Highlights
-
•
The kinetics of N-butylbenzenesulfonamide (NBBS) was investigated in rodents.
-
•
NBBS was rapidly absorbed following gavage exposure in rodents.
-
•
It was rapidly eliminated with a half-life ≤3.5 h.
-
•
Bioavailability following gavage administration was high.
-
•
Systemic exposure was higher following gavage exposure than following via feed.
Keywords: N-butylbenzenesulfonamide, plasticizer, systemic exposure, half-life, clearance, rodents
Abstract
N-Butylbenzenesulfonamide (NBBS) is a widely used plasticizer and hence there is potential for human exposure via oral routes. This work investigates the toxicokinetic behavior of NBBS in rodents following a single gavage (20, 60, and 200 mg/kg body weight) or multi-day feed administration (500, 1000, and 2000 ppm). In male and female rats following gavage administration, maximum plasma NBBS concentration, Cmax, was reached at ≤0.539 h. Cmax increased proportionally to the dose. Area under the curve (AUC) increased more than proportionally to the dose and was 4- to 5-fold higher in females than in males. In mice, plasma Cmax was reached at ≤0.136 h and increased proportionally to the dose in female mice and more than proportionally to the dose in males. AUC increased more than proportionally to the dose with no apparent sex difference. Elimination of NBBS in plasma was faster in mice (half-life (h); mice ≤0.432, rat ≤3.55). Oral bioavailability was higher in female rats (≥60%) than males (23-52%) with apparent saturation of clearance at ∼200 mg/kg body weight in females. In mice, bioavailability (5-14%) was lower with no apparent sex difference. NBBS was detected in brains of rats and mice but with low brain:plasma ratios (rats, ≤5; mice, ≤1) suggesting low potential to cross the blood brain barrier. Systemic exposure in male rats and mice following a single gavage administration was ≥48-fold higher than multi-day feed exposure. These data demonstrate potential species, sex, dose- and route-related difference in toxicokinetics of NBBS in rodents.
Introduction
N-Butylbenzenesulfonamide (NBBS) is a plasticizer with a US production volume of 1-10 million lbs in 2015 (EPA, 2019). It is used primarily in polyamides (e.g., Nylon 6, 11, and 12) with some reported use in medical devices (Wypych, 2017). NBBS is not listed as a priority pollutant by the Environmental Protection Agency and, thus, is not routinely monitored. However, there are various reports demonstrating the presence of NBBS in the environment, and in some cases at significant levels (Huppert et al., 1998, Oros et al., 2003, Dsikowitzky et al., 2004a, Dsikowitzky et al., 2004b, Pedersen et al., 2005, Grigoriadou et al., 2008, Plumlee et al., 2012, Di Carro et al., 2018). For example, in secondary effluent from a water reclamation plant in California, up to 16 μg/L of NBBS has been detected (Soliman et al., 2007) and in a groundwater sample from an agricultural land-use area in the United Kingdom, concentration of 4000 μg/L NBBS has been reported (Manamsa et al., 2016). NBBS has been detected as a contaminant in wine with concentrations up to 2 μg/L (Duffield et al., 1994) and identified as a leachate from polyamide cooking utensils (Skjevrak et al., 2005). Collectively, these data demonstrate potential for human exposure to NBBS via oral routes.
Despite potential for human exposure, there are limited toxicity data for NBBS for evaluating the hazard associated with exposure. An early study suggested that NBBS elicited neurotoxic effects in rabbits following exposure via intracisternal injection of leached NBBS from a plastic dosing vial (Strong et al., 1990). Subsequent studies demonstrated similar findings following intraperitoneal injection of NBBS in rabbits (Strong et al., 1991) and in Wistar rats at 300 mg/kg body weight (Lee et al., 1995). However, in a more recent 27-day study following gavage administration in Sprague-Dawley (SD) rats with doses up to 300 mg/kg body weight, no signs of neurotoxicity were observed (Rider et al., 2012). In a 28-day study evaluating the immunotoxicity of NBBS in mice following dermal exposure to 0 (control) or 100% NBBS (neat NBBS), authors reported significant increases in liver and kidney weights, although no effects on the immune parameters measured were observed (Marrocco et al., 2015).
Due to limited data available for hazard characterization of NBBS, the National Toxicology Program (NTP) is investigating the toxicity of NBBS following oral perinatal exposure in rats and adult exposure in mice (NTP, 2020). Absorption, distribution, metabolism, and excretion (ADME) and toxicokinetic (TK) data are essential for the design of toxicology studies and interpretation of study data. We have previously investigated the ADME behavior of [14C]NBBS in Hsd:Sprague Dawley® SD® (HSD) rats and B6C3F1/N mice of both sexes following gavage administration (Waidyanatha et al., 2020). [14C]NBBS was well-absorbed in male and female rats and excreted extensively in urine (70-83%) and feces (11-14%) 72 h following a single gavage administration of 2, 20, or 200 mg/kg body weight. In addition, 25% of the dose was excreted in 24 h bile following gavage administration of [14C]NBBS, suggesting that the administered dose recovered in feces was absorbed dose. The radioactivity was distributed to all tissues examined with 14 and 8% of the administered dose remaining in tissues at 24 and 72 h, respectively, after dose administration of 20 mg/kg body weight, suggesting significant distribution and retention in tissues. There was no apparent dose-, species-, or sex-related differences in disposition of [14C]NBBS in rodents. Urinary radiochemical profiles were similar between doses, routes, species, and sexes. Among numerous metabolites identified, oxidative metabolites of NBBS predominated (Waidyanatha et al., 2020).
There are limited studies investigating the TK behavior of NBBS. Following a single intravenous administration (IV) of 1 mg/kg body weight [13C6]NBBS in male Sprague Dawley (SD) rats, the elimination of NBBS in plasma was triphasic with respective half-lives of 0.78, 11, and 1036 min (Kumar et al., 2007). NBBS was detected in the liver, kidney, muscle, fat, and brain. However, 4 h after the administration, levels decreased dramatically with tissue:plasma ratios generally lower than 1. In female Wistar rats, following a single IV administration of 1 mg/kg body weight, the maximum NBBS concentration was reached within 2 min in liver and skeletal muscle and within 5 min in kidney and fat (Kumar et al., 2007). In female rats, the oral bioavailability following administration of 1 mg/kg body weight was reported as 52% to 79%; the plasma elimination was triphasic with respective half-lives of 0.32, 27, and 500 min (NTP, 2010).
TK data are important to design and interpret toxicology study data. However, as summarized above, there are limited TK data in the literature for NBBS. Hence, we have undertaken a comprehensive investigation of plasma and brain TK of NBBS following a single gavage administration in male or female HSD rats and B6C3F1/N mice. The two rodent models used are the ones used in NTP NBBS toxicity studies. Brain was included since NBBS is considered as a potential neurotoxicant, as mentioned above. The NTP has selected to test NBBS in rodent models following multiple exposure via feed. Hence, we have also undertaken limited TK studies in rodents following multiple feed exposure.
Materials and methods
Chemicals and reagents
NBBS (Figure S1) was obtained from Ivy Fine Chemicals (Lot IF10505; Cherry Hill, NJ). Identity of NBBS was confirmed by 1H and 13C NMR and mass spectrometry (MS) and the purity (>99.9%) was determined by gas chromatography (GC) with flame ionization detection. d9-NBBS with the label on the butyl group was obtained from Synfine Research (Richmond Hill, Ontario, Canada). Control HSD and SD rat matrices for analytical method validation and matrix calibration curves were obtained from BioIVT Inc. (Westbury, NY). All other chemicals and reagents were from commercial sources.
Quantitation of NBBS
Methods of liquid-liquid extraction followed by GC-MS was used to quantitate NBBS in plasma and brain. Brain was prepared by homogenizing 1:9 brain:deionized water (w/w) using a polytron homogenizer. Methods were fully validated in SD rat plasma and brain and cross validated to B6C3F1 mouse plasma and brain. The validation included an assessment of linearity, selectivity, inter- and intra-day precision (estimated as percent relative standard deviation, RSD), inter- and intra-day accuracy (estimated as percent relative error, RE), absolute recovery, experimental limits of quantitation (LOQ), and carryover in the analytical system. Selectivity was assessed by analyzing six matrix blanks for background interferences at the retention time of NBBS. The LOQ was set as the lowest NBBS standard that could be accurately quantified with a RE ≤ 20% of the nominal concentration and with RSD ≤ 20%. Recovery was expressed as the percentage of the response of the analyte in matrix to that in solvent. Intra-day precision and accuracy were evaluated using quality control (QC) samples prepared at three concentrations on the same day. Inter-day precision and accuracy were evaluated at the same three concentration levels, using multiple sets of independent QC samples prepared and analyzed over at least three analysis days. Methods were qualified for secondary matrices, HSD rat and B6C3F1/N mouse plasma and brain, using QC samples prepared at three concentrations.
Stock solutions of NBBS were prepared in acetone and further diluted in water to generate standards in the working range. A stock solution of d9-NBBS, to be used as internal standard, was prepared in acetone and diluted either in acetone or dichloromethane to generate a working internal standard solution at 600 ng/mL. Solvent calibration curves were prepared from 5 to 1,000 ng/mL for plasma validation or 50 to 10,000 ng/mL for brain validation. Matrix calibration standards and QC samples were prepared by spiking 100 μL of plasma or brain homogenate with appropriate NBBS standards prepared from alternate stock solutions. Matrix blanks were prepared similarly except acetone or dichloromethane was added in place of the NBBS standard. Eight-point matrix calibration curves were prepared to achieve final concentrations in plasma from 5 to 1000 ng/mL and in brain homogenate from 25 to 5000 ng/g. Six replicates of LOQ samples were prepared at 5 ng/mL plasma or 25 ng/g brain. QC samples to cover the low, mid, and high end of the matrix calibration curves, were prepared similarly to matrix standards but using an independent stock solution from above. QC samples were also prepared at concentrations up to 40,000 ng/mL in plasma and brain to assess whether the concentrations outside the calibrated range could successfully be quantified after diluting into the validated range with respective blank matrix.
