tukey’s test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
RNA isolation and qPCR
Analysis of senescence marker expression in tissues was performed as described in (Yousefzadeh et al. 2019). Tissues were harvested from euthanized animals and snap frozen in liquid nitrogen. Total RNA was isolated by Trizol extraction according to manufacturer’s specifications (Thermo Fisher, Waltham, MA). Gene expression changes in p16Ink4a, p21Cip1, Il1β, Il6, Mcp1, and Tnfα were quantified by qRT-PCR and analyzed by ΔΔCt method. Expression was normalized to Gapdh. Primer sequences were as follows: Cdkn1a (p21Cip1) Fwd 5’-GTCAGGCTGGTCTGCCTCCG-3′, Cdkn1a (p21Cip1) Rev. 5’-CGGTCCCGTGGACAGTGAGCAG-3′; Cdkn2a (p16Ink4a) Fwd 5′- CCCAACGCCCCGAACT-3′, Cdkn2a (p16Ink4a) Rev. 5’-GCAGAAGAGCTGCTACGTGAA-3′; Gapdh Fwd 5’-AAGGTCATCCCAGAGCTGAA-3′, Gapdh Rev. 5’-CTGCTTCACCACCTTCTTGA-3′; Il1β Fwd 5′- CACAGCAGCACATCAACAAG-3′, Il1β Rev. 5’-GTGCTCATGTCCTCATCCTG-3′; Il6 Fwd 5’-CTGGGAAATCGTGGAAT-3′, Il6 Rev. 5’-CCAGTTTGGTAGCATCCATC-3′; Mcp1 Fwd 5’-GCATCCACGTGTTGGCTCA-3′, Mcp1 Rev. 5’-CTCCAGCCTACTCATTGGGATCA-3′; Tnfα Fwd 5’-ATGAGAAGTTCCCAAATGGC-3′, Tnfα Rev. 5’-CTCCACTTGGTGGTTTGCTA-3′.
Chemokine analysis
Tissue levels of MCP-1 were measured by ELISA (RayBiotech, Norcross, GA) according to manufacturer’s specifications.
Geropathology
The GGP provides a grading system to assess biological aging in mice by measuring the pathological status of a wide range of tissues in a standardized scoring system. The grading system generates a numerical score for the total lesions in each tissue, which when averaged over the mice in the cohort provides a composite lesion score (CLS) for each tissue and mouse. Mouse tissues (n = 8 per tissue per group) were collected at the time of necropsy. Tissues were fixed in 10% neutral-buffered formalin for at least 24 h and stored in 70% ethanol until being processed and embedded into paraffin blocks. Tissues were sectioned and stained with hematoxylin and eosin and scored for both the presence and severity of age-related lesions by a veterinary pathologist to generate a CLS for each tissue (see Supplemental Table 1 for a list of age-related lesions scored in each tissue). This score has been confirmed to faithfully rise with chronological age in diverse mouse strains and is reflective of health span (Ladiges 2016).
Statistics
All data are expressed as the mean ± standard deviation (SD). Statistical differences among the groups were measured using one-way analysis of variance (ANOVA) with Tukey’s test. A p value of ≤ 0.05 was considered statistically significant. Asterisks in the figure represent the following: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. All statistical analyses were performed with Prism software version 8.3 (GraphPad, San Diego, CA).
Results
To investigate the impact of circulating factors on the senescent cell burden and age-related pathology of old and young mice, 4- and 18-month-old male animals were surgically paired for ~ 8 weeks before euthanasia and tissue collection (Fig. 1A). Isochronic pairings of young and old mice were included as controls. Initially, the levels of mRNA expression of senescence markers p16Ink4a and p21Cip1 were measured by qPCR in order to quantitatively determine the extent of the senescent cell burden (Sharpless and Sherr 2015; Yousefzadeh et al. 2019). Significantly elevated p16Ink4a expression was present in multiple tissues (forebrain, liver, lung, kidney, and pancreas) of old isochronic parabionts (OO) compared to their younger counterparts (YY) (Fig. 1B), but not in cerebellum, heart, and muscle tissue, similar to the pattern observed in natural aging (Yousefzadeh et al. 2020). Interestingly, a significant reduction in p16Ink4a expression was observed in multiple tissues of old mice that were paired to young mice (OY). Conversely, young heterochronic parabionts (YO) had increased p16Ink4a expression in the liver, kidney, and pancreas, but not in the forebrain or lung.
