Abstract
Background
Anal precancers and cancers can be detected during screening with high-resolution anoscopy (HRA). The sensitivity of HRA depends on the burden and duration of human papillomavirus (HPV) among those screened as well as anoscopist proficiency, which is highly correlated with prior screening experience. Our objective was to compare the identification and type of HPV and the likelihood of HRA-detected precancer for men who have sex with men (MSM) undergoing their first HRA-screening in Nigeria.
Methods
MSM were recruited from an HIV test-and-treat cohort, TRUST/RV368, into a new anal cancer screening program. Anal swabs obtained during screening underwent Ion Torrent next-generation sequencing using barcoded HPV PCR broad-spectrum primers 5+/6+ to detect up to 161 HPVs. All high-risk (HR) HPVs and the most abundant low-risk (LR)-HPVs were evaluated as type-specific infections with some categorized as belonging to a multiple infection. HRA screening results included benign, low-grade squamous intraepithelial lesions (LSIL), or HSIL as detected by cytology or histology. Multivariable logistic regression was used to assess the association of HPV and other cofactors with any SIL.
Results
Among 342 MSM, 60% were HIV-infected, 89% were under 35 years of age, and 51% had 8 or more years since anal coital debut. Of those with SIL, 89% had LSIL and only 11% had HSIL. Prevalence of any HPV and high-risk (HR)-HPV was 92% and 74%, respectively. The most prevalent genotypes in rank order were HPV6 (31%), HPV16 (23%), HPV42 (20%), HPV11 (18%), HPV45 (18%), and HPV51 (17%). For multiple HR-HPVs, 31% had a single HR-HPV, 32% had 2-3, and 10% had 4 or more. Low-risk HPVs, type 6 and/or 11, were common (42%) and were significantly associated with SIL (adjusted odds ratio [aOR]:1.8, 95% confidence interval [CI]: 1.1–3.1) together with perianal warts (aOR:6.7, 95% CI: 3.3–13.5). In contrast, HR-HPV and multiple HR-HPVs were not significantly associated with SIL (all p > 0.05).
Conclusions
Detection of HSIL was low. Although HR-HPV was abundant, HSIL development also depends on the duration of HR-HPV infections and the anoscopist's level of experience. As our cohort ages and the anoscopist becomes more skilled, detection of HSIL will likely improve.
Keywords: HPV in MSM, Anal cancer screening, Sub-Saharan Africa, Next-generation sequencing
1. Introduction
HIV-infected men who have sex with men (MSM) are at higher risk of developing human papillomavirus (HPV)-associated malignancies including high-grade squamous intraepithelial lesions (HSILs) and anal cancer as compared to HIV-uninfected MSM [1]. For HIV-infected MSM, HSIL prevalence ranges from 20 to 40% in high-income countries with established HRA clinics and approximately 2% of HSIL progress to invasive anal cancer in 5 years [[2], [3], [4], [5], [6], [7]]. Oncogenic progression is highest for those with persistent high-risk HPV16. HPV16 is the most prevalent (75–82%) in the anal canal and the single most common genotype (59–76%) detected in anal cancer biopsy specimens from men and women in high-income countries [[8], [9], [10]].
HIV contributes to the persistence of multiple HPV infections [[11], [12], [13]]. For older American MSM with controlled HIV viremia, detection of multiple high-risk [HR]-HPV types significantly increased with severity of precancerous lesions (Benign: 31%, LSIL-anal intraepithelial neoplasia [AIN]1: 46%, HSIL-AIN2: 64%, HSIL-AIN3:76%) [14]. Multiple HPVs were more likely to be detected in anal cancer biopsies from HIV-infected as compared to HIV-uninfected (42% vs 10%, respectively) [15]. Furthermore, non-16/18 oncogenic HPVs were 3-fold more prevalent in anal cancers in these individuals [15]. For a small proportion of anal cancers, HPV 6 or 11 were the sole types detected, reinforcing that low-risk types still carry a risk of oncogenic progression [14,16]. Because HIV may facilitate increased persistence of a variety of HPVs, co-infection with multiple types increases the risk of developing anal precancer and cancer.