All samples for validation were prepared for analysis as follows. To 100 μL of plasma or brain homogenate 50 μL of working internal standard solution (600 ng/mL d9-NBBS) was added. Samples were extracted with 0.5 mL of dichloromethane and the supernatant was collected for analysis by GC-MS as described below.
Stability of NBBS in extracted samples was evaluated in samples stored at ambient temperature for 7 d. Stability of the NBBS in respective matrices was evaluated following three freeze-thaw cycles over 24 h intervals and in samples stored at −70 °C for up to 200 d to cover the study sample storage condition and duration.
All samples were analyzed by GC-MS using an Agilent (Santa Clara, CA) 6890 GC coupled to a 5975 Mass Selective Detector. The column used was a Rtx-5MS (30 m X0.32 mm, 1 μm film thickness, Restek, Bellefonte, PA). The initial oven temperature 100 °C was increased at 20 °C/min to 300 °C, where it was held for 5 min. Carrier gas (helium) flow was ∼2 mL/min. The injector temperature was 300 °C and samples were injected in splitless mode. The ion source, auxiliary, and quadrupole temperatures were 240, 280, and 150 °C, respectively. Ions monitored in electron ionization (70 eV) were m/z 170 (M-C3H7 = 213-43) and 172 (M-C3D7 = 222-50) for NBBS and d9-NBBS, respectively; approximate retention time for the NBBS and d9-NBBS was 7.6 min.
Calibration curves relating GC-MS peak area response ratio of NBBS to d9-NBBS and concentration of analyte in matrix were constructed using a quadratic regression with 1/X weighting for plasma and 1/X2 weighting for brain homogenate. The concentration of analyte was calculated using response ratio, the regression equation, initial sample volume and dilution when applicable. The concentration of NBBS in plasma was expressed as ng/mL of plasma. For brain, the concentration determined for ng/g homogenate was converted to ng/g brain using the weight of brain sample used.
Animals and animal maintenance
Studies were conducted at Battelle Memorial Institute (Columbus, OH or West Jefferson, OH) and were approved by the Institutional Animal Care and Use Committee. Animals were housed in facilities that are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (NRC, 2011). Studies were conducted in compliance with the Food and Drug Administration Good Laboratory Practice Regulation.
Male and female HSD rats (∼ 14 weeks old) were obtained from Harlan Laboratories (Indianapolis, IN). B6C3F1/N mice (∼ 11 weeks old) were obtained from Taconic Farms (Hudson, NY). Animals were quarantined for 4 d before being used in a study. Animals were randomized into dosing groups using Xybion Next Generation PATH/TOX SYSTEM (Xybion Medical Systems Corporation, Version 1.7.2) for single administration studies and Provantis (Version 8.6.1.2) for multiple administration studies. Animals were individually housed in solid bottom polycarbonate cages suspended on stainless steel racks with Sani-Chips® hard wood bedding (P.J. Murphy Forest Products Corp., Montville, NJ). Animals had ad libitum access to certified, irradiated NTP 2000 feed (Ziegler Bros, Inc., Gardners, PA) and city (Columbus, OH) tap water. Water was analyzed annually per Battelle’s standard operating protocol. No known contaminants that would interfere with the study were found in feed or water. During the quarantine and study periods, room temperature was maintained between 69 to 75 °F and relative humidity was maintained within 35 to 65%. A 12-h light/dark cycle was maintained during the quarantine and study period.
Study design and dose administration
Study design is given in Table 1. The doses used for single administration gavage studies were 20, 60 or 200 mg/kg body weight. Exposure duration selected was 7 d and exposure concentrations used were 500, 1000, and 2000 ppm. Doses and exposure concentrations were selected after taking into consideration the oral gavage LD50 of NBBS in rats (1725–2050 mg/kg body weight) (NTP, 2010), the doses used in ADME studies (2-200 mg/kg body weight) (Waidyanatha et al., 2020), and doses used in NTP dosed-feed toxicity studies (500, 1000, 2000 ppm in feed). To aid in the interpretation of oral data, IV studies were conducted in male and female rats and mice following administration of a 20 mg/kg body weight dose.
Table 1.
Single gavage and intravenous (IV) and multiple feed administration TK study design of NBBS.
| Species (Sex) | Route | Dose (mg/kg or ppm) |
Blood collection time points (h)a |
|---|---|---|---|
| Single administration (mg/kg) | |||
| Rat (Male, Female) | Gavage | 20, 60, 200 | 20 mg/kg: 0 (predose), 0.0333, 0.0833, 0.166, 0.25, 0.333, 0.5, 0.75, 1, 2, 4, 8 60 and 200 mg/kg: 0 (predose), 0.0833, 0.166, 0.333, 0.5, 0.75, 1, 2, 4, 8, 12, 24 |
| Mouse (Male, Female) | Gavage | 20, 60, 200 | 20 mg/kg: 0 (pre-dose), 0.0333, 0.0833, 0.166, 0.25, 0.333, 0.5, 0.75, 1, 2, 4, 8 60 and 200 mg/kg: 0 (pre-dose), 0.0833, 0.166, 0.333, 0.5, 0.75, 1, 2, 4, 8, 12, 24 |
| Rat (Male, Female) | IV | 20 | 0 (pre-dose), 0.0333, 0.0833, 0.166, 0.25, 0.333, 0.5, 0.75, 1, 2, 3, 4 |
| Mouse (Male, Female) | IV | 20 | 0 (pre-dose), 0.0333, 0.0833, 0.166, 0.25, 0.333, 0.5, 0.75, 1, 2, 3, 4 |
| Multiple administration (ppm)b | |||
| Rat, Male | Feed | 500, 1000, 2000 | 0c, 0.5, 1, 2, 4, 6, 10, 12, 18, 24 |
| Mouse, Male | Feed | 500, 1000, 2000 | 0c, 0.5, 1, 2, 4, 6, 10, 12, 18, 24 |
N = 3 animals per time point.
Animals were administered dosed-feed for 7d. On day 8, when lights were turned on, dosed-feed was removed and control feed was provided.
Blood was collected immediately after removal of dosed feed (0 h) and at the time points indicated.
Gavage dose formulations of NBBS (2, 6, and 20 mg/mL for mice and 4, 12, and 40 mg/mL for rats) were prepared in 0.5% methyl cellulose and analyzed using a validated high-performance liquid chromatography (HPLC) method with ultra violet (UV) detection (linear range, 0.006 to 0.5 mg/mL, equivalent to 0.6 to 50 mg/mL formulations diluted 1:100 for analysis; r ≥0.99; RSD ≤ 5%; RE ≤±10%). IV dose formulations (5 mg/mL for mice and 10 mg/mL for rats) were prepared in water:Cremophor:ethanol (8:1:1) and analyzed using a validated HPLC-UV method (linear range, 3.2 to 100 mg/mL r ≥ 0.99; RSD ≤ 5%; RE ≤ ±10%).
Dosed-feed formulations were prepared in NTP 2000 feed at 500, 1000, and 2000 ppm by adding NBBS into feed in a 6-quart planetary mixer set at a slow speed and mixing for up to 10 minutes. Dosed-feed was extracted by refluxing for 1 h with acidified methanol and the extract was analyzed using a validated HPLC-UV method (linear range, 100 to 3125 ppm; r ≥ 0.99; RSD ≤ 5%; RE ≤ ±10%). The homogeneity of feed formulations was confirmed by sampling (n = 3 from each location) from the top, middle and the bottom of the blender and analyzing for NBBS concentration; RSD of samples taken from 3 locations (n = 9 samples) were ≤ 5% demonstrating formulation homogeneity.
All gavage, IV, and feed formulations were within 10% of the target concentration. Prior to study initiation, stability (≤ 10% of day 0) in gavage, IV and feed formulations was confirmed for up to 42 d at ambient or refrigerated conditions.
Male and female rats were 14-15 weeks old and male and female mice were 12 weeks old at the time of exposure to NBBS. Each animal was weighed prior to dosing and its weight was recorded to determine the dosing volume. Single gavage doses were administered at 20, 60, and 200 mg/kg body weight. Dose formulations were administered in a volume of 5 mL/kg for rat and 10 mL/kg for mouse. Single IV dose was administered at 20 mg/kg body weight; dose was administered into a lateral tail vein in a volume of 2 mL/kg for rats and 4 mL/kg for mice. In multiple administration feed studies, during exposure period, animals had ad libitum access to dosed feed at 500, 1000, and 2000 ppm for 7 d. On day 8, when lights were turned on, dosed-feed was removed and control feed was provided until the end of study duration.
Following dose administration or last day of feed exposure to NBBS, blood samples were collected from 3 animals per timepoint at target times as given in Table 1. In gavage and IV studies, time points were selected based on a preliminary TK study (data not shown). Time points for the feed study were selected based on the half-life of gavage preliminary TK study. Blood samples were obtained via abdominal aorta (gavage and IV studies) and retro-orbital plexus (dosed feed study) in rats and cardiac puncture in mice while under CO2:O2 (70:30) anesthesia. The actual times for blood collection were recorded at each time point. Immediately following collection, blood was dispensed into tubes containing K3EDTA, mixed by inversion and placed on ice. Plasma was prepared by centrifugation within 1 h of blood collection. Immediately following blood collection, each animal was humanely terminated via exsanguination and brains collected and weighed. All samples were stored at −70 °C.
Study sample preparation and analysis
Study samples were prepared similarly to matrix standards as described above. Study samples that exceeded the matrix calibration range were diluted into the validated range using respective control matrix. Each study sample set was bracketed by method blanks, matrix calibration standards, and QC samples prepared at low and high ends of the calibration curve. The concentration of NBBS was calculated, similarly to that described above, using response ratio, the regression equation, initial sample volume and dilution when applicable. All concentrations above LOQ (5 ng/mL for plasma and 25 ng/g for brain) of the assay were reported. Data from study samples were considered valid if: the matrix calibration curve was linear (r ≥ 0.99); at least 75% of matrix standards were within 15% of nominal values (except at the LOQ where it was 20%); at least 67% of the QC samples were within 15% of nominal values. All QCs were within 15% of nominal value. To improve the sensitivity of detection, limited plasma samples from the feed study was analyzed after concentrating the extract to 50 μL and using a curve range of 0.5 to 25 ng/mL. The LOQ for the modified assay was 0.5 ng/mL.
