Abstract
Food processing generates side streams that are not fully utilized and typically treated as waste materials. One of such food by-product, brewers’ spent grains (BSG) are disposed in huge quantities from the beer industry annually. Submerged fermentation of BSG using Bacillus subtilis WX-17, without supplementary components, is herein employed. The fermentation products were extracted in the liquid phase, resulting in a potential novel nutritional beverage containing Bacillus subtilis WX-17. Bacillus subtilis WX-17, was still viable after a period of 6 weeks with a final cell count of 9.86 log CFU/mL. Gas chromatography-mass spectrophotometry (GC-MS) was employed for identification of the metabolites produced from the growth of Bacillus subtilis WX-17. Seven essential amino acids and citric acid cycle (TCA) intermediates were found to have increased significantly (p < 0.05) whereas all carbohydrates decreased significantly (p < 0.05) in the beverage after submerged fermentation. Additionally, antioxidant activity quantified using DPPH radical scavenging activity, increased by 2.08-fold while total phenolic content increased from 125.7 ± 0.74 μg/mL to 446.74 ± 1.26 μg/mL. The results proved the potential of employing submerged fermentation of BSG using Bacillus subtilis WX-17 to produce a novel and highly nutritious beverage.
Keywords: Food science, Submerged fermentation, Brewers' spent grain, Nutritional beverage, Antioxidant activity
Food science, Submerged fermentation, Brewers spent grain, Nutritional beverage, Antioxidant activity.
1. Introduction
Brewers’ spent grains (BSG) are generated as waste side streams during beer production process, where global yield is estimated at up to 38.6 × 106 tonnes per year [1]. Currently, they are commonly used as either animal feed (e.g. for cattle) [2, 3] or disposed in landfills [4]. In parallel, BSG have been reported to contain proteins, fibres and phenolic compounds in abundant amounts [1, 5]. It is therefore worth exploring the development of high-value BSG-based products by harvesting their remaining nutrients, especially from the perspective of food security, economics as well as environment sustainability. Fermentation is a promising approach to increase nutrient content, thereby achieving a high-value BSG-based products. At the same time, this technique would enhance the utilization of BSG.
Microbial fermentation provides a feasible way to re-utilize or add value to bio-waste/food-products. Bacillus subtilis (B. subtilis) is one of the most noticeable bacterial workhorse for fermentation, since it can produce many health benefits and anti-microbial compounds [6]. Specifically in food processing, B. subtilis has been used for the production of traditional soya-based natto for centuries [7]. Although fermentation using BSG has been proven to be viable for the cultivation of several microorganisms [8, 9], studies are rare on submerged fermenting of B. subtilis without additional supplementation. It is well established that B. subtilis can secrete abundant amounts of extracellular enzymes, including amylases and cellulases [10]. As such, B. subtilis is able to release the nutrients of BSG by breaking down the material, which is rich in protein and cellulose, in turn releasing or producing antioxidants such as phenols. Considering that no submerged fermentation of B. subtilis with BSG and B. subtilis drink have been reported till date, development of a nutritional B. subtilis beverage from BSG will be intriguing and worth exploring. According to [11], B. subtilis is a common species present in health beneficial products that is widely used as supplements and improving human health.
This work studies the possibility of utilizing BSG as a substrate in submerged fermentation to enable the growth of Bacillus subtilis WX-17 (B. subtilis WX-17) without additional supplements yet producing nutritional components in the liquid. The microorganism involved is B. subtilis WX-17, which was isolated previously from natto. Antioxidant assays and metabolomics using gas chromatography-mass spectrophotometry (GC-MS), coupled with testing of B. subtilis WX-17 viability across time would be employed to evaluate the fermented liquid product as a nutritional beverage.
2. Methods and materials
2.1. Submerged fermentation of B. subtilis WX-17 with BSG
B. subtilis WX-17 was previously isolated from natto according to [12] and subsequently cultured in nutrient broth for a day at 37 °C. BSG were provided by Asia Pacific Breweries (Singapore) Pte. Ltd. and stored at −80 °C. With minor modifications from [13], BSG were grinded into 5 mm mesh size using ceramic mortar and pestle. Ten grams of autoclaved, grinded BSG were inoculated with B. subtilis WX-17 (106 CFU/g) and 50 mL sterile water in an Erlenmeyer flask. Submerged fermentation was carried out for 72 h at 37 °C, 200 rpm. Samplings of both unfermented and fermented BSG were carried out at 0 h and 72 h. The samples were filtered using vacuum filtration with filter paper of 40 μm particle retention size. The supernatant collected was stored in -20 °C until further analysis.
