Short abstract
Objective
Idiopathic dilated cardiomyopathy (IDCM) is a primary myocardial disease resulting in symptoms of heart failure. Right bundle branch block (RBBB) is associated with increased cardiovascular risk and all-cause mortality. Therefore, the present study was performed to identify the prognostic impact of RBBB in patients with IDCM.
Methods
In total, 165 hospitalized patients with IDCM were evaluated. Receiver operating characteristic curve analysis was used to determine the cutoff point, and Cox regression was used to assess risk factors.
Results
After a median follow-up of 73.1 months (interquartile range, 36.1–88.7 months), 59 (35.8%) patients had died. All-cause mortality was significantly higher in patients with than without RBBB (log-rank χ2 = 9.400), P<0.05. Significant independent predictors of all-cause mortality in patients with IDCM were RBBB (hazard ratio, 2.898; 95% confidence interval, 1.201–6.995) and the left ventricular end-diastolic dimension (LVEDD) (hazard ratio, 1.034; 95% confidence interval, 1.004–1.066) at admission. Patients with RBBB and an LVEDD of ≥63 mm had the highest mortality (log-rank χ2 = 14.854), P<0.05.
Conclusion
RBBB was an independent predictor of all-cause mortality, and the combination of RBBB and LVEDD provided more clinically relevant information than RBBB alone for assessing the risk of all-cause mortality in patients with IDCM.
Keywords: Idiopathic dilated cardiomyopathy, right bundle branch block, mortality, prognosis, left ventricular end-diastolic dimension, cardiovascular risk
Introduction
Idiopathic dilated cardiomyopathy (IDCM) is a myocardial disorder characterized by a dilated left ventricular chamber and systolic dysfunction that commonly results in congestive heart failure.1,2 IDCM is currently the leading indication for heart transplantation and remains the third most common cause of heart failure after coronary artery disease and hypertension.2,3 With an incidence of 5.5 cases per 100,000 individuals per year, IDCM is the most common form of cardiomyopathy, and it is diagnosed when no other cause of congestive heart failure is apparent.4,5
Right bundle branch block (RBBB) is defined as a QRS duration of ≥120 ms in adults according to the American Heart Association recommendations for standardization of the electrocardiogram (ECG).6 On the ECG, RBBB presents with a wide, blunt R or R’ wave in lead V1 or aVR and a wide, blunt S wave in leads V5, I, and aVL.7 The prevalence of RBBB in the general population ranges from 0.5% to 1.5%, shows male predominance (1.4% in men and 0.5% in women), and increases with age to 2.2% in patients aged >55 years.8,9 Some previous studies have shown that RBBB is associated with right ventricular dysfunction in patients with congenital heart diseases and has been proven to be independently associated with adverse outcomes.10–12 However, the relationship between RBBB and the prognosis of IDCM is unclear. Therefore, the aim of the present study was to define the clinical value of RBBB in predicting the outcome of IDCM.
Patients and methods
Patients
We retrospectively reviewed the data of patients admitted to our cardiology department with IDCM from February 2004 to July 2011. All patients recruited into this study provided verbal informed consent. This research was approved by the Ethics Committee of Fuwai Hospital. For all patients, we obtained an extensive baseline clinical history, performed a physical examination, and obtained a 12-lead ECG and 2-dimensional Doppler echocardiogram. The definition of IDCM was based on the World Health Organization criteria: presentation with both a left ventricular end-diastolic dimension (LVEDD) of ≥55 mm and a left ventricular ejection fraction (LVEF) of <50% in the absence of a possible known cause.13 The exclusion criteria were as follows:
A history of severe systemic hypertension (>160/100 mmHg), diabetic heart diseases, and other cardiomyopathies with LVEDD dilation, including alcoholic cardiomyopathy and peripartum cardiomyopathy
An ischemic etiology (ruled out by coronary angiography or computed tomography angiography)
Organic valve diseases, tachycardia-induced cardiomyopathy, congenital heart diseases, hyperthyroidism, and advanced systemic diseases
Echocardiography
Because the pulmonary artery systolic pressure (PASP) is equal to the right ventricular systolic pressure in the absence of pulmonary stenosis, the PASP was estimated using Doppler echocardiography by calculating the right ventricular to right atrial pressure gradient during systole (approximated by the modified Bernoulli equation as 4v2, where v is the velocity of the tricuspid regurgitation jet in m/s). The right atrial pressure, estimated based on the echocardiographic characteristics of the inferior vena cava and assigned a standardized value, was then added to the calculated gradient to give the PASP. According to the new guideline, the presence of a PASP of ≥40 mmHg was likely to be pulmonary hypertension.14
Follow-up and endpoint
Follow-up was performed until November 2016. Data on the occurrence of all-cause mortality at follow-up were collected by reviewing the medical records (outpatient clinic attendance and hospitalization), telephone interviews, and retrieval of survival status through the police station. The primary endpoint in this study was all-cause mortality. This study was approved by the Ethics Commission of Fuwai Hospital.
