Abstract
The addition of n-dodecane (between 1–3%) to the Escherichia coli fermentation broth in a mechanically agitated and aerated bioreactor revealed improved DO (dissolved oxygen) levels induced during fermentation which lead to an increase in biomass productivity and faster glucose consumption. The maximum values for enzyme activity (increased with 43% compared with the control) and kLa (the volumetric mass transfer coefficient) were obtained for the addition of 2% v/v n-dodecane in the bioreactor, due to the fact that oxygen limitation during the exponential growth phase of the bacterium can repress β-galactosidase production. The oxygen vector addition increased the available dissolved oxygen and activated a redox-sensitive regulation and an elevated intracellular oxidative metabolism that lead to the enhancement in E. coli biomass accumulation and a more accurate protein folding of β-galactosidase that would increase its activity. In addition to the experimental analysis, a complex model, developed using an improved version of Bacterial Foraging Algorithm and Artificial Neural Networks, was proposed, with a good average absolute value (6.2% in the training phase and 7.28% in the testing phase) between the process dynamic and the predictions generated by the model.
Keywords: Aerobic fermentation, β-Galactosidase, Biosynthesis, Oxygen vector
Introduction
β-Galactosidase, EC 3.2.1.23, is an important enzyme in dairy and medical industry. It is used for obtaining dairy products with very low lactose content used for condensed milk or ice-cream, for speeding the coagulation time for cheese and yoghurt and also for valorization of whey (Nivetha and Mohanasrinivasan 2016; Kokkiligadda et al. 2016). In medical industry, this enzyme facilitates the production of lactose biosensors and treatment of lactose malabsorption and intolerance, and it is proposed as a solution for the synthesis of β-galactosides from nucleosides and acyclic nucleoside analogues from lactose in a simple two-step process (Blazek et al. 2012; Tomizawa et al. 2016).
Escherichia coli is a coliform bacterium that is used for the biosynthesis of several metabolic products at industrial level; as it is easy to cultivate, it requires an inexpensive medium and high product titer can be achieved. It can produce β-galactosidase and the process is very well known: the biosynthesis is controlled by the lacZ gene from the lac operon (Anisha 2017). The enzyme biosynthesis is strongly influenced by the growth conditions: nutrient availability [carbon and nitrogen sources but also growth factors—thiamine, quinones (Khedr et al. 2013)], and the intracellular microenvironment. E. coli submerged fermentation is a highly aerobic process, requiring large quantity of oxygen (Losen et al. 2004). The carbon source conversion for biomass growth involves a large number of enzymes and cofactors implied in the central metabolic pathways, oxygen being the terminal electron acceptor in the respiratory chain (extremely important in the energy metabolism). Taking into account the low aqueous solubility of oxygen and its high demand, the addition of a non-aqueous oxygen vector could increase the process productivity. Several advantages, such as higher oxygen solubility, lack of toxicity to microorganisms, antifoaming action, a decreased energy consumption used for mixing intensifications, and easy separation from the fermentation medium, are important for their use in biotechnology, especially for industrial large-scale fermentation (Narta et al. 2011; Zhang et al. 2018).
To increase the productivity of an aerobic microbial process, it is extremely important to optimize the oxygen transfer. Oxygen vectors are water immiscible organic compounds: liquid hydrocarbons, perfluorocarbons, and silicone or plant oils (palm, castor, olive, soybean, and sunflower seed) that provide a 15–20-fold higher solubility for oxygen (compared to water) and, therefore, can facilitate oxygen transport in different types of microbial cell cultures, by acting like an active intermediate in the oxygen transport from gas bubbles to the aqueous phase (Folescu and Blaga 2013). Oxygen vectors can improve oxygen transfer towards the bacteria by an increased driving force due to higher oxygen solubility or by forming a new interfacial area between the air and the liquid phase (Feng et al. 2016a, b; Xia et al. 2017).