2.2. Metabolomics analysis
Metabolomics analysis was conducted according to the method in [14] with some minor modifications. 100 μL of Methoxamine (MOX) hydrochloride in pyridine (20 mg/mL) was added for derivatizating1 mL of freeze dried samples and incubated for 1 h at 37 °C. Next, 200 μL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) with 1% trimethylchlorosilane (TMCS) silylation reagent was added to the samples and incubated at 70 °C for 30 min. 100 μL was drawn from each sample and measured thrice. The column specifications and GC-MS method were used according to [14].
2.3. Phenolic content analysis
According to a protocol in [15], total phenolic content was analysed and expressed in terms of gallic acids equivalent (GAE). 1 mL of sample was added to 5 mL of purified water and 0.5 mL of Folin-Ciocalteu reagent before being vortexed. Samples were left to sit for 5 min. 1.5 mL of 20% sodium carbonate was added to each sample and tubes were filled up to 10 mL using purified water. The samples were incubated at 20 °C in dark condition for 2 h. Subsequently, the optical density was measured using Thermo Fisher NanoDrop 2000c spectrophotometer at wavelength of 750 nm. Deionized water was used as blank.
2.4. Antioxidant activity analysis
1,1,-diphenyl-2-picryl-hydrazil (DPPH) free radical-scavenging capacity was investigated according to [16] with some modifications. 600 μL of sample was added to 600 μL of 0.1 mM DPPH solution prepared in ethanol. The samples in tubes were afterwards incubated for 20 min in the dark condition at 20 °C. Samples were measured at a wavelength of 515 nm using spectrophotometer. The antioxidant activity was obtained from the conversion of absorbance readings and quantification in terms of weight of Trolox.
2.5. Evaluation of B. subtilis WX-17 viability
Ten times dilution was carried out by adding liquid sample to sterile water in an Eppendorf tube. The dilution process was performed 11 times consecutively. 100 μL from each tube was plated onto nutrient agar petri dishes and incubated for 24 h at 37 °C. Thereafter, the cell counts in colonies forming units (CFU) were recorded. This is repeated weekly for a duration of 6 weeks.
2.6. Statistical analysis
Samples were statistically analyzed by partial least square-discriminant analysis (PLS-DA) and heatmap using MetaboAnalyst 4.0 [17]. Heatmap was generated with Euclidean distance calculation together with ward clustering algorithm. Statistical differences between samples were deduced based on Student's t-test with p < 0.05 as the benchmark for statistical significance.
3. Results & discussion
3.1. Evaluation of B. subtilis WX-17 viability
The changes in B. subtilis WX-17 cell count in colonies forming units/mL (CFU/mL) were recorded over 6 weeks at 4 °C (Figure 1). The count decreased slightly across 6 weeks without much variations, with an initial cell count of 10.48 log CFU/mL in week 0 to a final cell count of 9.86 log CFU/mL in week 6 (Figure 1). The relatively high survival rate of B. subtilis WX-17 after 6 weeks could be due to the formation of endospores by Bacillus species, which have been found to be metabolically dormant life forms and extremely resistant to various external conditions such as gamma radiation, UV as well as dessication [18, 19]. The viability cell count amount of B. subtilis WX-17 obtained is relatively higher or similar to other common beneficial microorganisms in beverages such as Bifidobacterium adolescentis and Lactobacillus casei, which are reported to be around 9 log CFU/mL and 8.72 log CFU/mL respectively [20]. B. subtilis was evaluated to be potentially beneficial for humans and biotherapeutic [21, 22]. Hence, it can be inferred that the viability of B. subtilis WX-17 in the fermented beverage after 6 weeks would provide beneficial properties. In addition, the fermented liquid with B. subtilis WX-17 can possibly serve as a feed supplement for domestic animals. According to [23], the presence of B. subtilis tends to have a positive effect on feed efficiency of Holstein calves.
Figure 1.
Changes in cell count of B. subitilis WX-17 grown in BSG media stored at 4 °C over 6 weeks.