p21Cip1 expression was significantly elevated in all tissues of old isochronically paired mice (OO) except for the cerebellum and heart (Fig. 1C). In contrast, expression of p21Cip1 was significantly reduced in multiple tissues of old mice heterochronically paired to young mice (OY). A reciprocal increase in p21Cip1 expression was observed in all tissues of the young heterochronic parabionts (YO) except for cerebellum, forebrain, lung, and heart. These findings demonstrate that there is an age-related increase in cellular senescence observed in multiple tissues of old mice that is reduced by exposure to young blood through heterochronic parabiosis. Conversely, exposure of young mice to old blood increases expression of similar markers of cellular senescence.
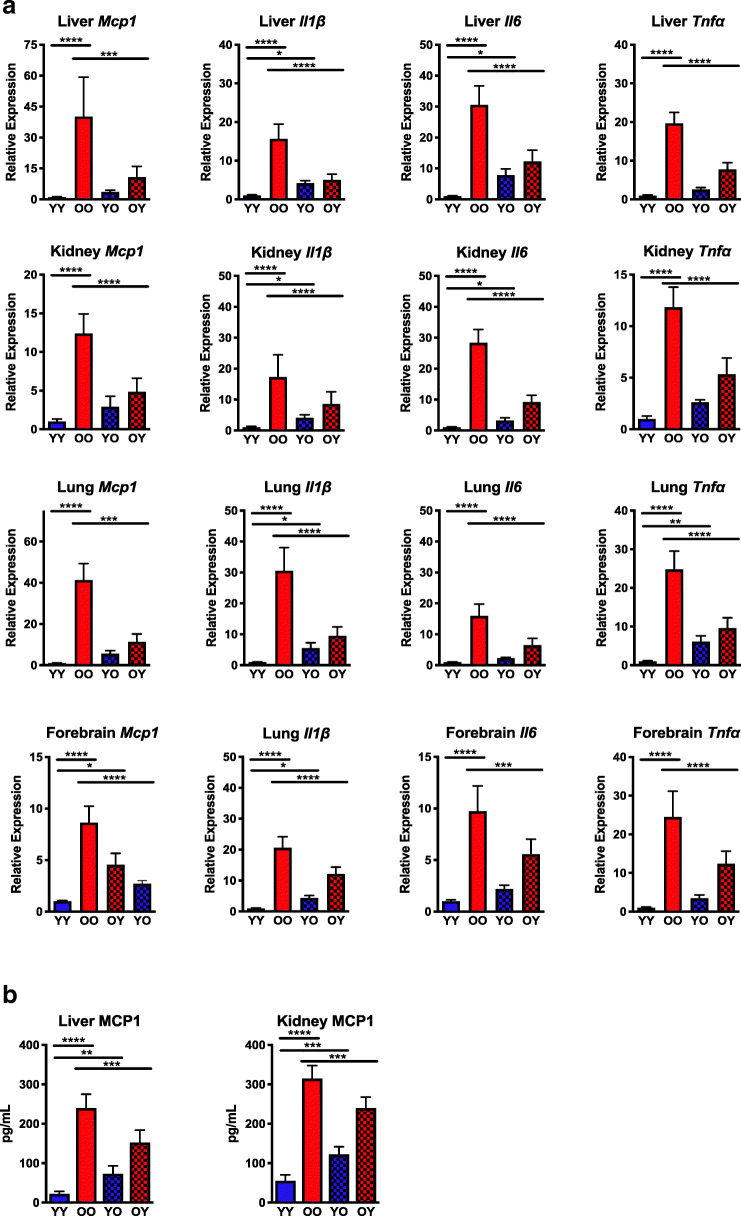
A common feature of cellular senescence is the release of soluble factors, termed the senescence-associated secretory phenotype (SASP), which can promote secondary senescence (Sharpless and Sherr 2015). These SASP factors are comprised of chemokines, cytokines, growth factors, and secreted proteases, which act on nearby cells in a paracrine manner or on distal cell populations in an endocrine manner (Coppe et al. 2010). To examine the effects of heterochronic parabiosis on SASP, mRNA expression of multiple SASP markers was quantified in tissues (Fig. 2A) with age-related increases in cellular senescence (Fig. 1). Expression of Mcp1, a chemokine that is responsible for monocyte recruitment and can serve as a surrogate biomarker for biological aging and frailty (Yousefzadeh et al. 2018a), was significantly increased in multiple tissues of isochronic old mice (OO) (Fig. 2A). Heterochronic pairing significantly reduced the Mcp1 expression in the forebrain, kidney, liver, and lung of old mice paired with young mice (OY). These gene expression findings were confirmed with analysis of tissue levels of MCP-1 protein by ELISA, which showed a similar pattern of age-related expression in the kidney and liver (Fig. 2B). Consistent with other markers, MCP-1 protein was significantly decreased in old heterochronic parabionts (OY) but was increased in young heterochronic parabionts (YO).