High-resolution anoscopy (HRA) is currently the gold standard for detection of precancer and cancer in the anal canal, but the procedure is challenging and hence there is a high likelihood of under-detection of lesions for an anocopist-in-training [30]. Detectability depends on the size, number, and HRA appearance of these HPV-associated lesions as well as the skill of the anoscopist. The International Anal Neoplasia Society (IANS) has issued guidelines for monitoring screening proficiency, which focus on the screening practice of the clinic and anoscopist [17]. IANS also highlights that many of the metrics outlined will vary depending on the burden of disease in a screened population. For high-income countries, cytology is used to refer at risk individuals to HRA. In the absence of referrals, HPV genotyping may inform the background prevalence of a screened population. HPV genotyping has a high negative predictive value (87%, 95% CI: 79–93%) capable of indicating who is unlikely to develop precancer [18]. Our objective was to deduce whether the population prevalence of HR-HPV increased the likelihood of HRA-detected precancer for Nigerian MSM undergoing their first HRA-screening.
2. Material and methods
2.1. Study population and sampling
TRUST/RV368 is a cohort of MSM, recruited through respondent-driven sampling for HIV testing and treatment at MSM-friendly clinics in Abuja and Lagos, Nigeria [20,21]. Participants who were at least 18 years of age and visiting the Abuja clinic for TRUST/RV368 follow-up visits were invited and consented to participate in a new anal cancer screening program. The anal cancer study enrolled 366 men between December 2016 and August 2017 and provided incentives for transport and time (Naira 1500 equivalent to USD 4) [19].
Procedures for the anal cancer study have been described previously [19]. In brief, MSM underwent swabbing for cytology and HPV testing prior to HRA. Two Copan ESwabs™ (Copan Diagnostic Inc., Murrieta, CA) were inserted by a doctor approximately 4–6 cm into the anorectum and rotated 360 degrees several times. The first swab was smeared on a glass slide and fixed in 100% alcohol for cytology. The second swab was placed in 1 mL of liquid Amies and stored at −80 °C until HPV testing. During HRA, 5% acetic acid and Lugol's iodine were used to visualize abnormalities and only men with acetowhite lesions were biopsied and evaluated by histology. Those without acetowhite lesions were classified as having a benign examination. The more severe diagnosis by cytology or histology was considered the final screening result and categorized as benign, low-grade squamous intraepithelial lesions (LSIL) or HSIL.
MSM who participated in the anal cancer study and had available anal swabs were included in this cross-sectional study. Demographic and sexual behavioral data were obtained from the TRUST/RV368 and anal cancer studies [[19], [20], [21]]. Data for laboratory-diagnosed HIV, rectal Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) were accessed from the TRUST/RV368 study.
This study was approved by the Federal Capital Territory Health Research Ethics Committee in Nigeria, the Clinical Research Committee at the University of Maryland Marlene and the Stewart Greenebaum Comprehensive Cancer Center, and the institutional review boards of the University of Maryland Baltimore and the University of California San Francisco.
2.2. HPV extraction and detection
HPV DNA was extracted from 100 μL of Amies transport medium using the QIAamp MinElute Media Kit (Qiagen, Valencia, CA). DNA was resuspended in 50 μL of Buffer AVE. Median DNA concentration was 89.9 ng/μl (interquartile range [IQR]: 75.6–111.7 ng/μl). High and low-risk HPV genotypes were detected from 5 μl of genomic DNA in a final reaction volume of 25 μl using the BSGP5+/6+ primer set in an Ion Torrent next-generation sequencing-based HPV genotyping assay [22,23]. This assay amplifies an approximately 140-bp fragment of the L1 consensus region. In brief, all samples were included in the sequencing pool at a standardized concentration of 500 pM, as determined by the BioAnalyzer (ThermoFisher, Waltham, MA). Samples without library product detection by the BioAnalyzer were included in separate pools at equal volumes. Pooled samples were purified and quantified for emulsion template preparation on the Qubit 2.0 Fluorometer (Agilent, Santa Clara, CA) and prepared using the Ion Chef (ThermoFisher, Waltham, MA). Sequencing was performed on the Ion S5 (ThermoFisher, Waltham, MA) using 400 base pair sequencing chemistry and 520 chips, with two sequencing runs per initialization. Quality and consistency of the sequencing runs were determined by reviewing the loading of ion sphere particles (ISPs) on the chips, the proportion of ISPs with more than one amplicon (polyclonal reads), and average read lengths. All assays included appropriate controls for HPV detection and contamination.
Data processing was performed by the Ion Torrent server. Only reads >100 base pairs in length were mapped to the full genomic sequences of 161 HPVs downloaded from the Papillomavirus Episteme (PaVE) database with a minimum score of AQ17. For samples with >5000 reads, HPV type-specific infections were considered positive if the number of type-specific reads accounted for more than 1% of the total number of reads. All samples negative for HPV were analyzed by PCR to identify the presence of an internal human control target (Isocitrate Dehydrogenase; IDH1), indicative of amplifiable human cellular DNA. The IDH assay is a human PCR assay used in the same clinical lab where the HPV sequencing was performed.
