Abstract
There is no effective serologic parameter to distinguish different types of pancreatitis now. To distinguish between acute pancreatitis (AP) and acute exacerbations of chronic pancreatitis (CP) and to determine whether fibrosis occurs in CP, we evaluated the ability to produce white blood cells (WBCs), the neutrophil-to-retinol-binding protein (RBP) ratio (called the WNR), the product of the gamma-glutamyl transpeptidase (GGT) level, and the 5′-nucleotide-to-RBP ratio (called the GNR). We evaluated the newly proposed difference index RBP and analyzed the effectiveness of the WNR and GNR in 691 patients with pancreatic diseases. We performed univariate and multivariate analyses of serological indices and their correlations with RBP and performed receiver operating characteristic (ROC) curve analyses of the WNR and GNR. The serum RBP level decreased markedly in AP compared with that in the acute stage of CP (p < 0.05). The GGT, alkaline phosphatase (ALP), total protein (TP), albumin (ALB), prealbumin (PA), 5′-nucleotide, and uric acid (UC) serum levels were significantly higher for fibrotic CP than for the acute stage of CP without fibrosis (p < 0.05). With progressing to pancreatic fibrosis, the liver injury-related indicators, prothrombin time (PT), activated partial thromboplastin time (APTT), D-Dimer, aspartate aminotransferase (AST), and GGT, gradually increased (p < 0.05). ROC curve analysis suggests that both the WNR (area under the curve [AUC] = 0.821) and GNR (AUC = 0.778) can be used to differentiate pancreatitis types.
Keywords: pancreatitis, WNR, GNR, pancreatic fibrosis
Graphical Abstract

To distinguish different types of pancreatitis by serological index, Luo and colleagues evaluated the ability to produce white blood cells (WBCs), the neutrophil-to-retinol-binding protein (RBP) ratio (called the WNR), the product of the gamma-glutamyl transpeptidase (GGT) level, and the 5′-nucleotide-to-RBP ratio (called the GNR). Both can be used to differentiate pancreatitis types.
Introduction
Acute pancreatitis (AP) is caused by proinflammatory factors, including alcohol consumption and pancreatic stones,1 which trigger the activation of trypsin and subsequent digestive tract damage.2 AP can be clearly diagnosed by the acute onset of abdominal pain, serum amylase, or lipase, three times above the normal upper limit, and imaging changes of the pancreas, indicating extravasation, edema, and fluid around the pancreas by abdominal ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). AP is often accompanied by transient or continuous multiple organ failure, which is the main cause of increased mortality by AP.3 Recurrent attacks to and sustained damage of the pancreatic parenchyma from AP-induced chronic inflammation can cause chronic pancreatitis (CP), leading to pancreatic exocrine insufficiency, diabetes, atrophy, fibrosis, and fatty replacement.2,4 CP patients in the stable phase of the disease did not have obvious discomfort, but they were admitted to the hospital for acute exacerbation in the context of CP. Due to common symptoms, such as abdominal pain and digestive dysfunction, AP and the acute stage of CP cannot be clearly distinguished using clinical symptoms. Differences in serological indicators have diagnostic value for many diseases, including AP and acute exacerbation in the context of CP. Studies have shown that AP is associated with a significant increase in the levels of serum lipase, amylase, C-reactive protein, white blood cells (WBCs), and neutrophils (NEs) during disease onset compared with CP.5,6 Moreover, AP is more likely to lead to renal dysfunction and increase levels of serum indicators of kidney disorder than CP.7 Although imaging findings can differentiate between these two types of disease, no serological markers are available to differentiate between AP and acute exacerbation in the context of CP.
CP is divided into two types: atrophic and fibrotic.8,9 In the early stages of CP, harmful stimuli, such as inflammatory factors and ethanol, increase the apoptotic rate of pancreatic parenchyma cells and cause pancreatic atrophy. However, as pancreatic stellate cells activate and proliferate, extracellular matrix deposition increases, resulting in pancreatic fibrosis.10,11 A diagnosis of CP mainly depends on the patient’s medical history, clinical symptoms and signs, laboratory examination, imaging modalities, and pathological evidence of pancreatic tissue biopsy.12 However, to date, no study has addressed whether differences in blood composition can be used to identify whether fibrosis has occurred in lesions from CP. Recently, we found a significant decrease in the serum levels of retinol-binding protein (RBP) in patients with AP and a significant increase in the serum levels of gamma-glutamyl transpeptidase (GGT) and 5′-nucleotide (5′-NT), which are associated with pancreatic cancer, in patients with pancreatic fibrosis, suggesting that pancreatic fibrosis patients possess similarities to pancreatic cancer patients.