Microbial processes are poly-phase systems: when an aerobic strain is used, the bioprocess involves at least three phases, gas–liquid–solid. By the oxygen vectors addition in the fermenter a four phase system is formed that is biomass (solid)/aqueous culture medium (liquid)/organic immiscible n-dodecane (liquid)/air (gas). For the oxygen transfer, it is assumed that the oxygen vector is adsorbed to the air bubbles surface and oxygen diffusion occurs through the oxygen vector and the culture medium or directly to the adsorbed cells on the oxygen vector. The formation of the cells–droplets–air bubbles associations and their stability depend on cells affinity for hydrocarbon phase, hydrocarbon droplets size, and mixing intensity (Amaral et al. 2006; Galaction et al. 2015). The oxygen vector`s addition in the fermentation broth improved the productivity for validamycin A, S-adenosylmethionine, ergosterol, poly(γ-glutamic acid), lovastin, l-sorbose, antroquinonol, etc., and offers several advantages, besides higher oxygen solubility, such as improved yield of secondary metabolites and antifoaming action (Blaga et al. 2018; Feng et al. 2016a, b; Li et al. 2012;Xia et al. 2019; Zhang et al. 2012).
During the past years, several studies have shown an enhanced enzymatic activity determined by the use of oxygen vectors for cellulase from rice straw produced by Trichoderma reesei, l-asparaginase produced by Bacillus brevis, fumarase in recombinant Escherichia coli related to an adequate oxygen supply in the broth, and an intracellular microenvironment of the submerged fermentation that facilitates the correct folding of the protein (Narta et al. 2011; Xia et al. 2017; Zhang et al. 2018).
In this communication, we are reporting the investigations of n-dodecane as oxygen vector for the enhancement of β-galactosidase activity and E. coli cell mass. Dodecane is easily separated from the fermentation broth due to its low melting point, and is reusable, non-toxic, and inexpensive. It has been chosen as it has been already successfully used for bacteria fermentation and is more environmental friendly then perfluorocarbons (which also are expensive, requiring high initial investment cost) (Zhang et al. 2018). This study is the first that analyzes the n-dodecane effect on β-galactosidase production and models its dynamic using Artificial Intelligence tools in the form of Artificial Neural Networks (ANNs) and Bacterial Foraging Optimization (BFO) algorithm (Passino 2002). These tools were selected based on their capability to solve the particularities of the problem at hand and on their advantages. ANNs are flexible, able of modelling complex non-linear relationships and were widely used to solve problems from various domains, including the influence of oxygen vector on various systems: simulated broths (carboxymethylcellulose sodium salt solutions) (Dragoi et al. 2011), Propionibacterium shermanii, and Saccharomyces cerevisiae cultures (Dragoi et al. 2013). As the structure of the ANN determines the manner in which the model elements are distributed and interconnected (Faris et al. 2019) and as these elements influence the performance, in this work, a simultaneous topology (architecture) and internal parameter (weights, biases, and activation function) optimization are performed by an improved version of BFO. BFO is inspired from the foraging behavior of E. coli and mimics not only the chemotactic strategy but also mechanisms such as swarming, reproduction, elimination, and dispersal (Niu et al. 2013).
Materials and methods
Bacterial strain, media, and culture conditions
Escherichia coli ATCC 15224, the mutant strain that does not require lactose in the medium for β-galactosidase production, was used for the study on the effects of growth conditions. All chemicals were purchased from Sigma-Aldrich Chemical Co. The culture media (1 l) used were glucose 3 g, peptone 10 g, meat extract 5 g, sodium chloride 5 g. Cells were grown at 37 °C, 150 rpm, 2 l/min air, and pH of 6.5 (corrected with NH4OH), and were harvested by centrifugation after 7 cultivation hours. After the inoculation (one colony harvested from an agar plate was suspended in 50 ml medium and grown overnight in an incubator), the oxygen vector, sterilized by filtration, was added in different volumetric fractions (1, 2, and 3%) in the broth. The batch fermentation of E. coli was carried out in 2 l autoclavable laboratory stirred bioreactor (Fermac, Electrolab), provided with an integrated control system. The bioreactor mixing system consists of one turbine impeller (d = 55 mm, 6 blades) and three baffles (s/d = 0.2).
