Abstract
Mammalian target of rapamycin inhibitors (mTORi) are used to treat a variety of malignancies and have an established role in organ transplantation. Interstitial lung disease (ILD) is one of the complications of mTORi and is believed to be a class effect. More cases of ILD have been reported with sirolimus than with everolimus in the literature, possibly due to earlier introduction and wider use of sirolimus. We report the case of a kidney transplant recipient who developed ILD secondary to sirolimus and improved rapidly after switching to everolimus.
Keywords: Sirolimus, Everolimus, Drug induced pneumonitis
1. Case report
A 67 your-old female patient with history of end stage renal disease, most likely secondary to chronic glomerulonephritis. She underwent a living unrelated kidney transplantation in July 1998. She received a triple immunosuppressive regimen consisting of cyclosporine, azathioprine and prednisone. She had no history of rejection episodes and had maintained excellent graft function with a serum creatinine (SCr) of 0.7–1 mg/dL and had no proteinuria.
In August 2016 she was diagnosed with aggressive diffuse large B cell lymphoma, plasmablastic subtype involving the left nostril, stage 1AE. As a result of the lymphoma her immunosuppressive agents were discontinued and she received lymphoma treatment with Etoposide, Prednisone, Vincristine, Doxorubicin and cyclophosphamide.
In February 2017, after completion of chemotherapy she was started on sirolimus 2mg daily. The level was maintained at 5–10 ng/ml. In July 2017, the patient presented with dry cough, fever and dyspnea on exertion for six weeks. There was no hemoptysis. Chest radiograph showed patchy infiltrates in the left lung.
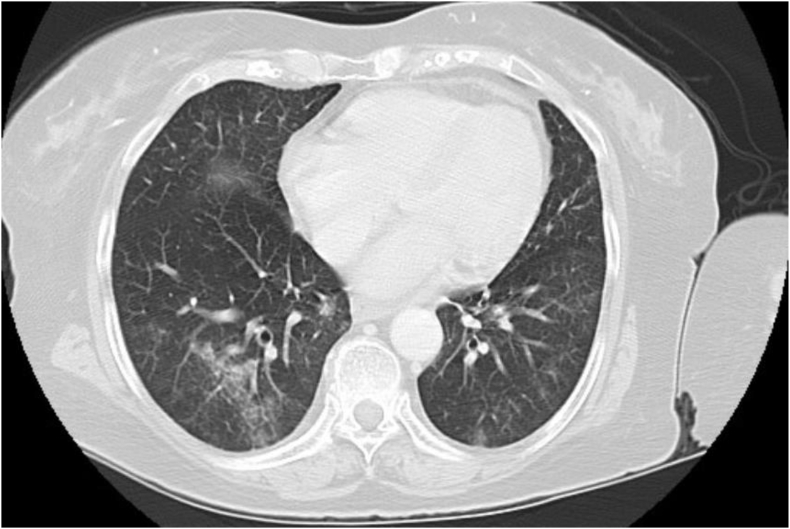
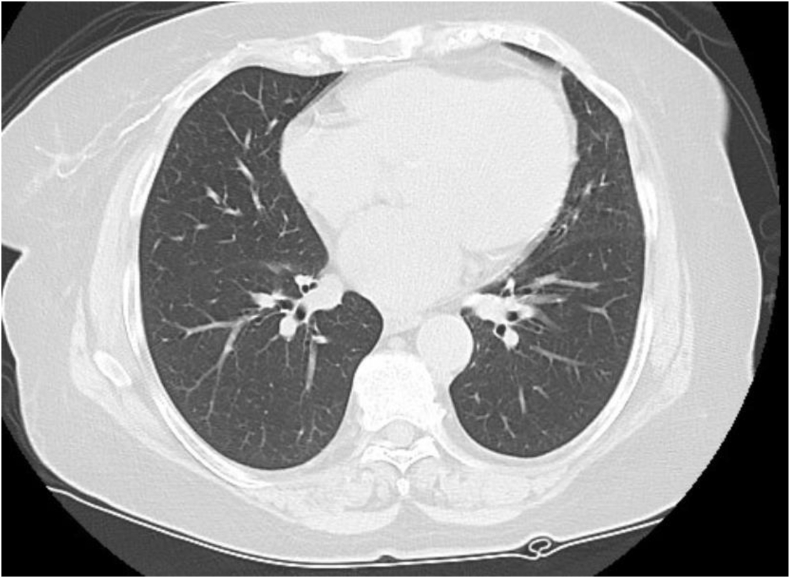
Chest computed tomography scans (CT) revealed multiple patchy, predominantly ground glass opacities scattered in a peribronchovascular distribution throughout the lung fields bilaterally, mainly involving the left upper and both lower lobes (Fig. 1). Broncho alveolar fluid analysis revealed cloudy fluid with WBC 310/mm3, 10% polymorphonuclears, 76% lymphocytes and 14% monocytes. The gram stain was negative and so was the bacterial, viral and fungal culture. After an exhaustive work-up to exclude infectious causes and other pulmonary diseases, the diagnosis of sirolimus-associated pulmonary toxicity was made. In November 2017, sirolimus was discontinued and she was switched to everolimus at 0.75 mg twice daily. The level was maintained at 4–8 ng/ml. Within one week the patient experienced improvement in her symptoms and she was back to her baseline level of activity after two months. A repeat chest CT scan revealed significant decrease of the interstitial infiltrates (Fig. 2). Two years after conversion to everolimus the patient has excellent graft function with a SCr of 1.0 mg/dL, no proteinuria, and no respiratory symptoms.