RBP is a lipocalin superfamily member that is mainly synthesized in the liver and transports retinol into peripheral tissues.13,14 Most RBP-retinol complexes cannot be filtered in the glomerulus by transthyretin (TTR) binding. A small amount of RBP-retinol complexes is degraded and absorbed by proximal glomerular tubules, thus increasing the levels of RBP in urine and decreasing the RBP levels in the blood. Pancreatitis is often accompanied by the dysfunction of multiple organs, including the liver and kidney; therefore, RBP is used as an important indicator of pancreatic disease progression. In this study, we developed new analytical methods using the serum levels of various indicators to study the progression of pancreatitis from the acute to fibrotic stages. We used the product of the WBC and the NE-to-RBP ratio (WNR) as a mechanism to distinguish and predict AP and acute exacerbation in the context of CP, as well as used the product of GGT and the 5′-nucleotide-to-RBP ratio (GNR) as an indicator of fibrosis during CP.
Results
Clinical Characteristics
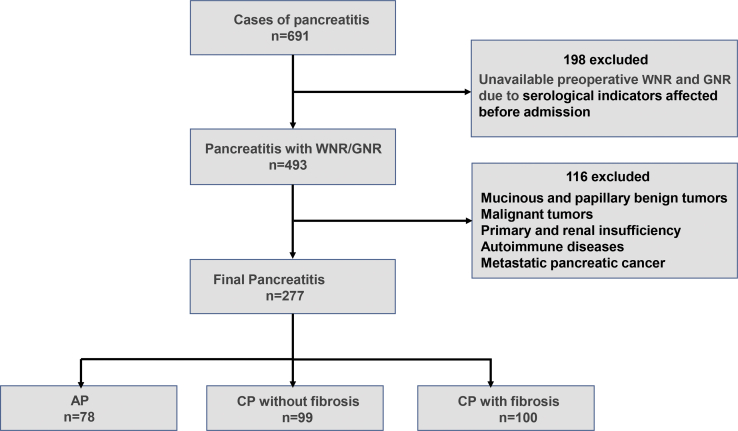
Two hundred seventy-seven cases of pancreatitis were studied, including 78 cases of AP and 199 cases of CP with acute exacerbation (99 cases of nonfibrous hyperplasia and 100 cases of fibrous hyperplasia) (Figure 1). The clinical characteristics of the subjects with AP and CP are shown in Table S1. Excluding significant differences in BMI, blood pressure, and sex, the serological indices for WBC, NE, prothrombin time (PT), D-Dimer, alanine aminotransferase (ALT), alkaline phosphatase (ALP), and other indicators were elevated during acute pancreatic inflammation but not in CP. However, the RBP levels were significantly increased in CP compared with that during acute pancreatic inflammation. Moreover, the WNR was significantly higher for AP. Our differential analysis of the serum indicators WBC, NE, RBP, and, WNR in AP and CP is shown in Figure S1. An analysis of serum parameters for CP with or without fibrous tissue hyperplasia in Table 1 indicates that, in addition to differences between BMI and alcohol consumption, the levels of serum indicators, such as GGT, ALP, prealbumin (PA), and 5′-nucleotides, were significantly lower in patients with CP without fibrosis than in those with fibrosis, but the RBP levels were lower in patients with pancreatic fibrosis than in those without fibrosis. Additionally, a statistically significant difference in GNR was found between the two types of CP. Differential analysis of the serum levels of GGT, 5′-nucleotides, RBP, and GNR for the two types of CP is shown in Figure 2.
Figure 1.
Flow Chart of Patient Selection in Cohort Study
AP, acute pancreatitis; CP, chronic pancreatitis.
Table 1.
