Abstract
Modern geroscience is divided as regards the validity of the free radical theory of aging. Thermodynamic arguments and observations from comparative zoology support it, whereas results from experimental manipulations in representative animal species sometimes strongly contradict it. From a comparison of the multi-step aging process with a linear metabolic pathway (glycolysis), we here argue that the identification of the rate-limiting kinetic steps of the aging cascade is essential to understand the overall flux through the cascade, i.e., the rate of aging. Examining free radical reactions as a case in point, these reactions usually occur as chain reactions with three kinetically independent steps: initiation, propagation, and termination, each of which can be rate-limiting. Revisiting the major arguments in favor and against a role of free radicals in aging, we find that the majority of arguments in favor point to radical propagation as relevant and rate-limiting, whereas almost all arguments in disfavor are based on experimental manipulations of radical initiation or radical termination which turned out to be ineffective. We conclude that the overall lack of efficacy of antioxidant supplementation (which fosters termination) and antioxidant enzyme overexpression (which inhibits initiation) in longevity studies is attributable to the fact that initiation and termination are not the rate-limiting steps of the aging cascade. The biological and evolutionary plausibility of this interpretation is discussed. In summary, radical propagation is predicted to be rate-limiting for aging and should be explored in more detail.
Keywords: Free radical theory of aging, Metabolic flux, Radical initiation, Radical propagation, Radical termination, Rate-limiting step
The rate-limiting step as concept in chemistry and metabolic control analysis
In a linear chain of causally dependent transformations, the slowest of the transformations predicts and determines the overall velocity of the chain and is thus rate-limiting. This concept of the existence of rate-limiting steps in linear chains of reactions has become highly instrumental in almost every branch of chemistry and beyond (Murdoch 1981; Boscá and Corredor 1984; Odian 2004). Applied to the analysis of metabolic pathways, however, is has soon become clear that chains of reversible equilibrium reactions are imprecisely described by the velocity of the slowest step under standard conditions, since dynamic adjustments of substrate concentrations occur (Murdoch 1981). In other words, more substrate accumulates in front of a slow enzyme, making its effective turnover rate faster, such that after a sufficiently long roll-in period, a steady state may emerge in which metabolite concentration may differ vastly, but in which all transformations occur at the same effective velocity. To accommodate for this shortcoming of the rate-limiting step concept, “metabolic control analysis” has largely superseded the simple search for the slowest step when analyzing linear metabolic pathways such as glycolysis (Rapoport et al. 1976; Kashiwaya et al. 1994; Marín-Hernández et al. 2006). As a result, the term “rate-limiting” has usually been substituted by the term “flux-controlling”, which describes in how far the relative acceleration of a certain enzymatic step accelerates the overall flux through a pathway (Rapoport et al. 1976).
Regarding the flux through glycolysis (Fig. 1), it has long been known that only a few of the essentially involved steps of this metabolic pathway largely determine the overall flux through the pathway (Rapoport et al. 1976; Boscá and Corredor 1984; Kashiwaya et al. 1994). Notably, the later-described flux-controlling steps were very often identical to the earlier-described rate-limiting steps as based on the maximum velocities vmax of the individual enzymes under saturating substrate conditions. For example, in an analysis of glycolysis in the working rat heart under different conditions, hexokinase (enzyme (1) in Fig. 1) did not only exhibit the lowest vmax value, but also the highest flux control coefficient (0.59 with glucose alone and 0.97 with glucose/insulin) (Kashiwaya et al. 1994). In contrast, the two enzymes with the highest vmax values (3-phosphoglycerate kinase (7) and 3-phosphoglycerate mutase (8)) exhibited flux control coefficients approaching zero (0.008 for both enzymes). It is interesting to note that flux-controlling steps are often the same in biologically different systems as long as the architecture of the metabolic pathway is the same. For instance, high flux control by hexokinase has also been found in human erythrocytes (Rapoport et al. 1976), in human tumor cells, even those with hexokinase overexpression (Marín-Hernández et al. 2006), and in tethered, wing-beating fruitflies (Eanes et al. 2006).
Fig. 1.