Toxicokinetic analysis
Individual animal data were evaluated for aberrant concentrations and time points. The actual blood collection times were within 5% of nominal values for time points ≤4 h and within 15 min for time points ≥4 h and hence, the actual collection time was used for plasma TK analysis. For brain data, blood collection timepoints were used. Based on these assessments, all quantified values were used in TK analysis.
WinNonlin software (Version 6.4 or 8.0, Certara, Princeton, NJ) was used for TK analysis. A variety of compartmental models were tested for NBBS plasma concentration versus time data sets from gavage and IV studies. For each compartmental model, individual animal data sets were analyzed with and without weighting. The model and the weighting factor that resulted in the best goodness of fit (evaluated using the Akaike Information Criterion and Schwarz Bayesian Criterion) was selected as the final model. Based on this, for rat gavage groups, a one-compartment model with first order input, first order output and 1/Y∧2 was used to calculate TK parameters (Model 3, equation 1) and for mouse groups, a two-compartment model with first order input, first order output with 1/Y∧2 weighting (Model 13, equation 2) was used to calculate TK parameters. For IV rat and mouse groups, a two-compartment model with bolus input and 1/Y∧2 weighting (Model 8, equation 3) was used to calculate TK parameters. NBBS brain mean concentration versus time data sets from gavage and IV groups and plasma data from feed male rat and mouse groups were evaluated using non-compartmental analysis (NCA). Glossary terms used for parameters calculated using compartmental models and NCA are given in Table S1.
| C(t)= A(e-k01t + e-k10t) | (1) |
| C(t) = Ae-αt + Be-βt + Ce-k01t | (2) |
| C(t) = Ae-αt + Be-βt | (3) |
Results
Analytical method validation
Analytical methods were validated in SD rat matrices and cross validated in B6C3F1 matrices. Subsequently, methods were assessed in the matrices from study-specific strains, HSD and B6F3F1/N. A summary of validation parameters and corresponding results are shown in Tables S2 and S3 for plasma and brain, respectively. In SD rat plasma, the method was linear with inter- and intra-day RE ≤±8.6% and RSD ≤ 7.9%; LOQ was 5 ng/mL. Standards as high as 30,000 ng/mL in plasma could successfully be diluted into the validated calibration range with estimated RE ≤±10.3% and RSD ≤ 2.7%. The method was cross-validated in B6C3F1 mouse plasma with intra-day RE ≤±12.8% and RSD ≤ 7.1%. The % RE and % RSD, respectively, in QCs prepared in HSD rat plasma were ≤ ±8.6 and ≤ 9.5 and in B6C3F1/N mouse plasma were ≤±12.5 and ≤5.4 determined using 3 QC levels prepared in respective matrices and analyzed with the calibration curve prepared in SD rat plasma (Table S2).
To improve the sensitivity of detection in plasma, the method was modified by concentrating the sample extract from 1 mL to 50 μL. The curve range used was 0.5 to 25 ng/mL. The method was linear (r ≥ 0.99) with RE ≤±10% and RSD ≤ 10%. The LOQ and LOD were 0.5 and 0.15 ng/mL, respectively.
In SD rat brain, the method was linear (r ≥ 0.99), with intra-day RE ≤±12.5% and RSD ≤ 10.0%; LOQ was 25 ng/g. Standards as high as 100,000 ng/g could successfully be diluted into the validated calibration range with estimated RE ≤±2.1% and RSD ≤ 0.8%. The method was cross-validated in B6C3F1 mouse brain with inter-day RE ≤±6.7% and RSD ≤ 13.0%. The %RE and %RSD, respectively, in HSD rat brain were ≤±12.9 and ≤19.1 and in B6C3F1/N mouse brain were ≤±3.3 and ≤10.2 determined using 3 QC levels prepared in respective matrix and analyzed with the calibration curve prepared in SD rat brain (Table S3).
In order to determine whether the NBBS is stable in extracted samples during the period of sample analysis, extracts prepared from plasma and brain at 3 QC levels were stored at ambient temperature up to 9 d and analyzed. The determined %RE in plasma and brain were ≤±24.3 and ≤±8.7, respectively (Tables S2 and S3). To investigate the stability of NBBS in matrix during freeze-thaw cycles and long-term storage, QC samples prepared at 3 concentrations underwent 4 freeze-thaw cycles and storage at ∼ −70 °C for up to ∼ 200 d to cover the period of study sample storage. For plasma and brain, respectively, the %RE determined during 4 freeze-thaw cycles were ≤±9.0 and ≤±7.8 and long-term storage were ≤±9.7 and ≤±6.3, of nominal concentrations for rat or mouse. Taken collectively, these data demonstrate the suitability of the analytical methods to quantitate NBBS in rat and moue plasma and brain and that NBBS is stable in both matrices when stored at ∼ −70 °C.
Toxicokinetic analysis of NBBS in rats following a single gavage and IV administration
All study data are available in the NTP Chemical Effects in Biological Systems (CEBS) database: : https://manticore.niehs.nih.gov/cebssearch/paper/14904. In male and female rats following gavage administration, NBBS in plasma was above LOQ of the assay only in samples from 0.0333 h through 8 h for the 20 mg/kg body weight males and females, from 0.0833 h through 12 h for the 60 mg/kg body weight groups except for one female rat at 12 h, and from 0.0833 h through 24 h for the 200 mg/kg body weight groups except for one male rat at 24 h. Data for two timepoints (0.0833 and 2 h, male rat 20 mg/kg body weight groups) were not available due to errors in dosing. Plasma NBBS concentration versus time data were fitted using a one-compartment model with first-order input and output, and 1/Y∧2 weighting. Model fits are shown in Fig. 1. TK parameters estimated are given in Table 2.
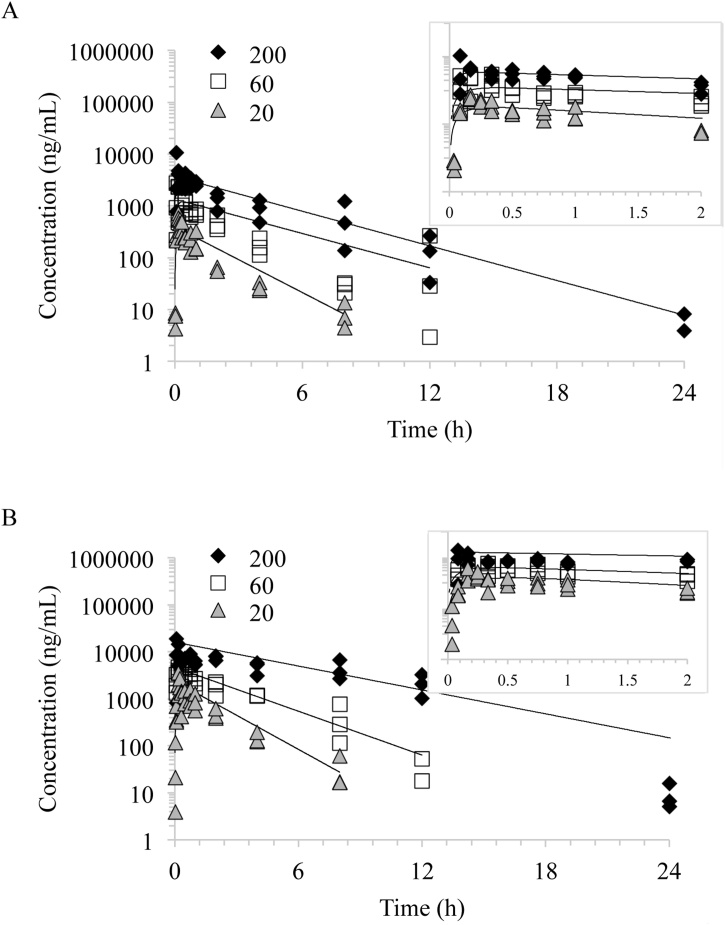
Fig. 1.
Plasma concentration versus time profiles of NBBS following a single gavage administration of 20, 60 (dashed line), and 200 mg/kg NBBS in A) male rats and B) female rats. Individual animal data were fitted using a one-compartment model with first order input, first order output and 1/Y∧2 weighting. Lines represent model fits.
Table 2.
Plasma toxicokinetic parameters of NBBS following a single gavage administration in male and female rats.a
| Parameterb | Dose (mg/kg) | ||
|---|---|---|---|
| Male | 20 | 60 | 200 |
| Cmax (ng/mL) | 316 ± 46 | 1,200 ± 290 | 3,440 ± 500 |
| Cmax /D (ng/mL)/(mg/kg) | 15.8 | 20.0 | 17.2 |
| Tmax (h) | 0.355 ± 0.105 | 0.378 ± 0.266 | 0.228 ± 0.183 |
| K01 half-Life (h) | 0.0809 ± 0.0353 | 0.0696 ± 0.0668 | 0.0361 ± 0.0373 |
| K10 half-Life (h) | 1.42 ± 0.17 | 2.72 ± 0.58 | 2.70 ± 0.21 |
| Cl1_F (mL/h/kg) | 26,000 ± 3,600 | 11,500 ± 2,400 | 14,100 ± 1,800 |
| V1_F (mL/kg) | 53,300 ± 9,900 | 45,400 ± 12,900 | 54,900 ± 8,800 |
| AUC (h*ng/mL) | 771 ± 107 | 5,190 ± 1100 | 14,200 ± 1,800 |
| AUC/D (h*ng/mL)/(mg/kg) | 38.6 | 86.5 | 71.0 |
| Female | |||
| Cmax (ng/mL) | 1,540 ± 250 | 3,890 ± 450 | 15,200 ± 1900 |
| Cmax /D (ng/mL)/(mg/kg) | 77.0 | 64.8 | 76.0 |
| Tmax (h) | 0.539 ± 0.134 | 0.392 ± 0.116 | 0.237 ± 0.175 |
| K01 half-Life (h) | 0.158 ± 0.065 | 0.0824 ± 0.0346 | 0.0353 ± 0.0330 |
| K10 half-Life (h) | 1.24 ± 0.16 | 1.93 ± 0.16 | 3.55 ± 0.28 |
| Cl1_F (mL/h/kg) | 5,370 ± 880 | 4,810 ± 500 | 2,460 ± 270 |
| V1_F (mL/kg) | 9,640 ± 2,250 | 13,400 ± 1,900 | 12,600 ± 1,800 |
| AUC (h*ng/mL) | 3,730 ± 610 | 12,500 ± 1,300 | 81,400 ± 9,100 |
| AUC/D (h*ng/mL)/(mg/kg) | 187 | 208 | 407 |
Based on one-compartment model with first order input, first order output and 1/Y∧2 weighting.