Univariate Analysis of Clinical Characteristics and Serological Indicators of CP with or without Fibrosis
| Variables | HC | CP without Fibrosis | CP with Fibrosis | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Number | 50 | 99 | 100 | |||
| Age, years | 43.25 ± 12.32 | 45.77 ± 14.78 | 48.74 ± 12.01 | 0.27 | 0.017 | 0.12 |
| Gender, F/M | 29/21 | 28/71 | 20/80 | <0.001 | <0.001 | 0.17 |
| Smoke, +/− | 13/37 | 39/60 | 53/47 | 0.11 | 0.002 | 0.054 |
| Drink, +/− | 14/36 | 35/64 | 50/50 | 0.37 | 0.01 | 0.037 |
| Blood pressure, +/− | 18/32 | 23/76 | 18/82 | 0.099 | 0.015 | 0.36 |
| BMI, kg/m2 | 23.87 ± 4.00 | 21.73 ± 3.19 | 22.80 ± 2.86 | <0.001 | 0.024 | <0.001 |
| WBC | 6.81 ± 1.02 | 5.84 ± 2.02 | 6.09 ± 2.03 | 0.003 | 0.028 | 0.33 |
| HBC | 138.46 ± 14.07 | 131.84 ± 16.05 | 134.180 ± 19.41 | 0.027 | 1.51 | 0.34 |
| PLT | 203.70 ± 48.94 | 202.13 ± 79.18 | 198.39 ± 78.58 | 0.90 | 0.68 | 0.72 |
| NE | 4.18 ± 1.07 | 3.65 ± 1.73 | 4.94 ± 3.53 | 0.23 | 0.087 | <0.001 |
| PT | 10.63 ± 0.58 | 11.48 ± 0.93 | 11.49 ± 1.38 | <0.001 | <0.001 | 0.97 |
| APTT | 26.15 ± 2.34 | 28.48 ± 3.57 | 28.13 ± 4.96 | 0.001 | 0.005 | 0.54 |
| D-Dimer | 0.26 ± 0.13 | 0.62 ± 0.96 | 0.74 ± 1.18 | 0.032 | 0.004 | 0.38 |
| ALT | 23.30 ± 8.18 | 36.38 ± 40.83 | 58.66 ± 64.82 | 0.12 | <0.001 | 0.001 |
| AST | 22.23 ± 5.03 | 34.50 ± 26.76 | 47.10 ± 46.97 | 0.04 | <0.001 | 0.01 |
| GGT | 29.48 ± 15.87 | 82.01 ± 151.90 | 191.77 ± 271.28 | 0.13 | <0.001 | <0.001 |
| ALP | 79.96 ± 15.37 | 110.09 ± 55.06 | 204.31 ± 222.01 | 0.23 | <0.001 | <0.001 |
| TP | 71.06 ± 3.86 | 69.41 ± 7.07 | 67.15 ± 7.17 | 0.13 | 0.01 | 0.04 |
| ALB | 43.79 ± 3.10 | 41.89 ± 5.08 | 40.05 ± 5.26 | 0.024 | <0.001 | 0.008 |
| GLO | 27.44 ± 2.63 | 27.53 ± 4.13 | 27.55 ± 4.68 | 0.91 | 0.88 | 0.97 |
| A/G | 1.61 ± 0.20 | 1.55 ± 0.28 | 1.50 ± 0.32 | 0.22 | 0.02 | 0.17 |
| TBIL | 12.14 ± 4.07 | 21.87 ± 53.66 | 29.73 ± 48.99 | 0.22 | 0.028 | 0.23 |
| DBIL | 2.59 ± 0.93 | 7.45 ± 27.79 | 12.85 ± 28.31 | 0.27 | 0.019 | 0.13 |
| TBA | 9.55 ± 3.41 | 13.09 ± 24.67 | 16.80 ± 21.27 | 0.32 | 0.044 | 0.21 |
| 5′-nucleotide | 6.46 ± 4.29 | 8.28 ± 11.88 | 27.92 ± 46.79 | 0.73 | <0.001 | <0.001 |
| α-fucose | 26.67 ± 5.85 | 27.01 ± 12.97 | 29.09 ± 14.97 | 0.88 | 0.27 | 0.25 |
| PA | 0.25 ± 0.043 | 0.19 ± 0.064 | 0.25 ± 0.13 | 0.001 | 0.88 | 0.001 |
| Adenylate deaminase | 8.87 ± 2.21 | 11.87 ± 4.17 | 13.25 ± 10.05 | 0.018 | <0.001 | 0.13 |
| BUN | 5.04 ± 0.91 | 5.16 ± 1.74 | 5.10 ± 5.57 | 0.85 | 0.93 | 0.91 |
| CR | 67.80 ± 13.36 | 65.24 ± 23.01 | 63.10 ± 18.28 | 0.45 | 0.17 | 0.44 |
| UC | 327.88 ± 67.63 | 336.41 ± 92.76 | 300.94 ± 110.04 | 0.61 | 0.11 | 0.01 |
| FBG | 5.34 ± 0.52 | 6.14 ± 2.97 | 12.56 ± 54.70 | 0.89 | 0.23 | 0.19 |
| Serum calcium | 2.34 ± 0.086 | 2.34 ± 0.13 | 2.55 ± 3.05 | 0.99 | 0.53 | 0.43 |
| RBP | 33.95 ± 4.95 | 27.09 ± 8.76 | 24.84 ± 9.14 | <0.001 | <0.001 | 0.028 |
| GNR | 6.26 ± 6.16 | 76.98 ± 417.88 | 536.50 ± 1,693.36 | 0.71 | 0.006 | <0.004 |
HC, health control; GNR, the product of GGT and the 5′-nucleotide-to-RBP ratio. P1, HC/CP without fibrosis; P2, HC/CP with fibrosis; P3, CP without fibrosis/CP with fibrosis; F, female; M, male; HBC, hemoglobin C; PLT, platelet; GLO, globulin; A/G, albumin/globulin; TBIL, total bilirubin; DBIL, direct bilirubin; TBA, total bile acids.