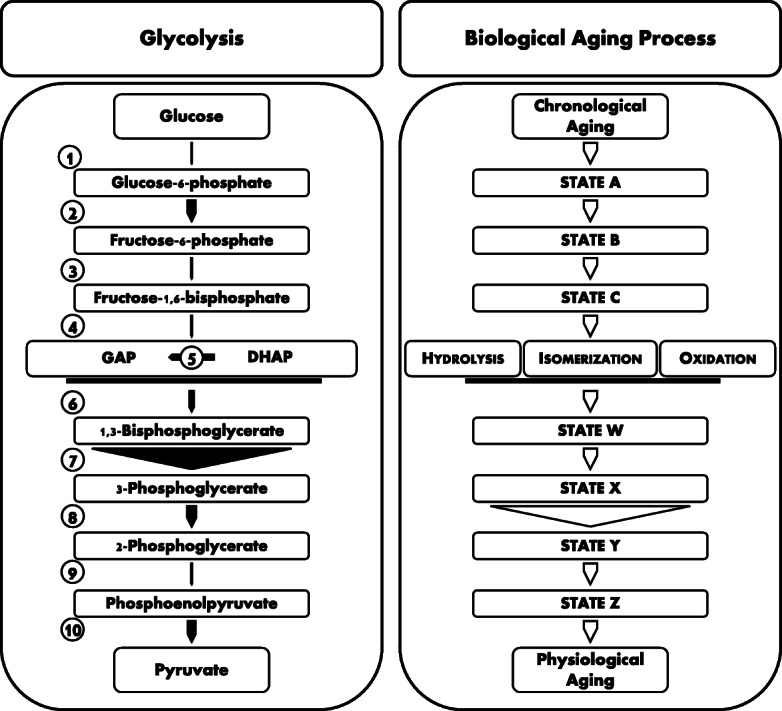
Glycolysis and aging, two multi-step kinetic processes. Glycolysis is a linear metabolic pathway for the degradation of glucose to pyruvate. The flux through this pathway is governed by a few rate-limiting, flux-controlling steps, whereas other steps, which are similarly indispensable for the overall pathway, are not rate-limiting. In the present representation, the thickness of the arrows (1)–(10) reflects the maximal reaction rates vmax of each individual step of glycolysis in the working rat heart, adopting data from Kashiwaya et al. (1994). Glycolysis in the rat heart is predominantly controlled by the slow hexokinase (1) and enolase (9) reactions, whereas the 3-phosphoglycerate kinase reaction (7) is never rate-limiting. As argued in the text, the biological aging process shares relevant aspects with linear metabolic pathways such as glycolysis, including the possible existence of rate-limiting steps. However, the reaction rates of the individual steps in the modeled aging cascade are unknown, as is the identity of the rate-limiting steps of the cascade. Spontaneous chemical transformations like oxidation, isomerization, or hydrolysis are likely involved in the aging cascade at some point, but it is unknown so far whether they are rate-limiting. In the depicted example, the enzyme catalyzing the transition from state X to state Y is not rate-limiting; hence, its essential role for aging can neither be determined by overexpression nor by knockdown, unless the knockdown is approaching 100% and no isozymes exist. GAP glyceraldehyde-3-phosphate, DHAP dihydroxyacetone phosphate
The key insight from these experiments is that many steps of glycolysis under entirely physiological conditions do not control the overall flux through the pathway; still, they are essentially involved in the pathway. In other words, even if these steps were accelerated substantially, the overall flux would not measurably change. In the above example of 3-phosphoglycerate kinase, a doubling of enzyme expression will only lead to a 0.8% acceleration of flux (Kashiwaya et al. 1994). In fruitflies, a 50% activity reduction of various glycolytic enzymes did not affect glycolysis-dependent wing-beating frequency, and even 90% knockdowns of certain enzymes (e.g., phosphoglucose isomerase) were phenotypically silent (Eanes et al. 2006). Hence, in similarly organized linear cascades of causally dependent transformations, there is a fair chance that many involved steps will not be recognized as such in simple overexpression or knockdown experiments. Their involvement can only be seen directly if the velocity of the said steps comes close to zero, which then results in the sudden collapse of the pathway. By analogy, the filling level of a car’s fuel tank only becomes velocity-limiting when it is close to empty; at any higher level, adding further fuel to the tank or removing fuel from the tank will neither modulate the car’s actual speed (controlled by the position of the accelerator) nor its maximum speed (controlled by the car’s construction and perhaps by the slope of the road). Visibly, at least three entirely different aspects may be rate-limiting in this simple example from real life. Focusing on the biological aging process, the question emerges whether this multi-step phenomenon may also be governed by discrete rate-limiting steps. Hence, can the above concepts from enzymology and chemical reaction kinetics also be applied to the aging process?
A comparison of glycolysis and aging
Biological aging is widely defined as the effect of time (chronological aging) on the functional capacity of a biological organism, which generally declines (physiological aging). Inspecting some of the phenotypic characteristics of aging such as the gradual loss of tissue-specific function in multiple tissues, the loss of repair capacity on the DNA, cell, and tissue level, or the accumulation of disordered material (Aunan et al. 2016; Korovila et al. 2017; Sebastiani et al. 2017), it is clear that many biochemical steps are necessary to produce such diverse manifestations. Almost inevitable as a consequence, some of these steps will be logically arranged in a linear hierarchy (Fig. 1). Hence, those steps effectively form an “aging cascade” whose properties can be compared to those of a linear metabolic pathway like glycolysis. Similarly as the overall flux through glycolysis determines the rate of pyruvate formation, the overall flux through the aging cascade then defines the rate of aging, our parameter of interest.