2.3. Statistical analysis
The primary outcome variable for all analyses was a binary categorization of having any SIL (LSIL or HSIL) because HSIL was an uncommon outcome. The main independent variables were presence of single and multiple HPVs. All HR-HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and any LR-HPV with at least 10% prevalence in the study population (6, 11, 42, 54, 67) were evaluated on a type-specific level. Low-risk (LR) HPVs, type 6 and/or 11, were grouped as wart-associated HPVs. Binomial models were used to estimate prevalence ratios with 95% confidence intervals (CIs) of HPV by HIV status.
Because HIV infection potentially modifies the detection of HPV and SIL, all variables were stratified by HIV infection status in order to systematically decide variable selection for further evaluation with SIL. Firstly, demographics, sexual behaviors, sexually transmitted infections (STIs) and HPV genotypes of the study population were described. Pearson's Chi-square and Fisher's exact tests were used to identify cofactors and HPV genotypes that were significantly different by HIV. Next, type-specific and groupings of HPV that differed by HIV status (p ≤ 0.05) were evaluated with SIL (any HPVs, any HR-HPVs, any wart-HPV, and type-specific infections [16, 35, 39, 45, 51, 58, 6, 11, 42, 67]). Pearson's Chi-square and Fisher's exact tests were used to test differences in HPV by SIL overall and after stratification by HIV. Next, HPVs that were significantly associated with SIL overall and after stratification were evaluated in a final multivariable model with behavioral and clinical confounders. Bivariate and multivariable logistic regression models were used to estimate odds ratios (ORs) with 95% CIs for the association between LR-HPV 6 and/or 11 and other risk factors of any SIL. Candidate variables identified in bivariate analyses with HIV (p < 0.20) (age, years since coital debut, lifetime number of receptive partners, any receptive anal sex, rectal NG, perianal warts) were entered into the multivariable model using a forward stepwise selection. A two-sided p-value less than 0.05 was considered statistically significant and a p-value <0.10 was considered suggestive of a trend. Significant, trending, and a priori covariates of SIL based on our prior work [19], were retained in the final model.
Model fit was evaluated using likelihood ratio tests and through a calibration plot of 9 bins that compared the agreement of the average predicted probabilities versus the average observed frequencies. High agreement is indicated by a slope of 1, a calibration-in-the-large (CITL) value close to zero, and a concordance index equivalent to area under the curve (AUC) for a binary outcome. A model with perfect discrimination has an AUC of 1 and one with an AUC of 0.5 predicts no better than chance. Data were analyzed using Stata Statistical Software, Release 13 (StataCorp, College Station, TX) and Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC).
3. Results
A total of 364 MSM from HRA screening had available anal swabs, including 22 (6%) with insufficient cellular material to assess HPV status. The remaining 342 participants with adequate swab specimens were included in the present analysis (Table 1). Of these, 89% were under 35 years of age and 89% were never married. Fifty-one percent had 8 or more years since anal coital debut, 32% had 11 or more lifetime receptive partners and 81% engaged in receptive anal intercourse in the past year. Prevalence of rectal STIs (CT and/or NG) was 22% (73/338) and of the men observed by the physician, 29% had perianal warts during screening. Sixty percent of the men (204/342) were HIV-infected, with a median CD4(+) count of 456 count/mm3 (IQR: 299–623 count/mm3) and 65% (133/204) with HIV RNA <1000 copies/mL. Many of the HIV-infected were older and reported more sexual practices as compared to the HIV-uninfected (Table 1).
Table 1.
Characteristics of HIV-infected and HIV-uninfected Men who Have Sex with Men who Underwent High-Resolution Anoscopy in Abuja, Nigeria.