Figure 2.
Boxplot Diagram Analysis of GGT, 5′-NT, RBP, and GNR Distributions in CP with or without Fibrosis
Relationships between the Serum RBP Levels and Other Biochemical Tests
We analyzed the correlations between various serum indicators and the RBP levels by Pearson’s correlation analysis (Table S2). The RBP levels were positively correlated with the levels of the renal injury-related indicators blood urea nitrogen (BUN), creatinine (CR), and uric acid (UC) and were negatively correlated with the levels of the coagulation function indicators PT, activated partial thromboplastin time (APTT), and D-Dimer during the differentiation of AP and CP. We tested for correlations between the RBP levels and indicators of fibrosis during CP and found that the RBP levels were positively correlated with the clinical characteristics of smoking, alcohol consumption, and BMI and the levels of the renal injury-related indicators BUN, CR, and UC but were negatively correlated with levels of the liver injury indicators PT, APTT, D-Dimer, aspartate aminotransferase (AST), and GGT (Table 2).
Table 2.
Correlation Coefficients for the Relationships between RBP and Various Parameters of CP with or without Fibrosis
| Factors | R | p Value |
|---|---|---|
| Smoking | 0.126 | 0.040 |
| Drinking | 0.161 | 0.012 |
| BMI | 0.121 | 0.044 |
| HBC | 0.131 | 0.033 |
| PT | −0.301 | 0.000 |
| APTT | −0.308 | 0.000 |
| D-Dimer | −0.229 | 0.001 |
| AST | −0.152 | 0.016 |
| GGT | −0.151 | 0.017 |
| TP | 0.253 | 0.000 |
| ALB | 0.383 | 0.000 |
| PA | 0.280 | 0.000 |
| BUN | 0.195 | 0.003 |
| CR | 0.304 | 0.000 |
| UC | 0.293 | 0.000 |
Ability of the WBC, NE, RBP, and WNR to Distinguish and Predict AP and Acute Exacerbations of CP
We analyzed the ability of the WNR, WBC, NE, and RBP values to distinguish AP and acute exacerbations of CP using receiver operating characteristic (ROC) curves (Figure S2). The area under the curve (AUC) value for distinguishing AP from CP patients by the WNR was 0.821, and the optimal sensitivity and specificity were 0.782 and 0.819, respectively. The AUC values of WBC, NE, and RBP were 0.767, 0.761, and 0.726, respectively, which were significantly lower than those obtained by AUC analysis of the WNR. Similarly, the sensitivity and specificity of the WBC, NE, and RBP in distinguishing between AP and acute exacerbations of CP were lower than those of the WNR. Multiple logistic regression analysis was employed to evaluate the role of related indicators in distinguishing between AP and acute exacerbations of CP (Table 3). Although many significant differences were found between AP and acute exacerbations of CP patients, as indicated by univariate analysis of clinical characteristics, the PT, total protein (TP), 5′-nucleotide, WBC, NE, and WNR values can be used to distinguish between AP and acute exacerbations of CP using multivariate analysis in a statistically meaningful manner. We also used a nomogram to predict and analyze the accuracy of the serum indexes WBC, D-Dimer, RBP, and WNR (Figure S3). The results suggest that the WBC, D-Dimer, WNR, and RBP were useful in predicting the type of disease.
Table 3.
Multivariate Analysis of Factors Associated with or without Fibrosis in Chronic Pancreatitis
| Factors | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Blood pressure | 0.208 | 0.063-0.688 | 0.01 |
| BMI | 1.250 | 1.055–1.481 | 0.01 |
| FBG | 1.159 | 1.016–1.321 | 0.028 |
| BUN | 0.633 | 0.456–0.879 | 0.006 |
| WBC | 0.523 | 0.312–0.879 | 0.014 |
| GGT | 1.004 | 1.001–1.007 | 0.005 |
| 5′-nucleotide | 2.707 | 1.525–4.804 | 0.001 |
| RBP | 0.906 | 0.834–0.984 | 0.019 |
| GNR | 0.998 | 0.997–1.000 | 0.016 |
Ability of GGT, 5′-Nucleotide, RBP, and GNR Values in Distinguishing Fibrotic from Nonfibrotic CP
We investigated the utility of the GGT, 5′-nucleotide, RBP, and GNR serum levels in distinguishing CP patients with or without fibrosis using ROC curve analysis (Figure 3). The AUC value for GNR was 0.782, and the optimal sensitivity and specificity were 0.72 and 0.727, respectively. The levels of GGT, 5′-nucleotide, and RBP also showed the ability to distinguish between fibrotic and nonfibrotic cases, with AUC values of 0.729, 0.723, and 0.577, respectively. Based on logistic analysis, GNR (odds ratio [OR]: 0.998; 95% confidence interval [CI]: 0.997–1.000), GGT (OR: 1.004; 95% CI: 1.001–1.007), 5′-nucleotide (OR: 2.707; 95% CI: 1.525–4.804), and RBP (OR: 0.906; 95% CI: 0.834–0.984) values proved to be independent, predictive markers of fibrosis in CP. Additionally, blood pressure (OR: 0.208; 95% CI; 0.063–0.688), BMI (OR: 1.250; 95% CI: 1.055–1.481), fasting blood glucose (FBG; OR: 1.159; 95% CI: 1.016–1.321), BUN (OR: 0.633; 95% CI: 0.456–0.879), and WBC (OR: 0.523; 95% CI: 0.312–0.879) have some significant value in predicting fibrosis (Table S3). We also analyzed the role of GGT, ALP, and the product of GGT and ALP in distinguishing fibrotic and nonfibrotic CP cases. The results indicate that the AUC values for GGT, ALP, and GGT × ALP were 0.729, 0.61, and 0.745, respectively (Figure S4).
