Abstract
Breast cancer is the most common malignancy affecting females, with over 260,000 new cases annually and over 3.1 million survivors in the United States alone. Exposure to potentially cardiotoxic therapies, including anthracyclines, trastuzumab, and radiation therapy, coupled with host factors, place patients at increased risk for the development of cardiovascular disease (CVD) compared to non-cancer controls. Overall survival outcomes are significantly worse in patients who develop CVD, and in certain breast cancer populations, cardiovascular death exceeds the risk of cancer death in the long-term. In order to mitigate the risk of CVD, there is a growing interest in the use of cardioprotective strategies at the time of cancer therapy initiation. In this review, we present a detailed evaluation of the evidence from recently completed as well as ongoing cardio-oncology clinical trials in pharmacologic cardioprotection in breast cancer patients. We focus primarily on the potential role of dexrazoxane, alterations in anthracycline dosing or formulation, neurohormonal antagonists, beta-blockers, and combination therapy. We also discuss ongoing studies in statin cardioprotection, radiation delivery strategies, use of risk-guided strategies and the study of specific cancer populations. We close with a discussion of the ongoing needs in the field of cardio-oncology in order to advance the clinical care of patients with rigorous, evidence-based medicine.
Introduction
Currently, there are over 3.1 million breast cancer survivors in the United States with over 260,000 new cases projected annually. Increases in breast cancer incidence, coupled with earlier detection rates and advances in cancer therapies, have resulted in a growing survivor population that is at increased risk for other comorbidities, and specifically, cardiovascular disease (CVD) (1). Breast cancer patients have an increased incidence of CVD compared to non-cancer controls, and after development of CVD, overall survival outcomes are significantly worse (2,3). In large administrative database studies, the cumulative incidence of major cardiac events is reported to be 4.1% at 5 years (4). Older age and the presence of traditional cardiovascular risk factors have also been associated with a higher rate of cardiac events, on the order of 8.9% at 5 years (4). CVD accounts for 16.3% of deaths in breast cancer patients, exceeding the mortality due to breast cancer in those with pre-existing cardiovascular risk factors at 10 year follow-up (3). Broadly speaking, all-cause mortality is increased 3.8-fold in cancer survivors who develop CVD compared to those who do not (2).
Moreover, during or soon after the completion of cancer therapy, the incidence of cancer therapeutics related cardiac dysfunction (CTRCD) ranges from 9 to 26% after treatment with doxorubicin, 13 to 17% with trastuzumab, and 27 to 34% with combination therapies (5–9). Detailed longitudinal phenotyping in a prospective cohort study of 277 breast cancer patients treated with doxorubicin and/or trastuzumab suggest that the median left ventricular ejection fraction (LVEF) decline with these therapies is to 43% with a median time to development of significant LVEF decline of 7 months (interquartile range 4–12), requiring treatment cessation or interruption in at least 33% of these patients (5). In a large study of 2625 patients treated with anthracyclines and followed for a median of 5.2 years, including 51% with breast cancer, 98% of the cases of cardiotoxicity were detected within 1 year after completion of chemotherapy (6).
Although there are a number of knowledge gaps in cardio-oncology, one key question is as follows: What is the role of pharmacologic cardioprotection in mitigating the risk of subsequent cardiac events in breast cancer patients treated with potentially cardiotoxic therapies, including anthracyclines, trastuzumab, and radiation therapy (2,10)? In this review, we perform a detailed evaluation of the evidence from ongoing and recently completed cardio-oncology clinical trials in cardioprotective strategies initiated at the time of cancer therapy. We present below the various Phase I and II clinical trials that evaluate the efficacy of prophylactic therapy with neurohormonal antagonists, beta blockers, or combination therapies in breast cancer patients receiving doxorubicin and/or trastuzumab therapy; trials that use risk-guided strategies to identify subgroups who may derive the most benefit from cardioprotection; and studies that are focused on specific populations within breast cancer (Table 1).
Table 1:
Recent and Ongoing Trials in Prophylactic Cardioprotection Interventions in Breast Cancer Patients
| Trial Number | Cancer Treatment Exposure | Trial Treatment/ Intervention | Outcomes | Results |
|---|---|---|---|---|
| Cardioprotection Trials Evaluating Modifications in Cancer Therapy in Breast Cancer Patients Receiving Anthracyclines | ||||
| Meta-analysis of 11 randomized controlled trials (12) | Anthracyclines | Dexrazoxane versus placebo or standard of care | Symptomatic heart failure, asymptomatic LVEF decline by multigated radionuclide angiography (MUGA) or echocardiogram, chemotherapy response rate | Lower occurrence of heart failure with dexrazoxane, no difference in chemotherapy response rate |
| Meta-analysis of 55 randomized controlled trials (13) | Anthracyclines | Dexrazoxane versus standard of care, liposomal doxorubicin versus standard formulation doxorubicin, continuous versus bolus infusion | Symptomatic heart failure, asymptomatic LVEF decline by MUGA or echocardiogram | Lower occurrence of heart failure and asymptomatic LVEF decline with dexrazoxane, continuous infusion of doxorubicin, and liposomal doxorubicin |
| Meta-analysis of 7 randomized controlled trials (14) | Anthracyclines | Liposomal doxorubicin versus standard formulation doxorubicin, epirubicin versus doxorubicin | Symptomatic heart failure, asymptomatic LVEF decline by MUGA or echocardiogram | Lower occurrence of heart failure with liposomal doxorubicin |
| Meta-analysis of 9 randomized controlled trials (15) | Anthracyclines | Liposomal doxorubicin versus standard formulation doxorubicin | Symptomatic heart failure, reduction in LVEF | Lower occurrence of heart failure and LVEF reduction with liposomal doxorubicin |
| Cardioprotection Trials Evaluating Neurohormonal Antagonists in Breast Cancer Patients Receiving Anthracyclines | ||||
| NCT03265574: PROACT (21) | Anthracyclines | Enalapril versus standard of care | LVEF by echocardiogram, Troponin T | Ongoing |
| NCT02053974 (19) | Anthracyclines | Spironolactone versus placebo | LVEF and E/e’ by echocardiogram | Less LVEF reduction and less E/e’ increase with spironolactone |
| NCT01708798 (20) | Anthracyclines | Eplerenone versus placebo | Average e’ by echocardiogram | No difference in average e’, terminated for futility |
| Cardioprotection Trials Evaluating Beta-Blockers in Breast Cancer Patients Receiving Anthracyclines | ||||
| NCT01724450: CECCY (22) | Anthracyclines | Carvedilol versus placebo | LVEF and diastolic function by echocardiogram, troponin I | No difference in LVEF; carvedilol attenuated diastolic dysfunction and Troponin I elevation |
| Cardioprotection Trials Evaluating ACEI/ARBs and Beta-Blockers Singly or in Combination in Breast Cancer Patients Receiving Anthracyclines and/or Trastuzumab | ||||
| NCT01016886: MANTICORE (23) | Trastuzumab ± Anthracyclines | Perindopril or Bisoprolol versus placebo | Indexed LV volume and LVEF by cMRI | No difference in indexed LV volume with perindopril or bisoprolol; less LVEF reduction with bisoprolol |
| NCT01434134: PRADA (24) | Anthracyclines ± Trastuzumab | Candesartan, Metoprolol, Candesartan +Metoprolol, versus placebo | LVEF by echocardiogram, biomarkers: troponin I and T, NTproBNP, BNP, c-reactive protein, galectin 3 | Less LVEF reduction with candesartan treatment, metoprolol attenuated troponin |
| NCT01009918 (26) | Trastuzumab ± Anthracyclines | Lisinopril or Carvedilol versus placebo | LVEF by echocardiogram | Less LVEF reduction with either lisinopril or carvedilol only in patients treated with combination trastuzumab plus doxorubicin |
| NCT00459771 (27) | Trastuzumab + Anthracyclines | Candesartan versus placebo | LVEF by echocardiogram, troponin T, BNP | No difference in LVEF, troponin T, or BNP with candesartan treatment |
| Cardioprotection Trials Evaluating Statins in Breast Cancer Patients Receiving Anthracyclines and/or Trastuzumab | ||||
| NCT01988571: PREVENT (28) | Anthracyclines | Atorvastatin versus placebo | LVEF by cMRI | Ongoing |
| NCT02096588 (29) | Anthracyclines ± Trastuzumab | Simvastatin versus standard of care | Global longitudinal strain by echocardiogram | Ongoing |
| Cardioprotection Trials Evaluating Beta-Blockers in Metastatic Her2-Positive Breast Cancer Patients | ||||
| NCT03418961: SWOG S1501 (30) | Trastuzumab | Carvedilol versus placebo | LVEF by echocardiogram, death, time to first cardiac event | Ongoing |
| Cardioprotection Trials Evaluating Radiation Therapy in Breast Cancer Patients | ||||
| NCT02603341: PCORI/RADCOMP (31) | ± adjuvant/ neoadjuvant chemotherapy | Proton versus photon radiation therapy | Cardiovascular and cancer mortality, quality of life | Ongoing |
| Cardioprotection Trials Evaluating Risk-Guided Strategies Using Imaging or Biomarkers in Breast Cancer Patients | ||||
| NCT01968200: ICOS-One (36) | Anthracyclines | Prophlyactic enalapril vs troponin-triggered enalapril | Troponin (various assay platforms), LVEF by echocardiogram | No difference in troponin elevation or LVEF between the preventive versus troponin-triggered initiation of enalapril treatment |
| EudraCT 2017-000896-99, ISRCTN 24439460: Cardiac-CARE (37) | Anthracyclines | Troponin-triggered ARB + beta-blocker versus standard of care | LVEF by cMRI, heart failure hospitalization | Ongoing |
| ACTRN12614000341628: SUCCOUR (41) | Anthracyclines and/or Trastuzumab | Global longitudinal strain-guided versus LVEF-guided ramipril and carvedilol | 3D LVEF by echocardiogram | Ongoing |
Cardioprotection Trials Evaluating Modifications in Cancer Therapy in Breast Cancer Patients Receiving Anthracyclines
Anthracycline chemotherapy is hypothesized to cause cardiomyocyte injury through an increase in oxidative stress, potentially via the inhibition of topoisomerase-2 beta. Specifically, the quinone moiety of doxorubicin undergoes redox cycling resulting in reactive oxygen species, and additionally anthracycline-iron complexes form which create toxic hydroxyl radicals that are cytotoxic to cardiomyocytes, disrupt mitochondrial biogenesis, and damage the cell membrane (11). Earlier studies have determined several treatment protocols that limit anthracycline cardiotoxicity, including the use of dexrazoxane, liposomal formulation, and continuous infusion. Dexrazoxane is an iron chelator that reduces reactive oxygen species and also, interestingly, interacts with topoisomerase-2 beta. Two published meta-analyses demonstrated heart failure risk reduction with use of dexrazoxane, with a risk ratio of 0.29 (0.20, 0.41) (12) and odds ratio (OR) of 0.21 (0.13, 0.33) (13). Subclinical cardiotoxicity, defined as either asymptomatic LVEF decline or abnormality in cardiac function determined using a diagnostic test, was also decreased, with a relative risk of 0.33 (0.20, 0.55) (13). These data are primarily derived from populations with metastatic breast cancer and exposure to high doses of anthracyclines.
Additionally, anthracycline continuous infusion (versus bolus dosing) protocols are also a potential cardioprotective strategy. A meta-analysis of four randomized controlled trials that compared bolus to infusion dosing of either epirubicin or doxorubicin showed a higher incidence of clinical cardiotoxicity with bolus dosing (OR 4.13; 1.75, 9.72) (13). Furthermore, liposomal doxorubicin changes tissue distribution by enlarging the molecular structure and prohibiting its passage through narrow capillary junctions in cardiac tissue, while still allowing unrestricted passage in areas of tumor angiogenesis. Three meta-analyses have demonstrated a reduction in the development of heart failure with no change in anti-neoplastic efficacy or overall survival with the use of liposomal doxorubicin: OR 0.18 (0.08, 0.38) (13), relative risk 0.2 (0.05, 0.75) (14), and OR 0.34 (0.24, 0.47) (15). The 2017 American Society of Clinical Oncology Clinical Practice Guidelines recommend consideration of the use of dexrazoxane, continuous infusion, and liposomal formulation for patients receiving high dose anthracycline treatment. Dexrazoxane is approved by the FDA for use in adult cancer patients who will receive a total dose of doxorubicin >300 mg/m2 or epirubicin >540 mg/m2 (16).
Cardioprotection Trials Evaluating Neurohormonal Antagonists in Breast Cancer Patients Receiving Anthracyclines
There are a number of studies ongoing evaluating the efficacy of prophylactic neurohormonal blockade with angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), or aldosterone antagonists. These medications, as well as beta-blockers, detailed below, have well-established roles in the treatment of heart failure with reduced LVEF and have been shown to promote ventricular recovery through inhibition of adverse remodeling that is mediated via adrenergic and neuroendocrine dysregulation. Interestingly, animal models have demonstrated anthracycline-induced angiotensin converting enzyme elevations in the serum and cardiac tissue in rats 20 days after exposure (17). Similar models in hamsters have demonstrated that lisinopril reduced angiotensin converting enzyme levels, left ventricular dilation, and overall mortality (18). Overall, these data provide some basic evidence to support the potential efficacy of these therapies in anthracycline cardiotoxicity prevention.
The role of aldosterone antagonists has been studied in small trials in breast cancer patients undergoing anthracycline chemotherapy. Akpek, et al. (2015) randomized 83 patients to receive a fixed dose of spironolactone 25 mg/day versus placebo, which was initiated one week before anthracycline administration and continued for an average of 24 weeks during chemotherapy (NCT02053974). In follow-up echocardiograms performed three weeks after completion of chemotherapy, the participants in the spironolactone group had smaller decreases in LVEF pre- and post-anthracycline treatment compared to the placebo group. The pre-and post-treatment LVEFs were noted to be 67.0 ± 6.1% and 65.7 ± 7.4% (p = 0.094) in the spironolactone group versus 67.7 ± 6.3% and 53.6 ± 6.8% (p < 0.001) in the placebo group (19). Diastolic function indices, including E/e’, were preserved over time in the spironolactone group (8.3 ± 1.6 and 8.5 ± 2.6, p = 0.625), while a significant increase was observed in the control group (8.3 ± 2.1 and 9.3 ± 2.8, p = 0.022).
Additional smaller studies have sought to evaluate the role of the aldosterone antagonist eplerenone for cardioprotection, as determined by changes in diastolic function parameters, in patients receiving doxorubicin. The University of British Columbia conducted a study (NCT01708798) of 44 early stage breast cancer patients with normal baseline LVEF treated with anthracyclines, who were randomized to treatment with eplerenone versus placebo. The primary outcome measure was change in diastolic function as measured by the average e’ by echocardiography at 6 months. Secondary outcomes included worsening of additional echocardiographic diastolic parameters, echocardiographic evidence of systolic dysfunction, biomarker abnormalities, and global longitudinal strain. This study was terminated in November, 2016 due to a negative primary endpoint and a reported determination of futility (20).
One ongoing open label study is investigating the efficacy of ACEIs (enalapril) for prophylactic cardioprotection in breast cancer patients receiving adjuvant anthracycline chemotherapy with epirubicin >300mg/m2 (NCT03265574: PROACT). In this trial, 170 breast cancer patients with normal baseline LVEF are being randomized to receive usual care with enalapril, initiated prior to the administration of anthracycline, or usual care alone. Patients will be evaluated by echocardiography pre- and post-treatment, and by blood biomarkers. In this study, cardiac troponin T is the primary outcome measure (21).
Overall, the role of neurohormonal blockade for prophylactic cardioprotection in breast cancer patients treated with anthracyclines alone remains an area of active investigation. There is conflicting evidence regarding the use of aldosterone antagonists for this purpose. Future larger trials are needed; moreover, using maximally tolerated medication dosages as well as both objective and symptom-driven endpoints will help to clarify the role of neurohormonal antagonists in mitigating cardiac dysfunction (Figure 1).
Figure 1:
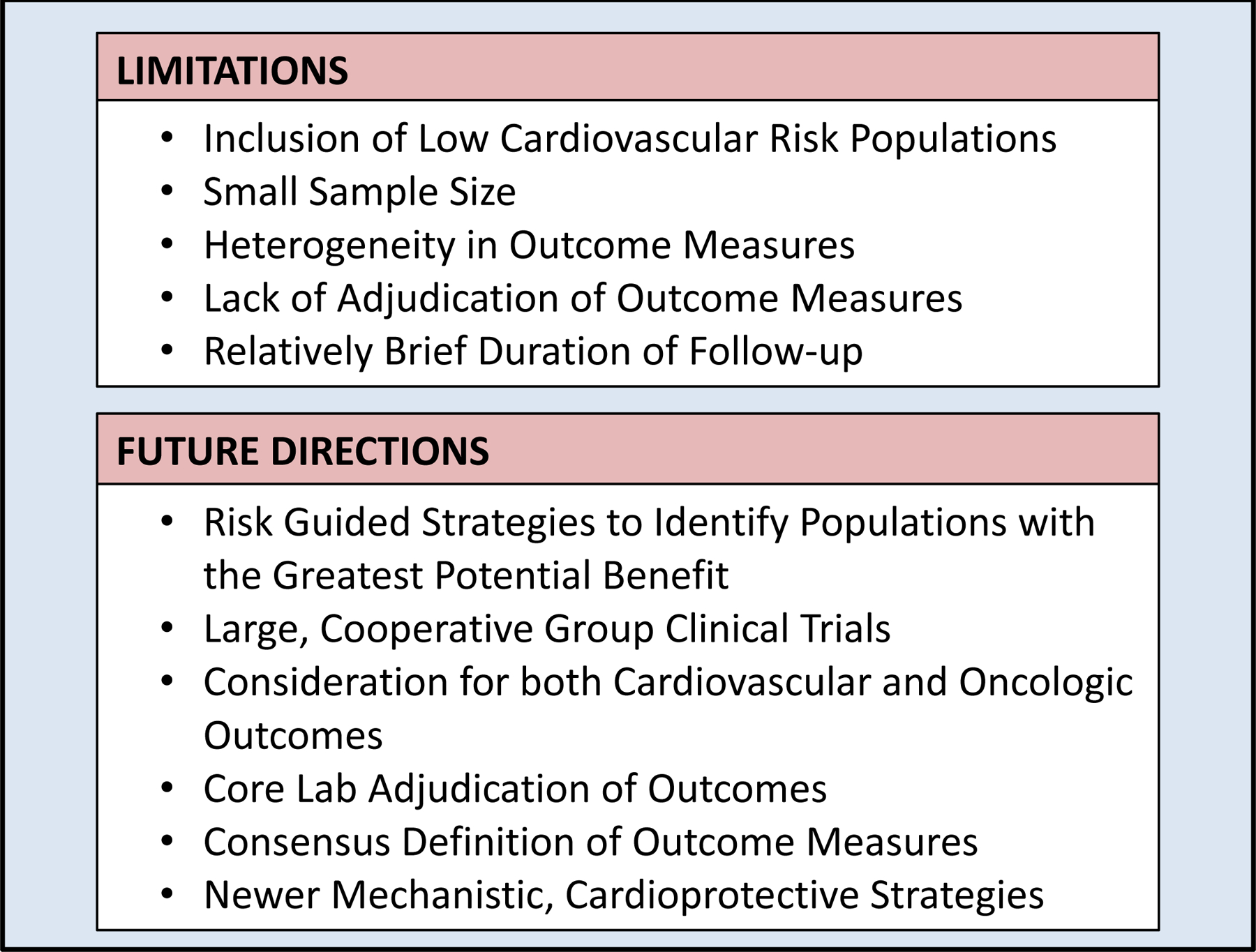
Limitations of Recent Pharmacologic Cardioprotection Trials in Breast Cancer and Proposed Future Directions
Cardioprotection Trials Evaluating Beta-Blockers in Breast Cancer Patients Receiving Anthracyclines
In addition to neurohormonal antagonists, beta-blockers, used widely in heart failure with reduced ejection fraction, have also been studied as a potential cardioprotectant in breast cancer patients treated with anthracyclines. Beta-blockers, such as carvedilol, have been shown to possess anti-oxidant properties, providing a potential mechanistic basis for its efficacy. This may counteract the cardiotoxic reactive oxygen species generated by various breast cancer therapies (e.g. anthracyclines, trastuzumab, and radiation therapy) (16,22). The Carvedilol for Prevention of Chemotherapy Cardiotoxicity trial (NCT01724450: CECCY), by Avila, et al. (2018), randomized 200 Her2-negative breast cancer patients with normal baseline LVEF to carvedilol treatment during anthracycline chemotherapy versus placebo. The primary endpoint was a 10% reduction in LVEF by echocardiogram at 6 months. This endpoint was reached by 14 patients (14.5%) in the carvedilol treatment group versus 13 patients (13.5%) in the control group (p = 1.0). In this study, carvedilol use did not demonstrate a statistically significant impact on the incidence of early onset of LVEF reduction. However, both secondary endpoints of troponin elevations and diastolic dysfunction parameters were attenuated in the carvedilol treatment group at 6 months. Troponin I levels were increased from baseline until the end of the study in both groups with sixty-five patients (33.8%) having elevated troponin I levels >0.04 ng/ml. Here, 25 (26%) participants in the carvedilol treated group and 40 (41.6%) in the placebo control group experienced elevations (p=0.03) (22). The incidence of diastolic dysfunction at the end of treatment was 24 (28.5%) patients in the carvedilol group versus 32 (37.2%) in the placebo group (p = 0.039).
While carvedilol did not prevent a reduction in LVEF, this population had limited cardiovascular risk factors and a lower than expected incidence of chemotherapy-related heart failure, limiting the trial’s power. The primary outcome measure was not core-lab quantitated, and LVEF is known to be highly variable in this setting. Moreover, follow-up time was relatively short and the majority of patients did not achieve the target dose of carvedilol. Nonetheless, the reduction in troponin elevation and diastolic dysfunction in the treatment group provide some evidence for a possible reduction in myocardial injury with carvedilol treatment during anthracycline chemotherapy.
Cardioprotection Trials Evaluating ACEI/ARBs and Beta-Blockers Singly or in Combination in Breast Cancer Patients Receiving Anthracyclines and/or Trastuzumab
Similar to attempts in patients treated with anthracyclines alone, primary prevention efforts with early beta-blocker or ACEI/ARB therapy have also shown mixed results in breast cancer patients treated with trastuzumab with or without anthracyclines. Trastuzumab is a humanized monoclonal antibody to the Her2-Neu receptor, present within approximately 25% of invasive ductal carcinomas. The Her-2 receptor is found on the cell surface of cardiomyocytes and vascular endothelial cells and is important in cardiovascular injury repair mechanisms and angiogenic signaling (5).
The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research trial (NCT01016886: MANTICORE) did not demonstrate prevention of left ventricular remodeling in 94 patients with early Her2-positive breast cancer receiving trastuzumab with or without anthracyclines treated with perindopril or bisoprolol, as compared to placebo. In this trial, patients received cardiac medications or placebo for the duration of trastuzumab therapy, and completed a cardiac magnetic resonance imaging (cMRI) scan at baseline and after cycle 17. Although there were no statistically significant differences amongst groups in the primary outcome of indexed left ventricular end diastolic volume, bisoprolol modestly attenuated a trastuzumab-mediated LVEF decline (absolute declines: bisoprolol: −1% [4,−6], perindopril: −3% [−1,7], placebo: −5% [0,10]). Additionally, the perindopril and bisoprolol groups had fewer asymptomatic LVEF reductions at cycle 4 compared to placebo, although this difference was not significant after cycle 17. Both perindopril and bisoprolol were found in multivariable regression to predict LVEF maintenance after exposure to cancer therapy (23).
The Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy trial (NCT01434134: PRADA) evaluated the use of a beta-blocker, ARB, or their combination versus placebo in prevention of cardiac dysfunction in patients receiving anthracyclines with or without trastuzumab. This was a 2×2 factorial design including 130 patients with early stage breast cancer. The primary outcome was change in LVEF by cMRI. In the candesartan group, the LVEF reduction was 0.8% (95% CI −0.4, 1.9) compared to 2.6% (95% CI 1.5, 3.8) in the placebo group (p = 0.026). There was no statistically significant effect of metoprolol on LVEF (24). Results of a 121 patient subgroup analysis showed that several biomarkers were increased after epirubicin administration, including troponin I and T, N-terminal pro brain natriuretic peptide (NTproBNP), BNP, c-reactive protein, and galectin 3 (25). Similar to results seen in the CECCY trial, troponin elevation was attenuated by metoprolol treatment but not candesartan; but there was no noted association between LVEF and biomarkers. A follow-up (PRADA-2) is currently underway to evaluate the use of the angiotensin neprilysin inhibitor sacubitril-valsartan for prophylactic cardioprotection (personal communication).
Guglin, et al. (2018) evaluated 468 Her2-positive early stage breast cancer patients treated with trastuzumab with or without anthracyclines, randomized to prophylactic cardioprotection with lisinopril, carvedilol, or placebo (NCT01009918). Outcomes included troponin and BNP elevation, LVEF by echocardiogram, and heart failure symptoms at 2 years follow-up. Overall, there was no significant difference in outcomes, with an LVEF reduction of >10% occurring in 30% of the lisinopril group, 29% of the carvedilol group, and 32% of the placebo group. However, patients treated with combination doxorubicin and trastuzumab experienced a lower incidence of LVEF reduction with either therapy; here, a >10% LVEF reduction occurred in 37% and 31% of patients in the lisinopril and carvedilol groups, respectively, compared to 47% of patients in the placebo group (p = 0.009) (26).
Additionally, Boekhout, et al. (2016) studied the effect of the ARB candesartan in 210 women with early stage Her2-positive breast cancer (NCT00459771). In this study all patients underwent sequential anthracyclines followed by trastuzumab chemotherapy. Patients were randomized to candesartan versus placebo for 78 weeks starting at treatment initiation. The primary outcome was an absolute LVEF reduction by 15% or to an LVEF value <45%. This study found no difference in the incidence of LVEF reduction between the treatment and placebo groups (19% and 16%, respectively), and also noted no association between candesartan treatment and NTproBNP or high-sensitivity troponin T elevations (27).
Overall, in patients receiving anthracyclines with or without trastuzumab, prophylactic treatment with ACEI/ARB and beta-blockers singly or in combination has shown equivocal results. One issue that limits direct comparison of each of these studies is differences in primary and secondary outcome definition and method of ascertainment. PRADA used cMRI for LVEF measurement and also had patients undergo echocardiography to assess additional parameters, such as diastolic function. Other studies have used echocardiography solely, and not relied upon core lab assessment. This has been associated with higher methodologic variability which precludes detection of significant differences that may be small in magnitude. Multiple trials have been limited by a lower than expected observed primary endpoint in the placebo or control group, which reduces study power. Overall, there is inconsistent evidence that treatment with ACEI/ARBs and beta-blockers provide substantial cardioprotection against LVEF declines; overall, this effect has been modest, likely given the unselected patient populations and treatment of low-risk individuals.
Cardioprotection Trials Evaluating Statins in Breast Cancer Patients Receiving Anthracyclines and/or Trastuzumab
Statins are widely used for their lipid-lowering effects but have also been proposed to have anti-inflammatory or pleiotropic effects that may prevent chemotherapy cardiotoxicity as well. A current Phase II randomized controlled trial is evaluating the use of statins for cardioprotection in breast cancer and lymphoma patients. The Preventing Anthracycline Cardiovascular Toxicity with Statins (NCT01988571: PREVENT) trial randomized 279 patients with early stage breast cancer or lymphoma treated with anthracyclines and normal baseline LVEF to receive atorvastatin 40mg or placebo at the initiation of chemotherapy and continued for 24 months. The primary endpoint includes LVEF maintenance at 24 months determined by cMRI. This study will also quantify measures of cardiac and vascular remodeling including strain, wall thickness, left ventricular volumes, fibrosis, and pulse wave velocity. Results from this study are expected in May 2020 (28). There is also a smaller ongoing study that is evaluating the use of simvastatin for prophylactic cardioprotection (NCT02096588) (29).
Cardioprotection Trials Evaluating Beta-Blockers in Metastatic Her2-Positive Breast Cancer Patients
Few trials have included patients with metastatic disease. However, these patients are often at higher risk for development of CTRCD given a higher lifetime cumulative exposure of several cardiotoxic treatments. There is an ongoing trial of prophylactic carvedilol use in the metastatic Her2-positive population receiving trastuzumab by the Southwest Oncology Group (NCT03418961: SWOG S1501). Enrolled patients must have Her2-positive metastatic disease currently receiving trastuzumab without anthracycline chemotherapy. Inclusion criteria also requires baseline LVEF >50% and presence of one cardiovascular risk factor, including previous anthracycline chemotherapy exposure. Eight-hundred ten patients are to undergo random assignment to placebo versus carvedilol for a 108-week duration. This study will report on time to LVEF decline by 10% to <50%, or 5% to <50%; death; first cardiac event; and medication tolerance (30).
Cardioprotection Trials Evaluating Radiation Therapy in Breast Cancer Patients
Radiation therapy results in various cardiotoxic effects, including valvular disease, coronary disease, heart failure, pericardial disease, and arrhythmias. Valvular heart disease develops secondary to radiation therapy induced increases in osteogenic factors; coronary artery disease results from both macrovascular lipid-rich plaque development and microvascular inflammation and endothelial cell dysfunction; and various phenotypes of cardiomyopathy, including systolic dysfunction or restrictive physiology, potentially result from cardiomyocyte damage and vascular endothelial dysfunction (11). Modern treatment strategies have become less cardiotoxic with advancements in cardiac-sparing techniques, improved localization of treatment, and 3D planning models. However, there remains uncertainty regarding the cardiovascular effects of radiation with proton versus photon therapy. The ongoing Pragmatic Randomized Trial of Proton Versus Photon Therapy for Patients With Non-Metastatic Breast Cancer: A Radiotherapy Comparative Effectiveness Consortium Trial (NCT02603341: PCORI RadComp) is currently evaluating this question. In this trial, patients with locally advanced breast cancer are randomized to proton versus photon therapy with planned 10 year follow-up of endpoints including cardiovascular morbidity and mortality, cancer outcomes, and quality of life metrics (31).
Cardioprotection Trials Evaluating Risk-Guided Strategies Using Imaging or Biomarkers in Breast Cancer Patients
While several studies have investigated the use of prophylactic cardioprotection based upon chemotherapy exposure alone (i.e., anthracyclines or trastuzumab), others have proposed risk-guided strategies targeting patients at higher risk for development of CTRCD. This has the potential to identify the subgroup of patients who will manifest the greatest magnitude of benefit from primary prevention. Several methods of risk stratification have been proposed including a troponin-guided and a strain-guided strategy.
Troponin Guided Strategy for Cardioprotection
Cardiac troponins are enzymes exclusively expressed in myocardial tissue used to detect myocardial injury. Several studies have demonstrated associations between troponin elevations before and during treatment with the development of CTRCD (32–35), providing a rationale for its use in identifying high risk patients. A group of investigators led by Cardinale, et al. (2018) pioneered a troponin strategy for guidance of primary prevention with ACEI treatment in The International Cardio-Oncology Society-One trial (NCT01968200: ICOS-One). In this trial of 273 cancer patients (73% breast cancer) receiving anthracycline chemotherapy, patients had troponin evaluated prior to and after each infusion. In the preventive arm all patients were given enalapril at study initiation whereas in the troponin-triggered group patients only received enalapril after developing an elevated troponin (measured on institution-specific platforms). Outcomes were not different between these two groups, including incidence of troponin elevation (23% versus 26% in the preventive and troponin-triggered groups, respectively) and cardiotoxicity, as defined by LVEF reduction (36). This group has hypothesized that the ACEI mechanism of cardioprotection may act after cardiomyocyte injury via protection against neurohormonally-mediated adverse remodeling. In this setting, troponin, which is released at the time of cardiomyocyte injury, would not be affected by ACEI administration. Additionally the timing of initial and peak troponin elevations after anthracycline exposure in this setting is not entirely clear.
Another trial examining outcomes using the troponin-guided strategy for initiation of cardioprotection is ongoing. The Cardiac-CARE trial (EudraCT 2017–000896-99, ISRCTN 24439460) is enrolling breast cancer patients receiving anthracycline chemotherapy and monitoring troponin I before each chemotherapy administration. The development of an elevated troponin I will result in randomization to receive either standard of care or beta-blocker and ARB combination treatment. This study will evaluate outcomes of LVEF by cMRI as well as heart failure hospitalizations (37).
Echocardiographic Strain Guided Strategy for Cardioprotection
Several studies have shown that echocardiographic strain, a measurement of myocardial deformation, predicts the development of reduced LVEF (38–40). Abnormal strain has also been suggested as a strategy to select patients who are high risk for the development of CTRCD.
The Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes trial (ACTRN12614000341628: SUCCOUR) is an ongoing study evaluating the use of global longitudinal strain compared to LVEF, both derived from echocardiography, to guide initiation of cardioprotective medications. This study includes patients receiving cardiotoxic chemotherapy who have at least one cardiovascular risk factor. Current study enrollment includes 88% breast cancer patients, and the most common chemotherapeutic regimen is anthracyclines plus trastuzumab. Patients are randomized to receive treatment with ramipril and carvedilol based on LVEF guidance versus global longitudinal strain guidance. The study is ongoing but preliminary results at one year follow-up demonstrate that 37.5% of patients in the strain-guided group versus 8.7% in the LVEF-guided group have been started on treatment. The primary endpoint of this study is the change in 3D LVEF from study initiation compared to at 3 years of follow-up (41).
Overall, more investigation is needed in the area of risk-guided patient selection for cardioprotective treatment. Identification of accurate and reliable methods of patient risk stratification is imperative to the success of this strategy.
Conclusions and Future Directions
Breast cancer patients are at increased risk of the development of CVD compared to non-cancer controls. Age, traditional cardiovascular risk factors, and treatment-specific exposures contribute to this increased risk. Once patients develop CVD, overall survival outcomes are significantly worse, and in certain breast cancer populations, cardiovascular death exceeds cancer death rates. It is therefore imperative to identify strategies of effective cardioprotection. Data from the trials summarized here suggest that medications that are the cornerstones of treatment of heart failure with reduced ejection fraction have a potential role in cancer therapy cardiotoxicity prevention. These data suggest that neurohormonal blockade and beta-blockers have a modest effect on mitigating declines in cardiac function, as determined by LVEF. These medications have also been associated with reduced rates of troponin elevation and diastolic dysfunction. Breast cancer patients comprise a heterogeneous population with unique cardiovascular and treatment-related risk factors, and at this time the best primary prevention treatment strategy may include beta blockers and neurohormonal agents in combination or alone. However, to rigorously determine what is the most effective therapy, a meta-analysis should be performed. As higher risk subpopulations are studied, more robust data in this area may help further define primary prevention treatment recommendations.
Current guidelines from multiple international oncologic societies advocate for individualized decision-making regarding treatment cessation including consideration of the availability of alternative cancer therapies, the potential opportunity to treat for curative intent, and severity of cardiac dysfunction. Most guidelines recommend consideration of treatment cessation or interruption for symptomatic heart failure, or for significant asymptomatic LVEF reductions (16,42,43). Rechallenging a patient with cardiotoxic chemotherapy after development of CTRCD requires weighing individualized risks and benefits.
Newer trials have expanded upon traditional endpoints of LVEF and troponins to include additional metrics of cardiac function, including multiple newer biomarkers, strain imaging and measures derived from cMRI. Additional methods of cardioprotection with statins or type of radiation therapy (e.g. proton) are areas of active investigation. Current trials also aim to demonstrate cardioprotection using selective treatment of high risk patients identified through imaging or circulating biomarkers. These areas of ongoing investigation and directions for future research are summarized in Figure 1. For example, robust strategies to identify high risk individuals, larger cooperative group trials, core lab adjudication and consensus definitions of cardiovascular outcomes, and mechanistic cardioprotective strategies are important advances for the field. As the field of cardio-oncology continues to make advances in defining the epidemiology and mechanisms of CTRCD, there is a continued need for improved risk stratification and newer cardioprotective strategies, in order to accomplish the ultimate goal of decreasing CVD morbidity and mortality in this growing population.
Funding sources
This work was supported by R01 HL 118018 (Ky)
Footnotes
Conflict of Interest:
None
References
- 1.American Cancer Society. How Common Is Breast Cancer? [cited 2018 Oct 29]. Available from: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html.
- 2.Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, et al. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol Off J Am Soc Clin Oncol. 2016. April 1;34(10):1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017. January 1;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 4.Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, et al. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J Clin Oncol Off J Am Soc Clin Oncol. 2016. 01;34(19):2239–46. [DOI] [PubMed] [Google Scholar]
- 5.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation. 2017. April 11;135(15):1397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation. 2015. June 2;131(22):1981–8. [DOI] [PubMed] [Google Scholar]
- 7.Drafts BC, Twomley KM, D’Agostino R, Lawrence J, Avis N, Ellis LR, et al. Low to Moderate Dose Anthracycline-Based Chemotherapy Is Associated With Early Noninvasive Imaging Evidence of Subclinical Cardiovascular Disease. JACC Cardiovasc Imaging. 2013. Aug;6(8):877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein JM. The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells. Biochem Pharmacol. 1975. September 15;24(18):1659–62. [DOI] [PubMed] [Google Scholar]
- 9.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol Off J Am Soc Clin Oncol. 2002. March 1;20(5):1215–21. [DOI] [PubMed] [Google Scholar]
- 10.Shelburne N, Adhikari B, Brell J, Davis M, Desvigne-Nickens P, Freedman A, et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014. September;106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.In: Ky, Bonnie Cardio-Oncology In: Zipes Douglas P; Libby Peter; Bonow Robert O; Mann Douglas L; Tomaselli Gordon F; Braunwald Eugene, Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine 11th Edition Philadelphia, PA: 2019. p 1641–1650. [Google Scholar]
- 12.van Dalen EC, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2005. January 25;(1):CD003917. [DOI] [PubMed] [Google Scholar]
- 13.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010. June 29;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010. May 12;(5):CD005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol. 2012. April 23;1(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017. March 10;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesan N, Ramesh CV, Jayakumar R, Chandrakasan G. Angiotensin I converting enzyme activity in adriamycin induced nephrosis in rats. Toxicology. 1993. December 31;85(2–3):137–48. [DOI] [PubMed] [Google Scholar]
- 18.Okumura K, Jin D, Takai S, Miyazaki M. Beneficial effects of angiotensin-converting enzyme inhibition in adriamycin-induced cardiomyopathy in hamsters. Jpn J Pharmacol. 2002. February;88(2):183–8. [DOI] [PubMed] [Google Scholar]
- 19.Akpek M, Ozdogru I, Sahin O, Inanc M, Dogan A, Yazici C, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy: Effects of spironolactone on anthracycline cardiomyopathy. Eur J Heart Fail. 2015. January;17(1):81–9. [DOI] [PubMed] [Google Scholar]
- 20.Virani SA, Davis M. NCT01708798 [Internet] Study of the Effect of Eplerenone on Heart Function in Women Receiving Anthracycline Chemotherapy for Breast Cancer. [cited 2018 Oct 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT01708798?term=NCT01708798&rank=1 [DOI] [PMC free article] [PubMed]
- 21.Chang L NCT03265574 [Internet] PROACT: Can we Prevent Chemotherapy-related Heart Damage in Patients With Breast Cancer? (PROACT). [cited 2018 Oct 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT03265574?term=NCT03265574&rank=1
- 22.Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J Am Coll Cardiol. 2018. May 22;71(20):2281–90. [DOI] [PubMed] [Google Scholar]
- 23.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol Off J Am Soc Clin Oncol. 2017. March 10;35(8):870–7. [DOI] [PubMed] [Google Scholar]
- 24.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016. 01;37(21):1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati G, Heck SL, Røsjø H, Ree AH, Hoffmann P, Hagve T- A, et al. Neurohormonal Blockade and Circulating Cardiovascular Biomarkers During Anthracycline Therapy in Breast Cancer Patients: Results From the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) Study. J Am Heart Assoc. 2017. November 8;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guglin ME. Lisinopril or Carvedilol for Prevention of Trastuzumab Induced Cardiotoxicity - Lisinopril or Carvedilol for Cardiotoxicity [Internet] Abstract presented at: American College of Cardiology Annual Scientific Session (ACC 2018); 2018. March 11 [cited 2018 Oct 29]; Orlando, Florida: Available from: https://www.acc.org/latest-in-cardiology/clinical-trials/2018/03/10/15/11/lisinopril-or-carvedilol-for-cardiotoxicity [Google Scholar]
- 27.Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, et al. Angiotensin II–Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016. August 1;2(8):1030. [DOI] [PubMed] [Google Scholar]
- 28.Hundley G NCT01988571 [Internet] Preventing Anthracycline Cardiovascular Toxicity With Statins (PREVENT). [cited 2018 Oct 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT01988571?term=NCT01988571&rank=1
- 29.Smith K NCT02096588 [Internet] Detection and Prevention of Anthracycline-Related Cardiac Toxicity With Concurrent Simvastatin. [cited 2018 Oct 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT02096588?term=NCT02096588&rank=1
- 30.Floyd J NCT03418961 [Internet] S1501 Carvedilol in Preventing Cardiac Toxicity in Patients With Metastatic HER-2-Positive Breast Cancer. [cited 2018 Oct 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT03418961?term=NCT03418961&rank=1
- 31.Bekelman J, Cahlon O, McDonald S. NCT02603341 [Internet] The RADCOMP Study. [cited 2018 Oct 29]. Available from: http://www.radcomp.org/
- 32.Auner HW, Tinchon C, Linkesch W, Tiran A, Quehenberger F, Link H, et al. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol. 2003. April;82(4):218–22. [DOI] [PubMed] [Google Scholar]
- 33.Blaes AH, Rehman A, Vock DM, Luo X, Menge M, Yee D, et al. Utility of high-sensitivity cardiac troponin T in patients receiving anthracycline chemotherapy. Vasc Health Risk Manag. 2015;11:591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol Off J Am Soc Clin Oncol. 2010. September 1;28(25):3910–6. [DOI] [PubMed] [Google Scholar]
- 35.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004. June 8;109(22):2749–54. [DOI] [PubMed] [Google Scholar]
- 36.Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer Oxf Engl 1990. 2018. May;94:126–37. [DOI] [PubMed] [Google Scholar]
- 37.The Cardiac CARE Trial – Can we prevent heart muscle injury related to chemotherapy? [cited 2018 Oct 29]. Available from: https://www.ed.ac.uk/usher/edinburgh-clinical-trials/our-studies/ukcrc-studies/cardiac-care/cardiac-care-trial
- 38.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014. October;15(10):1063–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2013. May;26(5):493–8. [DOI] [PubMed] [Google Scholar]
- 40.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2017. March 10;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 41.Negishi T, Thavendiranathan P, Negishi K, Marwick TH, SUCCOUR investigators. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc Imaging. 2018. August;11(8):1098–105. [DOI] [PubMed] [Google Scholar]
- 42.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016. 21;37(36):2768–801. [DOI] [PubMed] [Google Scholar]
- 43.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol Off J Eur Soc Med Oncol. 2012. October;23 Suppl 7:vii155–166. [DOI] [PubMed] [Google Scholar]
- 44.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation [Internet]. 2017. August 8 [cited 2018 Dec 24];136(6). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]