Measurement and analysis methods
Cell density was monitored by measuring the optical density (OD) at 600 nm with a spectrophotometer (UV–Vis CAMSPEC M550) using 10 mm cuvettes and a 1/10 dilution. The dissolved oxygen values at different time intervals during fermentation was determined by oxygen electrode of InPro 6800 series (Mettler Toledo, Switzerland) installed in the bioreactor. The values of oxygen transfer rate, quantified by means of kLa, have been calculated using the dynamic method. The glucose analysis was performed by HPLC, using a Dionex system equipped with refractive index detector and a HyperRez carbohydrate column (300 × 7.7 mm, 8 μm), with water as mobile phase at 0.6 ml/min and oven temperature of 80 °C. Protein concentration was determined using the Bradford assay (colorimetric protein determination with coomassie blue, under acidic conditions quantified at 595 nm), and the enzyme activity was determined using o-nitrophenyl β-D-galactopyranoside (ONPG), prepared in sodium acetate buffer, as substrate and measuring the absorbance of the product o-nitrophenol (after enzyme inactivation with Na2CO3 1 M), at 415 nm (UV–Vis CAMSPEC M550). The unit of enzyme activity was considered the amount of enzyme in 1 ml that is necessary to catalyze the hydrolysis of 1 μmol of ONPG in 1 min under the conditions of the test (at room temperature and pH 4.5 in buffer).The β-galactosidase specific activity was calculated as the ratio between the enzymatic activity and the protein concentration in the sample. Each experiment has been carried out three times; under identical conditions, the average value of measured parameters being used with a maximum experimental error varied between of 5.22 and 7.08%.
Modelling
After gathering the experimental data, a modelling methodology combining ANNs and an improved version of BFO was applied. In this approach, which will be further referred as iBFO-ANN (Fig. 1), ANN acts as a model for the considered process and iBFO is the optimizer that determines the optimum values for the parameters of the model (number of hidden layers, number of neurons in each hidden layer, weights, biases, activation functions, and their parameters). The type of ANN used is represented by the classic feed forward multilayer perceptron neural network, where all the processing elements (also called neurons) are organized in layers and the all neurons from each layer are linked with all the neurons from the next layer. To optimize them, all the parameters considered for are included into the structures representing the bacteria from the iBFO algorithm (in an encoded form—group of real number values).
Fig. 1.
General schema of the modelling methodology
iBFO has the same structure, parameters, and steps as the classic BFO, which is a population-based metaheuristic algorithm. The steps of the algorithm include chemotaxis (where the bacteria tumbles and swims in the environment), swarming [where the social behavior is simulated using the cell-to-cell attraction and repelling effect (Feng et al. 2016a, b)], reproduction, and elimination dispersal (where each bacteria is eliminated with a probability ped). The main difference between iBFO and BFO consists in how ped is set. In BFO is fixed, while, in iBFO, it is adapted (Eq. 1) based on the average fitness of the bacteria in the colony:
| 1 |
Results and discussion
For aerobic strains, an adequate supply of dispersed oxygen is essential for a high productivity and the use of oxygen vectors can increase aerobic bioprocesses productivity (Amaral et al. 2006; Anisha 2017; Galaction et al. 2015; Lai et al. 2012; Qoronfleh 1999; Wang 2000).
In the E. coli strain, more than 200 genes have their expression dependent of oxygen availability due to cells metabolism adjustment, with negative effect on optimal growth (Unden et al. 1995; Rosano and Ceccarelli 2014), and therefore, aeration control is extremely important. Since oxygen limitation in the growing phase will strongly decrease both biomass yield and growth rate, the dissolved oxygen needs to be controlled above 20% of air saturation by changing agitation (from 150 to 400 rpm) and/or aeration rate (Qoronfleh 1999), but the mixing intensification can affect the cells by the shear forces developed and increase the energy consumption. The purpose of the study was to produce the maximum quantity of enzyme with high enzymatic activity, without increasing the aeration rate or the stirrer rotation speed which will increase the energy costs.
The experimental results for E. coli broths (Fig. 2) indicated a significant increase of DO, by adding n-dodecane in a proportion between 1 and 3%, without intensification of mixing or aeration compared with the control (same conditions without oxygen vector). When n-dodecane volumetric fraction was greater than 2% (v/v), some of the oxygen vector was not dispersed completely in the fermentation broth, and formed droplets that accumulated on the surface of the medium, decreasing the surface area available for oxygen transfer.
Fig. 2.

Influence of n-dodecane concentration on dissolved oxygen profile for E. coli fermentation (Glucose initial concentration 3 g/l). Error bars represent standard deviation
It can be observed that the values of dissolved oxygen concentration are significantly higher in the presence of hydrocarbon, due to its positive effect on oxygen transfer from air bubble to liquid phase, effect that is maintained for all the duration of the fermentation. The data for dissolved oxygen are consistent with an increased ability of the cells to consume the additional oxygen supplied, similar results being reported for other strains and the use of other oxygen vectors (Amaral et al. 2006; Blaga et al. 2018; Narta et al. 2011).