Fig. 1.
Chest CT showing peribronchovascular ground glass opacities scattered throughout the lung fields.
Fig. 2.
Repeat CT after switching to everolimus showing significant improvement.
2. Discussion
Sirolimus, the first approved mammalian target of rapamycin inhibitor (mTORi) was introduced into clinical transplantation in the late 1990's. Sirolimus may cause proteinuria, but unlike calcineurin inhibitors (CNIs), it is generally considered non-nephrotoxic. It has been used alone or in combination therapy with low dose CNIs in several settings to avoid nephrotoxicity, such as in cases of delayed graft function and chronic allograft nephropathy [1]. The main adverse effects of sirolimus, beside infectious complications, are thrombocytopenia, hyperlipidemia, stomatitis, development of proteinuria and delayed wound healing. Pulmonary toxicity in the form of bronchiolitis obliterans and interstitial lung disease (ILD), was recognized early after introduction of sirolimus into clinical transplantation [[2], [3], [4]].
Similar to sirolimus, everolimus, another mTORi, exhibits antiproliferative properties. Everolimus was approved for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer, neuroendocrine tumors of pancreatic origin, advanced renal cell carcinoma after failure of treatment with sunitinib or sorafenib, angiomyolipoma and tuberous sclerosis complex, and subependymal giant cell astrocytoma. In 2010 the US Food and Drug Administration approved the use of everolimus for prevention of organ rejection in adult kidney transplant patients. Similar to sirolimus, everolimus may cause and/or worsen preexisting proteinuria in renal transplant recipients, but otherwise has a good renal safety profile [5]. Everolimus has been found to cause ILD among cancer patients and solid organ transplant recipients [[6], [7], [8]]. More cases of ILD have been reported with sirolimus than with everolimus in the literature, possibly due to earlier introduction and wider use of sirolimus, especially in kidney transplant recipients. Similarly, Temsirolimus, another mTORi, has been found to cause ILD among patients with metastatic renal carcinoma [9,10]. Mostly, mTORi‐induced ILD remains asymptomatic or mildly symptomatic, but it can lead to severe morbidity and even mortality. The diagnosis of mTORi induced ILD is often difficult as clinical, radiological and pathological features are nonspecific. Due to the nonspecific clinical features of ILD, a broad differential diagnosis that includes opportunistic infections and malignancies should be considered before the diagnosis of ILD is made. To differentiate between ILD and infection, a thorough diagnostic workup to rule out infectious processes and malignancies, often including broncho-alveolar lavage, with or without lung biopsy, is indicated. The incidence rate of ILD associated with mTORi in cancer patients has been reported between 0.03 and 0.11 per patient [11,12]. Lack of uniform diagnostic criteria and active surveillance may explain the variation in the reported incidence. The underlying mechanisms of ILD by mTORi remain uncertain. Two types of pulmonary toxicity have been described: a lymphocytic pneumonitis without hemorrhage, and alveolar hemorrhage without lymphocytic alveolitis [13]. Both dose related direct toxicity, and immunological response have been proposed [11]. Development of pneumonitis after insertion of everolimus eluting coronary stents, where systemic exposure is very limited, supports the notion that immunological processes play a major role in the development of ILD [14].
It is reasonable to think that mTORi induced ILD is a class effect and is not specific to one agent. However, resolution of ILD after switching from sirolimus to everolimus has been reported in a few cases previously [15,16]. Despite having a similar mechanism of action, both everolimus and sirolimus have distinct pharmacokinetic, pharmacodynamic and toxicodynamic properties. Everolimus is more hydrophilic because it has an additional hydroxyethyl group at the C (40) of the molecule which results in different tissue distribution, different affinities to drug transporters and metabolizing enzymes, as well as differences in drug-target protein interactions [17]. It is also more potent in terms of interaction with the mTOR complex 2 than sirolimus [17]. Our case supports the idea that in cases of sirolimus induced ILD, switching to everolimus is a viable option in order to benefit from the antiproliferative effect of mTORi and to avoid CNIs side effects.
CRediT authorship contribution statement
Ahmed M. Alkhunaizi: Conceptualization, Writing - original draft. Thamer H. Al-Khouzaie: Writing - review & editing, Data curation. Ahmed I. Alsagheir: Writing - review & editing, Investigation.