Statistical analysis
Baseline demographic data, risk factors, and clinical variables are descriptively summarized. Variables with a normal distribution are presented as mean ± standard deviation, whereas those with a non-normal distribution are presented as median and interquartile range (IQR). Categorical variables are presented as percentage frequency. The t test was used to compare differences in continuous variables, and the chi-square (χ2) test was used to compare differences in categorical variables.
To predict all-cause mortality from the baseline variables, a univariate Cox regression screening of all parameters at enrollment was initially performed. For the multivariable Cox proportional hazards analysis, we included only those variables with a p value of <0.05 in the univariate analysis as well as the N-terminal fragment pro-brain natriuretic peptide (NT-pro BNP) level because it is a well-known prognostic indicator in patients with IDCM.
On the basis of the multivariate Cox regression analysis, continuous variables were categorized by receiver operating characteristic (ROC) curve analysis, which was used to optimize the cutoff level of continuous variables. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. The level of statistical significance was set at p < 0.05. All hypothesis tests were two-sided. The entire analysis was performed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Study population
In total, 165 patients who were hospitalized from February 2004 to July 2011 were included in this study. Twenty patients were lost to follow-up, which was performed until November 2016. Of the 165 patients included in the study, 107 (64.8%) were men and 58 (35.1%) were women. The age of the patients at diagnosis of IDCM ranged from 19 to 80 years (mean, 56.9 ± 12.3 years), and the median disease duration was 2 years (IQR, 0.35–5 years). One hundred two (61.8%) patients had New York Heart Association (NYHA) functional class III/IV heart failure. Forty-six patients had a QRS duration of ≥150 mm and 83 had a duration of ≥120 mm, among whom 12 patients had RBBB. During a median follow-up time of 73.1 months (IQR, 36.1–88.7 months), 59 patients with IDCM (35.8%) died.
The baseline clinical, ECG, and echocardiographic characteristics of patients with IDCM are shown in Table 1. Among all patients with IDCM, differences in the baseline age, diastolic blood pressure (DBP), RBBB, and atrial fibrillation were observed between those who died and those who survived. Age and DBP at admission were higher in patients who died than in those who survived. RBBB and atrial fibrillation were more frequent in patients who died. Table 2 shows the baseline clinical, ECG, and echocardiographic characteristics of patients divided by RBBB. Patients with RBBB had a higher NT-pro BNP level, QRS duration, and right ventricular (RV) diameter. The frequency of left bundle branch block was lower in the RBBB-positive than -negative group.
Table 1.
Patient characteristics categorized according to survival and death.
| All patients (n = 165) | Death (n = 59) | Survival (n = 106) | p value | |
|---|---|---|---|---|
| Mean age, years | 56.9 ± 12.3 | 60.9 ± 11.6 | 54.7 ± 12.1 | 0.002 |
| Disease duration, years | 2 (0.35–5) | 3 (0.5–7) | 2 (0.3–5) | 0.365 |
| Smoking | 67 (40.6) | 25 (42.4) | 42 (39.6) | 0.897 |
| Syncope | 9 (5.5) | 4 (6.8) | 5 (4.7) | 0.576 |
| Heart rate, bpm | 77.9 ± 15.9 | 75.2 ± 16.8 | 79.4 ± 15.4 | 0.105 |
| Sex | 0.668 | |||
| Male | 107 (64.8) | 37 (62.7) | 70 (66.0) | |
| Female | 58 (35.2) | 22 (37.3) | 36 (34.0) | |
| NYHA functional class | 0.122 | |||
| I | 6 (3.6) | 0 (0.0) | 6 (5.7) | |