| Total N = 342 n (%) |
HIV-infected N = 204 n (%) |
HIV-uninfected N = 138 n (%) |
P† | |
|---|---|---|---|---|
| Age (years) | <0.01 | |||
| ≤24 | 148 (43.3) | 70 (34.3) | 78 (56.5) | |
| 25–34 | 156 (45.6) | 111 (54.4) | 45 (32.6) | |
| ≥35 | 38 (11.1) | 23 (11.3) | 15 (10.9) | |
| Ever married | 0.51 | |||
| No | 302 (89.1) | 179 (88.2) | 123 (90.4) | |
| Yes | 37 (10.9) | 24 (11.8) | 13 (9.6) | |
| Years since anal coital debut | <0.01 | |||
| ≤7 | 159 (49.1) | 82 (42.1) | 77 (59.7) | |
| ≥8 | 165 (50.9) | 113 (58.0) | 52 (40.3) | |
| Lifetime No. of receptive partners | <0.01 | |||
| ≤10 | 227 (68.0) | 114 (57.0) | 113 (84.3) | |
| ≥11 | 107 (32.0) | 86 (43.0) | 21 (15.7) | |
| Any receptive anal sex in past year | <0.01 | |||
| No | 64 (19.0) | 25 (12.4) | 39 (28.7) | |
| Yes | 273 (81.0) | 176 (87.6) | 97 (71.3) | |
| Transactional sex in past year | 0.56 | |||
| No | 208 (64.4) | 128 (65.6) | 80 (62.5) | |
| Yes | 115 (35.6) | 67 (34.4) | 48 (37.5) | |
| Rectal Neisseria gonorrhoeae | 0.07 | |||
| No | 291 (85.8) | 180 (88.7) | 111 (81.6) | |
| Yes | 48 (14.2) | 23 (11.3) | 25 (18.4) | |
| Rectal Chlamydia trachomatis | 0.24 | |||
| No | 300 (88.5) | 183 (90.2) | 117 (86.0) | |
| Yes | 39 (11.5) | 20 (9.9) | 19 (14.0) | |
| Perianal warts | <0.01 | |||
| No | 242 (71.0) | 128 (62.8) | 114 (83.2) | |
| Yes | 99 (29.0) | 76 (37.3) | 23 (16.8) | |
| Anal Cancer Screening Results* | <0.01 | |||
| Benign | 146 (42.7) | 68 (33.3) | 78 (56.5) | |
| LSIL | 174 (50.9) | 120 (58.8) | 54 (39.1) | |
| HSIL | 22 (6.4) | 16 (7.8) | 6 (4.4) | |
Abbreviations: p, p-value; No., number; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
*Worst diagnosis between cytology or histology.
†Pearson's Chi-square test.
Bold type indicates statistically significant (p < 0.05) differences in behavioral and clinical characteristics by HIV status.
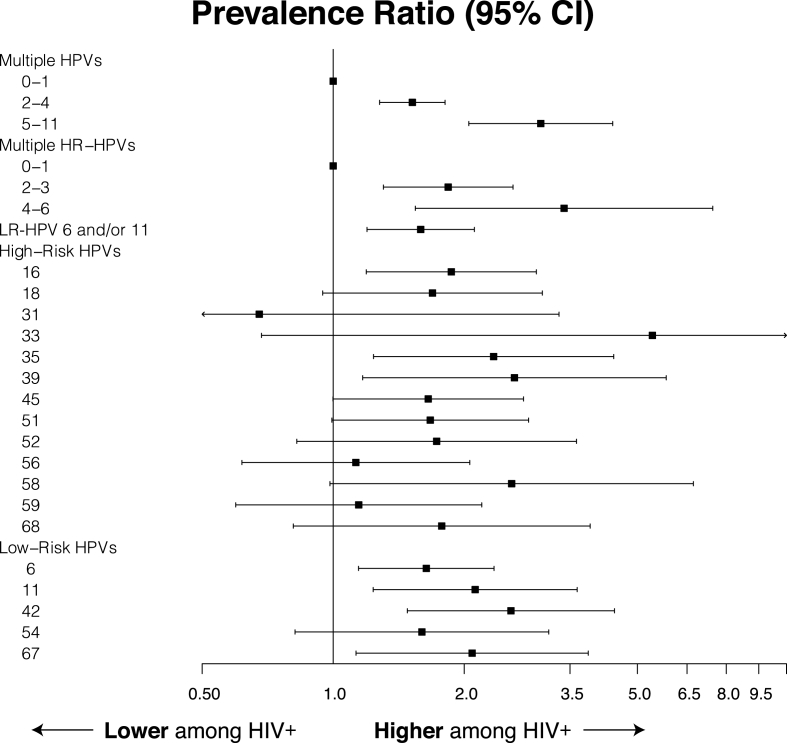
Prevalence of any HPV and high-risk (HR)-HPV was 92% and 74%, respectively. The median and maximum number of genotypes detected among all participants were 3 and 11, respectively. The most prevalent HPVs in rank order were HPV6 (31%), HPV16 (23%), HPV42 (20%), HPV11 (18%), HPV45 (18%), and HPV51 (17%); these percentages of type-specific infections are not exclusive as a result of multiple co-infected individuals. (Table 2). For multiple HR-HPVs, 31% had a single HR-HPV, 32% had 2-3, and 10% had 4 or more. Forty-two percent of participants had either HPV6 or 11, the wart-associated type-specific HPVs. HIV-infected men were 2-fold more likely to have any or multiple HPVs detected (Fig. 1).