Fig. 2.
Analysis of the senescence-associated secretory phenotype in parabiotic mice. A Total RNA was isolated from snap-frozen tissues collected from parabiotic mice (n = 5–8 mice per group). Expression of senescence-associated secretory phenotype (SASP) genes Il1β, Il6, Mcp1, and Tnfα was measured by qPCR using the ∆∆Ct method and normalized to Gapdh expression. B Hepatic and renal levels of the SASP factor MCP-1 were quantified by ELISA (n = 7 mice per group). Values represent the mean ± SD and one-way ANOVA with Tukey’s test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Expression of pro-inflammatory cytokines (Il1β, Il6, and Tnfα) was also significantly increased in old isochronically paired mice (OO) compared with young isochronic controls (YY) (Fig. 2A). Old heterochronic parabionts (OY) experienced significant reductions in expression of these cytokines in all tissues measured. Significant increases in Il1β (forebrain, liver, lung, kidney), Il6 (liver, kidney), and Tnfα (lung) expression were observed in young heterochronic parabionts (YO) relative to young isochronic parabiont controls (YY). Concomitant with p16Ink4a and p21Cip1 expression (Fig. 1), SASP marker expression elevated in old isochronic pairings (OO) was significantly reduced in old heterochronic parabionts (OY).
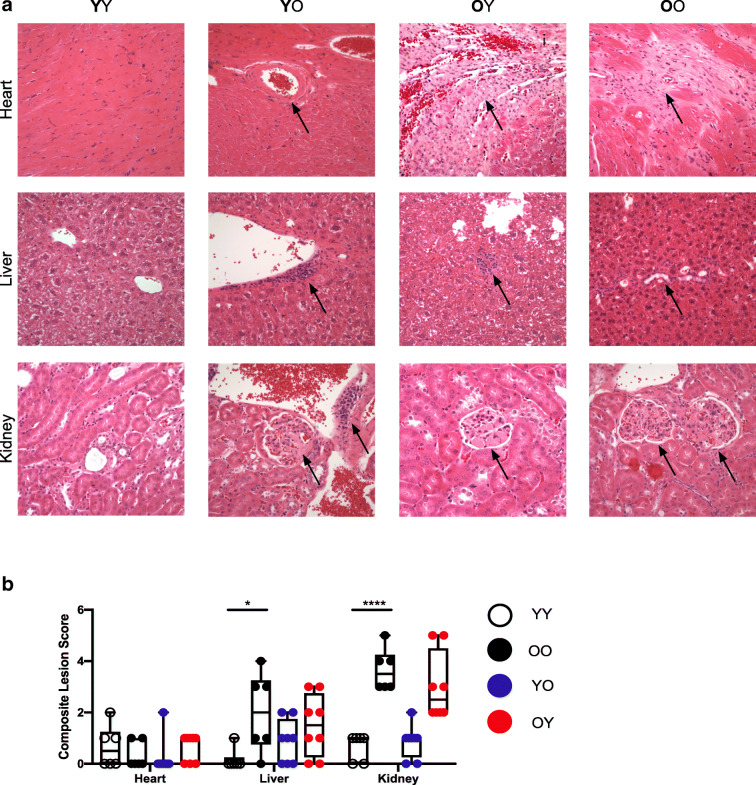
The accumulation of senescent cells with aging impairs tissue homeostasis (Baker et al. 2016; Krishnamurthy et al. 2004; Wang et al. 2009; Xu et al. 2018). To determine if the transposition of senescent phenotypes was associated with histopathologic changes in the heterochronically paired mice, tissues were assessed via the Geropathology Grading Platform (GGP) as recently described to generate a composite lesion score (CSL) for each tissue (Ladiges 2016) (Fig. 3). Age-related CLSs were significantly increased in the livers and kidneys of old isochronically paired mice (OO) relative to controls (YO) (Fig. 3B). No difference was found in CLSs from the hearts of young (YY) and old (OO) isochronic mouse pairings, consistent with p16Ink4a and p21Cip1 expression data collected in the heart (Fig. 1). Age-related lesions were modestly, but not significantly, reduced in livers from old heterochronic parabionts (OY) compared with isochronic parabionts (OO). Similarly, hepatic lesions were slightly, albeit not significantly elevated in young mice that were paired with old mice (YO) (Fig. 3B). These findings show that age-related lesions are increased in some tissues of parabiotic mice and that relatively short-term pairings of 2 months have only a modest effect on age-specific lesions.