Values given are mean ± standard error for up to 3 animals.
NBBS was absorbed rapidly in both male and female rats following gavage administration with plasma Tmax values between 0.228 and 0.378 h for males and 0.237 and 0.539 h for females (Table 2). Cmax values in females were higher than in males and increased proportional to the dose in both males and females as demonstrated by the dose-normalized values (males 15.8, 20.0, and 17.2 and females 77.0, 64.8, 76.0 (ng/mL/(mg/kg body weight) for 20, 60, and 200 mg/kg body weight, respectively) (Table 2). The volume of distribution was higher in males (45,400 to 54,900 mL/kg) than females (9,640 to 13,400 mL/kg) and was higher in both sexes than the reported body water volume in rats (688 mL/kg) suggesting distribution of NBBS to tissues (Davies and Morris, 1993). Clearance of NBBS was lower in females than in males. Consequently, AUC was higher in females (3,730 to 81,400 h*ng/mL) than in males (771 to 14,200 h*ng/mL) and increased more than proportional to the dose in both sexes as evidenced by the dose-normalized values, AUC/D (Table 2). NBBS was eliminated rapidly with half-lives (K10 half-lives) ranging from 1.42 to 2.72 h in males and 1.24 to 3.55 h in females with no apparent sex difference.
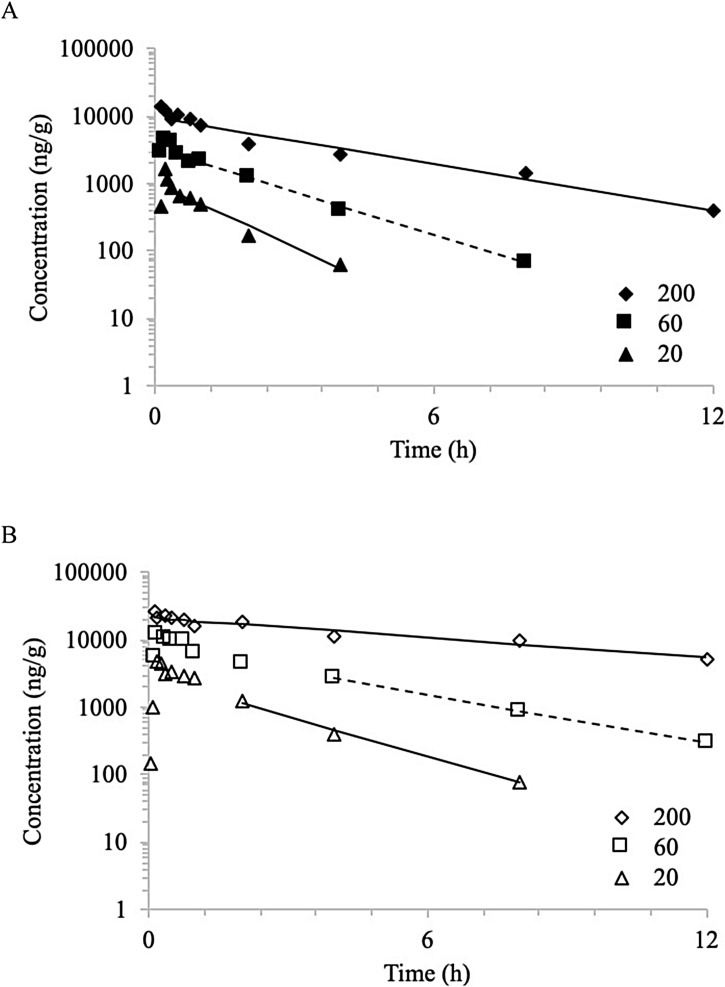
Following gavage administration in rats, NBBS was distributed to the brain in both males and females. NBBS was measurable only in samples from 0.0833 h through 8 h for the males and from 0.0333 through 8 h for the females in the 20 mg/kg body weight, from 0.0833 h through 12 h for the males and females in the 60 mg/kg body weight, and from 0.0833 h through 12 h for the males and through 24 h for the females in the 200 mg/kg body weight. Similar to plasma, data for two timepoints (0.0833 and 2 h, male rat 20 mg/kg body weight groups) were not available due to errors in dosing. Concentration versus time data were evaluated using NCA; plots are shown in Fig. 2. TK parameters estimated are given in Table 3. In brain, observed Tmax ≤0.204 h in both sexes. Half-life of elimination was higher in females than in males and increased with dose at 0.960, 1.42, and 2.64 h for males and 1.55, 2.47, and 6.11 h for females, for 20, 60, and 200 mg/kg body weight, respectively. Systemic exposure parameters, Cmax and AUC, were higher in females than in males. In both sexes, Cmax increased less than proportional to the dose, while AUC increased more than proportional to the dose as demonstrated by dose-normalized values (Table 3).
Fig. 2.
Brain concentration versus time profiles of NBBS following a single gavage administration of 20, 60 (dashed line), and 200 mg/kg NBBS in A) male rats and B) female rats. Mean data were analyzed using non-compartmental analysis. Lines represent model fits.
Table 3.
Brain toxicokinetic parameters of NBBS following a single gavage administration in male and female rats.a
| Parameter | Dose (mg/kg) | ||
|---|---|---|---|
| Male | 20 | 60 | 200 |
| Cmax (ng/g) | 1,680 | 4,560 | 13,400 |
| Cmax /D (ng/g)/(mg/kg) | 84.0 | 76.0 | 67.0 |
| Tmax (h) | 0.204 | 0.202 | 0.116 |
| Half-life (h) | 0.960 | 1.42 | 2.64 |
| AUC (h*ng/g) | 1,370 | 7,220 | 35,100 |
| AUC/D (h*ng/g)/(mg/kg) | 68.5 | 120 | 176 |
| Female | |||
| Cmax (ng/g) | 4,780 | 11,700 | 25,800 |
| Cmax /D (ng/g)/(mg/kg) | 239 | 195 | 129 |
| Tmax (h) | 0.198 | 0.196 | 0.113 |
| Half-life (h) | 1.55 | 2.47 | 6.11 |
| AUC (h*ng/g) | 7,580 | 32,000 | 187,000 |
| AUC/D (h*ng/g)/(mg/kg) | 379 | 533 | 935 |
Based on non-compartmental analysis. Values from up to 3 animals are given.
Following IV administration of 20 mg/kg body weight in male and female rats, NBBS in plasma was measurable at the earliest time point of 0.0333 h through 4 h in both sexes. Plasma NBBS concentration versus time data were fitted using a two-compartment model with 1/Y∧2 weighting and brain concentration versus time data were analyzed using NCA. Corresponding fits are shown in Figures S2A and S2B for plasma and brain, respectively. TK parameters estimated are given in Table S4. Plasma Cmax was similar between males and females although AUC was slightly higher in females (6170 h*ng/mL) than in males (3300 h*ng/mL). NBBS was eliminated rapidly in plasma with males showing faster elimination than females (K10 half-life; males, 0.221 h; females, 0.431 h). NBBS was rapidly distributed to the brain with no apparent sex difference in TK parameters (for males and females, respectively, Tmax 0.0679 and 0.0674 h; Cmax 61,200 and 42,600 ng/g; AUC 17,500 and 19,200 h*ng/g; half-life 0.434 and 0.714 h).
Toxicokinetic analysis of NBBS in mice following gavage and IV administration
All study data are available in the NTP Chemical Effects in Biological Systems (CEBS) database: : https://manticore.niehs.nih.gov/cebssearch/paper/14904. In male and female mice following gavage administration, NBBS was measurable in plasma from 0.0333 h through 4 h for the 20 mg/kg body weight males and females, from 0.0833 h through 4 h for the 60 mg/kg body weight except one female, and from 0.0833 h through 8 h for the 200 mg/kg body weight group except two females. NBBS was below LOQ in all other timepoints. Data from one animal was not used (male 0.5 h) due to an error in dosing. Plasma NBBS concentration versus time data were fitted using a two-compartment model with first-order input and output, and 1/y∧2 weighting. Model fits for male mice are shown in Fig. 3 as an example. TK parameters estimated are given in Table 4.