Figure 3.
The GNR Is Effective for Distinguishing between CP with or without Fibrosis
(A–D) ROC curve for the discrimination of CP patients with and without fibrosis using GGT (A), 5′-nucleotide (B), RBP (C), and GNR (D) values. AUC, area under the curve.
Discussion
Clinical manifestations do not distinguish and predict AP and acute exacerbations of CP because of the commonality of symptoms, including abdominal pain and indigestion.15 AP and acute exacerbations of CP can lead to obvious inflammatory reactions, immune system disorders, and functional degeneration of multiple organs during disease progression;16, 17, 18 therefore, the serum component levels are used to distinguish and predict AP and acute exacerbations of CP. Previous studies have found that the levels of D-Dimer, natural anticoagulants, and other routine coagulation parameters can be used as indicators to evaluate the degree of AP.19 In distinguishing between AP and acute exacerbations of CP, monocyte chemoattractant protein 1, transforming growth factor β1, and hyaluronic acid are also considered to be valid serum predictors.20 Our study performed statistical analyses of serological indices related to clinical characteristics and liver and kidney function. We found that the levels of many serological indicators, such as the WBC, NE, TP, D-Dimer, PA, and UC, were significantly higher in AP than CP. These results showed that AP is more likely to lead to functional lesions in multiple organs than CP, a phenomenon that may be closely related to its stimulation of explosive inflammatory responses, oxidative stress responses, and immune system activation.21 Therefore, the serological indicators related to multiple organ injuries may provide predictive power for distinguishing between AP and acute exacerbations of CP. To better distinguish between AP and acute exacerbations of the CP, WBC, NE, and RBP values were utilized as the WNR, calculated by multiplying the WBC and NE-to-RBP ratio. We found that the WNR is significantly different for AP and CP. To distinguish between patients with the same symptoms during AP or acute phase of CP, we also used a nomogram by analyzing the difference in the serum indexes WBC, D-Dimer, RBP, and WNR. The results suggested that a patient with a higher WBC, WNR, and level of D-Dimer and a lower level of RBP is more likely to have AP; conversely, a patient with a lower WBC, WNR, and level of D-Dimer and a higher level of RBP is more likely to have CP.
We also analyzed the ability of the serological indicators to determine whether fibrosis has occurred during CP cases. Our results showed that the biliary obstruction indicators GGT, ALP, and 5′-nucleotides; the liver damage indicators TP, ALB, and PA; and the renal injury index UC were significantly higher for CP with fibrosis than for CP without fibrosis. In these early stages, because the pancreatic and total bile ducts are not obstructed, the changes in serum indicators are mainly restricted to those associated with injury to the pancreas, liver, and kidney. Because inflammation becomes delayed and collagen is deposited in the pancreas, fiber tissue hyperplasia intensifies, resulting in pancreatic stenosis and choledochal obstruction. Although changes occur in serological indicators related to organ injury at this stage, the main elevated serological indicators are GGT, ALP, and 5′-nucleotides associated with bile duct obstruction. To make a more obvious distinction between fibrotic and nonfibrotic CP, we calculated the GNR, defined as the product of the GGT and NE-to-RBP ratio, which exacerbated differences and made it easier to distinguish between fibrotic and nonfibrotic cases of CP. Our results demonstrated that the WNR and GNR are effective in differentiating between pancreatic diseases at different stages of development. With the consideration that GGT and ALP are significantly elevated in fibrotic CP cases, our study analyzed whether GGT, ALP, and GGT × ALP were more useful in differentiating CP with or without fibrosis. In terms of the AUC value and sensitivity and specificity of the prediction, the GNR is a better predictor to distinguish between fibrotic and nonfibrotic CP cases than GGT × ALP.