Cell growth (respectively enzyme production) is strongly influenced by the n-dodecane addition in the medium, as it can be observed from Fig. 3: in 6 h of fermentation, an optimum increase with approximate 48% was obtained for the 2% n-dodecane addition compared to the control (in which a decreased oxygenation leads to a decreased growth rate). The increase in biomass concentration with a higher rate in the early phase and a lower rate in the late phase is correlated to an increased availability of oxygen in the fermentation broth, fact confirmed by the superior values of the dissolved oxygen in the presence of n-dodecane, hence stating its positive role in oxygen solubility and oxygen transfer rate to the rapidly growing bacterium. The highest cell density was attained for 2% n-dodecane in the broth, for which rapid growth of bacteria with a shorted lag phase allowed it to enter the logarithmic phase earlier leading to an increased productivity. Lower n-dodecane quantity added in the medium 1% oxygen vector provided oxygen concentrations close to the control that leads to limited growth and low oxidation rates, while more oxygen vector (3%) affected the biomass accumulation, probably due to an inhibitory effect generated by oxygen radicals or oxygen peroxide that leads to membrane cell damage by oxidative stress or by an accelerated aerobic catabolic rate for the carbon source that can generate inhibitory compounds as formate or acetate (Anisha 2017). A similar decline in the performance of aerobic fermentation with increasing oxygen-vector concentration beyond an optimum level has been also observed for B. brevis using liquid paraffin as oxygen vector (Narta et al. 2011) and S. cerevisiae using n-dodecane as oxygen vector (Blaga et al. 2018).
Fig. 3.
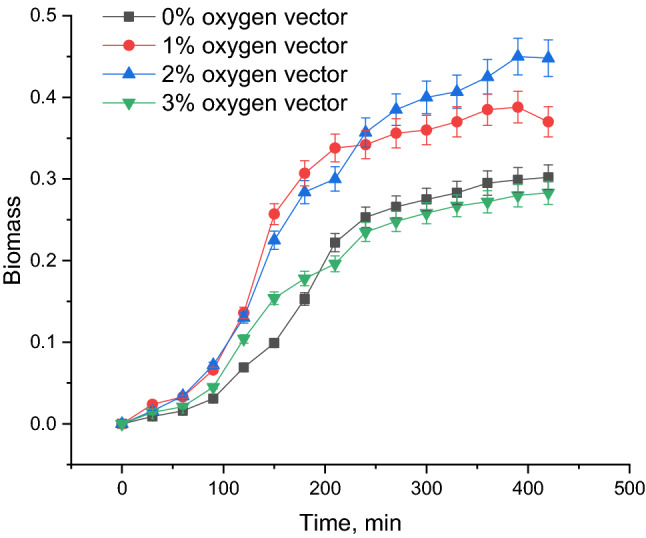
Influence of n-dodecane concentration on biomass accumulation for E. coli fermentation (OD600 nm, dilution 1/10)
The use of n-dodecane in E. coli fermentation, as it can be observed from Fig. 4, leads to an accelerated glucose consumption (in the control fermentation, all the glucose was consumed after 425 min, while in the case of oxygen vector addition, the glucose reached 0 g/l after 350 min). The level of oxygen concentration controls both bacterial cells growth, and metabolic equilibrium for the utilization of glucose for growth and enzyme production. The n-dodecane addition leads to a superior rate of glucose consumption for 2% oxygen vector, due to an increased rate of bacterial growth under higher dissolved oxygen concentration. For 1 and 3%, the final glucose consumption was above the value obtained in the absence of oxygen vector, proving the positive effect of its addition in the fermentation broth. A number of other studies have also obtained enhanced cell mass production using different oxygen vectors (Amaral et al. 2006; Blaga et al. 2018; Narta et al. 2011; Xia et al. 2019).
Fig. 4.

Influence of n-dodecane concentration on glucose consumption for E. coli fermentation process
The maximum for enzyme activity and also kLa (the volumetric mass transfer coefficient) was obtained for the addition of 2% v/v n-dodecane in the bioreactor (Fig. 5), due to the fact that oxygen limitation during the exponential growth phase of the bacterium can repress β-galactosidase production during the exponential and stationary phases. These results show that optimum dissolved oxygen proportion will increase both the biomass concentration and the enzyme-specific activity, similar to the results obtained for other enzymes (Table 1).