Table 2.
Prevalence of HPV for HIV-infected and HIV-uninfected Nigerian MSM who Underwent High-Resolution Anoscopy in Abuja, Nigeria.
| HPV genotype | Total n = 342 n (%) |
HIV-infected n = 204 n (%) |
HIV-uninfected n = 138 n (%) |
P* |
|---|---|---|---|---|
| Multiple HPVs | <0.01 | |||
| 0 | 29 (8.5) | 2 (1.0) | 27 (19.6) | |
| 1 | 38 (11.1) | 14 (6.9) | 24 (17.4) | |
| 2–4 | 168 (49.1) | 101 (49.5) | 67 (48.6) | |
| 5–11 | 107 (31.3) | 87 (42.7) | 20 (14.5) | |
| Multiple High-Risk HPVs | <0.01 | |||
| 0 | 90 (26.3) | 29 (14.2) | 61 (44.2) | |
| 1 | 106 (31.0) | 68 (33.3) | 38 (27.5) | |
| 2–3 | 111 (32.5) | 79 (38.7) | 32 (23.2) | |
| 4–6 | 35 (10.2) | 28 (13.7) | 7 (5.1) | |
| Low-Risk HPV 6 and/or 11 | <0.01 | |||
| No | 198 (57.9) | 103 (50.5) | 95 (68.8) | |
| Yes | 144 (42.1) | 101 (49.5) | 43 (31.2) | |
| High-risk HPVs | ||||
| 16 | 79 (23.1) | 58 (28.4) | 21 (15.2) | <0.01 |
| 18 | 49 (14.3) | 35 (17.2) | 14 (10.1) | 0.07 |
| 31 | 6 (1.8) | 3 (1.5) | 3 (2.2) | 0.69 |
| 33 | 9 (2.6) | 8 (3.9) | 1 (0.7) | 0.09 |
| 35 | 49 (14.3) | 38 (18.6) | 11 (8.0) | <0.01 |
| 39 | 34 (9.9) | 27 (13.2) | 7 (5.1) | 0.01 |
| 45 | 62 (18.1) | 44 (21.6) | 18 (13.0) | 0.05 |
| 51 | 59 (17.3) | 42 (20.6) | 17 (12.3) | 0.05 |
| 52 | 32 (9.4) | 23 (11.3) | 9 (6.5) | 0.14 |
| 56 | 40 (11.7) | 25 (12.3) | 15 (10.9) | 0.70 |
| 58 | 24 (7.0) | 19 (9.3) | 5 (3.6) | 0.05 |
| 59 | 35 (10.2) | 22 (10.9) | 13 (9.4) | 0.68 |
| 68 | 29 (8.5) | 21 (10.3) | 8 (5.8) | 0.14 |
| Low-risk HPVs | ||||
| 6 | 106 (31.0) | 75 (36.8) | 31 (22.5) | <0.01 |
| 11 | 62 (18.1) | 47 (23.0) | 15 (10.9) | <0.01 |
| 42 | 67 (19.6) | 53 (26.0) | 14 (10.1) | <0.01 |
| 54 | 37 (10.8) | 26 (12.8) | 11 (8.0) | 0.16 |
| 67 | 49 (14.3) | 37 (18.1) | 12 (8.7) | 0.01 |
Abbreviations: p, p-value.
*Pearson's Chi-square and Fisher's exact tests. Bold type indicates statistically significant (p < 0.05) differences in HPV prevalence by HIV infection status.
Fig. 1.
HPV prevalence ratios and 95% confidence intervals by HIV status.
The overall prevalence of any SIL (LSIL or HSIL) was 57% (196/342). HSIL comprised only 6% of those screened (11% of the SILs) and was pooled with LSIL to improve power. The only HPVs significantly associated with detection of any SIL during anal cancer screening were the wart-related HPVs, specifically HPV6 (Table 3). These findings were consistent for the overall sampled population and after stratification by HIV infection. For the LSIL cases strictly diagnosed by histology, the pathologist indicated that 78% (102/131) had condyloma/HPV. There was increased detection of SIL as the number of HPVs increased both overall and among HIV-uninfected. Detection of SIL was similar regardless of HPV16 infection or increasing numbers of HR-HPV for all men and after stratification by HIV (all p > 0.05). For HIV-infected MSM, in whom the majority of HSIL cases were diagnosed, prevalence of HR-HPVs did not increase with severity of lesions (Supplemental Fig. A1).