3.2. Statistical analysis on detected metabolites
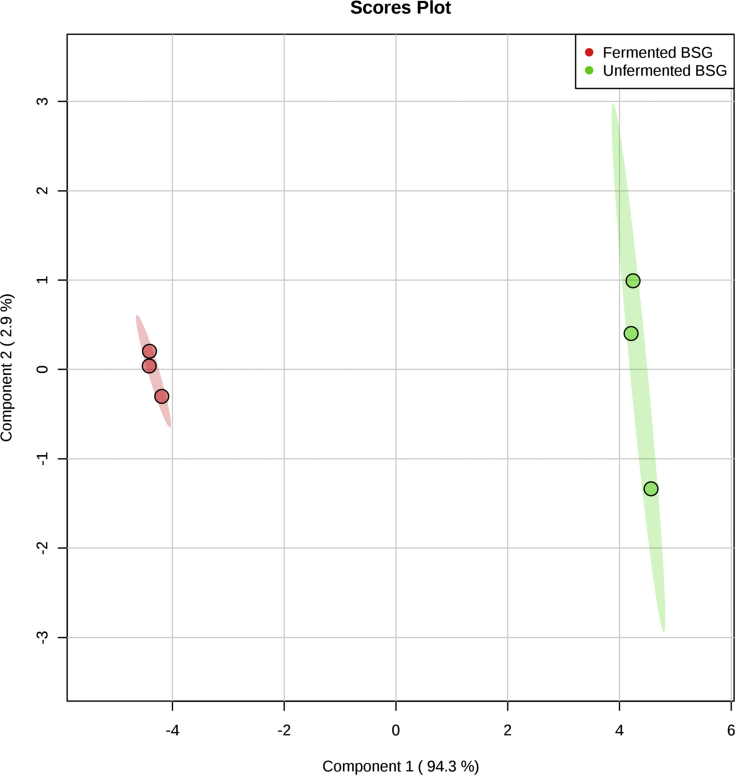
An untargeted metabolomics analysis was employed to gain insights into the submerged fermentation process. A total of 23 significantly different metabolites (p < 0.05) were observed. Using a statistical analysis, PLS-DA, a distinct separation between the metabolites in fermented and unfermented BSG was obtained (Figure 2). The variances of the principal components namely, PC1 and PC2 on the axes were 94.3% and 2.9% respectively. The clear difference between the samples is mainly due to the first principal component. The PLS-DA plot can be evaluated using R2 value and Q2 value, which represents the explained variance and the predictive capability of the model respectively [24]. With a R2 value and Q2 value of 0.99, it can be considered to be significantly substantial [25].
Figure 2.
PLS-DA plot for different metabolites detected for control (unfermented BSG) samples in green and fermented BSG samples in red as shown in the legend. Each cluster was presented with 95% confidence interval and explained variances are indicated in the brackets. Each dot represents metabolites in each replicate.
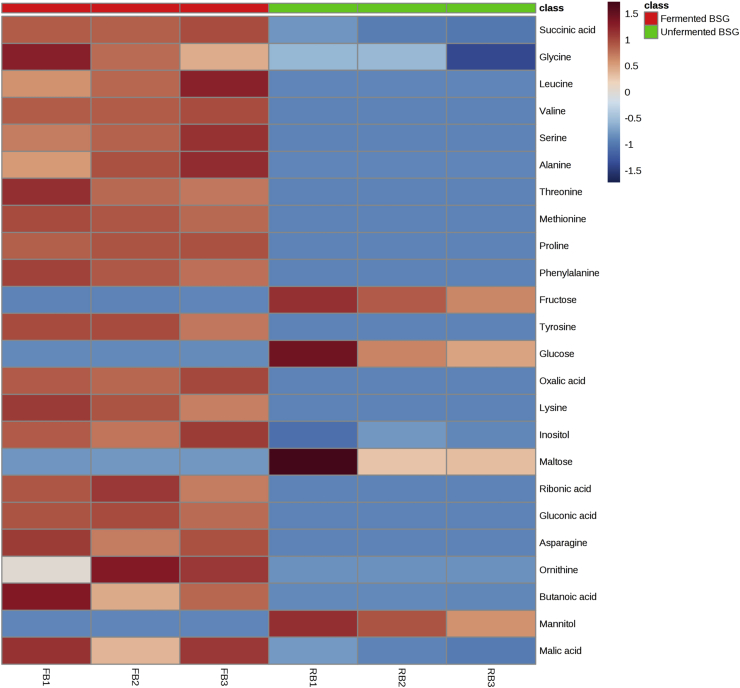
The detected metabolites were mapped onto a clutstering heatmap and can be mainly classified into types of amino acids, carbohydrates, and the citric acid (TCA) cycle intermediates (Figure 3). Heatmap provides a visual overview and uses a color gradient to indicate the changes in the metabolites after fermentation [26]. There were a total of 13 amino acids detected by GC-MS that were upregulated after fermentation of BSG. Increased levels of amino acids would enhance the nutritional value of the fermented beverage. During the fermentation process, B. subtilis could possibly produce enzymes such as proteases, which allows proteolysis to take place and produce various amino acids. This hydrolysis of protein complexes present in BSG into simple amino acids is in line with an observation in another fermented food study [27].