Fig. 5.

Influence of oxygen-vector concentration on kLa (volumetric mass transfer coefficient) and enzyme-specific activity (the ratio between the enzymatic activity and the protein concentration)
Table 1.
Results obtained for the use of oxygen vectors in different enzyme production
| No. | Microorganism | Enzyme | Oxygen vector | Increase of activity or productivity | Refs. |
|---|---|---|---|---|---|
| 1 | Bacillus brevis | L-asparaginase | n-Dodecane, 4% | 34% increase in enzyme activity | Narta et al. (2011) |
| 2 | Yarowia lipolitica | Lipase | Perfluorodecalin 20% | 23-fold increase in enzyme productivity | Amaral et al. (2006) |
| 3 | Escherichia coli | Fumarase | n-Dodecane, 2.5% | 24% increase in enzyme activity | Zhang et al. (2018) |
| 4 | Tricoderma reesei | Xylanase | n-Dodecane, 1% | 29.2% higher enzyme activity | Xia et al. (2017) |
The oxygen vector added in the fermentation medium enhanced the gas mass transfer rate, improving the gas liquid contact: kLa has increased at low volumes of n-dodecane: 1 and 2%, but at higher volumes (3%), kLa decreased, due to poor dispersion and reduced contact area. In relation to the enzymatic activity: the positive effect of n-dodecane on the available oxygen can change the intracellular metabolism (into a more oxidative one through a different oxygen transport in the respiratory chain) of the bacteria that could help a more accurate folding of the enzyme generating higher activity, similar to fumarase biosynthesis (Zhang et al. 2018). The experimental results showed that the n-dodecane addition determined an increase on the β-galactosidase specific activity (for 2% v/v enzymatic activity increased with 43% compared with the control). At 3% oxygen vector added, catabolite inhibition as a consequence of growth inhibition generates acetic and formic acid that have an inhibitory effect on protein expression (Losen et al. 2004; Qoronfleh 1999; Zhang et al. 2018).
In Table 2, the values of kLa in oxygen vector presence are reported to kLa in the absence of the oxygen vector. For 2% n-dodecane in the fermentation broth, an increase in the kLa with a factor of 1.23 can be observed, while, for 3%, a decrease by 0.23 was recorded, showing that oxygenation inside the fermentation broth is subject to constrains. Based on these data, the optimum ratio of oxygen vector is 2%; over this value, its addition had no effect (or it produces a slightly decrease) on the biomass accumulation or enzyme activity. This dose-dependent effect of n-dodecane was also noted in other application, such as in Streptomyces hygroscopicus fermentation for Validamycin A biosynthesis, in Aspergillus niger fermentation for citric acid production and Streptococcus zooepidemicus for high-molecular-weight hyaluronic acid (Feng et al. 2016a, b; Lai et al. 2012; Xia et al. 2017).
Table 2.
Effect of n-dodecane addition on the volumetric mass transfer coefficient and enzyme activity
| Oxygen vector content, % v/v | 0 | 1 | 2 | 3 |
| kLav/kLa0 | 1 | 1.12 | 1.23 | 0.77 |
| Enzyme-specific activity increase, % | – | 19 | 42 | 6 |
The results represented in Fig. 6 showed an improved yield coefficient, Yx/s, (expressed as the mass of cells formed per unit mass of substrate consumed) and protein content under n-dodecane addition, higher than that under the control, due to improved oxygen transfer, except for 3% oxygen vector, in which case the decrease in protein content was correlated with a reduced cell accumulation. Highest levels of biomass (Yx/s) and protein content were achieved after the addition of 2% n-dodecane, corresponding to optimum dissolved oxygen concentration and no inhibitory products in the fermentation medium although glucose consumption was very high in the system with and without oxygen vector. Primary metabolism of E. coli responds quickly to oxygen limitation, changes in pH, and acetate concentration (Losen et al. 2004), which hinder optimal growth conditions. When confronted with an increased oxidative metabolism, its metabolic activity would be stimulated to keep a balance of internal environment, and thus, an increased quantity of protein could be obtained. However, by increasing the oxygen vector quantity above 2%, it affects the bacterial metabolism (generate oxidative stress with a negative impact on biomass accumulation) and it increases the apparent viscosity of the broth with a negative impact on oxygen mass transfer. The addition of 2% oxygen vector in the fermentation broth increased both biomass concentration, specific enzyme activity, and yield coefficient (Yx/s), proving that for these optimum conditions, less substrate is necessary to obtain an increased quantity of cells and enzyme.