Table 3.
Overall and HIV stratified proportions of any squamous intraepithelial lesions by anal HPV genotypes (row percentages of number of SIL cases/person with or without HPV).
| Any LSIL/HSIL |
|||
|---|---|---|---|
| Overall n/N (%) |
HIV-infected n/N (%) |
HIV-uninfected n/N (%) |
|
| Multiple HPVs | |||
| 0 | 5/29 (17.2) | 0/2 (0.0) | 5/27 (18.5) |
| 1 | 23/38 (60.5) | 10/14 (71.4) | 13/24 (54.2) |
| 2–4 | 102/168 (60.7) | 69/101 (68.3) | 33/67 (49.3) |
| 5–11 | 66/107 (61.7) | 57/87 (65.5) | 9/20 (45.0) |
| Multiple High-Risk HPVs | |||
| 0 | 44/90 (48.9) | 21/29 (72.4) | 23/61 (37.7) |
| 1 | 64/106 (60.4) | 41/68 (60.3) | 23/38 (60.5) |
| 2–3 | 68/111 (61.3) | 56/79 (70.9) | 12/32 (37.5) |
| 4–6 | 20/35 (57.1) | 18/28 (64.3) | 2/7 (28.6) |
| Low-Risk HPV 6 and/or 11 | |||
| No | 90/198 (45.5) | 60/103 (58.3) | 30/95 (31.6) |
| Yes | 106/144 (73.6) | 76/101 (75.3) | 30/43 (69.8) |
| High-Risk HPVs | |||
| HPV16 | |||
| No | 148/263 (56.3) | 98/146 (67.1) | 50/117 (42.7) |
| Yes | 48/79 (60.8) | 38/58 (65.5) | 10/21 (47.6) |
| HPV35 | |||
| No | 170/293 (58.0) | 114/166 (68.7) | 56/127 (44.1) |
| Yes | 26/49 (53.1) | 22/38 (57.9) | 4/11 (36.4) |
| HPV39 | |||
| No | 174/308 (56.5) | 117/177 (66.1) | 57/131 (43.5) |
| Yes | 22/34 (64.7) | 19/27 (70.4) | 3/7 (42.9) |
| HPV45 | |||
| No | 154/280 (55.0) | 102/160 (63.8) | 52/120 (43.3) |
| Yes | 42/62 (67.7) | 34/44 (77.3) | 8/18 (44.4) |
| HPV51 | |||
| No | 159/283 (56.2) | 107/162 (66.1) | 52/121 (43.0) |
| Yes | 37/59 (62.7) | 29/42 (69.1) | 8/17 (47.1) |
| HPV58 | |||
| No | 183/318 (57.6) | 125/185 (67.6) | 58/133 (43.6) |
| Yes | 13/24 (54.2) | 11/19 (57.9) | 2/5 (40.0) |
| Low-Risk HPVs | |||
| HPV6 | |||
| No | 116/236 (49.2) | 77/129 (59.7) | 39/107 (36.5) |
| Yes | 80/106 (75.5) | 59/75 (78.7) | 21/31 (67.7) |
| HPV11 | |||
| No | 150/280 (53.6) | 101/157 (64.3) | 49/123 (39.8) |
| Yes | 46/62 (74.2) | 35/47 (74.5) | 11/15 (73.3) |
| HPV42 | |||
| No | 153/275 (55.6) | 100/151 (66.2) | 53/124 (42.7) |
| Yes | 43/67 (64.2) | 36/53 (67.9) | 7/14 (50.0) |
| HPV67 | |||
| No | 166/293 (56.7) | 112/167 (67.1) | 54/126 (42.9) |
| Yes | 30/49 (61.2) | 24/37 (64.9) | 6/12 (50.0) |
Abbreviations: SIL, squamous intraepithelial lesions; L/HSIL, low-grade/high-grade squamous intraepithelial lesions.
Bold type indicates statistical significance (p < 0.05) differences in the prevalence of SIL by HPVs overall and stratified by HIV using Pearson's Chi-Square and Fisher's exact tests.