Figure 3.
A heatmap analysis of metabolites detected in both control and submerged fermented BSG. The three columns from the left denotes the metabolites obtained from submerged fermented BSG and subsequently the remaining three columns representing the metabolites in unfermented BSG samples. Higher amounts of specific metabolites were shown in red, whereas blue indicated lower amounts of the specific metabolites.
Amino acids are required nutrients for human functions, growth and health [28]. As proteins are made up of amino acids, the increased levels of amino acids would also provide better health benefits such as improved skeletal muscle protein synthesis [29]. The various amino acids can be further categorized into essential and non-essential amino acids. Essential amino acids are non-producible by the human body and only can be obtained from dietary intake [30]. The essential amino acids detected in this study include lysine, threonine, methionine, valine, leucine, tyrosine, phenylalanine [31]. The increase in the concentrations of 7 essential amino acids would improve the nutritional value of the beverage as a supplement for humans (Table 1).
Table 1.
Essential Amino acids results (μg/mL) for control and fermented BSG.
| Essential amino acids | Unfermented BSG | Fermented BSG |
|---|---|---|
| Leucine | N.D. | 4.11 ± 0.74 |
| Valine | N.D. | 0.82 ± 0.02 |
| Threonine | N.D. | 0.69 ± 0.09 |
| Phenylalanine | N.D. | 31.2 ± 2.87 |
| Methionine | N.D. | 5.21 ± 0.27 |
| Lysine | 0.01 ± 2E-04 | 44.92 ± 4.28 |
| Tyrosine | N.D. | 28.11 ± 1.89 |
N.D. – Not detected.
Based on the heatmap, various carbohydrates detected substantially decreased after submerged fermentation (Figure 3). It can be inferred that remaining carbohydrates present in BSG served as carbon sources for B. subtilis WX-17 growth. It is postulated that the fermentation using B. subtilis WX-17 produced various types of microbial enzymes that hydrolyze polysaccharides into simpler carbohydrates [32, 33, 34]. Metabolites categorized under the TCA cycle intermediates, which are succinic acid and malic acid, were observed to have increased after submerged fermentation (Figure 3). In the growth of microorganisms, the TCA cycle is a series of chemical reactions that generate energy to aerobic microorganisms. The upregulation in TCA cycle intermediates is in line with previous studies [35, 36], which showed that increase in TCA cycle metabolites stimulate growth of microorganisms. Overall, in both heatmap and PLS-DA, several significantly different metabolites (p < 0.05) were obtained after submerged fermentation.
3.3. Antioxidant and phenolic content assay
Based on DPPH radical scavenging activity results from this study, submerged fermented BSG produced approximately 2.08 times more antioxidants in terms of Trolox equivalent compared to the unfermented BSG (Table 2). A small portion of inhaled oxygen in humans would naturally transform into free radicals, which might cause damage to the body. As long as there is a balance between free radicals transformation and eradication, these free radicals will not cause any harm [37]. However, external conditions such as stress and smoking could lead to an increase in levels of free radicals and might result in several human diseases [38]. Adequate amounts of antioxidant intake would be able to control the excessive amounts of free radical production [39]. With an increase in DPPH radical scavenging activity after submerged fermentation, the antioxidant compounds present would further enhance the nutritional value of the beverage.
Table 2.
Antioxidant activity based on weight of Trolox and GAE (μg/mL).
| Samples | Weight of Trolox (μg/mL) | GAE (μg/mL) |
|---|---|---|
| Unfermented BSG (Control) | 3.51 ± 0.15 | 125.7 ± 0.74 |
| Fermented BSG (Day 3) | 7.31 ± 0.11 | 446.74 ± 1.26 |
In this study, there was an increase in phenolic content from 125.7 ± 0.74 μg GAE/mL to 446.74 ± 1.26 μg GAE/mL after submerged fermentation, which is in agreement with a study that reported microbial fermentation serving as a powerful method for extracting phenolic compounds (Table 2) [40]. BSG had been found to contain phenolic compounds that are bounded to the cell wall [41, 42]. Cell wall degrading enzymes would be required to extract the components [40]. Hence, it is postulated that B. subtilis WX-17 was able to produce enzymes such as cellulases to degrade the cell wall and extract the phenolic compounds through submerged fermentation [43].