| II | 57 (34.4) | 18 (30.5) | 39 (36.8) | |
| III | 73 (44.2) | 27 (45.8) | 46 (43.4) | |
| IV | 29 (17.8) | 14 (23.7) | 15 (14.1) | |
| Blood pressure | ||||
| SBP, mmHg | 118.3 ± 17.1 | 116.6 ± 16.7 | 119.2 ± 17.2 | 0.348 |
| DBP, mmHg | 74.8 ± 13.2 | 71.2 ± 12.2 | 76.8 ± 13.4 | 0.008 |
| Blood test results | ||||
| Cr, µmol/L | 87 (70.6–98.1) | 89.6 (75.5–102.9) | 85.4 (68.5–97.4) | 0.098 |
| BUN, µmol/L | 6.7 (5.5–8.7) | 6.57 (5.6–8.9) | 6.67 (5.4–8.35) | 0.450 |
| NT-pro BNP, fmol/mL | 1173.1 (602.5–2186.1) | 1278.6 (627–2305.9) | 1087.8 (575.9–2057.6) | 0.263 |
| cTnI | 0.05 (0.32–0.05) | 0.05 (0.05–0.1) | 0.05 (0.025–0.05) | 0.363 |
| ECG test results | ||||
| QRS duration, ms | 126.3 ± 32.8 | 132.8 ± 32.3 | 122.8 ± 32.7 | 0.066 |
| RBBB | 12 (7.3) | 8 (13.6) | 4 (3.7) | 0.020 |
| LBBB | 53 (32.1) | 19 (32.2) | 34 (32.1) | 0.987 |
| AF | 9 (5.5) | 6 (10.2) | 3 (2.8) | 0.047 |
| Echocardiography results | ||||
| LVEF, % | 34.6 ± 8.9 | 33.6 ± 8.5 | 35.2 ± 9.1 | 0.250 |
| LVEDD, mm | 65.4 ± 8.6 | 67.1 ± 8.8 | 64.5 ± 8.3 | 0.066 |
| RV, mm | 21.9 ± 4.0 | 22.5 ± 3.8 | 21.5 ± 4.1 | 0.124 |
| LA, mmPH, mmHg | 43.2 ± 7.919 (11.5) | 44.4 ± 8.29 (15.3) | 42.5 ± 7.810 (9.4) | 0.1610.946 |
Data are expressed as mean ± standard deviation, median (interquartile range), or n (%); p values were calculated from independent-samples t-tests or χ2 tests for categorical data. Bold data indicate p < 0.05.
Five patients lacked LVEDD data, 16 lacked RV data, 7 lacked LA data, 4 lacked LVEF data, 33 lacked NT-pro-BNP data, 7 lacked Cr data, 8 lacked BUN data, and 103 lacked cTnI data.
RBBB, right bundle branch block; NYHA, New York Heart Association; SBP, systolic blood pressure; DBP, diastolic blood pressure; Cr, creatinine; BUN, blood urea nitrogen; NT-pro BNP, N-terminal fragment pro-brain natriuretic peptide; cTnI, cardiac troponin; LBBB, left bundle branch block; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; RV, right ventricle; LA, left atrium; PH, pulmonary hypertension.
Table 2.
Patient characteristics categorized according to RBBB.
| RBBB-positive (n = 12) | RBBB-negative (n = 153) | p value | |
|---|---|---|---|
| Mean age, years | 58.2 ± 14.8 | 56.8 ± 12.1 | 0.712 |
| Disease duration, years | 3.5 (1–5) | 2 (0.3–5.5) | 0.656 |
| Smoking | 6 (60.0) | 61 (39.8) | 0.491 |
| Syncope | 1 (8.33) | 8 (5.22) | 0.648 |
| Heart rate, bpm | 75.7 ± 16.8 | 78.1 ± 15.9 | 0.618 |
| Sex | 0.444 | ||
| Male | 9 (75.0) | 98 (64.1.0) | |
| Female | 3 (25.0) | 55 (35.9.0) | |
| NYHA functional class | 0.060 | ||
| I | 0 (0.0) | 6 (3.9) | |
| II | 1 (30.5) | 56 (36.6) | |
| III | 8 (45.8) | 65 (42.5) | |
| IV | 3 (23.7) | 26 (17.0) | |
| Blood pressure | |||
| SBP, mmHg | 110.7 ± 17.1 | 118.9 ± 16.9 | 0.108 |
| DBP, mmHg | 71.2 ± 13.3 | 75.1 ± 13.2 | 0.326 |
| Blood test results | |||
| Cr, µmol/L | 99.9 ± 28.7 | 86.5 ± 24.5 | 0.073 |
| BUN, µmol/L | 8.2 ± 1.9 | 7.1 ± 2.3 | 0.101 |
| NT-proBNP, fmol/mL | 2443 (1742.3–3970.4) | 1078.7 (574.7–2061.6) | 0.001 |
| cTnI | 0.05 (0.0115–0.115) | 0.05 (0.0335–0.05) | 0.733 |
| ECG test results | |||
| QRS duration, ms | 155.8 ± 32.318.4 | 123.8 ± 32.6 | <0.001 |
| LBBB | 0 (0.0) | 53 (34.6) | 0.014 |
| AF | 1 (8.3) | 8 (5.2) | 0.648 |
| Echocardiography results | |||
| LVEF, % | 31.8 ± 9.3 | 34.8 ± 8.9 | 0.260 |
| LVEDD, mm | 69.4 ± 8.5 | 65.1 ± 8.5 | 0.093 |
| RV, mm | 24.1 ± 4.4 | 21.7 ± 3.9 | 0.046 |
| LA, mm | 46.1 ± 8.7 | 42.9 ± 7.9 | 0.188 |
Data are expressed as mean ± standard deviation, median (interquartile range), or n (%); p values were calculated from independent-samples t-tests or χ2 tests for categorical data. Bold data indicate p < 0.05.