Fig. 3.
Geropathological scoring of parabiotic mice. A Representative images of heart, liver, and kidney histologic sections from parabiotic mice. All images were taken at × 40. Heart (arteriosclerosis, YO; cardiomyopathy, OY, OO), liver (lymphoid aggregate, YO, OY; bile duct hyperplasia/cysts, OO), and kidney (nephropathy, YO, OY, OO; lymphoid aggregate, YO) pathologies are indicated by arrow. B Histopathologic composite lesion scores (CLS) for age-related pathology in tissues determined using the Geropathology Grading Platform (n = 6–8 tissues per group). Values represent the mean ± SD and one-way ANOVA with Tukey’s test. *p < 0.05, ***p < 0.001
Discussion
In this study, we examined the extent of cellular senescence in multiple tissues in both isochronic and heterochronic parabiotic mice to determine whether pro- and/or anti-geronic circulating factors can modulate senescent burden that accumulates with age. Our results demonstrate that senescence was enhanced in an age-dependent manner with tissues from old isochronic pairings (OO) showing significantly higher levels of p16Ink4a and p21Cip1 expression relative to samples from young isochronically paired mice (YY) except for the cerebellum, heart, and skeletal muscle. Similarly, SASP marker expression was elevated (Fig. 2A) in all the tissues from aged mice (OO) with increased senescence marker, p16Ink4a and p21Cip1, expression. Tissue levels of the SASP factor, MCP-1, which can serve as a surrogate biomarker for biological age (Yousefzadeh et al. 2018a), increased in the livers and kidneys of aged mice (OO). The elevated senescence marker expression was reduced in tissues taken from old heterochronic parabionts (OY) after exposure to a young circulation for 2 months as was SASP marker expression. In addition, the tissue levels of MCP-1 were significantly reduced in tissues from old heterochronic parabionts (OY). Taken together, these findings suggest that one mechanism through which exposure to a young circulatory system can confer beneficial effects is through reduction in cellular senescence.
Conversely, exposure to an old circulatory system enhanced cellular senescence. Expression of p16Ink4a and p21Cip1 was enhanced in multiple tissues in young mice paired with old mice (YO). Similarly, expression of individual SASP markers was increased in tissues from young heterochronic parabionts (YO). Consistent with our panel of senescence marker gene expression data, increased MCP-1 protein was present in young tissues of the heterochronic pairings (YO). We also have examined the effect of heterochronic parabiosis specifically on intervertebral disc degeneration (IDD), demonstrating that exposure to old blood accelerates disc matrix degeneration in young mice (Lei et al, submitted). Conversely, old mice paired with young mice (OY) exhibited a significant decrease in expression of cellular senescence and SASP markers but showed only a marginal decrease in the levels of disc MMP-13 and ADAMTS4 and aggrecan fragmentation. Taken together, these findings suggest that one mechanism in which exposure to an old circulatory system drives aging in multiple tissues is through an increase in cellular senescence.
