Abstract
Objective
Bleomycin is an important chemotherapeutic drug that activates premature senescence to decrease the tumorigenic process. We aimed to investigate the role of phosphatase and tensin homolog deleted on chromosome ten (PTEN) in bleomycin-induced premature senescence in lung cancer cells.
Methods
Human lung cancer A549 cells were incubated in the presence of different concentrations of bleomycin for 5 days. A lentivirus vector was used to silence the PTEN gene, followed by stimulation with bleomycin (1 µg/mL). Changes were evaluated by senescence-associated β-galactosidase staining, reverse transcription-polymerase chain reaction, and western blot.
Results
Treatment with bleomycin induced premature senescence. PTEN expression was decreased and key downstream molecules in the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway were gradually activated following bleomycin treatment. Silencing PTEN reduced autophagy and accelerated senescence of A549 cells. Autophagy levels were also increased and senescence markers were reduced after inhibiting mTOR.
Conclusions
Downregulation of PTEN mediates bleomycin-induced premature senescence in lung cancer cells by suppressing autophagy via the PI3K/Akt/mTOR pathway. These findings provide new insights into the potential role of PTEN as a molecular target for cancer chemotherapy.
Keywords: PTEN, bleomycin, autophagy, premature senescence, PI3K/Akt/mTOR pathway, cancer cell
Introduction
Cellular senescence refers to the gradual deterioration of cell proliferation, differentiation ability, and physiological function in response to extracellular environmental factors or changes in internal gene expression. Hayflick first introduced the concept of cellular senescence to describe the state in which normal human fibroblasts entered permanent growth arrest following extensive serial passaging in culture.1 This senescence caused by excessive cell proliferation is known as replicative senescence, while an alternative form of senescence, referred to as premature senescence, is caused by diverse stimuli, including DNA lesions, telomere erosion, and reactive oxygen species,2 and is irrelevant of either telomere shortening or the duration of cellular proliferation. Cell senescence is characterized by large, flat cell morphology, increased senescence-associated (SA)-β-galactosidase (gal) and DNA damage response protein expression, telomere dysfunction, and increased expression of p16 and p21 cell cycle proteins.3–5 Cellular senescence is also associated with the activation of tumor suppressor pathways, namely the p16INK4a/retinoblastoma (Rb) and p53/p21WAF/Cip1 pathways.6,7
Tumor cell proliferation is unlimited. Previous studies have identified specific mutations in tumor suppressor genes such as p21WAF/Cip1, Rb, and p53 in tumor cells, leading to excessive cell proliferation.8 Moreover, telomerase activity occurs in 90% of malignant tumor tissues.9 These studies suggested a strong association between cell senescence and tumorigenesis. Schmitt et al.10 demonstrated that chemotherapeutic drugs could affect the aging process in tumor cells, and the percentage of aging tumor cells was closely associated with disease prognosis. These results indicated that tumor cells could enter a state of cell cycle arrest in response to chemotherapeutic agents, suggesting that cellular senescence is a standard process that can be used to evaluate the efficacy of chemotherapy.
Bleomycin is an effective anti-cancer drug currently used in chemotherapeutic regimens for the treatment of lymphoma, squamous cell carcinoma, and germ cell tumors, and for inducing chemical pleurodesis in malignant effusions.11 It acts by inducing DNA damage, leading to premature senescence.12 Previous studies showed that bleomycin induced cellular senescence in alveolar epithelial cells in vitro and in vivo, leading to inhibition of tumor growth.13 However, the molecular mechanism of this process is not clear.
The anti-oncogene protein phosphatase and tensin homolog deleted on chromosome ten (PTEN) was initially identified in 1997, and was shown to possess dual-specificity phosphatase activity.14 By dephosphorylating phosphatidylinositol 3,4,5-triphosphate to phosphatidylinositol 4,5-bisphosphate, PTEN reverses the action of phosphoinositide 3-kinase (PI3K), thereby hampering all downstream functions controlled by the Akt/mammalian target of rapamycin (mTOR) axis, leading to the inhibition of cellular proliferation and the induction of apoptosis.15–17 Previous studies have shown that PTEN is closely associated with cellular senescence, and mice with additional genomic copies of PTEN had significantly extended lifespans.17 Loss of PTEN induced epithelial cell growth arrest and accelerated aging in some cancer types.18,19 However, the detailed mechanism by which PTEN affects cellular senescence in lung cancer cells remains poorly understood.