We also found that the RBP is significantly lower in AP than in CP and is lower in fibrotic CP than in nonfibrotic CP. Pearson’s correlation analysis of RBP and other serological indices in AP and CP showed that the RBP level is positively correlated with renal injury-related indicators but is negatively correlated with coagulation function indicators. In the analysis of CP with or without fibrosis, RBP was found to correlate with serological indicators related to both renal injury and liver injury, indicating that a large number of inflammatory factors flow into the kidneys and other organs through the blood, although they are more likely to cause inflammatory damage to kidneys during the early stages of pancreatic disease. However, as fibrosis progresses, bile duct obstruction may cause serious damage to liver function. Therefore, based on the pathological characteristics of pancreatitis progression, the WNR and GNR used to classify different types of pancreatitis may be a relatively effective method for diagnosis.
Numerous have shown that the combination of multiple variables, rather than a single variable, may be more effective for diagnosing different types of diseases. For example, the potential invasiveness of intraductal papillary mucinous neoplasms of the pancreas can be effectively and rapidly diagnosed by the NE-to-lymphocyte ratio.22 Moreover, the NE-to-lymphocyte ratio can be used to effectively predict the survival rates of breast cancer patients.15 We examined whether the WNR and GNR can be used to effectively diagnose different pancreatic disease types via ROC curves. Although the WBC, NE, GGT, and RBP have some utility in diagnosing pancreatitis, the WNR and GNR have higher sensitivity and specificity for diagnoses, because they have higher AUC values, sensitivity, and specificity. In patients with pancreatitis, due to differences in pancreatitis progression, the types of organs affected by injury can vary, thus increasing or decreasing the levels of different serological indicators. Compared with pancreatitis cases with no obvious changes in the levels of individual serum factors, the products of different variables whose serum levels rise simultaneously can show obvious changes in patients, further indicating that a combination of different variables is more effective than a single variable for diagnosing diseases. Multivariate logistic regression analysis of multiple serological indicators revealed that both the WNR and GNR are effective parameters for distinguishing pancreatitis types. Therefore, with the combination of these results with those from ROC curves and multifactor analysis, we propose that the WNR and GNR may be more effective indicators for diagnosing different types of pancreatitis.
We could not obtain completely accurate clinical data due to the limitations of hospital examinations and patient treatments. Patient data were collected after the patients were admitted to the hospital; however, a small number of patients may have been ill for a relatively long period before they were admitted to the hospital, and diseases may have been previously treated at other hospitals. Moreover, patients were not immediately examined after hospital admission, and differences in wait times and examination times could have led to inaccurate clinical data. Herein, we report a retrospective study of patients admitted up to 5 years ago due to a lack of sufficient numbers of more recent patients. Therefore, this article does not specifically include the case data of newly admitted patients and their levels of various indicators.
Our study indicates that an elevated WNR is an independent predictive factor for differentiating AP from acute exacerbations of CP and that an elevated GNR can also distinguish fibrotic CP from nonfibrotic CP (Figure S5). With the distinction between AP and CP, both the WNR values and changes in renal injury indicators may increase diagnostic accuracy. For the identification of chronic pancreatic fibrosis, the GNR, combined with assessments of liver and kidney function damage, likely increases accurate diagnosis rates. Future research into the classification of pancreatitis diseases will help to evaluate and improve the accuracy of our predictions.
Materials and Methods
Patient Selection
This study was a retrospective study of hospitalized patients with a definitive diagnosis of AP, CP, and CP with fibrosis, according to the diagnostic criteria for AP and CP, inquiring about the etiology and history—for example, heavy drinking and recurrent AP (sustained pathologic response >6 months)—and discarding case data that did not distinguish the type of pancreatitis to prevent patients with CP from being mistaken for those with AP. In total, 691 patients were admitted to the Southwest Hospital of the Army Military Medical University from January 2015 to April 2019, due to pancreatitis symptoms, including abdominal pain, nausea, and vomiting. The biochemical parameters collected were the results of the first biochemical test after admission. To exclude patients who did not meet the inclusion criteria, we inquired about the patient’s medical history and checked the medical records before admission. After