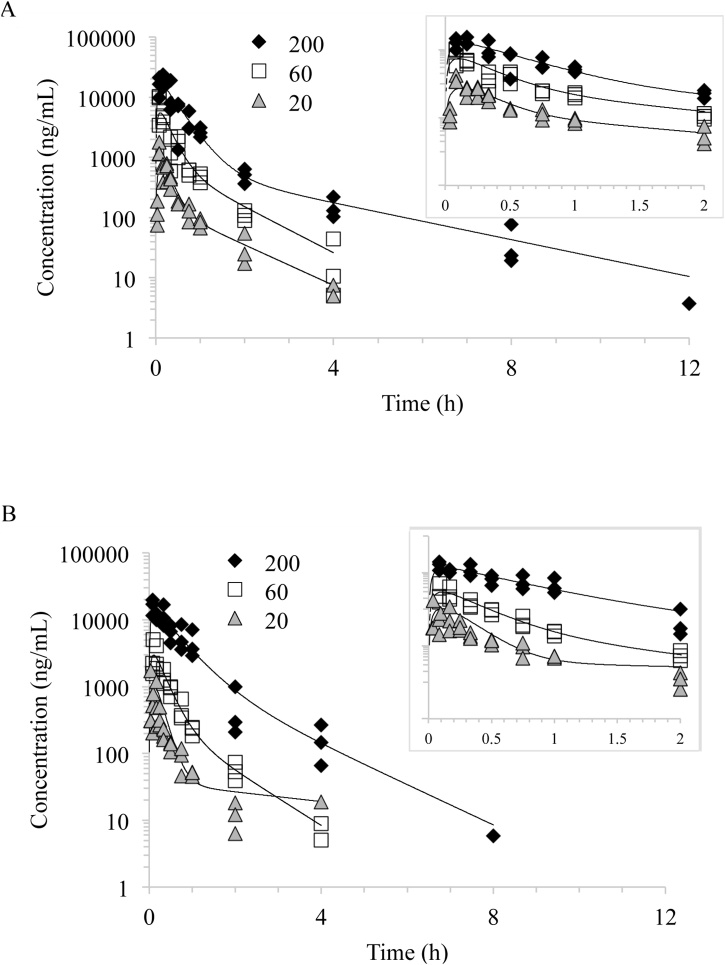
Fig. 3.
Plasma concentration versus time profiles of NBBS following a single gavage administration of 20, 60 (dashed line), and 200 mg/kg NBBS in A) male mice and B) female mice. Individual animal data were fitted using a two-compartment model with first order input, first order output and 1/Y∧2 weighting. Lines represent model fits.
Table 4.
Plasma toxicokinetic parameters of NBBS following a single gavage administration in mice.a
| Parameterb | Dose (mg/kg) | ||
|---|---|---|---|
| Male | 20 | 60 | 200 |
| Cmax (ng/mL) | 756 ± 119 | 5,530 ± 1250 | 15,500 ± 2400 |
| Cmax/D (ng/mL)/(mg/kg) | 37.8 | 92.1 | 77.5 |
| Tmax (h) | 0.131 ± 0.030 | 0.103 ± 0.057 | 0.136 ± 0.051 |
| K01 half-Life (h) | 0.0631 ± 0.0655 | 0.0358 ± 0.0381 | 0.0431 ± 0.0281 |
| K12 (1/h) | 1.82 ± 2.68 | 0.922 ± 0.814 | 0.384 ± 0.161 |
| K21 (1/h) | 1.30 ± 0.64 | 1.23 ± 0.49 | 0.426 ± 0.084 |
| Alpha half-life (h) | 0.119 ± 0.118 | 0.156 ± 0.071 | 0.262 ± 0.055 |
| Beta half-life (h) | 0.895 ± 0.247 | 0.789 ± 0.185 | 1.97 ± 0.31 |
| K10 half-Life (h) | 0.201 ± 0.160 | 0.220 ± 0.073 | 0.317 ± 0.058 |
| Cl1_F (mL/h/kg) | 46,800 ± 4900 | 22,700 ± 2700 | 19,900 ± 1900 |
| Cl2_F (mL/h /kg) | 24,700 ± 18400 | 6,620 ± 4250 | 3,500 ± 1170 |
| V1_F (mL/kg) | 13,600 ± 11100 | 7,180 ± 2670 | 9,120 ± 2020 |
| V2_F (mL/kg) | 19,000 ± 7400 | 5,360 ± 1900 | 8,200 ± 1940 |
| AUC (h*ng/mL) | 427 ± 45 | 2,650 ± 310 | 10,000 ± 900 |
| AUC/D (h*ng/mL)/(mg/kg) | 21.4 | 44.2 | 50.0 |
| Female | |||
| Cmax (ng/mL) | 1,040 ± 190 | 2,960 ± 430 | 13,700 ± 2300 |
| Cmax/D (ng/mL)/(mg/kg) | 52.0 | 49.3 | 68.5 |
| Tmax (h) | 0.107 ± 0.027 | 0.107 ± 0.038 | 0.133 ± 0.075 |
| K01 half-Life (h) | 0.0477 ± 0.0303 | 0.0362 ± 0.0236 | 0.0347 ± 0.0314 |
| K12 (1/h) | 2.14 ± 1.49 | 0.544 ± 0.300 | 0.200 ± 0.206 |
| K21 (1/h) | 0.276 ± 0.386 | 1.17 ± 0.31 | 0.844 ± 0.316 |
| Alpha half-life (h) | 0.121 ± 0.045 | 0.177 ± 0.042 | 0.354 ± 0.119 |
| Beta half-life (h) | 4.13 ± 7.60 | 0.737 ± 0.135 | 1.00 ± 0.23 |
| K10 half-Life (h) | 0.199 ± 0.144 | 0.221 ± 0.039 | 0.432 ± 0.093 |
| Cl1_F (mL/h/kg) | 36,900 ± 18,900 | 43,000 ± 3500 | 18,500 ± 1900 |
| Cl2_F (mL/h /kg) | 22,800 ± 17,700 | 7,460 ± 3250 | 2,310 ± 2030 |
| V1_F (mL/kg) | 10,600 ± 4,100 | 13,700 ± 2900 | 11,500 ± 2700 |
| V2_F (mL/kg) | 82,400 ± 17,4000 | 6,360 ± 1650 | 2,730 ± 1610 |
| AUC (h*ng/mL) | 541 ± 277 | 1,390 ± 110 | 10,800 ± 1100 |
| AUC/D (h*ng/mL)/(mg/kg) | 27.1 | 23.2 | 54.0 |
Based on two-compartment model with first order input, first order output and 1/Y∧2 weighting.
Values given are mean ± standard error for up to 3 animals.
NBBS was absorbed rapidly in both male and female mice following gavage administration with plasma Tmax values between 0.103 and 0.136 h (Table 4). Cmax increased with the dose in both sexes with no apparent sex difference; however, the dose-normalized Cmax (Cmax/D) in males were 37.8, 92.1, and 77.5 for 20, 60, and 200 mg/kg body weight suggesting some dose non-linearity (Table 4). In both males and female, the volume of distribution to the peripheral compartment (V2_F, 2,730 to 82,400 mL/kg) was much higher than the reported body water volume in mice (725 mL/kg) (Davies and Morris, 1993) demonstrating significant distribution to tissues. There was a general trend that the distribution (V2_F) to and the clearance (Cl2_F) from the peripheral compartment decreased with the dose in both sexes. NBBS was eliminated rapidly in plasma with K10 half-lives ≤0.432 h; values increased with the dose in both males (0.201 to 0.317 h) and females (0.199 to 0.432 h) with no apparent sex difference. The clearance of NBBS from the central compartment (Cl1_F) decreased with the dose in both males (46,800, 22,700, 19,900 mL/h/kg for 20, 60, and 200 mg/kg body weight, respectively) and females (36,900, 43,000, 18,500 mL/h/kg, for 20, 60, and 200 mg/kg body weight, respectively). Consequently, AUC increased more than proportional to the dose at 200 mg/kg body weight in both sexes with dose-normalized (AUC/D) values of 21.4, 44.2, and 50.0 (h*ng/mL)/(mg/kg body weight) for males and 27.1, 23.2, and 54.0 (h*ng/mL)/(mg/kg body weight) for females, for 20, 60, and 200 mg/kg body weight, respectively, with no apparent sex difference.
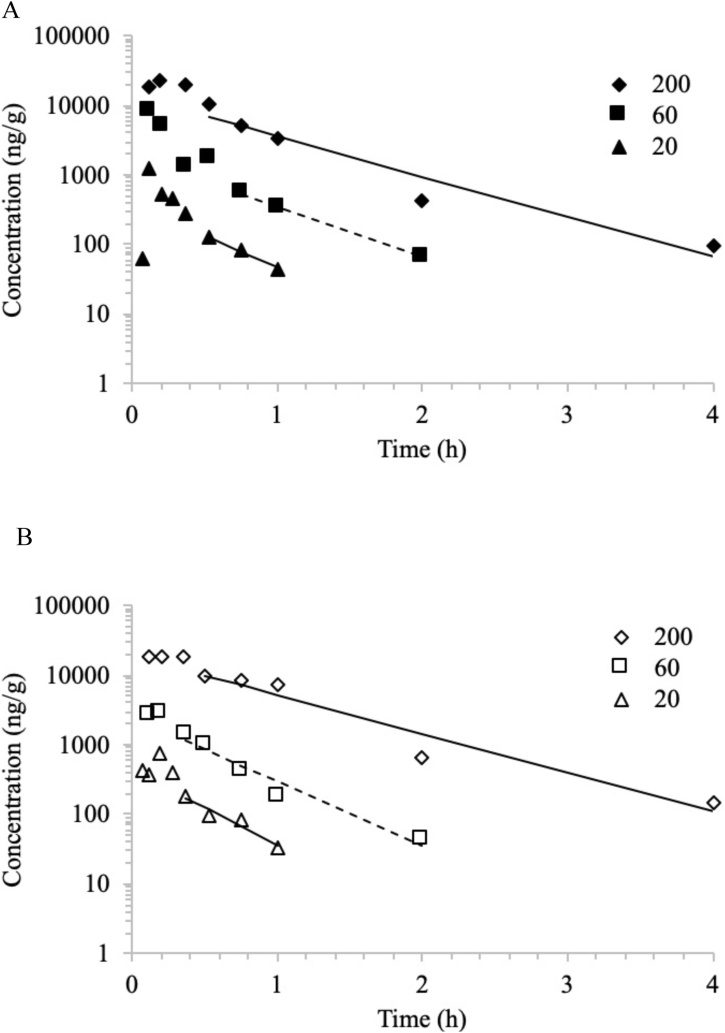
Following gavage administration, NBBS was distributed to the brain in both male and female mice. NBBS was measurable in samples from 0.0333 h through 2 h for the males and through 1 h for females in the 20 mg/kg body weight group, from 0.0833 h through 2 h for the males and females in the 60 mg/kg body weight group, and from 0.0833 h through 8 h for most males and through 4 h for the females in 200 mg/kg body weight. Data from one animal was not used (male 0.5 h, 60 mg/kg body weight) due to error in dosing. Concentration versus time data were evaluated using NCA and plots are shown in Fig. 4. TK parameters estimated are given in Table 5. In brain, Tmax was reached at ≤0.362 h in mice of both sexes. NBBS was eliminated rapidly in the brain; half-life was similar in males and females with apparent increase with the dose in both sexes (0.315, 0.417, and 0.524 h for males and 0.278, 0.329, and 0.545 h for females for 20, 60, and 200 mg/kg body weight, respectively). Systemic exposure parameters, Cmax and AUC, were in general similar between males and females and increased more than proportional to the dose in both sexes as evidenced by the dose-normalized values (Cmax/D and AUC/D) (Table 5).