The present study aimed to investigate the effects of PTEN on bleomycin-induced premature senescence in lung cancer cells and to explore its precise mechanism of action.
Materials and methods
Cell culture and treatment with mTOR inhibitors
The A549 human lung adenocarcinoma cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM growth medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and antibiotics (100 units/mL penicillin and 100 mg/mL streptomycin) at 37°C in a humidified air atmosphere containing 5% CO2. Bleomycin was purchased from Nippon Kayaku Co. Ltd. (Tokyo, Japan), dissolved in 0.9% phosphate-buffered saline (PBS), and stored at −20°C. A549 cells were incubated with bleomycin (0, 0.1, 1, 5, 10 and 50 µg/mL) for 120 hours in culture medium.20 The same volume of PBS was added to the culture medium as a negative control. The mTOR inhibitor rapamycin was purchased from Sigma (St. Louis, MO, USA), dissolved in dimethylsulfoxide (DMSO) (Sigma), and stored at 4°C. The cells were treated with 100 mM rapamycin for 24 hour in the presence or absence of bleomycin. The same volume of DMSO was added to the culture medium as a positive control.
Cell viability assay
Cell viability was assessed by MTT assay. The cells were plated at a density of 3,000 to 3,500 cells/well in 96-well sterile plastic plates and allowed to attach overnight. The cells were subsequently exposed to different concentrations of bleomycin for 120 hours, and 20 µL of thiazolyl blue (MTT, Sigma) was then added to each well. Following incubation for 4 hours at 37°C, 150 µL of DMSO (Sigma) was added to each well for 10 minutes with gentle shaking at room temperature to dissolve the formazan product. The absorbance of each sample was measured at 490 nm. The average of three repeated experiments was calculated.
Measurement of mRNA levels
Total RNA was extracted from A549 cells using TRIzol reagent (Takara, Japan) according to the manufacturer’s instructions. Nucleic acid stability and concentration were determined by agarose gel electrophoresis. The absorbance (A value) was measured using an ultraviolet spectrophotometer, with an optimal 260/280 ratio of 1.8 to 2.0. For standard and semi-quantitative polymerase chain reaction (PCR), first-strand cDNA synthesis was performed using a reverse transcription kit (TaKaRa, Japan), and the synthesized DNA was stored at −20°C. Real-time PCR was conducted using an ABI StepOnePlus with SYBR mix agents (Takara Bio, Inc., Otsu, Japan). All results were measured in relation to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA for each sample. The primers used were as follows: PTEN forward, 5′-ACCAGTGGCACTGTTGTTTCAC-3′, and reverse, 5′-TTCCTCTGGTCCTGGTATGAAG-3′; p16 forward, 5′-AGCCTTCGGCTGACTGGCTGG-3′, and reverse, 5′-GCGCTGCCCATCATCATGAC-3′; p21 forward, 5′-GCACTCAGAGGAGGCGCCATGTCA-3′, and reverse, 5′-CTGTCCCCTGCAGCAGAGCAGGT-3′; GAPDH forward, 5′-GTCACCAGGGCTGCTTTTAAC-3′, and reverse, ′-TGATGGGATTTCCATTGATGA-3′. The reaction conditions were as follows: 94°C for 5 minutes, 94°C for 35s, 57°C for 50s, and 72°C for 50s. This program was repeated 40 times. The dissociation curve for the PCR products was estimated based on the PCR results obtained by the following amplification conditions: 94°C for 35s, 57°C for 50s, and 72°C for 50s.