4. Conclusion
This work demonstrates that submerged fermentation with BSG as the sole substrate was feasible to grow B. subtilis WX-17 on BSG. Growth of B. subtilis WX-17, in the beverage had a final cell count of 9.86 log CFU/mL after a period of 6 weeks when stored at 4 °C. It produced a nutritious beverage, tested to contain an increased amount of amino acids, total phenolic content and antioxidant activity. The combination of higher nutritional content together with presence of viable B. subtilis in the liquid would justify the potential of using the liquid as a novel nutritional beverage for human health. Further investigations on consumer acceptance, flavour of the beverage or different nutritional component analysis can be executed.
Declarations
Author contribution statement
Yong X. Tan, Wai K. Mok: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wei N. Chen: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Interdisciplinary Graduate School (IGS) at Nanyang Technological University and Zhong Feng International (M4062121.120.703012).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Nanyang Environment and Water Research Institute (NEWRI), Singapore, and the Interdisciplinary Graduate School (IGS), Nanyang Technological University, Singapore, for providing the facilities and support for this research.
References
- 1.Mussatto S.I. Brewer's spent grain: a valuable feedstock for industrial applications. J. Sci. Food Agric. 2014;94(7):1264–1275. doi: 10.1002/jsfa.6486. [DOI] [PubMed] [Google Scholar]
- 2.Mussatto S.I., Dragone G., Roberto I.C. Brewers' spent grain: generation, characteristics and potential applications. J. Cereal. Sci. 2006;43(1):1–14. [Google Scholar]
- 3.Lynch K.M., Steffen E.J., Arendt E.K. Brewers' spent grain: a review with an emphasis on food and health. J. Inst. Brew. 2016;122(4):553–568. [Google Scholar]
- 4.Buffington J. The economic potential of brewer’s spent grain (BSG) as a biomass feedstock. 2014;4:308–318. [Google Scholar]
- 5.Ikram S. Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 2017;82(10):2232–2242. doi: 10.1111/1750-3841.13794. [DOI] [PubMed] [Google Scholar]
- 6.Caulier S. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019;10(302) doi: 10.3389/fmicb.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu R.-L. Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J. Agric. Food Chem. 2009;57(2):503–508. doi: 10.1021/jf803072r. [DOI] [PubMed] [Google Scholar]
- 8.Patel H.M. Proliferation of Lactobacillus plantarum in solid-state fermentation of oats. Biotechnol. Prog. 2004;20(1):110–116. doi: 10.1021/bp034176r. [DOI] [PubMed] [Google Scholar]
- 9.Charalampopoulos D., Pandiella S.S., Webb C. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. J. Appl. Microbiol. 2002;92(5):851–859. doi: 10.1046/j.1365-2672.2002.01592.x. [DOI] [PubMed] [Google Scholar]
- 10.Schallmey M., Singh A., Ward O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004;50(1):1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H.-W., Jiang S., Hu J. IntechOpen; 2019. Gut-Brain Axis: Probiotic, Bacillus Subtilis, Prevents Aggression via the Modification of the Central Serotonergic System, in Oral Health by Using Probiotic Products. [Google Scholar]
- 12.Tan Y.X. Solid state fermentation of brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation. 2019;5(3):52. [Google Scholar]
- 13.Zdanowska P. An evaluation of the quality and microstructure of biodegradable composites as contribution towards better management of food industry wastes. Sustainability. 2019;11(5):1504. [Google Scholar]
- 14.Chen L., Chen W.N. Metabolite and fatty acid analysis of yeast cells and culture supernatants. Bio-protocol. 2014;4(17):e1219. [Google Scholar]
- 15.Kamtekar S., Keer V., Patil V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharmaceut. Sci. 2014;4:61–65. [Google Scholar]
- 16.Wan C. Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Phcog. Mag. 2011;7(25):40–45. doi: 10.4103/0973-1296.75900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486–w494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casula G., Cutting S.M. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002;68(5):2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman W.H. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 2007;189(23):8458. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shori A.B. Influence of food matrix on the viability of probiotic bacteria: a review based on dairy and non-dairy beverages. Food Bioscience. 2016;13:1–8. [Google Scholar]