In reality, aging might probably constitute a branched or anastomotic pathway with multiple endpoints representing different aspects of cellular, tissue, and organismal functional loss. However, the additional complexity of a branched pathway does not affect the central conclusion from linear pathway analysis that a few specific steps, potentially just one, may determine the overall velocity of each viable and complete linear branch ending at a specific aging phenotype. In other words, cardiac aging might not be controlled by the same rate-limiting steps as immune aging, but each phenomenon might indeed be governed by just a few kinetically limiting steps. In consequence, it is very well possible that steps are essentially involved in aging (like step X → Y would be in Fig. 1) whose influence cannot be seen even under fortunate experimental conditions, for example during a tenfold overexpression or 90% knockdown of an enzymatic activity (here representing step X → Y). A sound interpretation of the results of genetic and feeding studies aimed at modulating lifespan thus requires at least a basic consideration of whether the manipulated step was likely to be rate-limiting. To get more specific on this key consideration, we will briefly review the kinetics of free radical reactions in the following and will then analyze whether previous tests of the free radical theory of aging were going for plausibly rate-limiting steps of the aging cascade.
Initiation, propagation, and termination are three kinetically independent steps of radical chain reactions
Spontaneous and enzymatically uncontrolled chain reactions involving short-lived intermediates are the predominant manifestation of free radical activity in biological systems (Negre-Salvayre et al. 2010; Yin et al. 2011; Zimniak 2011). Even if stable radicals also exist that possess long lifetimes and specific reactivities towards only a few targets, these stable radicals are very much unlikely to contribute to the widespread stochastic damage that typically occurs during aging (Gladyshev 2016; Golubev et al. 2017). Hence, if free radicals were involved in the loss of homeostatic control and the inoperable molecular diversification that are hallmarks of aging (Avanesov et al. 2014; Gladyshev 2016), then free radical chain reactions involving highly reactive and short-lived intermediates are certainly more relevant than stoichiometric 1:1 radical reactions.
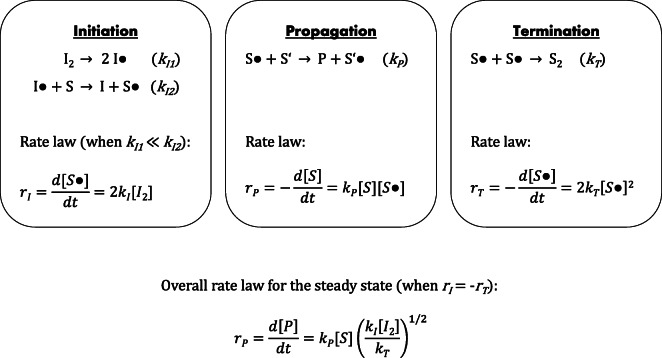
Like any radical reaction in polymer chemistry (Odian 2004), biological free radical chain reactions are characterized by three separate and kinetically independent steps, namely initiation, propagation, and termination (Doktorov et al. 2008; Yin et al. 2011) (Fig. 2). The most important biological chain reaction in quantitative terms is the lipid peroxidation reaction (Negre-Salvayre et al. 2010). One reason for this is the pre-accumulation of readily reactive hydrophobic substrates within the lipid bilayer, as separating layers of water between the individual reactive substrates are missing in this compartment. Admittedly, membrane proteins are relevant co-players in the lipid peroxidation reaction and may not only be preferentially affected victims (Schindeldecker et al. 2011; Granold et al. 2015), but may also act as active modulators or inhibitors of this reaction (Hajieva et al. 2015).
Fig. 2.