Fig. 6.

Influence of oxygen-vector concentration on yield coefficient and protein content
The data presented in Fig. 7 showed that, for 2% oxygen vector addition in the fermentation broth, which was found to be optimum for the analyzed process, the maximum biomass was obtained, taking into account the glucose consumed, at 390 min, moment that correspond to the maximum value for enzyme activity as well, this being the optimum moment to stop the fermentation.
Fig. 7.

Fermentation yield (enzymatic activity/biomass and biomass produced/glucose consumption) for 2% oxygen vector fermentation
In an aerobic process, the oxygen mass transfer can be improved by increasing aeration and agitation rate, but this is associated with higher production cost. The use of the oxygen vector can significantly improve the performance of the process by decreasing the cost related to the power consumption for the increased agitation or the compressed air delivery and their antifoaming action. Taking also into account the fact that n-dodecane is non-toxic, low cost, and biological inert, it could be an alternative for several aerobic processes.
Modelling
To determine the best model for each parameter, after the experimental data were gathered, the data underwent a pre-processing stage, where it was first normalized using the Min–Max strategy and then randomly assigned to the training (75%) and testing (25%) sets. All the pre-processing and simulations were performed using the described methodology implemented by the authors in.Net Visual Studio C#.
Next, due to the random nature of the iBFO, for each case, 50 simulations were performed, the statistics obtained being presented in Table 3, where MSE represents the Mean Squared Error and Topology is presented under the notation Inputs: NeuronsHiddenLayer1: NeuronsHiddenLayer2: Outputs. The data from Table 3 are obtained with the following settings for the iBFO algorithm: Nc = 20, Ns= 5, Nre = 8, Ned = 20, and initial value for ped = 0.25. The average absolute values for the best model obtained are 6.2% for the training dataset and 7.28% for the testing dataset. These low errors indicate that the methodology was able to determine a good model of the process that can efficiently replace the experiments when the new parameters are within the confines of the minimum and maximum values used in this study: time (0–450 min), oxygen vector concentration (0–3%v/v), dissolved oxygen (7–100), biomass (0.001–0.45), and glucose concentration (0.001–3.4 g/l). These good results also indicate the effectiveness of the methodology used, the optimization algorithm determining solutions with acceptable errors.
Table 3.
Characteristics of the ANN models obtained
| Parameter | Statistic | Fitness | MSE training | MSE testing | Correlation training | Topology |
|---|---|---|---|---|---|---|
| Enzyme activity | Average | 798.2606 | 0.001302 | 0.158421 | 0.990198 | – |
| Best | 1288.468 | 0.000776 | 0.157542 | 0.99417 | 5:07:06:01 | |
| Worst | 529.7118 | 0.001888 | 0.163846 | 0.98575 | 5:04:01 |
The best model of the enzyme activity (written in a Python format) is represented by the Eqs. (2)–(22). By changing the values of the inputs:
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
| 15 |
| 16 |
| 17 |
| 18 |
| 19 |
| 20 |
| 21 |
| 22 |
For the best models obtained in each case, for the testing data, a plot of experimental data versus ANN predictions was realized (Fig. 8).
Fig. 8.

Experimental vs ANN predictions for the testing data for enzyme activity (R2 = 0.95482)
As it can be observed from Fig. 8, for the best ANN models, the experimental vs the predictions, the R2 is close to 1, indicating that there is a strong correlation between the two.
Conclusions
The effect of n-dodecane as oxygen vector on the E. coli fermentation revealed that the relatively high DO levels induced during fermentation lead to an increase in biomass productivity and faster glucose consumption. Results on β-galactosidase biosynthesis suggest that n-dodecane addition markedly promotes biomass and enzyme biosynthesis compared with the control. Dodecane offers several advantages compared to other oxygen vectors: it is non-toxic for the biomass, chemically inert and stable (can be easily recovered), and inexpensive (requiring low initial investment costs). Under relatively high DO conditions, E. coli may modulate the metabolic flux in favor of biomass growth. Addition of n-dodecane in E. coli medium created a high dissolved oxygen fermentation environment which elevated intracellular oxidative metabolism that will promote a biomass accumulation and a more accurate protein folding of β-galactosidase that would increase its activity. In addition, the process was modelled using iBFO-ANN, a methodology that combines Artificial Neural Networks with an improved version on Bacterial Foraging Optimization being used. The results obtained have both good correlations (close to 1) and average absolute errors (7.28% in the testing phase), indicating that the developed model can successfully replace the experiments when working on the same intervals for the process parameters as in the current study.