Western blot analysis
Total cell proteins were extracted using a whole cell lysis assay kit (KeyGEN, Nanjing, China). The cells were lysed in RIPA buffer containing proteinase and phosphatase inhibitor cocktail (KeyGEN) on ice for 20 minutes. The protein concentration and purity were measured using a BCA protein assay kit (Millipore Corp., Billerica, MA, USA). Total proteins (20–30 µg) were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk in TBST for 1 hour at room temperature and immunoblotted overnight at 4°C with antibodies against PTEN (138G6, rabbit monoclonal antibody (mAb), #9559, CST, Beverly, MA, USA), Akt (11E7, rabbit mAb, #4685, CST), phospho (p)-Akt (Ser473, rabbit mAb, #4060, CST), FoxO3a (D19A7, rabbit mAb, #12829, CST), p-FoxO3a (Ser253, #9466, CST), mTOR (7C10, rabbit mAb, #2983, CST), p-mTOR (Ser2448, rabbit mAb, #5536, CST), p16 INK4A (D7C1M, rabbit mAb, #80772, CST), p21 Waf1/Cip1 (12D1, rabbit mAb, #2947, CST), β-actin (13E5, rabbit mAb, #4970, CST), SQSTM1/p62 (ab91526, Abcam, Cambridge, UK), and LC3A/B (ab128025, Abcam). The antibodies were used at a 1:1,000 dilution in 5% milk. Following incubation with horseradish peroxidase-conjugated secondary antibody for 1.5 hours, protein expression was detected by enhanced chemiluminescence (Millipore Corp.) using an automatic detection system (Tanon Science and Technology Co., Ltd., Shanghai, China).
Senescence-associated β-gal staining for senescence
The cells were stained using a senescence β-gal staining kit (Beyotime Biotech, Jiangsu, China). Briefly, following drug treatment, cell samples on six-well chamber slides were rinsed three times in PBS for 5 minutes each and fixed with 4% formaldehyde for 10 minutes at room temperature. Following washing three more times with PBS, the cells were mixed with 1 mL of dye from the above kit and incubated overnight at 37°C. The following day, the cells were rinsed with PBS and examined under a bright-field microscope. Senescence-associated (SA)-β-gal-positive (blue-stained) flattened cells with increased granularity were regarded as senescent. The percentage of senescent cells was determined by counting in 10 randomly selected fields (approximately 400 cells).
Cell transfection
Stable silencing and overexpression of PTEN was performed by lentiviral transfection. The lentiviral vectors were constructed by GENCHEM, Inc. (CA, USA) and loaded with the targeting gene and non-targeting control sequences for silencing PTEN, including PTEN small interfering (si) RNA (siPTEN). Lentiviruses were added to 1 mL of complete medium per well in a six-well slide supplemented with 5 µg/mL Polybrene (Sigma) and incubated for 8 hours. The medium was changed to fresh DMEM medium for continuous culture for 72 hours. Green fluorescence was observed to estimate the transfection efficiency, and cell proteins were then extracted for further analysis.
Statistical analysis