Generalized kinetics of free radical chain reactions. Free radical chain reactions are characterized by their three essential steps: initiation, propagation, and termination. These reactions exhibit different rate constants k and reaction rates r as linked in their rate laws. During initiation, initiator radicals I● are formed which attack substrate molecules S. In general, the subsequent attack on S is much faster than I● formation, resulting in the depicted, simplified rate law for initiation. Propagation is characterized by the formation of the product P out of S●. Termination involves the recombination of two radicals or the disproportionation of two radicals. The latter variant is not shown, but intended to be included in the overall rate law of termination with termination constant kT. Chain-transfer agents offering alternative reaction pathways for propagation or chain-breaking antioxidants offering alternative reaction pathways for termination were omitted for clarity. These agents are usually catalysts and do not change the shape of the corresponding rate laws but increase the apparent rate constants of propagation and termination. In the combined rate law for the overall process, the substrate concentration [S] and the propagation constant kP enter linearly, whereas the initiator concentration [I2], the initiation constant kI, and the reciprocal termination constant 1/kT only enter as square roots
Inspecting the chemical rate laws of typical reactions of radical initiation, radical propagation and radical termination more closely (Fig. 2), two relevant conclusions can be drawn. First, each reaction is indeed governed by its own rate constant, and these rate constants may vary by many orders of magnitude. Second, in the combined rate law for the steady state, the substrate concentration [S] and the propagation constant kP contribute linearly, while the initiator concentration [I2], the initiation constant kI, and the termination constant kT leave their marks as square roots. Hence, for the overall reaction rate, the influence of the propagation constant is higher than the influence of the initiation constant, and the influence of the substrate concentration is higher than the influence of the initiator concentration. Both of these conclusions arguably run counter to intuition but can hardly be denied. Clearly, the depicted reactions represent the simplest possible examples of radical reactions, and the above-derived conclusions are formally valid only for the steady state, implying that the total number of radicals does not continuously increase or decrease during the course of the reaction. Still, as a starting point, these simplifications appear well justified (Odian 2004), such that the mentioned conclusions should be taken into account when one intends to assess the role of free radicals in aging.
Evidence that propagation, but not initiation or termination, is rate-limiting for aging
If initiation, propagation, and termination represent three kinetically independent steps of radical reactions, which of these steps have actually been probed in experimental or correlative aging studies? Very frequently, antioxidant enzymes have been overexpressed or reduced in experimental aging studies, with mostly limited effects on longevity (Hulbert et al. 2007), especially when mice were investigated (Pérez et al. 2009b) (Table 1). Antioxidant enzymes such as superoxide dismutase (SOD), catalase, or glutathione peroxidase (GPx) are specific inhibitors of radical initiation, but not of propagation, and they also do not interfere with termination. GPx4 might also be viewed to be a repair enzyme, as it removes lipid hydroperoxides, which are products of an already running or terminated radical chain reaction. However, the specific danger of these lipid hydroperoxides comes from their potential to re-initiate further chain reactions (Maiorino et al. 2018), such that the classification of GPx4 as primary inhibitor of initiation is justified.
Table 1.
Lifespan effects of the modification of radical initiation, radical propagation, and radical termination
| Radical step | Experimental variable | Correlative variable | Effect on lifespan | Reference |
|---|---|---|---|---|
| Initiation | Cu/Zn-SOD | None (mouse) | Pérez et al. (2009a) | |
| Mn-SOD | None (mouse) | Jang et al. (2009) | ||
| Catalase | None (mouse) | Pérez et al. (2009a) | ||
| GPx4 | None (mouse) | Pérez et al. (2009b) | ||
| Cu/Zn-SOD | None (vertebrates) | Tolmasoff et al. (1980), Lopez-Torres et al. (1993), Page et al. (2010) | ||
| Mn-SOD | Increase (vertebrates) | Page et al. (2010) | ||
| Catalase | Conflicting (vertebrates) | Lopez-Torres et al. (1993), Page et al. (2010) | ||
| GPx | None (vertebrates) | Lopez-Torres et al. (1993), Page et al. (2010) | ||
| Propagation | Decrease in lipid unsaturation | Increase (C. elegans strains) | Shmookler Reis et al. (2011) | |
| Decrease in lipid unsaturation | Increase (mammals, birds, invertebrates) | Pamplona et al. (1998), Hulbert et al. (2014), Cortie et al. (2015), Galván et al. (2015) | ||
| Decrease in mitochondrial cysteine | Increase (vertebrates and invertebrates) | Moosmann and Behl (2008), Schindeldecker et al. (2011) | ||
| Termination | Vitamin E | None (vertebrates and invertebrates) | Ernst et al. (2013) | |
| Ascorbate | None (vertebrates and invertebrates) | Pallauf et al. (2013) | ||
| Ubiquinone | None (mouse and rat) | Lönnrot et al. (1998) | ||
| Carotenoids | None (mouse) | Massie et al. (1986) | ||
| Vitamin E | Increase (mammals) | Cutler (1991) | ||
| Ascorbate | None (vertebrates) | Cutler (1984a), Lopez-Torres et al. (1993) | ||
| Ubiquinone | None (vertebrates) | Lass et al. (1997) | ||
| Carotenoids | Increase (mammals) | Cutler (1984b) |
Unless otherwise stated, the cited effects on lifespan refer to increases in the experimental variable, e.g., increases in Cu/Zn-SOD or vitamin E. Regarding the selection of experimental studies, mouse studies were cited preferentially in this table when available. Comprehensive references on the effects of a modulation of antioxidant enzymes or low-molecular mass antioxidants on animal lifespan are provided in Hulbert et al. (2007), Pérez et al. (2009b), and Sadowska-Bartosz and Bartosz (2014)
Even more frequently than antioxidant enzymes, low-molecular mass antioxidants have been supplemented or depleted in animals to test their effect on lifespan. Again, the yielded effects were not very substantial and mostly occurred in the very shortest-lived of animals, with no effect in mice (Hulbert et al. 2007; Sadowska-Bartosz and Bartosz 2014). The employed substances have in common that they represent chain-breaking antioxidants, for which vitamin E and its recycling factor vitamin C, numerous phenolic compounds, and carotenoids are prominent examples. Notably, these compounds generally lack the capacity to reduce the rate of initiation or to modulate propagation; they can only catch propagating radicals and offer alternative reaction pathways of termination, thereby stopping the chain reaction. In summary, the majority of intervention studies and correlative studies on the effect of free radicals on aging (Table 1) have modulated the radical initiation step or the radical termination step and have been largely unsuccessful.