Fig. 4.
Brain concentration versus time profiles of NBBS following a single gavage administration of 20, 60 (dashed line), and 200 mg/kg NBBS in A) male mice and B) female mice. Mean data were analyzed using non-compartmental analysis. Lines represent model fits.
Table 5.
Brain toxicokinetic parameters of NBBS following a single gavage administration in male and female micea
| Parameterb | Dose (mg/kg) | ||
|---|---|---|---|
| Male | 20 | 60 | 200 |
| Cmax (ng/g) | 1,260 | 8,280 | 23,400 |
| Cmax/D (ng/g)/(mg/kg) | 63.0 | 138 | 117 |
| Tmax (h) | 0.118 | 0.120 | 0.198 |
| Half-life (h) | 0.315 | 0.417 | 0.524 |
| AUC (h*ng/g) | 279 | 2,430 | 14,200 |
| AUC/D (h*ng/g)/(mg/kg) | 14.0 | 40.5 | 71.0 |
| Female | |||
| Cmax (ng/g) | 733 | 2,830 | 18,700 |
| Cmax/D (ng/g)/(mg/kg) | 36.7 | 47.2 | 93.5 |
| Tmax (h) | 0.199 | 0.198 | 0.362 |
| Half-life (h) | 0.278 | 0.329 | 0.545 |
| AUC (h*ng/g) | 222 | 1,280 | 16,600 |
| AUC/D (h*ng/g)/(mg/kg) | 11.1 | 21.3 | 83.0 |
Based on non-compartmental analysis animals. Values from up to 3 animals are given.
Following IV administration in male and female mice of 20 mg/kg body weight, NBBS in plasma was measurable at the earliest time point (0.0333 h) through 4 h in all males and most females. Data from one animal were not used (female 4 h, 20 mg/kg body weight) due to an error in dosing. Plasma NBBS concentration versus time data were fitted using a two-compartment model with 1/Y∧2 weighting and brain concentration versus time data were evaluated using NCA. Corresponding fits are shown in Figures S3A and S3B for plasma and brain, respectively. TK parameters estimated are given in Table S5. In plasma, systemic exposure parameters, Cmax (male, 62,500; female, 60,800 ng/mL) and AUC (male, 7,180; female, 10,200 h*ng/mL), were similar between sexes. NBBS was eliminated rapidly in plasma with no apparent sex difference (K10 half-life male, 0.0796 h; female, 0.117 h). NBBS was rapidly distributed to the brain with males showing higher systemic exposure than females (Cmax 66,800 and 15,700 ng/g; AUC 20,500 and 4,150h*ng/g for males and females, respectively). Half-life of elimination of NBBS in brain was 0.183 and 0.352 h, for males and females, respectively (Table S5).
Bioavailability of NBBS in rats and mice following gavage administration
Bioavailability of NBBS following a single gavage administration in rats and mice was estimated using AUC following oral and IV administration, adjusted for dose administered (%F = AUC/Dose (oral) ÷ AUC/Dose (IV) x 100) (Table 6). In male rats at 20 mg/kg body weight, bioavailability was 23%; values increased to 43-52% at higher doses. In female rats, bioavailability was higher than in males with estimated values of 60 to 68% for 20 and 60 mg/kg body weight, respectively; the estimated value exceeded 100% at 200 mg/kg body weight suggesting saturation of metabolism and clearance processes ∼ 200 mg/kg body weight in females. In male and female mice bioavailability was low (5-14%) with no apparent sex- or dose-dependent difference (Table 6).
Table 6.
Bioavailabilitya of NBBS following a single oral administration in rats and mice
| Dose (mg/kg) | Male Rats | Female Rats | Male Mice | Female Mice |
|---|---|---|---|---|
| 20 | 23 | 60 | 6 | 5 |
| 60 | 52 | 68 | 12 | 5 |
| 200 | 43 | 132 | 14 | 11 |
Bioavailability (%F) was calculated as AUC/Dose (oral) ÷ AUC/Dose (IV) x 100.
Toxicokinetic analysis of NBBS following exposure via feed
Blood was collected at 10 timepoints up to 24 h following the last day of exposure. Average NBBS consumed (mg/day) was estimated using average food consumed over the 7-d exposure period and corresponding exposure concentration. Subsequently, the average dose received per day in mg NBBS/kg was estimated using average animal body weights (kg) over the 7-d period and are shown in Table S6 as mg/kg body weight/day. The estimated dose in mouse was approximately 2-fold higher than that in rat across all exposure concentrations. In both rats and mice, the dose (mg/kg body weight) increased proportional to the exposure concentration (ppm).
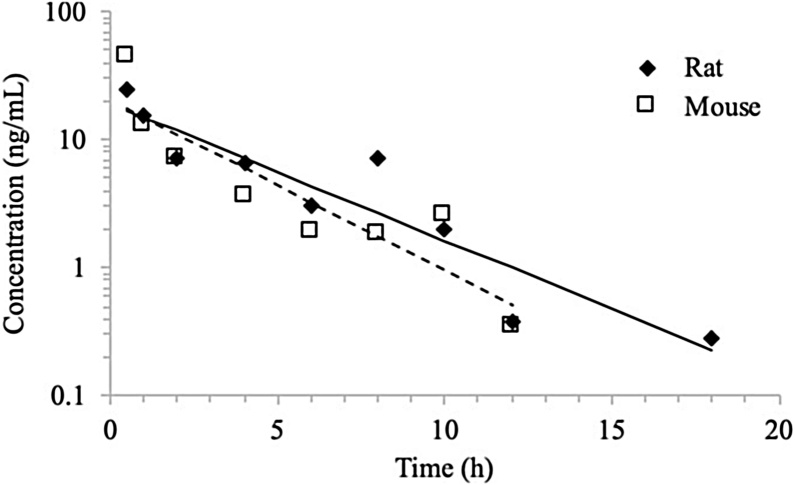
In rats, NBBS was measurable only in samples through 10 h in 500 and 1000 ppm groups and through 18 h in the 2000 ppm group. In mice, NBBS was detected in samples through 8 h in 500 ppm group and through 18 h in 1,000 and 2,000 ppm groups. Examination of the terminal elimination phases for the 500 ppm group in rats and the 500, 1000, and 2000 ppm groups in mice revealed marked increases in concentrations at the last measurable time points. The concentration versus time data was reevaluated without the 8 h sample for the 500 ppm group in mice and without the 18 h samples for the 1000 and 2000 ppm groups in mice and the 500 ppm group in rats. The corresponding fits after reevaluation are shown in Fig. 5 for the 2000 ppm rat and mouse exposure groups, as an example. Plasma NBBS concentration versus time data were evaluated with NCA and TK parameters estimated are given in Table 7.
Fig. 5.
Plasma concentration versus time profiles of NBBS following multiple feed exposure to 2000 ppm NBBS in male rats (solid line) and mice (dashed line). Mean data were analyzed using non-compartmental analysis. Lines represent model fits.
Table 7.
Plasma toxicokinetic parameters of NBBS in male rats and mice following exposure via feed for 7 d.
| Parametera | Exposure concentration (ppm) | ||
|---|---|---|---|
| Rats | 500 (42)b | 1000 (72) | 2000 (145) |
| Cmax (ng/mL) | 17.5 | 24.3 | 47.9 |
| Cmax/D (ng/mL)/(ppm) | 0.035 | 0.024 | 0.024 |
| Cmax/D (ng/mL)/(mg/kg) | 0.416 | 0.338 | 0.330 |
| Half-life (h) | 1.61 | 3.48 | 2.81 |
| AUC (h*ng/mL) | 38.2 | 58.6 | 87.8 |
| AUC/D (h*ng/mL)/(ppm) | 0.076 | 0.059 | 0.044 |
| AUC/D (h*ng/mL)/(mg/kg) | 0.909 | 0.814 | 0.606 |
| Exposure concentration (ppm, mg/kg) | |||
| Mice | 500 (82)b | 1000 (168) | 2000 (325) |
| Cmax (ng/mL) | 26.7 | 44.6 | 186 |
| Cmax/D (ng/mL)/(ppm) | 0.0534 | 0.0446 | 0.0930 |
| Cmax/D (ng/mL)/(mg/kg) | 0.326 | 0.265 | 0.572 |
| Half-life (h) | 1.06 | 2.09 | 2.28 |
| AUC (h*ng/mL) | 25.8 | 68.1 | 111 |
| AUC/D (h*ng/mL)/(ppm) | 0.052 | 0.068 | 0.056 |
| AUC/D (h*ng/mL)/(mg/kg) | 0.315 | 0.405 | 0.342 |
Based on non-compartmental analysis. Values from up to 3 animals are given.
Values in parenthesis are mg NBBS/kg body weight estimated using food consumption and subsequently chemical consumption.