The clearance of senescent cells has been reported to improve the histopathology of multiple tissues. Similarly, histological analysis of tissues in heterochronic parabiosis has been reported to improve the pathology of certain tissues such as heart and brain. The Geropathology Grading Platform (GGP) was developed by the Geropathology Research Network to detect the presence or absence as well as severity of age-related lesions (Supplemental Table 1) in histologic sections of murine tissues (Ladiges 2016; Ladiges et al. 2016; Ladiges et al. 2017). Lesion scores for each tissue are scored from each mouse in a cohort and averaged to generate a CLS, which appears to strongly correlate with the age of the animal. Using this platform, heart, liver, lung, and kidney sections from parabiotic mice were scored for lesions (Fig. 3A). Overall, age-associated lesions were significantly higher in old (OO) versus young (YY) isochronic pairings. The one exception was in heart tissue from aged isochronically paired mice (OO) (Fig. 3B) where there was no change in the CLS. A modest reduction in liver lesion scores occurred in old heterochronic parabionts (OY), but was not statistically significant. Also, we did not observe a histologic improvement in disc aging in old mice (OY) using a non-GGP score system (Lei et al, submitted). It is important to note that using the GGP to provide an unbiased composite lesion score did not result in an improvement in cardiac tissue pathology as previously reported (Loffredo et al. 2013). However, longer pairings may be required to observe significant changes in age-related lesions as scored using the GGP.
Many studies have provided strong evidence that soluble factors have a potent influence on aging, and some of this data has arisen from parabiosis studies. Our results demonstrated that the age-related increase in senescence presents in old isochronic parabiosis and that senescence was modulated in old (reduced) and young (increased) mice by circulating factors. SASP marker mRNA and protein expression in tissues confirmed these findings. These results are consistent with a model where certain SASP components produced by senescent cells in the old mice contribute to driving senescence in young heterochronic parabionts. Overall, these findings from heterochronically paired mice establish that certain tissues, with an increased senescent cell burden with age, would benefit by treatment with yet to be identified factors in young serum or interventions that may promote a more youthful circulation to reduce the senescent cell burden and thus improve health span, similar to the effects observed with senotherapeutics (Chang et al. 2016; Xu et al. 2018; Yousefzadeh et al. 2018b).
It now will be important to identify the specific circulating geronic factors that play key roles in driving or suppressing the senescent cell burden. Those factors important for driving senescence could include circulating SASP factors such as TNF-α, IL-1ß, IL-1α, IL-6, or HMGB1. Future analysis of emerging markers of senescence (Haque et al. 2020; Lawrence et al. 2018; Lewis et al. 2018; Liendl et al. 2019) would provide additional insight into more comprehensively determining the senescent cell burden in parabiotic mice beyond just the classical markers (p16Ink4a and p21Cip1 expression). In this regard, our preliminary analyses suggest that old murine and human serum-derived extracellular vesicles, potentially secreted by senescent cells, are able to induce senescence in cell culture. Also, CCL11 (Villeda et al. 2011) and ß2M (Smith et al. 2015), enriched in aged serum, have been implicated in driving certain age-related pathologies, but their effects on senescence are unknown. Unfortunately, even less is known about the factors in young serum important for suppressing senescence. GDF-11 (Katsimpardi et al. 2014; Loffredo et al. 2013), TIMP-2, and CSF-2 (Castellano et al. 2017) in young serum or cord blood have been reported to improve certain age-related pathologies, but whether they regulate senescence has not been reported.
In summary, these data provide evidence that markers of cellular senescence, typically associated as a manifestation of advanced age, can be rapidly induced by relatively short-term exposure to an old environment and lessened by exposure to a young environment. While we provide evidence across several distinct organs, it will be important to interrogate whether similar bidirectional effects are observed in other sites and to what extent these occur, including aorta, which was shown to have hyper-elevated levels of senescence (Khan et al. 2017; Yousefzadeh et al. 2020), adipose tissue, which is known to harbor a substantial number of senescent cells (Palmer et al. 2019; Xu et al. 2015) as well the brain, where senescence appears to occur particularly in endothelial cells and other cells to drive neurodegenerative changes (Bussian et al. 2018; Musi et al. 2018; Zhang et al. 2019). Comprehensive analysis into what particular cell types are affected by senescence in the brains of parabiotic mice, using spatial genomics or mass imaging, could provide a better understanding of the beneficial effects of parabiosis on cognition. For example, the senescent cell burden in astrocytes, a cell type implicated in causing cognitive decline (Csipo et al. 2020), is likely to be altered by the rejuvenating effects of parabiosis. Ultimately, a better understanding of the cell autonomous and non-autonomous drivers of senescence in diverse tissues, their interplay, and potential to be regulated should help to facilitate progress in the development of interventions to effectively target senescence as a means to delay aging.
Electronic supplementary material
(DOCX 12 kb).