What about propagation? Propagation has the highest kinetic influence of all three steps in prototypic radical chain reactions (Fig. 2), but at the same time, it is the most difficult step to manipulate. To modify propagation, there are essentially just two strategies: the lowering of the substrate concentration or the lowering of the effective propagation constant. The first strategy would imply that especially polyunsaturated fatty acids, which are the most reactive substrates of radical chain reactions in biology, would have to be reduced. The second strategy would imply that anything which catalyzes radical propagation would need to be removed from the membrane. As it turns out, the two best-established and most robust correlatives of longevity in animals precisely embody these two strategies (Table 1), as follows.
The avoidance of polyunsaturated fatty acids is without doubt the most widely reproduced biochemical idiosyncrasy of long-lived animals in comparison with short-lived animals. It has been confirmed in mammals (Pamplona et al. 1998; Hulbert et al. 2007; Cortie et al. 2015), birds (Hulbert et al. 2007; Galván et al. 2015), and invertebrates (Hulbert et al. 2014) as well as different strains of artificially long-lived nematodes (Shmookler Reis et al. 2011). Notably, direct experimental reduction of membrane unsaturation has also resulted in increased longevity in these nematodes (Shmookler Reis et al. 2011). In addition, various other, more pleiotropic manipulations to increase lifespan have been described to involve a lowered content of polyunsaturated fatty acids (Zimniak 2011).
As pertains to the avoidance of anything which catalyzes radical propagation, the surprising correlation of a low mitochondrially encoded cysteine content with lifespan first described about a decade ago (Moosmann and Behl 2008) seems to directly realize this demand in preventing “chain-transfer catalysis” (Smith 1946; Odian 2004) as detailed below. The cysteine-lifespan correlation has emerged as the most robust association of a single inherited molecular parameter with longevity in animals (Moosmann and Behl 2008). It has been shown to have a large effect size (~ 80% cysteine depletion in long-lived mammals as compared to the cytosolic expectation value), to apply to vertebrates and invertebrates within the same coordinate system, and to be entirely lost upon transition of an animal to an anaerobic-parasitic lifestyle, implicating oxidative stress as shaping factor (Moosmann and Behl 2008). Notably, the cysteine-lifespan correlation has been found to be predominantly carried by intramembrane cysteine residues, which excludes polar reaction mechanisms (such as thiolate-dependent nucleophilic reactions) as explanatory factors (Schindeldecker et al. 2011). From a more detailed comparison of the differential frequency and reactivity of cysteine and methionine in respiratory chain complexes, it was concluded that homolytic thiyl radical formation (RSH + X● → RS● + HX) was the most likely reason for the avoidance of cysteine residues in long-lived animals (Moosmann 2011). Thiyl radicals exhibit a wide range of unorthodox reactivities useful for organic synthesis (Dénès et al. 2014), but they have also been recognized for their induction of lipid peroxidation (Schöneich et al. 1989), lipid isomerization (Chatgilialoglu and Ferreri 2005), and their epimerization and oxidation of amino acids and proteins (Schöneich 2008). Most intriguingly though, it is hydrophobic thiols like dodecylthiol that are the most ancient (Smith 1946) and still widely employed chain-transfer catalysts for the enhancement of synthetic free radical polymerizations (Odian 2004). Per that property, in technical chemistry, they alleviate the need to use high amounts of radical initiator to achieve a complete turnover of the monomer by making radical propagation more efficient, sometimes by orders of magnitude. Evidently, chain-transfer catalysis by intramembrane thiols would be a highly adverse reactivity in an unsaturated biological membrane.