admission, the results of routine tests were further screened to exclude patients with other diseases that affected the biochemical markers. Of these patients, 493 had not been treated with drugs or had not undergone treatments or examinations that could change the levels of serum indicators before admission. To ensure that the WBC, NE, RBP, 5′-nucleotide, and GGT counts could be used as accurate serological indicators of CP and fibrosis, the following strict exclusion criteria were adopted: benign and malignant pancreatic tumors and metastatic pancreatic cancer; acute and chronic hepatitis, liver cirrhosis, liver cancer, liver hemangioma, and other liver diseases; cholecystolithiasis, cholecystitis, gallbladder cancer, and other gallbladder lesions; acute and chronic nephritis, renal failure, benign and malignant kidney tumors, kidney stones, and other kidney diseases; autoimmune diseases, such as systemic lupus erythematosus (SLE) and HIV; and chronic wasting diseases, such as malignant tumors and tuberculosis. We excluded 116 patients, according to the criteria (Figure 1). The diagnostic criteria for AP and CP are described above. AP can be diagnosed by the acute onset of abdominal pain, serum amylase, or lipase, three times above the normal upper limit, and imaging changes of the pancreas, indicating extravasation, edema, and fluid around the pancreas by abdominal ultrasound, CT, and MRI. CP is diagnosed mainly depending on the patient’s medical history, clinical symptoms and signs, laboratory examination, imaging modalities, and pathological evidence of pancreatic tissue biopsy. Imaging modalities include ultrasound, CT, MRI, endoscopic retrograde cholangiopancreatography (ERCP), and endoscopic ultrasonography (Eus). However, the definitive diagnostic criteria are pathological evidence of pancreatic tissue biopsy. Pancreatic biopsy methods include CT-guided or ultrasound-guided percutaneous biopsy of the pancreas and Eus-guided biopsy of the pancreas. When CP causes abdominal pain that cannot be treated with medication or when pancreatic duct stones need to be removed, local pancreatic tissue is removed, and a biopsy can determine whether the patient has CP or pancreatic fibrosis. A diagnosis of CP with or without fibrosis is based on gold-standard biopsy analysis: postoperative pathologic findings suggesting fibrous hyperplasia. All of the patients presented with upper abdominal pain. The study was conducted following the Declaration of Helsinki of the World Medical Association and was approved by the Ethics Committee of the Army Medical University. All of the patients signed informed consent forms.
Data Collection and Definitions and Quantitative Determination of the WNR and GNR
The results of the first routine blood examination and liver and kidney function examinations within 2 days of admission were used in the statistical analysis of data for this paper to standardize the blood test results. The laboratory test results, clinical features, and epidemiological and pathological test results of patients were collected from the official website of Southwest Hospital and were analyzed independently by two physicians. The blood WNR was calculated by multiplying the WBC and NE-to-RBP ratio, and the blood GNR was calculated by multiplying the GGT level and 5′-nucleotide-to-RBP ratio. The normal range of the WBCs is 4 × 109–10 × 109 cells/L, and that of the NE count is 2 × 109–7 × 109 cells/L. The normal range of 5′-nucleotide levels is 0–10 U/L, and that of GGT values is 4–50 U/L. The normal reference value of RBP is 25–70 mg/L. The biological parameters of all of the statistical analysis data in this paper are consistent with the experimental data of hospitals:
Statistical Analysis
All data obtained were analyzed using the SPSS 22.0 statistical software package and GraphPad Prism 6.0 software package. Continuous variables with normal distributions are shown as mean ± standard deviation (SD). Counting data were analyzed by the χ2 test, and multivariate analysis was performed using multivariate logistics analysis. ORs and 95% CIs were determined for variables of interest. Spearman R correlations were used to analyze correlations between RBP and other serum indexes. ROC curves were used to analyze the efficacy of WNR and GNR for diagnosis of pancreatitis types. p values of less than 0.05 were regarded as statistically significant.
Author Contributions
L.L., J.Z., J.Y., H.Z., and Y.T. collected and interpreted the data. L.L., D.Y., and Y.W. analyzed the data. L.L. and H.W. wrote the manuscript. B.N. and Z.T. revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Key Research and Development Project (grant/award numbers 2016YFA0502200, 2016YFA0502203, and 2017YFC1308600), Research Program of Foundation Science and Application Technology of Chongqing (grant/award numbers cstc2018jcyjA1826 and cstc2015jcyjA10105), and Basic Medical College Foundation of Army Medical University (2019JCZX10).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.010.
Contributor Information
Yuzhang Wu, Email: wuyuzhang@tmmu.edu.cn.
Huaizhi Wang, Email: whuaizhi@gmail.com.
Bing Ni, Email: nibing@tmmu.edu.cn.
Zhiqiang Tian, Email: tzhiq009@163.com.