Acknowledgement
The authors acknowledge the use of Johns Hopkins Aramco Healthcare (JHAH) facilities for research data used in this article. Opinions expressed in this article are those of the authors and not necessarily of JHAH.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101109.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Pham P.T., Pham P.C., Danovitch G.M., Ross D.J., Gritsch H.A., Kendrick E.A. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77(8):1215–1220. doi: 10.1097/01.tp.0000118413.92211.b6. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez J., Mahalati K., Kiberd B., McAlister V.C., MacDonald A.S. Conversion to rapamycin immunosuppression in renal transplant recipients: report of an initial experience. Transplantation. 2000;70(8):1244–1247. doi: 10.1097/00007890-200010270-00021. [DOI] [PubMed] [Google Scholar]
- 3.Morelon E., Stern M., Kreis H. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N. Engl. J. Med. 2000;343(3):225–226. doi: 10.1056/NEJM200007203430317. [DOI] [PubMed] [Google Scholar]
- 4.West M.L. Bronchiolitis obliterans and organizing pneumonia IN renal transplant recipients. Transplantation. 2000;69(7):1531. [Google Scholar]
- 5.Tedesco-Silva H., Jr., Vitko S., Pascual J., Eris J., Magee J.C., Whelchel J. 12-month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transpl. Int. 2007;20(1):27–36. doi: 10.1111/j.1432-2277.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 6.Iacovelli R., Palazzo A., Mezi S., Morano F., Naso G., Cortesi E. Incidence and risk of pulmonary toxicity in patients treated with mTOR inhibitors for malignancy. A meta-analysis of published trials. Acta Oncol. 2012;51(7):873–879. doi: 10.3109/0284186X.2012.705019. [DOI] [PubMed] [Google Scholar]
- 7.Lopez P., Kohler S., Dimri S. Interstitial lung disease associated with mTOR inhibitors in solid organ transplant recipients: results from a large phase III clinical trial program of everolimus and review of the literature. J. Transpl. 2014;2014:305931. doi: 10.1155/2014/305931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willemsen A., Tol J., van Erp N.P., Jonker M.A., de Boer M., Meek B. Prospective study of drug-induced interstitial lung disease in advanced breast cancer patients receiving everolimus plus exemestane. Targeted Oncol. 2019;14(4):441–451. doi: 10.1007/s11523-019-00656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eto M., Motoshima T., Sugiyama Y., Fujimoto K., Ohyama C., Mita K. Temsirolimus-induced interstitial lung disease as an on-target effect in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2017;35(15_suppl) e16086-e. [Google Scholar]
- 10.Matsuki R., Okuda K., Mitani A., Yamauchi Y., Tanaka G., Kume H. A case of delayed exacerbation of interstitial lung disease after discontinuation of temsirolimus. Respir. Med. Case Rep. 2017;22:158–163. doi: 10.1016/j.rmcr.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemsen A.E.C.A.B., Grutters J.C., Gerritsen W.R., van Erp N.P., van Herpen C.M.L., Tol J. mTOR inhibitor-induced interstitial lung disease in cancer patients: comprehensive review and a practical management algorithm. Int. J. Canc. 2016;138(10):2312–2321. doi: 10.1002/ijc.29887. [DOI] [PubMed] [Google Scholar]
- 12.Gartrell B.A., Ying J., Sivendran S., Boucher K.M., Choueiri T.K., Sonpavde G. Pulmonary complications with the use of mTOR inhibitors in targeted cancer therapy: a systematic review and meta-analysis. Targeted Oncol. 2014;9(3):195–204. doi: 10.1007/s11523-013-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlahakis N.E., Rickman O.B., Morgenthaler T. Sirolimus-associated diffuse alveolar hemorrhage. Mayo Clin. Proc. 2004;79(4):541–545. doi: 10.4065/79.4.541. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto S., Kikuchi N., Ichikawa A., Sano G., Satoh K., Sugino K. Everolimus-induced pneumonitis after drug-eluting stent implantation: a case report. Cardiovasc. Intervent. Radiol. 2013;36(4):1151–1154. doi: 10.1007/s00270-012-0477-y. [DOI] [PubMed] [Google Scholar]
- 15.Rehm B., Mayer J., Stracke S. Resolution of sirolimus-induced pneumonitis after conversion to everolimus. Transplant. Proc. 2006;38:711–713. doi: 10.1016/j.transproceed.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 16.De Simone P., Petruccelli S., Precisi A., Carrai P., Doria R., Menichetti F. Switch to everolimus for sirolimus-induced pneumonitis in a liver transplant recipient--not all proliferation signal inhibitors are the same: a case report. Transplant. Proc. 2007;39(10):3500–3501. doi: 10.1016/j.transproceed.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Klawitter J., Nashan B., Christians U. Everolimus and sirolimus in transplantation-related but different. Expet Opin. Drug Saf. 2015;14(7):1055–1070. doi: 10.1517/14740338.2015.1040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.