In summary, the two most robust biochemical correlates of longevity in animals, namely fewer unsaturated fatty acids and fewer intramembrane cysteine residues, converge on a single mechanism and coherently point to radical propagation as kinetically rate-limiting for aging: the first strategy reduces the substrate, and the second strategy reduces the chain-transfer catalyst.
On the evolutionary plausibility of the idea that radical propagation limits lifespan
Is the above conclusion that only radical propagation was rate-limiting for aging plausible? In the following, we argue that from a biochemical point of view, it is rather easy to suppress initiation and to enhance termination without inevitable side effects, but it is rather difficult to suppress propagation without inevitable side effects. In consequence, we claim that evolution rapidly optimizes radical initiation and termination to such an extent that both are far from being rate-limiting, whereas propagation may hardly be optimized without tradeoffs for the cell and is therefore likely to be rate-limiting.
Initiation by initiator radicals such as hydroxyl radical may be readily suppressed by lowering the concentrations of its originators, namely superoxide, hydrogen peroxide, or free iron. This can be achieved to almost any level by a heightened expression of antioxidant enzymes. Mutations in cis-regulatory regions like promotors which heighten protein expression occur rapidly and frequently and represent a major pathway of trait evolution in animals (Wray 2007). Hence, if increased longevity of an extant animal was achievable simply by a higher expression of an antioxidant enzyme like SOD, one might wonder why nature would not have already embodied such a simple solution. In other words, it is highly likely that the expression levels of the aging-relevant antioxidant enzymes are already optimized for each species such that they do not represent rate-limiting players anymore. This can be expected since a longer lifespan without tradeoffs is certainly a positively selected trait (Kirkwood and Austad 2000; Sahm et al. 2018).
A very similar argument applies to termination. The biosynthesis of terminators such as ubiquinone is clearly under genetic control and could be readily induced (Dallner and Sindelar 2000). Moreover, the uptake and distribution of the major external terminator, vitamin E, is tightly regulated by the expression of transport proteins like the α-tocopherol transfer protein (α-TTP). Notably, there is clear indication that mammals do not make maximum use of the tocopherol provided to them by their diet, as they largely ignore other tocopherols than α-tocopherol and poorly respond to heightened levels of α-tocopherol in the diet (Traber and Arai 1999). The same is true for ubiquinone (Dallner and Sindelar 2000). The most plausible interpretation of these observations is that the levels of terminators are already sufficiently high under most conditions as to make termination a non-rate-limiting step for aging.
The modulation of propagation, in contrast, appears to be a much more demanding task. A reduction in the amount of polyunsaturated fatty acids may be readily achieved to begin with. However, fatty acid unsaturation is the major mechanism to maintain membrane homoviscosity, which is essential for the proper functioning of membrane proteins, the establishment of the membrane permeability barrier, and the formation and trafficking of vesicles, just to name a few examples (Los and Murata 1998; Pamplona et al. 2002; Hulbert et al. 2014). Any reduction in unsaturation must therefore be compensated by either structural changes in membrane proteins or an adaptive change in fatty acid length, branching, or the general lipid bilayer composition beyond fatty acids (Pamplona et al. 2002). Hence, complex structural adaptations are required. As regards the removal of catalysts that foster radical propagation, this may also be readily accomplished if those were low-molecular mass compounds. For example, their degradation or functional replacement might be induced. As it turns out, though, it is membrane protein thiol groups that currently appear to represent the most relevant biological chain-transfer agents (Moosmann 2011). In consequence, what had to be achieved by evolution was a selective avoidance of surface-exposed, intramembrane thiol groups by avoiding protein cysteine residues without compromising protein function (Moosmann and Behl 2008; Moosmann 2011; Schindeldecker et al. 2011). Evidently, this was a more complex adaptation than a few simple promotor mutations would have been.
In view of the complexity of all identified cellular adaptations suppressing propagation, but the simplicity of most cellular adaptations suppressing initiation or fostering termination, we conclude that propagation will generally be the least optimized and thus rate-limiting step for aging. Propagation, as difficult as it may be to influence it from the outside, should be investigated more thoroughly as harbinger of aging.
Conclusion
Applying quantitative rate laws from chemical reaction kinetics to the free radical theory of aging, a model emerges that predicts a priori that (i) antioxidant enzymes are unlikely to limit lifespan, (ii) chain-breaking antioxidants are unlikely to limit lifespan, (iii) polyunsaturated fatty acids are likely to limit lifespan, and (iv) intramembrane thiols are likely to limit lifespan. For all of these four predictions, there is substantial experimental or comparative support. Hence, these four pieces of evidence need not be viewed to be contradictory any longer, but should rather be taken as indication that specific rate-limiting steps govern the rate of aging as induced by free radicals.