After adjusting for years since anal coital debut, HIV, rectal NG, and perianal warts, men with wart-related HPVs had a 2-fold increased odds of SIL detection as compared to those uninfected with HPV6 and/or 11 (adjusted OR [aOR]: 1.8, 95% CI: 1.1–3.1) (Table 4). Perianal warts were also independently associated with SIL detection (aOR: 6.7, 95% CI: 3.3–13.5). HIV and more years since anal coital debut trended towards a positive association (aOR: 1.6, 95% CI: 1.0–2.7, aOR: 1.7, 95% CI: 1.0–2.7, respectively) while rectal NG trended towards an inverse association (aOR: 0.5, 95% CI: 0.3–1.1) with SIL. Receptive anal sex was highly correlated with HIV infection and could not be retained in the final model. Using our final model, the binned predicted probabilities of the binary outcome SIL aligned linearly with the observed frequencies of SIL (slope = 0.991, CITL = 0.009, AUC = 0.752), suggesting this was a well-calibrated model (Supplemental Fig. B1).
Table 4.
Low-risk HPV 6 and/or 11 and Other Factors Associated with Any Squamous Intraepithelial Lesions.
| Characteristic | Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|---|---|---|
| Low-Risk HPV 6 and/or 11 | ||
| No | Ref. | Ref. |
| Yes | 3.35 (2.1–5.3) | 1.81 (1.1–3.1) |
| Age (years) | ||
| ≤24 | Ref. | |
| 25–34 | 1.5 (1.0–2.4) | |
| ≥35 | 1.6 (0.8–3.4) | |
| Years since anal coital debut | ||
| ≤7 | Ref. | Ref. |
| ≥8 | 1.56 (1.0–2.4) | 1.65 (1.0–2.7) |
| Lifetime No. of receptive partners | ||
| ≤10 | Ref. | |
| ≥11 | 1.24 (0.8–2.0) | |
| Receptive anal sex in past year | ||
| No | Ref. | |
| Yes | 1.79 (1.0–3.1) | |
| Transactional sex in past year | ||
| No | Ref. | |
| Yes | 1.06 (0.7–1.7) | |
| HIV infection | ||
| No | Ref. | Ref. |
| Yes | 2.60 (1.7–4.1) | 1.63 (1.0–2.7) |
| Rectal Neisseria gonorrhoeae | ||
| No | Ref. | Ref. |
| Yes | 0.63 (0.3–1.2) | 0.52 (0.3–1.1) |
| Rectal Chlamydia trachomatis | ||
| No | Ref. | |
| Yes | 0.67 (0.3–1.3) | |
| Perianal warts | ||
| No | Ref. | Ref. |
| Yes | 8.85 (4.6–17.0) | 6.66 (3.3–13.5) |
Abbreviations: HPV, human papillomavirus; OR, odds ratio; CI, confidence interval; No., number.
*The final model was adjusted for years since sexual debut, HIV, rectal Neisseria gonorrhoeae and perianal warts.
Bold type indicates statistically significant (p < 0.05).
4. Discussion
Our HPV-genotyping findings confirm that many Nigerian MSM undergoing screening with high-resolution anoscopy especially those with HIV, were co-infected with HPV16 and multiple HR-HPVs. Presence of any HPV16, HPV18, or multiple HR-HPVs, even after stratification by HIV, was not associated with LSIL or HSIL. Prior studies have shown that the presence of HPV16 and HPV18 increases the likelihood HSIL will be detected [24,25] and this association is independent of HIV status [[26], [27], [28]]. In addition, multiple HPV infections have been linked to increasing severity of SIL [29]. Despite creating a broad categorization of SIL by pooling LSIL and HSIL, SIL prevalence was the same in those with many (5–11) HR-HPVs as compared to those with very few (0–1) HR-HPVs. After stratifying by HIV, SIL detection did not differ by the number or type of HR-HPVs. Indirectly, not having a higher prevalence of SIL in those infected with HR-HPV suggests either our anoscopist was still relatively inexperienced and/or the HR-HPV infections did not have sufficient time to develop into lesions [19].
The lesions that were detected by the anoscopist were primarily LSIL and more abundant among MSM infected with LR-HPV 6 and/or 11. LR-HPV 6 and/or 11 cause 90% of anogenital warts (AGWs) [31,32] and if biopsied would be diagnosed by a pathologist as LSIL. AGWs can develop within 2–3 months after HPV infection and remain unchanged, become larger, or regress [[33], [34], [35]]. Incidence of AGWs peaks for men in their mid-twenties and transmission of AGWs to an uninfected partner can be as high as 64% [36,37]. To verify LR-HPV 6 and/or 11 was truly associated with LSIL independent of previously identified risk factors such as HIV and perianal warts, we evaluated the performance of our final model and found the predicted values agreed with the observed values, suggesting no systematic differences and a good model fit.