In male rats, Cmax increased only slightly from 17.5 to 24.3 ng/mL when exposure concentration was increased from 500 to 1000 ppm but increased proportional to the exposure concentration at 2000 ppm. AUC values also increased less than proportionally to the dose with values increased from 38.2 to 87.8 h*ng/mL. In mice, Cmax increased with the exposure and was more-than dose proportional at 2,000 ppm. AUC increased proportional to the exposure concentration as evident by the dose-normalized values (Table 7). In general, Cmax was only slightly higher in mice and AUC was similar in both species, despite the higher dose received in mice (82 to 325 mg/kg body weight) compared to rats (42 to 145 mg/kg body weight). The half-life for elimination of NBBS in plasma following exposure to NBBS via feed of male rats (1.61 to 3.48 h) and male mice (1.06 to 2.28 h) were similar.
Discussion
To the best of our knowledge, this is the first comprehensive study investigating the TK behavior of parent NBBS in rodents following oral administration. NBBS was rapidly absorbed in both male and female rats following a single gavage administration with plasma Tmax ≤0.539 h and with no clear dose-related effect. In mice (≤0.136 h), plasma Tmax was reached faster than in rats. In rats, Cmax increased proportional to the dose in both males and females as demonstrated by the dose-normalized (Cmax /D) values, and was 4- to 5-fold higher in females than in males. In mice, there was no apparent sex difference in plasma Cmax although Cmax increased more than proportional to the dose in males at the two highest doses and was dose-proportional in females. When compared between species, male mice (756 to 15,500 ng/mL) had higher plasma Cmax than male rats (316 to 3,440 ng/mL) but there was no clear difference in Cmax between female mice (1,040 to 13,700 ng/mL) and female rats (1,540 to 15,200 ng/mL).
Unlike Cmax, plasma AUC increased more than proportional to the dose in rats of both sexes at the two highest doses, suggesting saturation of metabolism and clearance processes. The dose normalized (AUC/D) values were 2- to 6-fold higher in female rats. Similar to rats, AUC increased more than proportional to the dose in male and female mice. However, unlike in rats, there was no apparent sex difference in AUC in mice. Mice had lower systemic exposure than rats based on the AUC and the effect was more pronounced in females compared to males.
The high volume of distribution estimated for parent NBBS for male and female rats and mice in the current investigation is consistent with our previous findings (Waidyanatha et al., 2020). Using [14C]NBBS, we showed that NBBS-derived radioactivity was well-distributed to all tissues examined, including the brain, in rats and mice following gavage and IV administration (Waidyanatha et al., 2020). Following gavage administration in male rats we showed that ∼14% and ∼8% of the total administered dose was remaining in tissues at 24 and 72 h, respectively, demonstrating significant tissue distribution/retention of NBBS-derived moieties (Waidyanatha et al., 2020). Distribution of parent NBBS to brain and other tissues following IV administration of 1 mg/kg body weight in male and female rats has also been demonstrated by other investigators (Kumar et al., 2007); however, four hours after administration, the levels of parent NBBS decreased significantly with tissue:plasma ratios reaching less than 1 which authors attributed to concentration-dependent plasma protein binding rather than lack of tissue retention (Kumar et al., 2007).
Since NBBS is a potential neurotoxicant (Strong et al., 1990, Strong et al., 1991), we investigated the TK behavior of parent NBBS in brains of rats and mice. Following a single gavage administration, NBBS was rapidly distributed to the brain in both rats and mice with Cmax reached ≤0.362 h following administration. In rats, Cmax/D decreased with the dose suggesting a less than dose proportional increase and was higher in females compared to males. On the other hand, similar to plasma, brain AUC increased more than proportional to the dose in both sexes in rats as evident by increasing AUC/D with the dose, and as with Cmax, was higher in females compared to males. Taken collectively, these data demonstrate higher exposure in the brains of females compared to males. In mice brains, both Cmax and AUC increased more than proportional to the dose and were similar between sexes.
Since the brains were not perfused prior to collection, brain:blood ratio should be evaluated in order to determine whether NBBS crosses the blood-brain barrier. Since blood levels of NBBS were not determined in our study, we used the brain-to-plasma ratio to determine the extent of distribution to and retention in the brain assuming a density of 1 g/mL for the brain for direct comparison of concentrations (Table 8). Using Cmax, brain:plasma ratios were ∼ 4-5 in male rats and ∼2-3 in female rats suggesting that NBBS may penetrate the blood-brain barrier in rats. This was less evident when AUC values were used for comparison where brain:plasma ratios were ∼1-3 for males and ∼2-3 for females. In mice, using either Cmax or AUC, brain:plasma ratios ranged from <1 to 2 (Table 8). We have previously shown, using [14C]NBBS, that NBBS preferentially partitions into red blood cells (RBC); less than 5% of total radioactivity in blood was associated with the plasma, although the fraction of parent NBBS in plasma and RBCs were not determined (Waidyanatha et al., 2020). Kumar et al. (2007) reported a similar finding in rats; using [13C]NBBS, they showed that NBBS readily partitioned into RBCs with a RBC:plasma ratio of 3 (Kumar et al., 2007). Hence, using brain:plasma ratio may overestimate the extent of NBBS distribution into the brain. Taken collectively, our data suggests that the penetration of the blood-brain barrier by NBBS is low in rats and mice.
Table 8.
Brain:plasma ratios of NBBS following gavage administration of NBBS in rats and mice based on the systemic exposure parameters.
| Dose (mg/kg) |
Male rat |
Female rat |
Male mouse |
Female mouse |
||||
|---|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmax | AUC | Cmax | AUC | Cmax | AUC | |
| 20 | 5.3 | 1.8 | 3.1 | 2.0 | 1.7 | 0.7 | 0.7 | 0.4 |
| 60 | 3.8 | 1.4 | 3.0 | 2.6 | 1.5 | 0.9 | 1.0 | 0.9 |
| 200 | 3.9 | 2.5 | 1.7 | 2.3 | 1.5 | 1.4 | 1.4 | 1.5 |
NBBS was rapidly eliminated from the central compartment with half-lives ≤3.55 h in rats with no apparent sex difference following gavage administration. Elimination was faster in mice with half-lives ≤0.432 h; similar to rats, there was no apparent sex difference. The half-life of elimination estimated in female rats in our study is similar to the terminal half-life reported in the literature following a single gavage administration of 1 mg/kg body weight in female rats (500 min) (NTP 2010). The plasma elimination half-lives increased and clearance decreased with increasing dose in both sexes in rats and mice. The estimated bioavailability (% F) of NBBS for female rats were higher (60, 68, and 132 for 20, 60, 200 mg/kg body weight, respectively) than in males (23, 52, 43, for 20, 60, 200 mg/kg body weight, respectively). These results point to some sex- and dose-related differences in bioavailability of NBBS in rats and that the saturation of metabolism and elimination processes of NBBS may begin at doses between 60 and 200 mg/kg body weight in female rats following a single gavage administration. Oral bioavailability observed in female rats in our study is similar to that reported in a previous investigation following gavage administration of 1 mg/kg body weight in female rats (52 to 79%) (NTP, 2010). In mice of both sexes, bioavailability (%F) of NBBS was much lower than that in rats (males, 6-14; females, 5-11), demonstrating a species difference; however, unlike in rats, there was no evidence of either dose- or sex-related differences in bioavailability. In a previous investigation, using [14C]NBBS, we reported that a gavage administered NBBS was fully absorbed in rats and mice (Waidyanatha et al., 2020). The less than 100% bioavailability observed in this investigation supports first pass metabolism of NBBS and is consistent with numerous urinary metabolites observed (Waidyanatha et al., 2020). In addition, lower bioavailability of NBBS for mice compared to rats suggest a potential higher first pass metabolism in mice compared to rats.
In order to bridge between different oral routes and provide more relevant TK data to aid in toxicology study design and interpretation, we also conducted a limited study in male rats and mice following exposure via feed and using the same exposure concentrations as those used in the NTP toxicology studies (500, 1000, and 2000 ppm). Based on the average daily food consumption and subsequently the average daily chemical consumption estimated from the 7-d exposure period, and the corresponding animal body weights, the daily dose of NBBS received by rats was 42, 72, and 145 mg/kg body weight for 500, 1000, and 2000 ppm exposure groups, respectively, and were within the range for the single gavage dose of 20, 60, and 200 mg/kg body weight. Similarly, in mice, the daily doses received via feed exposure were 82, 168, and 325 mg/kg body weight for 500, 1000, and 2000 ppm exposure groups, respectively (Table S6). The two lower doses were within the gavage doses of 20 and 60 mg/kg body weight in mice; however, the high dose was ∼1.5X higher than the highest gavage dose of 200 mg/kg body weight. Male mice received a mg/kg body weight dose approximately 2-fold higher than male rats. The dose in both species increased proportional to exposure concentration (Table S6).
During exposure via feed to 500, 1,000, and 2,000 ppm NBBS, mice had slightly higher Cmax than rats demonstrating higher overall exposure likely due to approximately 2-fold higher dose received by mice. However, per unit dose of exposure (Cmax/D, (ng/mL)/(mg/kg body weight)), rats and mice had similar exposure. In both rats and mice, AUC increased with the exposure concentration although the increase was more evident in mice (Table 7). On the other hand, AUC per unit mg/kg body weight dose (AUC/D) was higher in male rats than in male mice (rats, 0.606 to 0.909; mice, 0.315 to 0.405((h*ng/mL)/(mg/kg body weight)) suggesting mice cleared NBBS faster than rats. A similar behavior was also observed following administration of a single gavage dose (Table 2, Table 4).
When systemic exposure parameters normalized to mg/kg body weight dose in feed studies were compared to that following gavage administration, exposure via feed provides much lower systemic exposure to NBBS than following a single gavage administration in rats and mice. Cmax per unit dose of NBBS exposure ((ng/mL)/(mg/kg body weight)) following gavage administration ranged from 15.8 to 20.0 in male rats and 37.8 to 92.1 for male mice (Table 2, Table 4) whereas that following feed exposure ranged from 0.330 to 0.416 for male rats and 0.265 to 0.572 for male mice (Table 7). Similarly, AUC per unit dose of NBBS exposure ((h*ng/mL)/(mg/kg body weight)) in male rats and male mice, respectively, ranged from 38.6 to 86.5 and 21.4 to 50.0 following gavage administration (Table 2, Table 4) and 0.606 to 0.909 and 0.315 to 0.405 following feed exposure (Table 7). This means that following a single gavage dose, based Cmax and AUC, respectively, male rats had approximately 48-fold (e.g., 15.8/0.330 to 20.0/0.416) and 64- to 95-fold (e.g., 38.6/0.606 to 86.5/0.909) and male mice had approximately 143- to 161-fold and 68- to 123-fold higher systemic exposure than following exposure to NBBS via feed.