Five patients lacked LVEDD data, 16 lacked RV data, 7 lacked LA data, 4 lacked LVEF data, 33 lacked NT-pro-BNP data, 7 lacked Cr data, 8 lacked BUN data, and 103 lacked cTnI data.
NYHA, New York Heart Association; SBP, systolic blood pressure; DBP, diastolic blood pressure; Cr, creatinine; BUN, blood urea nitrogen; NT-pro BNP, N-terminal fragment pro-brain natriuretic peptide; cTnI, cardiac troponin; RBBB, right bundle branch block; LBBB, left bundle branch block; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; RV, right ventricle; LA, left atrium.
Relationship between RBBB and all-cause mortality
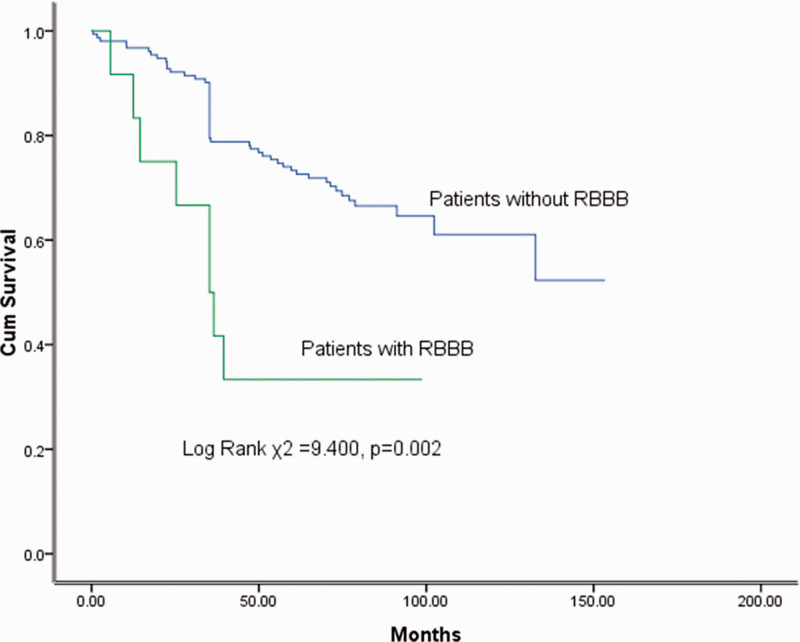
The Kaplan–Meier survival probability estimate was 96.4% at 1 year and 70.4% at 5 years. In our IDCM cohort, patients with RBBB had higher all-cause mortality than patients without RBBB (log-rank χ2 = 9.400, p = 0.002) (Figure 1).
Figure 1.
Kaplan–Meier curves demonstrating differences in groups divided according to the presence of RBBB. Cum, cumulative; RBBB, right bundle branch block.
ROC analysis of LVEDD and QRS
According to the ROC curve analysis, the optimal cutoff value of the LVEDD and QRS was 63 mm [area under the curve (AUC), 0.616; 95% confidence interval (CI), 0.523–0.709; p = 0.017] and 115 ms (AUC, 0.610; 95% CI, 0.519–0.701; p = 0.024), respectively (Figure 2).
Figure 2.