Acknowledgements
This work was supported by the “NMFZ” of the University of Mainz.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103:e29–e46. doi: 10.1002/bjs.10053. [DOI] [PubMed] [Google Scholar]
- Avanesov AS, Ma S, Pierce KA, Yim SH, Lee BC, Clish CB, Gladyshev VN. Age- and diet-associated metabolome remodeling characterizes the aging process driven by damage accumulation. Elife. 2014;3:e02077. doi: 10.7554/eLife.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscá L, Corredor C. Is phosphofructokinase the rate-limiting step of glycolysis? Trends Biochem Sci. 1984;9:372–373. [Google Scholar]
- Chatgilialoglu C, Ferreri C. Trans lipids: the free radical path. Acc Chem Res. 2005;38:441–448. doi: 10.1021/ar0400847. [DOI] [PubMed] [Google Scholar]
- Cortie CH, Hulbert AJ, Hancock SE, Mitchell TW, McAndrew D, Else PL. Of mice, pigs and humans: an analysis of mitochondrial phospholipids from mammals with very different maximal lifespans. Exp Gerontol. 2015;70:135–143. doi: 10.1016/j.exger.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Cutler RG. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr. 1984;3:321–348. doi: 10.1016/0167-4943(84)90033-5. [DOI] [PubMed] [Google Scholar]
- Cutler RG. Carotenoids and retinol: their possible importance in determining longevity of primate species. Proc Natl Acad Sci U S A. 1984;81:7627–7631. doi: 10.1073/pnas.81.23.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG. Antioxidants and aging. Am J Clin Nutr. 1991;53:373S–379S. doi: 10.1093/ajcn/53.1.373S. [DOI] [PubMed] [Google Scholar]
- Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- Dénès F, Pichowicz M, Povie G, Renaud P. Thiyl radicals in organic synthesis. Chem Rev. 2014;114:2587–2693. doi: 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- Doktorov AB, Lukzen NN, Pedersen JB. Analysis of lipid peroxidation kinetics. 1. Role of recombination of alkyl and peroxyl radicals. J Phys Chem B. 2008;112:11854–11861. doi: 10.1021/jp709921m. [DOI] [PubMed] [Google Scholar]
- Eanes WF, Merritt TJ, Flowers JM, Kumagai S, Sezgin E, Zhu CT. Flux control and excess capacity in the enzymes of glycolysis and their relationship to flight metabolism in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:19413–19418. doi: 10.1073/pnas.0607095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst IM, Pallauf K, Bendall JK, Paulsen L, Nikolai S, Huebbe P, Roeder T, Rimbach G. Vitamin E supplementation and lifespan in model organisms. Ageing Res Rev. 2013;12:365–375. doi: 10.1016/j.arr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Galván I, Naudí A, Erritzøe J, Møller AP, Barja G, Pamplona R. Long lifespans have evolved with long and monounsaturated fatty acids in birds. Evolution. 2015;69:2776–2784. doi: 10.1111/evo.12754. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell. 2016;15:594–602. doi: 10.1111/acel.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubev A, Hanson AD, Gladyshev VN. Non-enzymatic molecular damage as a prototypic driver of aging. J Biol Chem. 2017;292:6029–6038. doi: 10.1074/jbc.R116.751164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granold M, Moosmann B, Staib-Lasarzik I, Arendt T, Del Rey A, Engelhard K, Behl C, Hajieva P. High membrane protein oxidation in the human cerebral cortex. Redox Biol. 2015;4:200–207. doi: 10.1016/j.redox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajieva P, Bayatti N, Granold M, Behl C, Moosmann B. Membrane protein oxidation determines neuronal degeneration. J Neurochem. 2015;133:352–367. doi: 10.1111/jnc.12987. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Kelly MA, Abbott SK. Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B. 2014;184:149–166. doi: 10.1007/s00360-013-0786-8. [DOI] [PubMed] [Google Scholar]
- Jang YC, Pérez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Korovila I, Hugo M, Castro JP, Weber D, Höhn A, Grune T, Jung T. Proteostasis, oxidative stress and aging. Redox Biol. 2017;13:550–567. doi: 10.1016/j.redox.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Agarwal S, Sohal RS. Mitochondrial ubiquinone homologues, superoxide radical generation, and longevity in different mammalian species. J Biol Chem. 1997;272:19199–19204. doi: 10.1074/jbc.272.31.19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnrot K, Holm P, Lagerstedt A, Huhtala H, Alho H. The effects of lifelong ubiquinone Q10 supplementation on the Q9 and Q10 tissue concentrations and life span of male rats and mice. Biochem Mol Biol Int. 1998;44:727–737. doi: 10.1080/15216549800201772. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Los DA, Murata N. Structure and expression of fatty acid desaturases. Biochim Biophys Acta. 1998;1394:3–15. doi: 10.1016/s0005-2760(98)00091-5. [DOI] [PubMed] [Google Scholar]