All experiments were performed at least three times. The data are expressed as the mean ± standard deviation (SD). Comparisons between groups were performed using paired t-tests or one-way analysis of variance (ANOVA). Statistical analysis was carried out and graphs were plotted using Prism (GraphPad Software, Inc., La Jolla, CA, USA). P-values < 0.05 were considered statistically significant.
Results
Bleomycin induced premature senescence in lung cancer cells
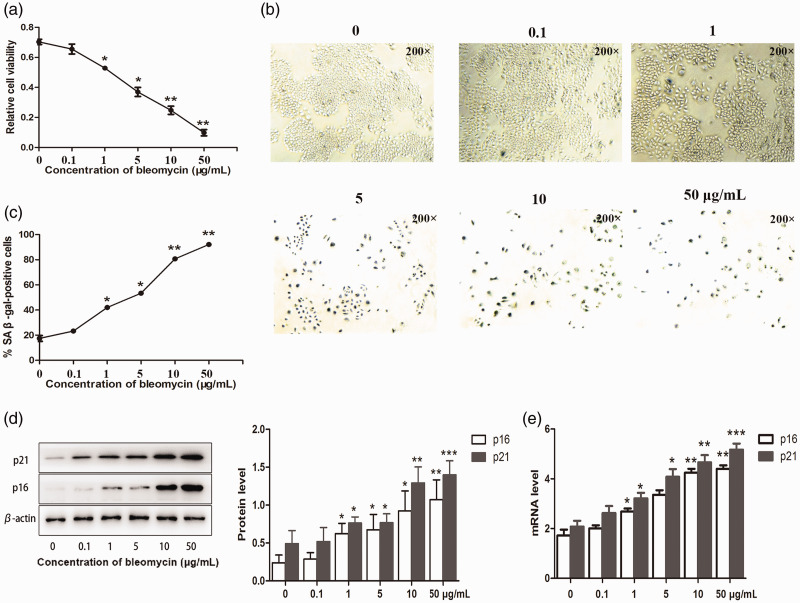
Cellular senescence is characterized by stable cell cycle arrest, enlarged and flattened cellular morphology, increased expression of p21 and p16, and enhanced SA-β-gal activity.4,5 Growth arrest is a necessary step in cell senescence. We therefore initially determined if bleomycin affected the proliferation of lung cancer cells. Bleomycin (0, 0.1, 1, 5, 10, and 50 µg/mL) significantly decreased the proliferation of A549 cells, as determined by MTT assay (P < 0.05 and < 0.01) (Figure 1a). A549 cells treated with bleomycin exhibited aging characteristics, including significantly intensified SA-β-gal staining (P < 0.05 and < 0.01), flattened cell morphology, and enlarged cell size (Figure 1b and c). These effects were noted within 5 days. Increasing the concentration of bleomycin significantly increased the protein and mRNA expression levels of p16 (P < 0.05 and < 0.01) and p21 (P < 0.05, < 0.01, and < 0.005) (Figure 1d and e).
Figure 1.
Bleomycin induced premature senescence of lung cancer cells. A549 cells were cultured with 0, 0.1, 1, 5, 10 and 50 µg/mL bleomycin, respectively, for 5 days. (a) Proliferation of A549 cells was assessed by MTT assay. (b) Bleomycin increased endogenous SA-β-gal activity. (d) p16 and p21 protein expression levels were determined by western blot analysis, and (e) gene expression levels were determined by polymerase chain reaction. Values given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005. (n = 3).
PTEN expression was reduced in lung cancer cells with bleomycin-induced premature senescence
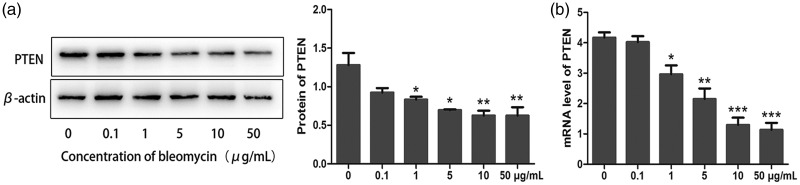
PTEN is closely associated with cellular senescence and has been reported to induce replicative senescence in epithelial prostate cancer cells.19 We determined if PTEN was involved in bleomycin-induced cellular senescence in lung cancer cells. PTEN protein and mRNA levels were significantly downregulated in line with increasing bleomycin concentration, as demonstrated by western blot analysis (P < 0.05 and < 0.01) and PCR (P < 0.05, < 0.01, and < 0.005) (Figure 2a and b). These results indicated that PTEN might be involved in the induction of premature senescence in lung cancer cells.
Figure 2.
PTEN levels were reduced in lung cancer cells with bleomycin-induced premature senescence. A549 cells were cultured with 0, 0.1, 1, 5, 10, and 50 µg/mL bleomycin, respectively, for 5 days. (a) PTEN protein levels were detected by western blot analysis. (b) PTEN mRNA levels were detected by polymerase chain reaction (original magnification ×200). Values given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005. (n = 3).