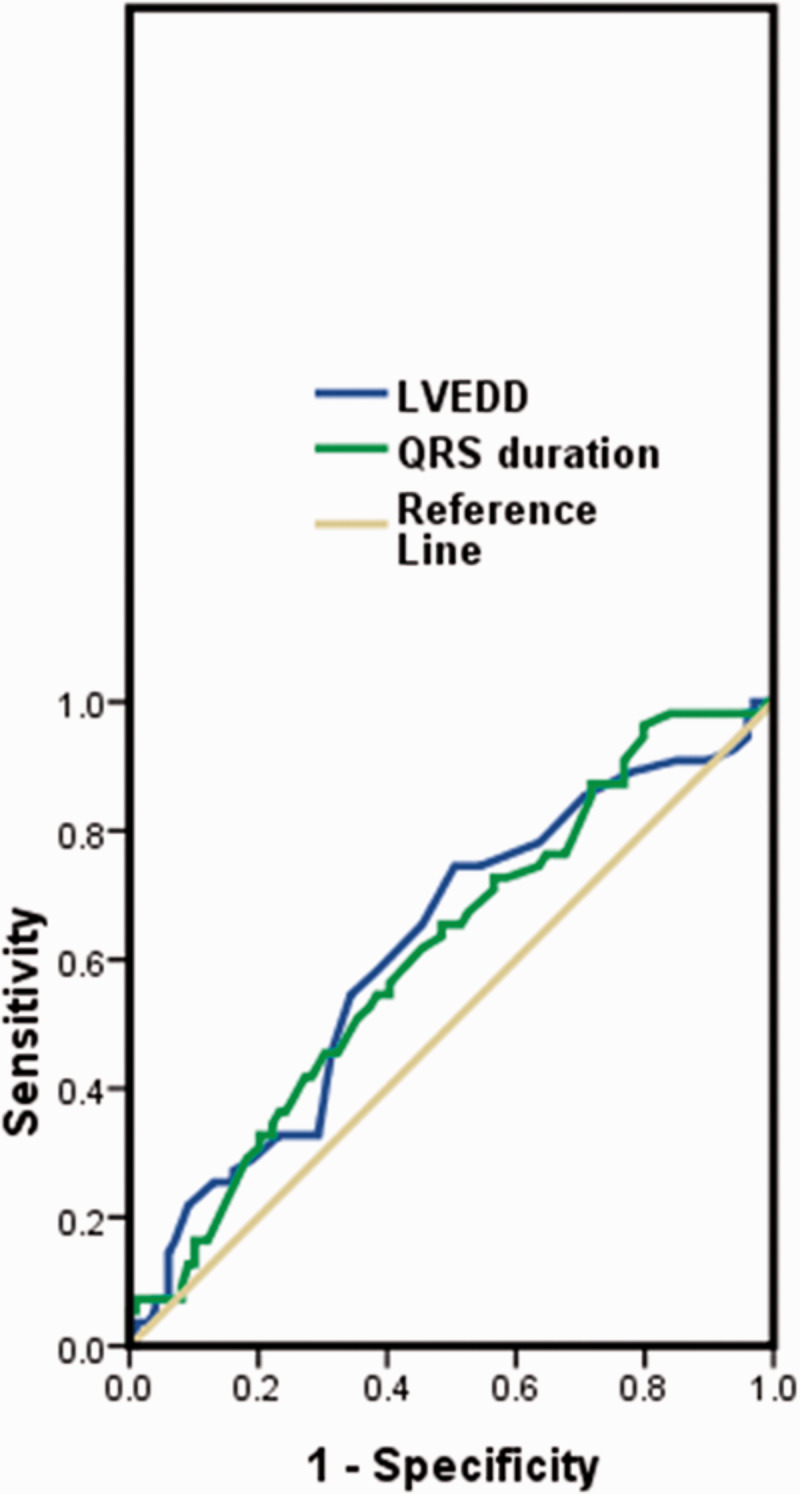
Receiver operating characteristic curve of LVEDD and QRS duration. LVEDD, left ventricular end-diastolic dimension.
Predictors of all-cause mortality in patients with IDCM
The univariate analysis revealed the following predictors of all-cause mortality in patients with IDCM: age (hazard ratio (HR), 1.031; 95% CI, 1.008–1.056; p = 0.009), NYHA class (HR, 1.486; 95% CI, 1.062–2.081; p = 0.021), DBP (HR, 0.974; 95% CI, 0.953–0.995; p = 0.016), RBBB (HR, 2.987; 95% CI, 1.411–6.323; p =0.004), LVEDD (HR, 1.029; 95% CI, 1.001–1.057; p = 0.042), and right atrial diameter (HR, 2.208; 95% CI, 1.037–4.702; p = 0.040). Independent predictors of all-cause mortality in the multiple Cox regression analysis were RBBB (HR, 2.898; 95% CI, 1.201–6.995; p = 0.018) and LVEDD (HR, 1.034; 95% CI, 1.004–1.066; p = 0.029) at admission (Table 3).
Table 3.
All-cause mortality in univariate and multivariable analyses.