- 21.Permpoonpattana P. Evaluation of Bacillus subtilis strains as probiotics and their potential as a food ingredient. Benef. Microbes. 2012;3(2):127–135. doi: 10.3920/BM2012.0002. [DOI] [PubMed] [Google Scholar]
- 22.Elshaghabee F.M.F. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01490. 1490-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenny B.F., Vandijk H.J., Collins J.A. Performance and fecal flora of calves fed a Bacillus subtilis concentrate. J. Dairy Sci. 1991;74(6):1968–1973. doi: 10.3168/jds.S0022-0302(91)78364-1. [DOI] [PubMed] [Google Scholar]
- 24.Kong X. Analysis of plasma metabolic biomarkers in the development of 4-nitroquinoline-1-oxide-induced oral carcinogenesis in rats. Oncology letters. 2015;9(1):283–289. doi: 10.3892/ol.2014.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henseler J., Ringle C.M., Sinkovics R.R. Emerald Group Publishing Limited; 2009. The Use of Partial Least Squares Path Modeling in International Marketing, in New Challenges to International Marketing; pp. 277–319. [Google Scholar]
- 26.Metsalu T., Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D.E. Metabolomics reveal that amino acids are the main contributors to antioxidant activity in wheat and rice gochujangs (Korean fermented red pepper paste) Food Res. Int. 2016;87:10–17. doi: 10.1016/j.foodres.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Wu G. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44(4):1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- 29.Ha E., Zemel M.B. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review) J. Nutr. Biochem. 2003;14(5):251–258. doi: 10.1016/s0955-2863(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 30.Galili G., Amir R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnology Journal. 2013;11(2):211–222. doi: 10.1111/pbi.12025. [DOI] [PubMed] [Google Scholar]
- 31.Tessari P., Lante A., Mosca G. Essential amino acids: master regulators of nutrition and environmental footprint? Sci. Rep. 2016;6 doi: 10.1038/srep26074. 26074-26074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlawat S. Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochem. 2009;44(5):521–526. [Google Scholar]
- 33.Raul D. Production and partial purification of alpha amylase from Bacillus subtilis (MTCC 121) using solid state fermentation. Biochemistry Research International. 2014;2014:5. doi: 10.1155/2014/568141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lincoln L., More S.S. Bacterial invertases: occurrence, production, biochemical characterization, and significance of transfructosylation. J. Basic Microbiol. 2017;57(10):803–813. doi: 10.1002/jobm.201700269. [DOI] [PubMed] [Google Scholar]
- 35.Song H., Lee S.Y. Production of succinic acid by bacterial fermentation. Enzym. Microb. Technol. 2006;39(3):352–361. [Google Scholar]
- 36.Clements L.D., Streips U.N., Miller B.S. Differential proteomic analysis of Bacillus subtilis nitrate respiration and fermentation in defined medium. Proteomics. 2002;2(12):1724–1734. doi: 10.1002/1615-9861(200212)2:12<1724::AID-PROT1724>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Hu C.-C. Antioxidant activity of fermented soybean extract. J. Agric. Food Chem. 2004;52(18):5735–5739. doi: 10.1021/jf035075b. [DOI] [PubMed] [Google Scholar]
- 38.Bandyopadhyay U., Das D., Banerjee R.K. Reactive oxygen species: oxidative damage and pathogenesis. Curr. Sci. 1999;77:658–666. https://www.jstor.org/stable/24102839 Bangalore. [Google Scholar]
- 39.Álvarez P. Diet supplementation for 5 weeks with polyphenol-rich cereals improves several functions and the redox state of mouse leucocytes. Eur. J. Nutr. 2006;45(8):428–438. doi: 10.1007/s00394-006-0616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhanja Dey T. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: a review. Trends Food Sci. Technol. 2016;53:60–74. [Google Scholar]
- 41.McCarthy A. Brewers' spent grain; Bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods. A review. 2012;72:1–9. doi: 10.1017/S0029665112002820. [DOI] [PubMed] [Google Scholar]
- 42.Meneses N.G.T. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Separ. Purif. Technol. 2013;108:152–158. [Google Scholar]
- 43.Deka D. Enhanced cellulase production from Bacillus subtilis by optimizing physical parameters for bioethanol production. ISRN Biotechnology. 2013;2013:11. doi: 10.5402/2013/965310. [DOI] [PMC free article] [PubMed] [Google Scholar]