PTEN played a key role in mediating bleomycin-induced premature senescence in lung cancer cells
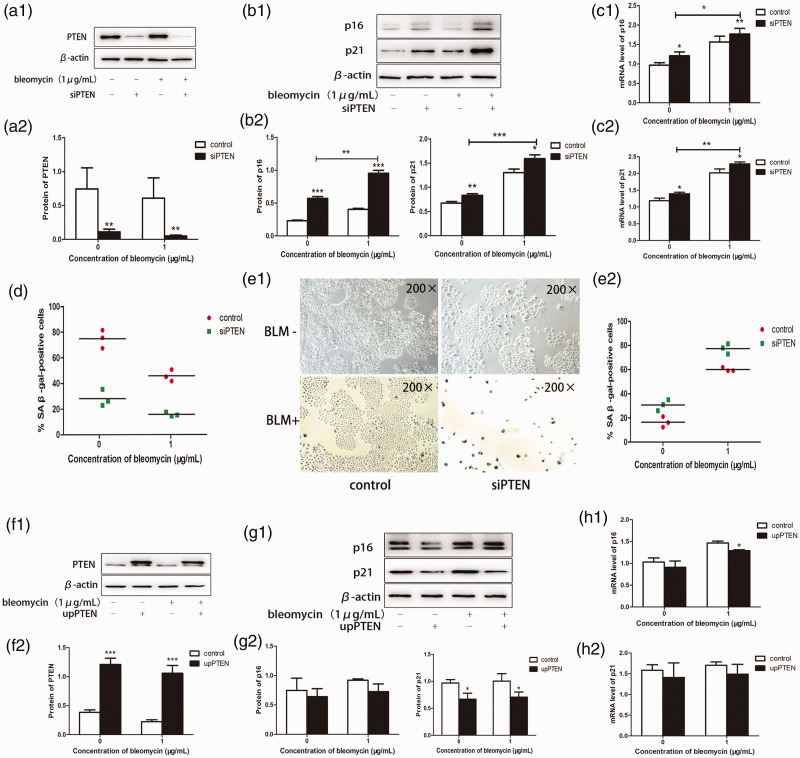
We transfected A549 cells with lentiviral vectors to produce PTEN-silenced and overexpressing cell lines, respectively, and characterized the function of bleomycin. According to the previous experiment (Figure 1), 1 µg/mL bleomycin induced cellular senescence with minimal cytotoxicity to cells, and we therefore used this concentration in subsequent experiments. PTEN protein levels were significantly reduced by transfection with silence of PTEN (siPTEN) as demonstrated by western blotting (P < 0.01) (Figure 3a). We also showed that siPTEN accelerated bleomycin-induced premature senescence, by significantly increasing p16 and p21 protein and mRNA expression levels, as confirmed by MTT assay and SA-β-gal staining (P < 0.01 and < 0.005) (Figure 3b–e). The opposite results were obtained following PTEN overexpression. There was a significant decrease in p16 mRNA (P <0.05), but the decrease in p16 protein level was not significant, while p21 protein was significantly decreased (P < 0.05), but no significant decrease in p21 mRNA (Figure 3f–h). These results confirmed the critical role of PTEN in bleomycin-induced cell senescence.
Figure 3.
PTEN played a key role in mediating bleomycin-induced premature senescence in lung cancer cells. Following transfection with PTEN siRNA or upregulation of PTEN, A549 cells were treated with bleomycin (1 µg/mL) for 5 days. (a) siRNA-mediated PTEN knockdown was confirmed by western blot analysis. (b) p16 and p21 protein expression levels were detected by western blot analysis, and (c) mRNA expression levels were detected by polymerase chain reaction (PCR). (d) siPTEN accelerated the inhibition of A549 cell proliferation by bleomycin. (e) Cells treated with bleomycin for 5 days were examined by SA-β-gal activity assay and SA-β-gal staining (original magnification ×200). (f) PTEN protein overexpression was confirmed by western blot analysis. (g, h) p16 and p21 protein and mRNA levels were measured by western blotting and reverse transcription-PCR, respectively. Values given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005. (n = 3). siPTEN, silence of PTEN; upPTEN, upregulation of PTEN; BLM, bleomycin.