Acknowledgements
The authors want to thank Ministère de l’Enseignement Supérieur et de la Recherche, Région Hauts-de-France (CPER ALIBIOTECH) for supporting and funding this work.
Author contributions
All authors have contributed equally to the article. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Amaral PFF, Rocha-Lea MHM, Marrucho IM, Coutinho JAP, Coelho MAZ. Improving lipase production using a perfluorocarbon as oxygen carrier. J Chem Technol Biotechnol. 2006;81:1368–1374. doi: 10.1002/jctb.1478. [DOI] [Google Scholar]
- Anisha GS. β-galactosidases, in Current Developments, pp 369–423. In: Pandey A, Negi S, Soccol C, editors. Biotechnology and bioengineering: production, isolation and purification of industrial products. Amsterdam: Elsevier Science Publisher; 2017. [Google Scholar]
- Blaga AC, Ciobanu C, Caşcaval D, Galaction AI. Enhancement of ergosterol production by Saccharomyces cerevisiae in batch and fed-batch fermentation processes using n-dodecane as oxygen-vector. Biochem Eng J. 2018;131:70–76. doi: 10.1016/j.bej.2017.12.010. [DOI] [Google Scholar]
- Blazek J, Jans P, Baszczynski O, Kaiser MM, Otmar M, Krecmerová M, Drancínsky M, Holy A, Králová B. An enzymatic glycosylation of nucleoside analogues using β-galactosidase from Escherichia coli. Bioorg Med Chem. 2012;20:3111–3118. doi: 10.1016/j.bmc.2012.02.062. [DOI] [PubMed] [Google Scholar]
- Dragoi EN, Curteanu S, Leon F, Galaction AI, Cascaval D. Modeling of oxygen mass transfer in the presence of oxygen-vectors using neural networks developed by differential evolution algorithm. Eng Appl Artif Intell. 2011;24:1214–1226. doi: 10.1016/j.engappai.2011.06.004. [DOI] [Google Scholar]
- Dragoi EN, Curteanu S, Galaction AI, Cascaval D. Optimization methodology based on neural networks and self-adaptive differential evolution algorithm applied to an aerobic fermentation process. Appl Soft Comput. 2013;13:222–238. doi: 10.1016/j.asoc.2012.08.004. [DOI] [Google Scholar]
- Faris H, Mirjalili S, Aljarah I. Automatic selection of hidden neurons and weights in neural networks using grey wolf optimizer based on a hybrid encoding scheme. Int J Mach Learn Cybern. 2019;10(10):2901–2920. doi: 10.1007/s13042-018-00913-2. [DOI] [Google Scholar]
- Feng X, He Y, Yang H, Juan Y. Self-adaptive bacterial foraging optimization algorithm based on evolution strategies. Revista Tecnica de la Facultad de Ingenieria Universidad del Zulia. 2016;39(8):350–358. [Google Scholar]
- Feng J, Jiang J, Liu Y, Li W, Azat R, Zheng X, Zhou WW. Significance of oxygen carriers and role of liquid paraffin in improving validamycin A production. J Ind Microbiol Biotechnol. 2016;43:1365–1372. doi: 10.1007/s10295-016-1822-y. [DOI] [PubMed] [Google Scholar]
- Folescu E, Blaga AC. Utilization of olive oil as a potential oxygen-vector in stirred bioreactors. Environ Eng Manag J. 2013;12:587–594. doi: 10.30638/eemj.2013.071. [DOI] [Google Scholar]
- Galaction AI, Blaga AC, Ciobanu CP, Turnea M, Caşcaval D. Distribution of oxygen transfer rates in stirred bioreactor for different fermentation broths—oxygen-vector dispersions. Environ Eng Manag J. 2015;14:433–447. doi: 10.30638/eemj.2015.045. [DOI] [Google Scholar]
- Khedr M, Desouky S, Badr U. Overproduction of β-galactosidase enzyme from Escherichia coli through genetic improvement. J Appl Sci. 2013;9:4809–4822. [Google Scholar]
- Kokkiligadda A, Beniwal A, Saini P, Vij S. Utilization of cheese whey using synergistic immobilization of β-galactosidase and Saccharomyces cerevisiae cells in dual matrices. Appl Biochem Biotechnol. 2016;179:1469–1484. doi: 10.1007/s12010-016-2078-8. [DOI] [PubMed] [Google Scholar]