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variable | Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value |
| Age | 1.031 (1.008–1.056) | 0.009 | 1.021 (0.995–1.048) | 0.118 |
| Sex | 1.031 (0.608–1.751) | 0.909 | – | – |
| NYHA class | 1.486 (1.062–2.081) | 0.021 | 1.295 (0.812–2.066) | 0.278 |
| Syncope | 1.168 (0.423–3.229) | 0.765 | – | – |
| Smoking | 1.179 (0.703–1.976) | 0.533 | – | – |
| SBP | 0.992 (0.977–1.007) | 0.299 | – | – |
| DBP | 0.974 (0.953–0.995) | 0.016 | 0.977 (0.952–1.002) | 0.075 |
| RBBB | 2.987 (1.411–6.323) | 0.004 | 2.898 (1.201–6.995) | 0.018 |
| LBBB | 0.902 (0.522–1.558) | 0.711 | – | – |
| QRS ≥ 115 | 1.626 (0.932–2.838) | 0.087 | – | – |
| AF | 0.477 (0.204–1.114) | 0.087 | – | – |
| LVEDD | 1.029 (1.001–1.057) | 0.042 | 1.034 (1.004–1.066) | 0.029 |
| LVEF | 0.979 (0.949–1.009) | 0.169 | – | – |
| RA diameter | 2.208 (1.037–4.702) | 0.040 | 1.848 (0.800–4.270) | 0.151 |
| NT-pro-BNP | 1.000 (1.000–1.000) | 0.398 | 1.000 (1.000–1.000) | 0.661 |
| FBG | 1.131 (0.982–1.303) | 0.087 | – | – |
| Cr | 1.007 (0.998–1.017) | 0.143 | – | – |
| cTnIPH | 1.071 (0.909–1.263)1.104 (0.393–3.105) | 0.4130.851 | – | – |
NYHA, New York Heart Association; SBP, systolic blood pressure; DBP, diastolic blood pressure; RBBB, right bundle branch block; LBBB, left bundle branch block; QRS, QRS duration; AF, atrial fibrillation; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; RA, right atrial; NT-pro BNP, N-terminal fragment pro-brain natriuretic peptide; FBG, fasting blood glucose; Cr, creatinine; cTnI, cardiac troponin; PH, pulmonary hypertension.
Bold data indicate p < 0.05.
Relationship between RBBB + increased LVEDD (≥63 mm) and survival
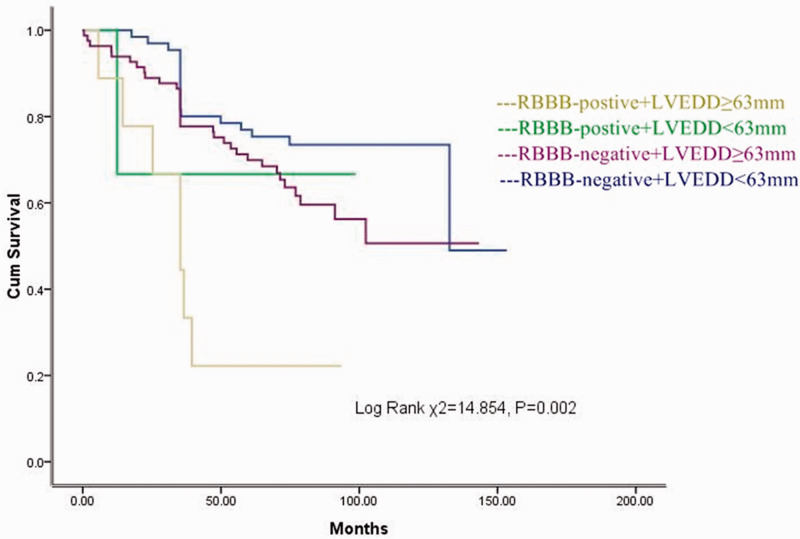
Twelve patients had RBBB (7.3%) and 91 patients had an LVEDD of ≥ 63 mm (55.2%). Figure 3 shows the long-term event-free survival of patients with or without RBBB and an LVEDD of ≥63 mm. Patients with RBBB + an LVEDD of ≥63 mm had the highest mortality (log-rank χ2 = 14.854, p = 0.002).
Figure 3.
Relationship between RBBB + increased LVEDD (≥63 mm) and survival. Cum, cumulative; RBBB, right bundle branch block; LVEDD, left ventricular end-diastolic dimension.
Discussion
In the present study, the most relevant finding was that RBBB at admission was an independent predictor of all-cause mortality in patients with IDCM, and the combination of RBBB and LVEDD provides more clinically relevant information than RBBB alone for assessing the risk of all-cause mortality in patients with IDCM.