Bleomycin-induced senescence was dependent on the PI3K/Akt/mTOR signaling pathway
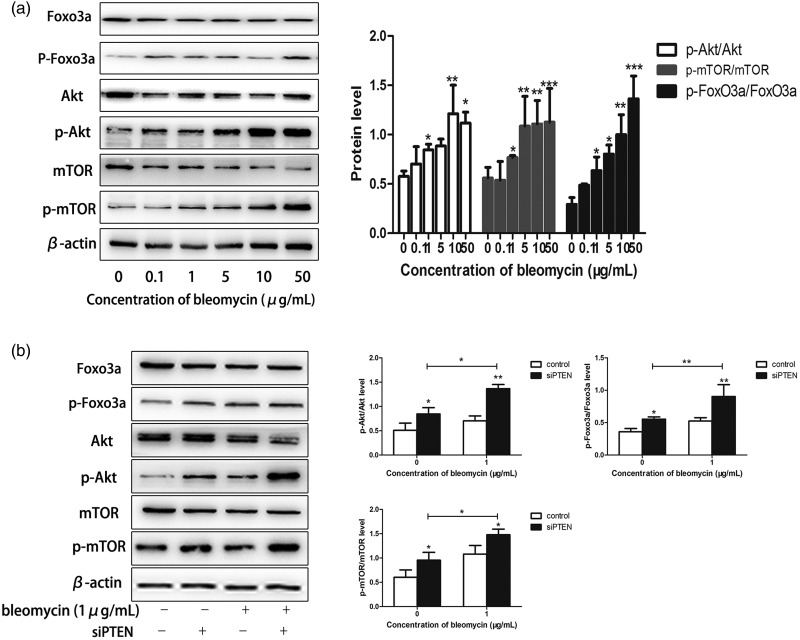
We further investigated the intracellular signaling pathways regulated by PTEN that were involved in bleomycin-induced senescence. Given that the PI3K/Akt/mTOR signaling pathway was reported to be inhibited by PTEN,21 we explored the involvement of this signaling pathway in lung cancer cell senescence. The PI3K/Akt/mTOR signaling pathway was activated in bleomycin-induced cells, as determined by western blotting (Figure 4a). Silencing of PTEN by transfection with siPTEN activated the PI3K/Akt/mTOR signaling pathway in lung cancer cells, as indicated by significantly increased phosphorylation of Akt, FoxO3a, and mTOR (P < 0.05, < 0.01, and < 0.005) (Figure 4b). These results suggested that the PI3K/Akt/mTOR pathway was activated by PTEN silencing and played an important role in modulating bleomycin-induced premature senescence.
Figure 4.
Bleomycin-induced senescence was dependent on the PI3K/Akt/mTOR signaling pathway. (a) The PI3K/Akt/mTOR signaling pathway was activated in bleomycin-treated cells, as determined by western blotting. (b) Akt and mTOR phosphorylation were significantly increased in lung cancer cells following transfection with PTEN siRNA. Values given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005 (n = 3).
PTEN accelerated premature senescence by inhibiting autophagy in bleomycin-treated lung cancer cells
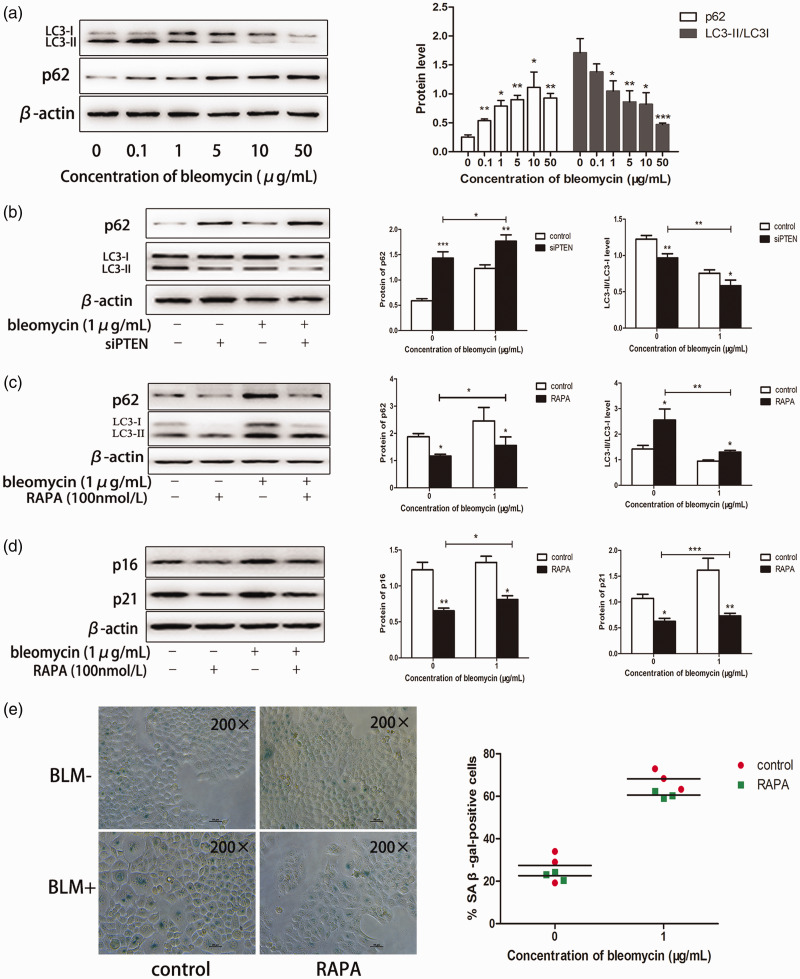
The PI3K/Akt/mTOR signaling pathway plays a key role in autophagy,22 as a mechanism by which cellular material is passed to lysosomes for degradation.23 Autophagy can regulate the process of cell aging. Enhanced autophagy in cells can clear accumulated senescent proteins from the body, and is thus an important mechanism for maintaining young cells.24 We hypothesized that autophagy might also play a key role in bleomycin-induced premature senescence. Treatment with bleomycin significantly decreased LC3-II levels and decreased the LC3-II/LC3-I ratio (P < 0.05, < 0.01, and < 0.005) (Figure 5a). Consistently, p62 expression increased significantly as the concentration of bleomycin increased (P < 0.05 and < 0.01) (Figure 5a). Similar results for LC3-II/LC3-I and p62 expression levels were noted following transfection with siPTEN (Figure 5b). mTOR is a serine/threonine kinase that is considered as a primary regulator of autophagy,25 and the mTOR inhibitor rapamycin can be used to induce autophagy. Exposure of A549 cells to rapamycin significantly increased the level of autophagy (Figure 5c) and significantly decreased the expression levels of senescence markers (P < 0.05) (Figure 5d, e).
Figure 5.
PTEN accelerated premature senescence by inhibiting autophagy in bleomycin-treated lung cancer cells. (a) Cells were treated with bleomycin, proteins were extracted, and LC3 and p62 expression were detected by western blotting. (b) Protein levels of LC3 and p62 were measured by western blotting following silencing of PTEN. (c) A549 cells were treated with 100 nmol/L rapamycin for 24 hours, and the medium was then changed to fresh DMEM with or without bleomycin (1 µg/mL) for 5 days. (d) p16 and p21 protein levels were measured by western blotting. (e) A549 cells were subjected to SA-β-gal activity assay and SA-β-gal staining (original magnification ×200). Values given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005. (n = 3). RAPA, rapamycin.
Discussion
Cellular senescence notably inhibits tumor development. Senescence plays a key role in protecting the body against tumorigenesis by suppressing cell proliferation, and is considered a hallmark of effective cancer therapy.26 The identification of molecules responsible for controlling premature senescence may thus aid the development of new cancer treatments. The tumor suppressor PTEN exerts diverse functions in several biological processes. Loss or mutation of PTEN can trigger stable growth arrest and cellular senescence, referred to as PTEN loss-induced cellular senescence.27 The current results confirmed that bleomycin was an effective chemotherapeutic drug that acted by inducing premature senescence in lung cancer cells. Moreover, we demonstrated that downregulation of PTEN could accelerate premature senescence in bleomycin-induced tumor cells by activating the PI3K/Akt/mTOR pathway. These results identified PTEN as a possible new molecular target for the chemotherapeutic drug bleomycin, and provide useful information for exploring viable cancer treatments based on inducing premature senescence, as well as the use of PTEN as a new reactive molecular target for cancer treatment.
Autophagy is a crucial self-renewal process in the growth and development of mammalian cells. The autophagy signaling pathway partially overlaps with the regulation of aging, and autophagy levels are thus closely associated with aging. Autophagy levels are reduced during the aging process, and low autophagy levels can accelerate aging, while high levels can slow the aging process.28,29 The molecular targets of inhibiting cell autophagy are activated by the PI3K/Akt/mTOR pathway.22 Our experiments indicated that autophagy levels were decreased following bleomycin-induced cellular senescence in lung cancer cells. Silencing of PTEN could activate the PI3K/Akt/mTOR pathway, decrease autophagy levels, and accelerate cellular senescence. Moreover, we inhibited mTOR expression to increase the levels of autophagy, resulting in delayed cellular senescence. These results suggest that autophagy played a key role in cellular senescence induced by bleomycin.