Supplemental Information
References
- 1.Wang G.J., Gao C.F., Wei D., Wang C., Ding S.Q. Acute pancreatitis: etiology and common pathogenesis. World J. Gastroenterol. 2009;15:1427–1430. doi: 10.3748/wjg.15.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan X., Wan J., Zhang G., Song L., Gui F., Zhang Y., Li Y., Guo J., Dawra R.K., Saluja A.K. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G816–G825. doi: 10.1152/ajpgi.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gougol A., Dugum M., Dudekula A., Greer P., Slivka A., Whitcomb D.C., Yadav D., Papachristou G.I. Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J. Gastroenterol. 2017;23:5431–5437. doi: 10.3748/wjg.v23.i29.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saluja A., Dudeja V., Dawra R., Sah R.P. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology. 2019;156:1979–1993. doi: 10.1053/j.gastro.2019.01.268. [DOI] [PubMed] [Google Scholar]
- 5.Ismail O.Z., Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin. Biochem. 2017;50:1275–1280. doi: 10.1016/j.clinbiochem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan M., Ates I., Akpinar M.Y., Yuksel M., Kuzu U.B., Kacar S., Coskun O., Kayacetin E. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2017;16:424–430. doi: 10.1016/S1499-3872(17)60007-9. [DOI] [PubMed] [Google Scholar]
- 7.Mao W., Wu J., Zhang H., Zhou J., Ye B., Li G., Gao L., Li X., Ke L., Tong Z. Increase in serum chloride and chloride exposure are associated with acute kidney injury in moderately severe and severe acute pancreatitis patients. Pancreatology. 2019;19:136–142. doi: 10.1016/j.pan.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Andersen P.L., Madzak A., Olesen S.S., Drewes A.M., Frøkjaer J.B. Quantification of parenchymal calcifications in chronic pancreatitis: relation to atrophy, ductal changes, fibrosis and clinical parameters. Scand. J. Gastroenterol. 2018;53:218–224. doi: 10.1080/00365521.2017.1415372. [DOI] [PubMed] [Google Scholar]
- 9.Diakopoulos K.N., Lesina M., Wormann S., Song L., Aichler M., Schild L., Artati A., Römisch-Margl W., Wartmann T., Fischer R. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638.e17. doi: 10.1053/j.gastro.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee A.T., Xu Z., Pothula S.P., Patel M.B., Pirola R.C., Wilson J.S., Apte M.V. Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol. Clin. Exp. Res. 2015;39:2123–2133. doi: 10.1111/acer.12882. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Yu X., Huang L., Yu C. GLP-1R agonist may activate pancreatic stellate cells to induce rat pancreatic tissue lesion. Pancreatology. 2013;13:498–501. doi: 10.1016/j.pan.2013.07.281. [DOI] [PubMed] [Google Scholar]
- 12.Majumder S., Chari S.T. Chronic pancreatitis. Lancet. 2016;387:1957–1966. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi C., Okuno M., Tannous L., Piantedosi R., Allan M., Goodman D.S., Blaner W.S. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 14.Blaner W.S. Retinol-binding protein: the serum transport protein for vitamin A. Endocr. Rev. 1989;10:308–316. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- 15.Imamura M., Morimoto T., Egawa C., Fukui R., Bun A., Ozawa H., Miyagawa Y., Fujimoto Y., Higuchi T., Miyoshi Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019;9:1811. doi: 10.1038/s41598-018-37633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zen Y., Deshpande V. Tumefactive Inflammatory Diseases of the Pancreas. Am. J. Pathol. 2019;189:82–93. doi: 10.1016/j.ajpath.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Del Fresno C., Saz-Leal P., Enamorado M., Wculek S.K., Martínez-Cano S., Blanco-Menéndez N., Schulz O., Gallizioli M., Miró-Mur F., Cano E. DNGR-1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science. 2018;362:351–356. doi: 10.1126/science.aan8423. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M., Ding L., Wang X., Hou J., Li M., Jiang Y., He X., Cui M., Hu F., Zhang X. Circulating CD14+CD163+CD115+ M2 monocytes are associated with the severity of new onset severe acute pancreatitis in Chinese patients. Int. Immunopharmacol. 2018;57:181–189. doi: 10.1016/j.intimp.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Badhal S.S., Sharma S., Saraya A., Mukhopadhyay A.K. Prognostic significance of D-dimer, natural anticoagulants and routine coagulation parameters in acute pancreatitis. Trop. Gastroenterol. 2012;33:193–199. doi: 10.7869/tg.2012.48. [DOI] [PubMed] [Google Scholar]
- 20.Kamath M.G., Pai C.G., Kamath A., Kurien A. Monocyte chemoattractant protein-1, transforming growth factor-beta1, nerve growth factor, resistin and hyaluronic acid as serum markers: comparison between recurrent acute and chronic pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2016;15:209–215. doi: 10.1016/s1499-3872(15)60029-7. [DOI] [PubMed] [Google Scholar]
- 21.Waldron R.T., Chen Y., Pham H., Go A., Su H.Y., Hu C., Wen L., Husain S.Z., Sugar C.A., Roos J. The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J. Physiol. 2019;597:3085–3105. doi: 10.1113/JP277856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gemenetzis G., Bagante F., Griffin J.F., Rezaee N., Javed A.A., Manos L.L., Lennon A.M., Wood L.D., Hruban R.H., Zheng L. Neutrophil-to-lymphocyte Ratio is a Predictive Marker for Invasive Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2017;266:339–345. doi: 10.1097/SLA.0000000000001988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.