- Maiorino M, Conrad M, Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29:61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- Marín-Hernández A, Rodríguez-Enríquez S, Vital-González PA, Flores-Rodríguez FL, Macías-Silva M, Sosa-Garrocho M, Moreno-Sánchez R. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006;273:1975–1988. doi: 10.1111/j.1742-4658.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Massie HR, Ferreira JR, Jr, DeWolfe LK. Effect of dietary beta-carotene on the survival of young and old mice. Gerontology. 1986;32:189–195. doi: 10.1159/000212788. [DOI] [PubMed] [Google Scholar]
- Moosmann B. Respiratory chain cysteine and methionine usage indicate a causal role for thiyl radicals in aging. Exp Gerontol. 2011;46:164–169. doi: 10.1016/j.exger.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. Mitochondrially encoded cysteine predicts animal lifespan. Aging Cell. 2008;7:32–46. doi: 10.1111/j.1474-9726.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Murdoch JR. What is the rate-limiting step of a multistep reaction? J Chem Educ. 1981;58:32–36. [Google Scholar]
- Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- Odian G. Principles of polymerization. 4. New York: John Wiley & Sons; 2004. [Google Scholar]
- Page MM, Richardson J, Wiens BE, Tiedtke E, Peters CW, Faure PA, Burness G, Stuart JA. Antioxidant enzyme activities are not broadly correlated with longevity in 14 vertebrate endotherm species. Age. 2010;32:255–270. doi: 10.1007/s11357-010-9131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallauf K, Bendall JK, Scheiermann C, Watschinger K, Hoffmann J, Roeder T, Rimbach G. Vitamin C and lifespan in model organisms. Food Chem Toxicol. 2013;58:255–263. doi: 10.1016/j.fct.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otín M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res. 1998;39:1989–1994. [PubMed] [Google Scholar]
- Pamplona R, Barja G, Portero-Otín M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA, Heinrich R, Rapoport SM. The regulatory principles of glycolysis in erythrocytes in vivo and in vitro. A minimal comprehensive model describing steady states, quasi-steady states and time-dependent processes. Biochem J. 1976;154:449–469. doi: 10.1042/bj1540449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska-Bartosz I, Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed Res Int. 2014;2014:404680. doi: 10.1155/2014/404680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm A, Bens M, Szafranski K, Holtze S, Groth M, Görlach M, Calkhoven C, Müller C, Schwab M, Kraus J, Kestler HA, Cellerino A, Burda H, Hildebrandt T, Dammann P, Platzer M. Long-lived rodents reveal signatures of positive selection in genes associated with lifespan. PLoS Genet. 2018;14:e1007272. doi: 10.1371/journal.pgen.1007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeldecker M, Stark M, Behl C, Moosmann B. Differential cysteine depletion in respiratory chain complexes enables the distinction of longevity from aerobicity. Mech Ageing Dev. 2011;132:171–179. doi: 10.1016/j.mad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Schöneich C. Mechanisms of protein damage induced by cysteine thiyl radical formation. Chem Res Toxicol. 2008;21:1175–1179. doi: 10.1021/tx800005u. [DOI] [PubMed] [Google Scholar]
- Schöneich C, Asmus KD, Dillinger U, von Bruchhausen F. Thiyl radical attack on polyunsaturated fatty acids: a possible route to lipid peroxidation. Biochem Biophys Res Commun. 1989;161:113–120. doi: 10.1016/0006-291x(89)91568-4. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, Thyagarajan B, Sun F, Schupf N, Newman AB, Montano M, Perls TT. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi: 10.1111/acel.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Xu L, Lee H, Chae M, Thaden JJ, Bharill P, Tazearslan C, Siegel E, Alla R, Zimniak P, Ayyadevara S. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging. 2011;3:125–147. doi: 10.18632/aging.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WV. Regulator theory in emulsion polymerization. I. Chain transfer of low molecular weight mercaptans in emulsion and oil-phase. J Am Chem Soc. 1946;68:2059–2064. doi: 10.1021/ja01214a055. [DOI] [PubMed] [Google Scholar]
- Tolmasoff JM, Ono T, Cutler RG. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Annu Rev Nutr. 1999;19:343–355. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Zimniak P. Relationship of electrophilic stress to aging. Free Radic Biol Med. 2011;51:1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]