At the present time, there are no human systemic exposure data for NBBS although it has been suggested that there is potential background exposure of humans via water and food (Duffield et al., 1994, Huppert et al., 1998, Oros et al., 2003, Dsikowitzky et al., 2004a, Dsikowitzky et al., 2004b, Pedersen et al., 2005, Skjevrak et al., 2005, Grigoriadou et al., 2008, Plumlee et al., 2012, Di Carro et al., 2018). In a previous investigation, we observed background levels of NBBS in control hepatocyte incubations suggesting potential leaching of NBBS from supplies used and hence potential ubiquitous existence of the compound (Waidyanatha et al., 2020). We have previously shown that human hepatocytes cleared NBBS much slower than rodent hepatocytes with half-lives >909 min for humans and 110 to 319 min in rodents suggesting that NBBS may bioaccumulate in humans more readily than in rodents (Waidyanatha et al., 2020). Based on the above information, background levels of NBBS must be present in human matrices such as urine, blood and plasma. Hence, future work will be aimed at investigating systemic exposure of NBBS in humans due to environmental exposures.
Conclusion
Our data demonstrated that NBBS was absorbed rapidly in rats and mice following gavage administration. The systemic exposure, AUC, increased more than proportional to the administered dose in both rats and mice, but was higher in rats compared to mice and was higher in female rats compared to male rats. Consistent with this, oral bioavailability of NBBS was higher in rats compared to mice and in female rats compared to males with apparent saturation of clearance beginning at doses between 60 and 200 mg/kg body weight in female rats. NBBS was detected in brains of rats and mice but with low brain:plasma ratios suggesting low potential of NBBS to cross the blood-brain barrier. Systemic exposure following a single gavage dose was much higher than 7-day feed exposure. These data are the first demonstration of species, sex, dose- and route-related difference in TK behavior of NBBS in rodents and subsequently will aid in the interpretation of potential species differences in toxicity.
Declaration of Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Transparency document
CRediT authorship contribution statement
Suramya Waidyanatha: Project administration, Conceptualization, Methodology, Supervision, Data curation, Formal analysis, Visualization, Writing - original draft. Seth Gibbs: Methodology, Formal analysis, Writing - review & editing, Data curation, Visualization. Natalie South: Methodology, Investigation, Writing - review & editing. Jeremy P. Smith: Methodology, Investigation, Writing - review & editing. Esra Mutlu: Project administration, Methodology, Writing - review & editing. Brian Burback: Supervision, Writing - review & editing. Yu Cao: Methodology, Writing - review & editing. Cynthia V. Rider: Writing - review & editing, Investigation.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors are grateful to Drs Madelyn Huang and Jason Stanko for their review of this manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-04, and performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract HHSN273201400027C (Battelle, Columbus, OH).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.05.005https://doi.org/10.1016/j.toxrep.2020.05.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- National Reserach Council . Eighth Edition. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Davies B., Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Di Carro M., Magi E., Massa F., Castellano M., Mirasole C., Tanwar S., Olivari E., Povero P. Untargeted approach for the evaluation of anthropic impact on the sheltered marine area of Portofino (Italy) Mar Pollut Bull. 2018;131:87–94. doi: 10.1016/j.marpolbul.2018.03.059. [DOI] [PubMed] [Google Scholar]
- Dsikowitzky L., Schwarzbauer J., Kronimus A., Littke R. The anthropogenic contribution to the organic load of the Lippe River (Germany). Part 1: qualitative characterisation of low-molecular weight organic compounds. Chemosphere. 2004;57:1275–1288. doi: 10.1016/j.chemosphere.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Dsikowitzky L., Schwarzbauer J., Littke R. The anthropogenic contribution to the organic load of the Lippe River (Germany). Part II: quantification of specific organic contaminants. Chemosphere. 2004;57:1289–1300. doi: 10.1016/j.chemosphere.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Duffield P., Bourne D., Tan K., Garruto R.M., Duncan M.W. Analysis of the neurotoxic plasticizer n-butylbenzenesulfonamide by gas chromatography combined with accurate mass selected ion monitoring. J Anal Toxicol. 1994;18:361–368. doi: 10.1093/jat/18.7.361. [DOI] [PubMed] [Google Scholar]
- EPA . 2019. ChemView data base.https://chemview.epa.gov/chemview Last accessed 9/6/2019. [Google Scholar]
- Grigoriadou A., Schwarzbauer J., Georgakopoulos A. Molecular indicators for pollution source identification in marine and terrestrial water of the industrial area of Kavala city, North Greece. Environ Pollut. 2008;151:231–242. doi: 10.1016/j.envpol.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Huppert N., Wurtele M., Hahn H.H. Determination of the plasticizer N-butylbenzenesulfonamide and the pharmaceutical Ibuprofen in wastewater using solid phase microextraction (SPME) Fresen J Anal Chem. 1998;362:529–536. [Google Scholar]
- Kumar G., Smith Q.R., Hokari M., Parepally J., Duncan M.W. Brain uptake, pharmacokinetics, and tissue distribution in the rat of neurotoxic N-butylbenzenesulfonamide. Toxicological Sciences. 2007;97:253–264. doi: 10.1093/toxsci/kfm057. [DOI] [PubMed] [Google Scholar]
- Lee W.Y., Hwang S.J., Cho D.H., Kim J.S. Behavioral-Changes with Alterations of Choline-Acetyltransferase Immunoreactivities Induced by N-Butyl Benzenesulfonamide. Vet Hum Toxicol. 1995;37:537–542. [PubMed] [Google Scholar]
- Manamsa K., Crane E., Stuart M., Talbot J., Lapworth D., Hart A. A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci Total Environ. 2016;568:712–726. doi: 10.1016/j.scitotenv.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Marrocco A., Meade B.J., Long C.M., Lukomska E., Marshall N.B., Anderson S.E. Investigations into the Immunotoxicity and Allergic Potential Induced by Topical Application of N-Butylbenzenesulfonamide (Nbbs) in a Murine Model. J Toxicol Env Heal A. 2015;78:1122–1132. doi: 10.1080/15287394.2015.1056898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP . 2010. Chemical Information Review Document for N-Butylbenzenesulfonamide [CAS No. 3622-3684-2]. Research Triangle Park: National Toxicology Program.https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/n_butylbenzensulfonamide_508.pdf Available at: [Google Scholar]
- NTP . 2020. Testing status of N-butylbenzenesulfonamide.https://ntp.niehs.nih.gov/testing/status/agents/ts-10057.html [Google Scholar]
- Oros D.R., Jarman W.M., Lowe T., David N., Lowe S., Davis J.A. Surveillance for previously unmonitored organic contaminants in the San Francisco Estuary (vol, 46, pg 1102, 2003) Mar Pollut Bull. 2003;46 doi: 10.1016/S0025-326X(03)00248-0. 1631-1631. [DOI] [PubMed] [Google Scholar]
- Pedersen J.A., Soliman M., Suffet I.H. Human pharmaceuticals, hormones, and personal care product ingredients in runoff from agricultural fields irrigated with treated wastewater. J Agr Food Chem. 2005;53:1625–1632. doi: 10.1021/jf049228m. [DOI] [PubMed] [Google Scholar]
- Plumlee M.H., Gurr C.J., Reinhard M. Recycled water for stream flow augmentation: Benefits, challenges, and the presence of wastewater-derived organic compounds. Sci Total Environ. 2012;438:541–548. doi: 10.1016/j.scitotenv.2012.08.062. [DOI] [PubMed] [Google Scholar]
- Rider C.V., Janardhan K.S., Rao D., Morrison J.P., McPherson C.A., Harry G.J. Evaluation of N-butylbenzenesulfonamide (NBBS) neurotoxicity in Sprague-Dawley male rats following 27-day oral exposure. Neurotoxicology. 2012;33:1528–1535. doi: 10.1016/j.neuro.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjevrak I., Brede C., Steffensen I.L., Mikalsen A., Alexander J., Fjeldal P., Herikstad H. Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit Contam. 2005;22:1012–1022. doi: 10.1080/02652030500090877. [DOI] [PubMed] [Google Scholar]
- Soliman M.A., Pedersen J.A., Park H., Castaneda-Jimenez A., Stenstrom M.K., Suffet I.H. Human pharmaceuticals, antioxidants, and plasticizers in wastewater treatment plant and water reclamation plant effluents. Water Environ Res. 2007;79:156–167. doi: 10.2175/106143006x111961. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Garruto R.M., Wolff A.V., Chou S.M., Fox S.D., Yanagihara R. N-Butyl Benzenesulfonamide - a Neurotoxic Plasticizer Inducing a Spastic Myelopathy in Rabbits. Acta Neuropathol. 1991;81:235–241. doi: 10.1007/BF00305863. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Garruto R.M., Wolff A.V., Yanagihara R., Chou S.M., Fox S.D. N-butylbenzenesulphonamide, a novel neurotoxic plasticising agent. Lancet. 1990;336:640. doi: 10.1016/0140-6736(90)93447-w. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S., Black S.R., Patel P.R., Rider C.V., Watson S.L., Snyder R.W., Fennel T.R. Disposition and metabolism of N-butylbenzenesulfonamide in Sprague Dawley rats and B6C3F1/N mice and in vitro in hepatocytes from rats, mice, and humans. Toxicology Letters. 2020 doi: 10.1016/j.toxlet.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych G. Plasticizer Types. In: Wypych G., editor. Handbook of Plasticizers. 3rd Edition. ChemTec Publishing; Toronto: 2017. pp. 7–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.