RBBB patterns are relatively rare in ECGs in the general population, and RBBB is generally considered a benign finding that does not imply increased risk when found in asymptomatic healthy individuals.8,15–17 A recent study indicated that a reduced LVEF was not associated with an increased likelihood of RBBB, and RBBB did not affect the prognosis among patients with RBBB without evidence of heart disease.18 However, among patients with heart failure, the presence of RBBB has been associated with a poor prognosis.19,20 The present study indicated that patients with IDCM with RBBB had enlarged RV dimensions (Table 2) and that RBBB was an independent predictor of a poor prognosis in patients with IDCM. Keller et al.21 revealed significant associations between RBBB and RV dysfunction and cardiac injury in their multivariable regression, and a recent study showed that RV dysfunction is being increasingly recognized in patients with heart failure and is an independent predictor of adverse outcomes in such patients.22 These studies indicate that RBBB may reflect the RV dimensions or RV dysfunction as well as the possibility that the prognostic impact of RBBB is related to the prognostic impact of RV dysfunction. This should be assessed in future explorations. Other researchers have stated that RBBB is associated with an increased risk of myocardial infarction and pacemaker insertion and that the prognosis of patients with acute myocardial infarction and RBBB at admission remains poor compared with patients who do not have bundle branch block.8,23,24 Additionally, in China, Li et al.25 reported that the presence of RBBB, but not LBBB or the QRS duration, was an independent predictor of all-cause mortality in patients with DCM; this is consistent with the findings of our study.
The effect of left ventricular function on the outcome of patients with heart failure has been well documented.26–28 However, LVEDD, an indicator of left ventricular systolic dysfunction, was a contradictory predictor of prognosis in patients with DCM. Previous studies have shown that a higher LVEDD is significantly related to an unfavorable outcome in patients with IDCM.29,30 Other studies have shown that the prognosis of patients with DCM with a mildly dilated left ventricle was similar to that of patients with a moderately to severely dilated left ventricle.31–33 In the present study, the LVEDD was an independent predictor of a poor prognosis in patients with IDCM, and the combination of the LVEDD and RBBB provides more clinically relevant information than RBBB alone for assessing the risk of all-cause mortality in patients with IDCM.
Study limitations
This study has several limitations. First, this was a single-center study performed in Fuwai Hospital in Beijing; therefore, the data described herein cannot be extrapolated to the entire population of patients with IDCM. Second, because all-cause mortality was the primary endpoint, we did not obtain complete data on the incidence of cardiac death, heart failure rehospitalization, and lethal ventricular arrhythmias. Such data could have provided more compelling and conclusive results than those of all-cause mortality alone. In addition, because the present study was a retrospective analysis, we did not collect precise information about medical therapy in patients with IDCM; thus, we did not include medication as an evaluation index in this study, which may have influenced the all-cause mortality.
Authors’ contributions
Xiaoping Li and Li Lai contributed to the conception of the study. Rong Luo and Chao Yan contributed significantly to the data analysis and manuscript preparation. Wei Fang and Rong Jiang performed the data analyses and wrote the manuscript. Yibin Tang and Wei Hua helped perform the analysis with constructive discussions.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by National Natural Science Foundation of China (No.81500297, No.81470521 and No.81770379).
References
- 1.Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health organization/international society and federation of cardiology. Task force on the definition and classification of cardiomyopathies. Circulation 1996; 93: 841–842. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the council on clinical cardiology, heart failure and transplantation Committee; quality of care and outcomes research and functional genomics and Translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 2006; 113: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 3.Codd MB, Sugrue DD, Gersh BJ, et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975‑1984. Circulation 1989; 80: 564–572. [DOI] [PubMed] [Google Scholar]
- 4.Tsirka AE, Trinkaus K, Chen SC, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol 2004; 44: 391–397. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 6.Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intra-ventricular conduction disturbances: a scientific statement from the American Heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society: endorsed by the international society for computerized electrocardiology. Circulation 2009; 119: e235–e240. [DOI] [PubMed] [Google Scholar]
- 7.Michowitz Y, Tovia-Brodie O, Heusler I, et al. Differentiating the QRS morphology of posterior fascicular ventricular tachycardia from right bundle branch block and left anterior hemiblock aberrancy. Circ Arrhythm Electrophysiol 2017; 10: e005074. [DOI] [PubMed] [Google Scholar]
- 8.Bussink BE, Holst AG, Jespersen L, et al. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J 2013; 34: 138–146. [DOI] [PubMed] [Google Scholar]
- 9.Haataja P, Nikus K, Kahonen M, et al. Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population:the Health 2000 Survey. Int J Cardiol 2013; 167: 1953–1960. [DOI] [PubMed] [Google Scholar]
- 10.Gatzoulis MA, Till JA, Somerville J, et al. Mechanoelectrical interaction in tetralogy of fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995; 92: 231–237. [DOI] [PubMed] [Google Scholar]
- 11.Book WM, Parks WJ, Hopkins KL, et al. Electrocardiographic predictors of right ventricular volume measured by magnetic resonance imaging late after total repair of tetralogy of Fallot. Clin Cardiol 1999; 22: 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egidy Assenza G, Valente AM, Geva T, et al. QRS duration and QRS fractionation on surface electrocardiogram are markers of right ventricular dysfunction and atrialization in patients with Ebstein anomaly. Eur Heart J 2013; 34: 191–200. [DOI] [PubMed] [Google Scholar]
- 13.Brandenburg RO, Chazov E, Cherian G, et al. ; WHO/ISFC Task Force. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J 1980; 44: 672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 15.Fahy GJ, Pinski SL, Miller DP, et al. Natural history of isolated bundle branch block. Am J Cardiol 1996; 77: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson P, Hansson PO, Eriksson H, et al. Bundle-branch block in a general male population: the study of men born 1913. Circulation 1998; 98: 2494–2500. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL, Das DN, Lakatta EG. Right bundle branch block: long-term prognosis in apparently healthy men. J Am Coll Cardiol 1983; 1: 887–892. [DOI] [PubMed] [Google Scholar]
- 18.Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117: 935–939. [DOI] [PubMed] [Google Scholar]
- 19.Barsheshet A, Goldenberg I, Garty M, et al. Relation of bundle branch block to long-term (four-year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol 2011; 107: 540–544. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Qadir HM, Tu JV, Austin PC, et al. Bundle branch block patterns and long-term outcomes in heart failure. Int J Cardiol 2011; 146: 213–218. [DOI] [PubMed] [Google Scholar]
- 21.Keller K, Beule J, Balzer JO, et al. Right bundle branch block and SIQIII-type patterns for risk stratification in acute pulmonary embolism. J Electrocardiol 2016; 49: 512–518. [DOI] [PubMed] [Google Scholar]
- 22.Oketona OA, Balogun MO, Akintomide AO, et al. Right ventricular systolic function in hypertensive heart failure. Vasc Health Risk Manag 2017; 13: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CK, Stewart RA, Gao W, et al. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial. Eur Heart J 2006; 27: 21–28. [DOI] [PubMed] [Google Scholar]
- 24.Vivas D, Pérez-Vizcayno MJ, Hernández-Antolín R, et al. Prognostic implica- tions of bundle branch block in patients undergoing primary coronary angi- oplasty in the stent era. Am J Cardiol 2010; 105: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Luo R, Fang W, et al. Effects of ventricular conduction block patterns on mortality in hospitalized patients with dilated cardiomyopathy: a single-center cohort study. BMC Cardiovasc Disord 2016; 16: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juilliere Y, Danchin N, Briancon S, et al. Dilated cardiomyopathy: long-term follow-up and predictors of survival. Int J Cardiol 1988; 21: 269–277. [DOI] [PubMed] [Google Scholar]
- 27.Grzybowski J, Bilińska ZT, Ruzyłło W, et al. Determinants of prognosis in non- ischemic dilated cardiomyopathy. J Card Fail 1996; 2: 77–85. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on card- iovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 29.Saad IA. Idiopathic dilated cardiomyopathy in children; Natural history and predictors of prognosis. Libyan J Med 2007; 2: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arola A, Tuominen J, Ruuskanen O, et al. Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics 1998; 101(3Pt1): 369–376. [DOI] [PubMed] [Google Scholar]
- 31.Gavazzi A, De Maria R, Renosto G, et al. The spectrum of left ventricular size in dilated cardiomyopathy: clinical correlates and prognostic implications. SPIC (Italian Multicenter Cardiomyopathy Study) Group. Am Heart J 1993; 125(2 Pt 1): 410–422. [DOI] [PubMed] [Google Scholar]
- 32.Kitaoka H, Matsumura Y, Yamasaki N, et al. Long-term prognosis of patients with mildly dilated cardiomyopathy. Circ J 2002; 66: 557–560. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Sharma P, Bahl A, et al. Left ventricular size as a predictor of outcome in patients of non-ischemic dilatedcardiomyopathy with severe left ventricular s-ystolic dysfunction. Int J Cardiol 2016; 221: 310–313. [DOI] [PubMed] [Google Scholar]