In the present study, we demonstrated that overexpression of PTEN reduced senescence; however, the effect was not significant. We speculated that this was because the high expression levels of PTEN in A549 cells meant that transfection with PTEN-overexpression vectors led to a non-significant change in expression levels. Furthermore, our data were derived from in vitro studies, and in vivo experiments are required to verify our conclusions.
In conclusion, the present study demonstrated that downregulation of PTEN played a key role in mediating bleomycin-induced premature senescence in lung cancer cells. We further demonstrated that the PI3K/Akt/mTOR signaling pathway was a major pathway involved in the inhibition of PTEN. Moreover, silencing of PTEN alone could cause premature senescence in lung cancer cells by suppressing autophagy via the PI3K/Akt/mTOR signaling pathway. These data suggest that PTEN might control cancer progression and might thus be a potential new molecular target for chemotherapeutic drugs.
Acknowledgements
We would like to express our sincere gratitude to Hourong Cai for his constructive comments.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by the Natural Science Foundation of Wuxi [grant number SBK2018022714], the project of Nanjing Medical University [grant number NMUB2018357], and Nanjing Medical University [project numbers NMUB2018357 and NMUB2018348].
ORCID iD
References
- 1.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614–636. [DOI] [PubMed] [Google Scholar]
- 2.Newgard CB, Sharpless NE. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest 2013; 123: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92: 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salama R, Sadaie M, Hoare M, et al. Cellular senescence and its effector programs. Genes Dev 2014; 28: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyapalan JC, Ferreira M, Sedivy JM, et al. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 2007; 128: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res 2014; 12: 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser 2015; 83: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynford-Thomas D. Proliferative lifespan checkpoints: cell-type specificity and influence on tumour biology. Eur J Cancer 1997; 33: 716–726. [DOI] [PubMed] [Google Scholar]
- 9.Cerella C, Grandjenette C, Dicato M, et al. Roles of apoptosis and cellular senescence in cancer and aging. Curr Drug Targets 2016; 17: 405–415. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109: 335–346. [DOI] [PubMed] [Google Scholar]
- 11.Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb 2010; 40: 213–215. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer 2005; 5: 102–112. [DOI] [PubMed] [Google Scholar]
- 13.Kasper M, Barth K. Bleomycin and its role in inducing apoptosis and senescence in lung cells - modulating effects of caveolin-1. Curr Cancer Drug Targets 2009; 9: 341–353. [DOI] [PubMed] [Google Scholar]
- 14.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell 2008; 133: 403–414. [DOI] [PubMed] [Google Scholar]
- 15.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 2004; 22: 2954–2963. [DOI] [PubMed] [Google Scholar]
- 16.Stiles B, Groszer M, Wang S, et al. PTENless means more. Dev Biol 2004; 273: 175–184. [DOI] [PubMed] [Google Scholar]
- 17.Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 2012; 15: 382–394. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005; 436: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad I, Patel R, Singh LB, et al. HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc Natl Acad Sci U S A 2011; 108: 16392–16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 2003; 22: 436–443. [DOI] [PubMed] [Google Scholar]
- 21.Alfieri R, Giovannetti E, Bonelli M, et al. New treatment opportunities in phosphatase and tensin homolog (PTEN)-deficient tumors: focus on PTEN/focal adhesion kinase pathway. Front Oncol 2017; 7: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Wang Z, Yakisich JS. Natural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agents. Anticancer Agents Med Chem 2013; 13: 1048–1056. [DOI] [PubMed] [Google Scholar]
- 23.Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol 2016; 12: 517–531. [DOI] [PubMed] [Google Scholar]
- 24.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011; 146: 682–695. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 2015; 125: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park C, Lee I, Kang WK. Influence of small interfering RNA corresponding to ets homologous factor on senescence-associated modulation of prostate carcinogenesis. Mol Cancer Ther 2006; 5: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 27.Alimonti A, Carracedo A, Clohessy JG, et al. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 2010; 42: 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol 2010; 12: 842–846. [DOI] [PubMed] [Google Scholar]
- 29.Pyo JO, Yoo SM, Ahn HH, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 2013; 4: 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.