Acknowledgments
This work was supported by R21 AG055026 (DMH), R01 AG044376 (NV), U19 AG056278 (LJN, WCL, PDR), PO1 AG043376 (PDR, LJN), R01 AG063543 (LJN), R56 AG059676 (LJN), R56 AG4059675 (PDR), P01 AG062412 (PDR, LJN) and the American Federation for Aging Research (DMH), as well as the Einstein Nathan Shock Center (P30 AG038072) and Einstein Cancer Center (P30 CA013330). MJY is supported by The Irene Diamond Fund/American Federation on Aging Research Postdoctoral Transition Award. The authors would like to thank Mariah Witt for editing the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical declaration
Animal studies were performed in accordance with the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Derek M. Huffman, Email: derek.huffman@einsteinmed.org
Paul D. Robbins, Email: probbins@umn.edu
References
- Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Carlson ME, et al. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Rebo J. Systemic problems: a perspective on stem cell aging and rejuvenation. Aging (Albany NY) 2015;7:754–765. doi: 10.18632/aging.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. Geroscience. 2020;42:51–55. doi: 10.1007/s11357-019-00140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Haque S, et al. circRNAs expressed in human peripheral blood are associated with human aging phenotypes, cellular senescence and mouse lifespan. Geroscience. 2020;42:183–199. doi: 10.1007/s11357-019-00120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017. 10.1038/nm.4324. [DOI] [PMC free article] [PubMed]
- Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SY, Awad EM, Oszwald A, Mayr M, Yin X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P, Breuss JM. Premature senescence of endothelial cells upon chronic exposure to TNFalpha can be prevented by N-acetyl cysteine and plumericin. Sci Rep. 2017;7:39501. doi: 10.1038/srep39501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiges W. Pathology assessment is necessary to validate translational endpoints in preclinical aging studies. Pathobiol Aging Age Relat Dis. 2016;6:31478. doi: 10.3402/pba.v6.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiges W, Ikeno Y, Niedernhofer L, McIndoe RA, Ciol MA, Ritchey J, Liggitt D. The Geropathology Research Network: An Interdisciplinary Approach for Integrating Pathology Into Research on Aging. J Gerontol A Biol Sci Med Sci. 2016;71:431–434. doi: 10.1093/gerona/glv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiges W, Snyder JM, Wilkinson E, Imai DM, Snider T, Ge X, Ciol M, Pettan-Brewer C, Pillai SPS, Morton J, Quarles E, Rabinovitch P, Niedernhofer L, Liggitt D. A New Preclinical Paradigm for Testing Anti-Aging Therapeutics. J Gerontol A Biol Sci Med Sci. 2017;72:760–762. doi: 10.1093/gerona/glx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence I, et al. Correlations between age, functional status, and the senescence-associated proteins HMGB2 and p16(INK4a) Geroscience. 2018;40:193–199. doi: 10.1007/s11357-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. doi: 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Rubinstein ND, Buffenstein R. A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence. Geroscience. 2018;40:105–121. doi: 10.1007/s11357-018-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liendl L, Grillari J, Schosserer M. Raman fingerprints as promising markers of cellular senescence and aging. Geroscience. 2019. 10.1007/s11357-019-00053-7. [DOI] [PMC free article] [PubMed]
- Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17:e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk T, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, von Zglinicki T, LeBrasseur N, Campisi J, Tchkonia T, Kirkland JL. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18:e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P, et al. Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18:e12927. doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. doi: 10.1038/ncomms13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen J, Kirkland JL, LeBrasseur N. Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes. 2016;65:1606–1615. doi: 10.2337/db15-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur N. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- Smith LK, et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijer ME, et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11:722–725. doi: 10.1111/j.1474-9726.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur N, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics enhance physical function in old age and extend post-treatment survival. Nat Med. 2018;8:1246–56. 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed]
- Yousef H, et al. Systemic attenuation of the TGF-beta pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget. 2015;6:11959–11978. doi: 10.18632/oncotarget.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2018a;17. 10.1111/acel.12706. [DOI] [PMC free article] [PubMed]
- Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Melos KI, Angelini L, Burd CE, Robbins PD, Niedernhofer LJ. Mouse Models of Accelerated Cellular Senescence. Methods Mol Biol. 2019;1896:203–230. doi: 10.1007/978-1-4939-8931-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020:e13094. 10.1111/acel.13094. [DOI] [PMC free article] [PubMed]
- Zhang P, et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci. 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb).