Abstract
Purpose
To report the optical coherence tomography angiography (OCT-A) findings in a patient with macular toxoplasma retinochoroiditis (TRC).
Observations
A 14-year old female presented with a 2-week history of decreased vision of her right eye. Upon presentation, on the right eye, visual acuity was 20/200 and the fundus revealed 2+ vitritis and an active parafoveal area of retinitis superotemporally. Toxoplasma serology revealed positive IgG and negative IgM. Initial treatment consisted of intravitreal clindamycin (1.0mg in 0.1 mL) with dexamethasone (1.0 mg in 0.1 mL) along with oral trimethoprim/sulfamethoxazole, azithromycin and prednisone; of which the trimethoprim/sulfamethoxazole was discontinued 4 days into therapy due to a pruritic rash. Six-weeks after the presentation, the oral prednisone had been tapered off, and all therapy discontinued. The patient had complete resolution of TRC and recovered 20/20 vision. OCT-A analysis of the right macula, performed after completion of treatment, revealed preservation of foveal perfusion along a parafoveal area of ischemia, superotemporally, at the superficial and deep retinal vascular complexes, including the choriocapillaris.
Conclusion and Importance
In macular TRC, OCT-A may help to assess therapeutic outcomes from a vascular perspective. To our knowledge, our case represents the first description in the medical literature of OCT-A findings in macular TRC.
Keywords: Optical coherence tomography angiography, Macula, Toxoplasma retinochoroiditis, Intravitreal clindamycin
1. Introduction
Toxoplasmosis is the most common retinal infection in the United States.1 The prevalence of Toxoplasma gondii infection in the United States population since 2009 is 14%; however, only 2% have ocular manifestations.1 The mean age for the presentation of symptomatic ocular infection is 29.5 years.2 The diagnosis of ocular toxoplasmosis is most commonly established on a clinical basis by the presence of distinctive clinical findings; most commonly a focal area of active retinochoroiditis at the edge of a pigmented chorioretinal scar.2 Complications which may present at any time during the clinical course include retinal detachment, cystoid macular edema, retinal vasculitis, and secondary glaucoma; however, blindness most commonly occurs by direct foveal involvement of the retinitis with subsequent scarring or by infarction of the foveal capillary networks.3, 4, 5, 6 This is of particular importance, as macular lesions in ocular toxoplasmosis have a disproportionately higher frequency than if the disease was randomly distributed among the retina.4,7
There is a myriad of systemic treatment alternatives for toxoplasma retinochoroiditis (TRC), including pyrimethamine with sulfadiazine, azithromycin, clindamycin, and trimethoprim/sulfamethoxazole and atovaquone, alone or in conjunction with systemic corticosteroids; all with various degrees of effectiveness and side effect profiles.8, 9, 10, 11, 12, 13 Intravitreal therapy with a combination of clindamycin and dexamethasone was shown in one randomized trial to be as effective as standard systemic therapy with pyrimethamine and sulfadiazine.12 The intravitreal clindamycin and dexamethasone combination approach delivers a high concentration of drug to the ocular tissues, reduces the likelihood of systemic complications, and improves proper treatment compliance as it is physician-administered.12 Most recently, trimethoprim/sulfamethoxazole with dexamethasone, has also been successfully used, at least in several patients, for the treatment of TRC.14
Optical coherence tomography angiography (OCT-A) is a novel imaging modality in which the variation of the optical coherence tomography (OCT) signal caused by moving particles, is used as the contrast mechanism for imaging blood flow.15, 16, 17 To differentiate moving particles from static tissue, repeated scans are done in the same location.15,18 OCT-A adds an additional component to OCT anatomical analysis as it renders multidimensional information with regards to anatomical status the macular capillary network providing further anatomical and physiological insight on diverse disease states.15, 16, 17, 18 This may be of particular utility in conditions such as macular TRC which may lead to vaso-occlusive damage.5
We report a case of macular TRC young adolescent female treated with intravitreal clindamycin and dexamethasone, and systemic azithromycin, along with the respective OCT-A analysis. To our knowledge, this is the first case of documenting OCT-A characteristics in a patient with macular TRC.