- Lai ZW, Rahim RA, Ariff AB, Mohamad R. Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. J Biosci Bioeng. 2012;114:286–291. doi: 10.1016/j.jbiosc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Li M, Meng X, Diao E, Dua F, Zhaoa X. Productivity enhancement of S-adenosylmethionine in Saccharomyces cerevisiae using n-hexadecane as oxygen vector. J Chem Technol Biotechnol. 2012;87:1379–1384. doi: 10.1002/jctb.3752. [DOI] [Google Scholar]
- Losen M, Frolich B, Pohl M, Buchs J. Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Prog. 2004;20:1062–1068. doi: 10.1021/bp034282t. [DOI] [PubMed] [Google Scholar]
- Narta U, Roy S, Kanwar SS, Azmi W. Improved production of l-asparaginase by Bacillus brevis cultivated in the presence of oxygen-vectors. Bioresour Technol. 2011;102:2083–2085. doi: 10.1016/j.biortech.2010.07.118. [DOI] [PubMed] [Google Scholar]
- Niu B, Wang H, Wang J, Tan L. Multi-objective bacterial foraging optimization. Neurocomputing. 2013;116:336–345. doi: 10.1016/j.neucom.2012.01.044. [DOI] [Google Scholar]
- Nivetha A, Mohanasrinivasan V. Mini review on role of β-galactosidase in lactose intolerance. Mater Sci Eng. 2016;263:022046. [Google Scholar]
- Passino KM. Biomimicry of bacterial foraging for distributed optimization and control. IEEE Control Syst. 2002;22:52–67. doi: 10.1109/MCS.2002.1004010. [DOI] [Google Scholar]
- Qoronfleh MW. Dissolved oxygen concentration affects the accumulation of HIV-1 recombinant proteins in Escherichia coli. Appl Biochem Biotechnol. 1999;80:107–119. doi: 10.1385/ABAB:80:2:107. [DOI] [PubMed] [Google Scholar]
- Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 2014;5:1–17. doi: 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa M, Tsumaki K, Sone M. Characterization of the activity of β-galactosidase from Escherichia coli and Drosophila melanogaster in fixed and non-fixed Drosophila tissues. Biochim Open. 2016;3:1–7. doi: 10.1016/j.biopen.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G, Becker S, Bongaerts J, Holighaus G, Schirawski J, Six S. O2-sensing and O2-dependent gene regulation in facultatively anaerobic bacteria. Arch Microbiol. 1995;164:81–90. [PubMed] [Google Scholar]
- Wang J. Enhancement of citric acid production by Aspergillus niger using n-dodecane as an oxygen-vector. Process Biochem. 2000;35:1079–1083. doi: 10.1016/S0032-9592(00)00142-4. [DOI] [Google Scholar]
- Xia J, He A, Li R, Zhang Y, Xu J, Liu X, Xu J. Enzymatic activity and protein expression of cellulase from rice straw produced by Trichoderma reesei in the presence of oxygen vectors. Ind Crops Prod. 2017;109:654–660. doi: 10.1016/j.indcrop.2017.09.017. [DOI] [Google Scholar]
- Xia Y, Chen Y, Liu X, Zhou X, Wang Z, Wang G, Xiong Z, Ai L. Enhancement of antroquinonol production during batch fermentation using pH control coupled with an oxygen vector. J Sci Food Agric. 2019;99:449–456. doi: 10.1002/jsfa.9206. [DOI] [PubMed] [Google Scholar]
- Zhang D, Feng X, Li S, Chen F, Xu H. Effects of oxygen vectors on the synthesis and molecular weight of poly(γ-glutamic acid) and the metabolic characterization of Bacillus subtilis NX-2. Process Biochem. 2012;47:2103–2109. doi: 10.1016/j.procbio.2012.07.029. [DOI] [Google Scholar]
- Zhang S, Song P, Li S. Application of n-dodecane as an oxygen vector to enhance the activity of fumarase in recombinant Escherichia coli: role of intracellular microenvironment. Braz J Microbiol. 2018;49:662–667. doi: 10.1016/j.bjm.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]