1.1. Case report
14- year old Hispanic female presented with a 2-week history of decreased visual acuity and a sensation of pressure on her right eye (OD). On examination, visual acuity was 20/200 OD and 20/20 in the left eye (OS), intraocular pressure IOP was of 48 mm Hg OD and 19 OS. The slit-lamp exam was unremarkable bilaterally. Fundus examination revealed 2+ vitritis and an active parafoveal area of retinitis superotemporally, with associated vasculitis of the arterioles and venules, on the right eye (Fig. 1A). Spectral-domain optical coherence tomography (SD-OCT) upon presentation revealed vitreous cells, increased reflectivity from the inner retinal layer, retinal thickening, and choroidal shadowing. (Fig. 2A). Toxoplasma serology revealed positive IgG (106.0 IU/mL, < 10 reference) and negative IgM. Syphilis serology by rapid plasma reagin method and Treponema pallidum antibodies were both negative. Westergren sedimentation rate was normal, 6mm/hr.
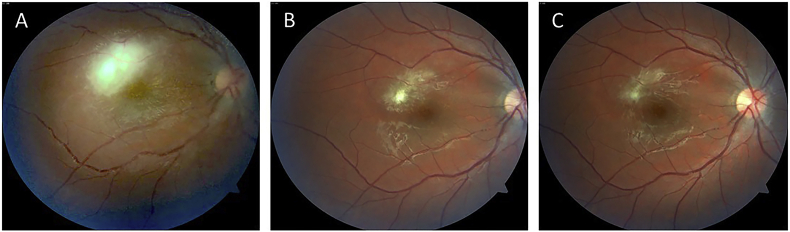
Fig. 1.
Color fundus photographs of the right posterior pole. A. Upon presentation, revealing an active area of parafoveal retinochoroiditis superotemporally along with vasculitis of the adjacent arterioles and venules. B. Four weeks after presentation, revealing a significant reduction in the area of retinochoroiditis involvement, yellow segmental intraarterial plaques (Kyrieleis' vasculitis), as well as mild residual perivenular sheathing. C. Six-weeks after presentation, upon completion of treatment, revealing a small residual perifoveal chorioretinal scar and total resolution of the retinal vasculitis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
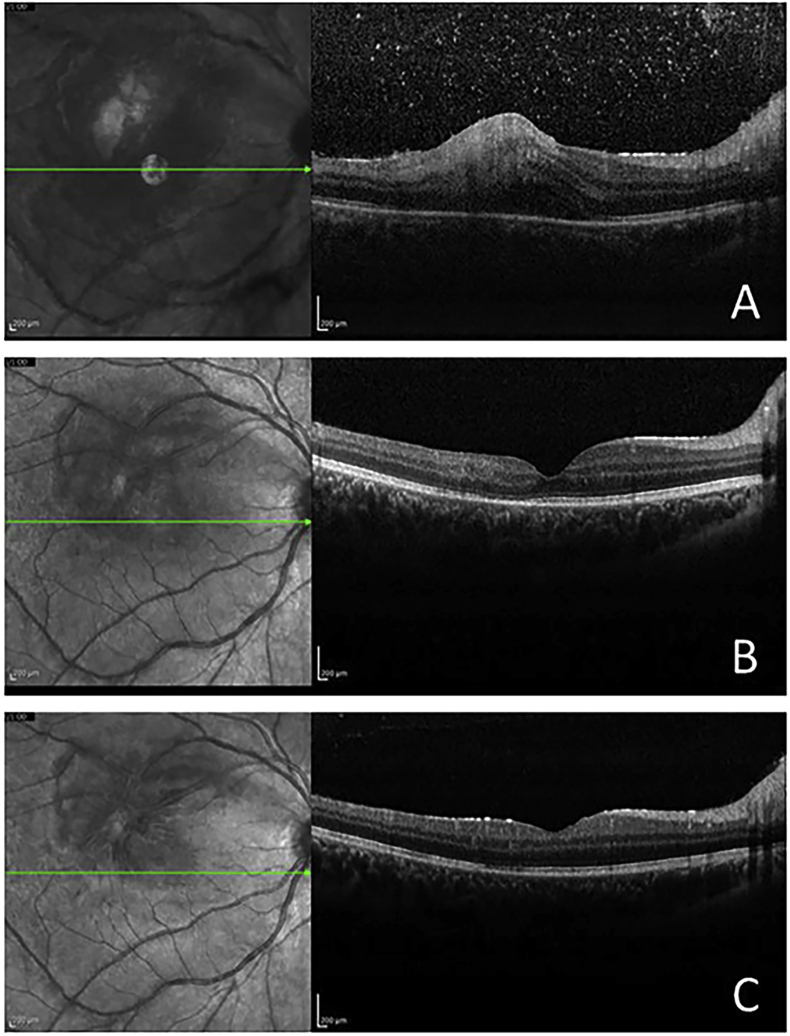
Fig. 2.
SD-OCT of the right eye. A. Upon presentation, revealing vitreous cells, increased reflectivity from the inner retinal layer, retinal thickening, and choroidal shadowing. B Four weeks after presentation, showing resolution of vitreous cells, along with substantial improvement of nerve fiber layer and retinal thickness. C. Upon completion of a 6-week course of therapy, there is normalization of retinal thickness and total resolution of the edema.
Treatment with intravitreal clindamycin (1.0mg in 0.1 mL) and dexamethasone (1.0 mg in 0.1 mL) was administered upon presentation. Concurrently, the patient was prescribed sulfamethoxazole/trimethoprim 800mg/160mg four times per day and oral azithromycin 500mg daily. Oral prednisone (1mg/kg) was started 48 hours after commencing the oral antimicrobial agents. Her ocular hypertension was treated with brimonidine/timolol 0.2%/0.5% ophthalmic solution. Four days after presentation, sulfamethoxazole/trimethoprim was discontinued due to systemic pruritic rash; however, treatment with azithromycin was continued for six additional weeks. Oral prednisone was tapered over a 6 weeks period.
At the four-week follow-up visit, her visual acuity had improved to 20/25 OD, the IOP normalized, and resolution of the vitritis was noted. The right fundus exam revealed significant improvement of the retinitis along with mild Kyrieleis’ vasculitis and perivenular sheathing (Fig. 1B), while the SD-OCT revealed substantial improvement of the nerve fiber layer and intraretinal thickening (Fig. 2B). Upon completion of a six weeks course of therapy, the patient recovered her baseline visual acuity of 20/20 on both eyes, the right fundus exam revealed resolution of the retinal vasculitis, along with residual parafoveal chorioretinal scarring superotemporally (Fig. 1C). SD-OCT of the right macula showed normalization of macular thickness and total resolution of the edema (Fig. 2C).
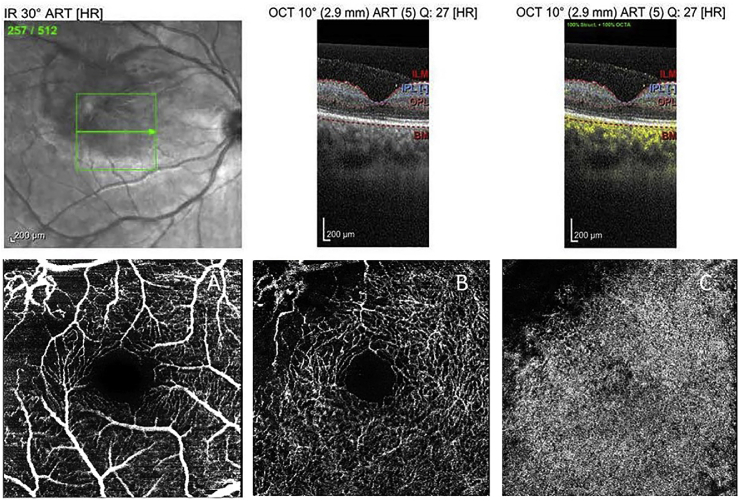
OCT-A analysis of the right macula, performed after completion of treatment, revealed a parafoveal area of absent perfusion, superotemporally, at the superficial and deep retinal vascular complexes, including the choriocapillaris. It did not reveal any foveal perfusion abnormalities (Fig. 3 A, B, and C).
Fig. 3.
OCT-A analysis of the right macula. Six-weeks after presentation and upon completion of therapy reveals an area of ischemia, superotemporally, encompassing: A the superficial vascular complex, B the deep retinal vascular complex, and C the choriocapillaris. Foveal perfusion appears preserved throughout all the analyzed layers.
2. Discussion
The retinal vascular endothelium has increased vulnerability to Toxoplasma gondii infection when compared with similar tissues elsewhere in the body.19 Although retinal toxoplasma infection most commonly manifests itself as focal necrotizing retinitis or retinochoroiditis, retinal vasculitis is a common feature, and vascular occlusions seem to play a significant role in the pathophysiology of this disease.2,5,6,20 Patients with macular TRC may be particularly vulnerable to clinically significant vascular damage from this condition.5,6 OCT-A may be used to non-invasively study macular perfusion at various depth levels of the capillary plexus.17,18 In our patient, OCT-A revealed a vascular perfusion defect that encompassed all retinal layers and the choriocapillaris (Fig. 3 A, B, and C), serving as further evidence of the role played by vascular occlusions in TRC. In particular, OCT-A in our patient revealed preservation of central macular perfusion, a feature that may very well help explain the good end visual outcome. One may hypothesize that the prompt and potent antimicrobial and anti-inflammatory effects of the intravitreal clindamycin and dexamethasone combination therapy, may help minimize the extent of end tissue damage from the necrotic process, and early stabilization of the vascular damage.
A careful selection of therapeutic agents and the route of administration is key when managing patients with macular toxoplasma retinochoroiditis (TRC). Although some studies have shown a smaller end lesion size with the use of pyrimethamine, sulfadiazine, and corticosteroids when compared with clindamycin or observation, other similar essays have failed to show a superior clinical outcome amongst any of the presently available means of systemic therapy.8, 9, 10,21,22 In 2017, a group from Indonesia reported results from a double-blind, randomized clinical trial in which quadruple therapy with trimethoprim/sulfamethoxazole, clindamycin and oral corticosteroids resulted in a more rapid resolution effect of TRC when compared with triple therapy with pyrimethamine, sulfadiazine and systemic corticosteroids.23
A therapeutic approach that rapidly delivers the highest possible level of medication to the affected tissue while minimizing systemic side effects such as intravitreal clindamycin and dexamethasone may be of particular value when treating patients with macular TRC.12 However, multiple intravitreal injections may be required during the full course of therapy.12,24, 25, 26 It is noteworthy, that a randomized clinical trial failed to show a statistically significant difference in final visual acuity or lesion size between pyrimethamine with sulfadiazine and oral corticosteroids versus intravitreal clindamycin with dexamethasone.12 However, the later approach is physician-administered, facilitating patient compliance in addition to minimizing systemic side effects.12,24,25 For the aforementioned reasons, our practice favors the use of intravitreal clindamycin plus dexamethasone for patients with TRC within 3000 μm of the fovea or 1500 μm of the optic disk.
Therefore, we emphasize a careful consideration to the risk and benefits of each therapeutic approach, while customizing therapy, taking into consideration multiple factors such as the anatomical location of the lesion, the prognosis for visual acuity improvement, the risk of macular involvement and the overall systemic health and therapeutic tolerance of each patient. For a young patient with a parafoveal lesion with associated vasculitis, we initially opted for a multitherapeutic approach with systemic pyrimethamine/sulfadiazine, azithromycin, corticosteroids along with intravitreal clindamycin with dexamethasone. The rationale for this combined approach is that it allows for the immediate intravitreal delivery of a therapeutic dose of medication to the affected tissue while minimizing the risks of recurrent intravitreal therapy. Although our patient developed a pruritic reaction to the pyrimethamine/sulfadiazine combination, she was able to comply with the rest of her therapy, which resulted in an excellent recovery of her foveal function.
3. Conclusion
Our case adds objective evidence to the notion that vascular occlusions may play a significant role in the pathophysiology of TCR. In cases of macular TRC, OCT-A may help to better assess the anatomical therapeutic outcomes, from a vascular perspective, as it simultaneously scans superficial and deep layers of the circulation.
3.1. Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support was used for this case report.
4. Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: AP, RL, AE, AO.
Acknowledgments
None.
Contributor Information
Alejandro L. Perez, Email: alejandro.perez3@upr.edu.
Rosa A. Lozada, Email: rosa.lozada21@gmail.com.
Andres Emanuelli, Email: andres.emanuelli@upr.edu.
Armando L. Oliver, Email: armando.oliver@upr.edu.
References
- 1.Jones J.L., Holland G.N. Short report: annual burden of ocular toxoplasmosis in the United States. Am J Trop Med Hyg. 2010 doi: 10.4269/ajtmh.2010.09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler N.J., Furtado J.M., Winthrop K.L., Smith J.R. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol. 2013 doi: 10.1111/j.1442-9071.2012.02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park Y.H., Nam H.W. Clinical features and treatment of ocular toxoplasmosis. Kor J Parasitol. 2013 doi: 10.3347/kjp.2013.51.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland G.N. Ocular toxoplasmosis: a global reassessment. Am J Ophthalmol. 2004 doi: 10.1016/j.ajo.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V., Takkar B., Chandra P., Kumar A. Macular infarction in a patient with Toxoplasma retinochoroditis. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-212596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggio F.B., Novelli FJ de, Rosa E.L., Nobrega M.J. Combined branch retinal vein and artery occlusion in toxoplasmosis. Arq Bras Oftalmol. 2016;79(3):189–191. doi: 10.5935/0004-2749.20160054. [DOI] [PubMed] [Google Scholar]
- 7.Friedmann C.T., Knox D.L. Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol. 1969 doi: 10.1001/archopht.1969.00990010483005. [DOI] [PubMed] [Google Scholar]
- 8.Rothova A., Meenken C., Buitenhuis H.J. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115(4):517–523. doi: 10.1016/s0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Lin X., Lu F. Current treatment of ocular toxoplasmosis in immunocompetent patients: a network meta-analysis. Acta Trop. 2018 doi: 10.1016/j.actatropica.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Lashay A., Mirshahi A., Parandin N. A prospective randomized trial of azithromycin versus trimethoprim/sulfamethoxazole in treatment of toxoplasmic retinochoroiditis. J Curr Ophthalmol. 2017;29(2):120–125. doi: 10.1016/j.joco.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothova A., Bosch-Driessen L.E.H., Van Loon N.H., Treffers W.F. Azithromycin for ocular toxoplasmosis. Br J Ophthalmol. 1998 doi: 10.1136/bjo.82.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soheilian M., Ramezani A., Azimzadeh A. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.J., Scott I.U., Brown G.C. Interventions for toxoplasma retinochoroiditis. Ophthalmology. 2013;120(2):371–378. doi: 10.1016/j.ophtha.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury H., Jindal A., Pathengay A., Bawdekar A., Albini T., Flynn H.W. The role of intravitreal trimethoprim/sulfamethoxazole in the treatment of toxoplasma retinochoroiditis. Ophthal. Surgery Lasers Img. Retin. 2015;46(1):137–140. doi: 10.3928/23258160-20150101-27. [DOI] [PubMed] [Google Scholar]
- 15.Wang R.K., Jacques S.L., Ma Z., Hurst S., Hanson S.R., Gruber A. Three dimensional optical angiography. Optic Express. 2007 doi: 10.1364/OE.15.004083. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A., Zhang Q., Chen C.-L., Wang R.K. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Optic. 2015 doi: 10.1117/1.jbo.20.10.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.-L., Wang R.K. Optical coherence tomography based angiography [Invited] Biomed Optic Express. 2017 doi: 10.1364/boe.8.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashani A.H., Chen C.L., Gahm J.K. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017 doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith J.R., Franc D.T., Carter N.S., Zamora D., Planck S.R., Rosenbaum J.T. Susceptibility of retinal vascular endothelium to infection witn Toxoplasma gondii tachyzoites. Investig Ophthalmol Vis Sci. 2004 doi: 10.1167/iovs.03-1105. [DOI] [PubMed] [Google Scholar]
- 20.Aleixo Alq do C., Curi A.L.L., Benchimol E.I., Amendoeira M.R.R. Toxoplasmic retinochoroiditis: clinical characteristics and visual outcome in a prospective study. PLoS Neglected Trop Dis. 2016 doi: 10.1371/journal.pntd.0004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell M., Carvounis P.E. Current treatment of toxoplasma retinochoroiditis: an evidence-based review. J Ophthalmol. 2014;2014:1–7. doi: 10.1155/2014/273506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soheilian M., Sadoughi M.-M., Ghajarnia M. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005;112(11):1876–1882. doi: 10.1016/j.ophtha.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Kartasasmita A., Muntur W., Enus S., Iskandar E. Rapid resolution of toxoplasma chorioretinitis treatment using quadruple therapy. Clin Ophthalmol. 2017;11:2133–2137. doi: 10.2147/OPTH.S148933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishore K., Conway M.D., Peyman G.A. Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Laser. 2001;32(3):183–192. [PubMed] [Google Scholar]
- 25.Martinez CE, Zhang D, Conway MD, Peyman GA. Successful management of ocular toxoplasmosis during pregnancy using combined intraocular clindamycin and dexamethasone with systemic sulfadiazine. Int Ophthalmol. 22(2):85-88. http://www.ncbi.nlm.nih.gov/pubmed/10472767. Accessed July 12, 2019. [DOI] [PubMed]
- 26.Bor’i A., Mahrous A., Al-Aswad M.A. Intravitreal clindamycin and dexamethasone combined with systemic oral antitoxoplasma therapy versus intravitreal therapy alone in the management of toxoplasma retinochoroiditis: a retrospective study. J Ophthalmol. 2018;2018 doi: 10.1155/2018/4160837. [DOI] [PMC free article] [PubMed] [Google Scholar]