During screening, our anoscopist noted a high prevalence of warts in the anal canal. AGWs are characterized by single or multiple papules that may draw the attention of a novice anoscopist to biopsy when learning high-resolution anoscopy (HRA). Nearly 80% of the LSILs detected by our pathologist were further characterized as being condyloma/HPV related. Frequent dialogue between the anoscopist and the pathologist about lesion characteristics would help any new anoscopist in the differentiation of SIL associated with HR-HPV versus LR-HPV and alter biopsy practices [38]. Adding p16 and/or Ki67 immunostaining during histology could also help distinguish HSIL from LSIL [39].
Although AGWs have historically be associated with benign disease, they should not be readily dismissed. Evidence is mounting that AGWs are common among those who develop anal cancer [[40], [41], [42]]. Studies have found AGWs co-infected with HR-HPV [43] and a proportion of AGWs contain HSIL [39,[44], [45], [46]]. There is no current consensus on treatment of AGWs [[47], [48]], but if they can be removed without sexual impairment, then it may diminish further transmission and improve visualization and detection of HSIL.
This study had some limitations. First, detection of HPV genotypes was from exfoliated cells of the anal canal and not from biopsies of the lesions, so the causal relationship between HPV 6 or 11 and LSIL could not be determined. Second, the majority of SIL cases were LSIL and power was limited to evaluate the association of HR-HPV infections with HSIL. Upon improved proficiency with HRA, future studies with more samples could type-specific HPVs from HSIL. Lastly, morphological characteristics of the lesions, such as homogenous terminal capillaries, would have helped confirm which LSILs were AGWs, but these data were not collected and should be considered when implementing future HRA screening [38,39].
In conclusion, this study confirmed a high abundance of HR-HPV and multiple HR-HPVs in our screened population. For any new screening program, understanding the characteristics of the screened population, such as age or frequency of HPV-related condyloma, are important for gauging expectations of an anoscopist-in-training. Our participants have sufficient HR-HPV and detection of HSIL will likely improve as they age and the anoscopist is supported with additional mentoring.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author. As a precautionary measure due to the criminalization of same-sex behavior in Nigeria, the research data is kept confidential.
Funding sources
Funding for this research was supported by the National Cancer Institute [2P30CA134274-09S2, 5P30CA134274] and jointly with the Office of AIDS Research [1K07CA225403]. Additional support was provided by the National Institutes of Health [R01 MH099001, R01 AI120913, R01 MH110358]; the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040]; Fogarty Epidemiology Research Training for Public Health Impact in Nigeria program [D43TW010051]; and the President's Emergency Plan for AIDS Relief through a cooperative agreement between the Department of Health and Human Services/Centers for Disease Control and Prevention, Global AIDS Program, and the Institute for Human Virology-Nigeria [NU2GGH002099]. The content is solely the responsibility of the authors and should not be construed to represent the positions of the National Institutes of Health, U.S. Army, the Department of Defense, the Department of Health and Human Services, or other funders. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
CRediT authorship contribution statement
Rebecca G. Nowak: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Lisa M. Schumaker: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Nicholas P. Ambulos: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Nicaise Ndembi: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Wuese Dauda: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Chinedu H. Nnaji: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Andrew Mitchell: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Trevor J. Mathias: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Paul Jibrin: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Teresa M. Darragh: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Oluwole Olaomi: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Trevor A. Crowell: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Stefan D. Baral: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Manhattan E. Charurat: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Søren M. Bentzen: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Joel M. Palefsky: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition. Kevin J. Cullen: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JMP has stock and ownership interests in Ubiome, Virion Therapeutics, VIR Biotechnology; has received honoraria from Janssen Pharmaceuticals, Vaccitech, Antiva Biosciences; has a consulting or advisory role for Antiva Biosciences, VIR Biotechnology, Vaccitech and Novan; receives research funding from Merck, Antiva Biosciences, VIR Biotechnology, CEL-SCI; and receives travel, accommodations and expenses from Merck, Vaccitech and Janssen. None of this relates to this specific work. All other authors have no declarations of interest.
Acknowledgements
The authors would like to thank all research participants, study staff and ICARH for their commitment to this study. We thank William A. Blattner, MD for his mentorship. We also give special thanks for Jing Yin and Li Tang for their availability and support in generating the HPV and IDH1 data as well as Lionhare Design for their graphic design support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2020.100200.
Contributor Information
Rebecca G. Nowak, Email: rnowak@ihv.umaryland.edu.
TRUST/RV368 Study Group:
Manhattan Charurat, Julie Ake, Aka Abayomi, Sylvia Adebajo, Stefan Baral, Trevor Crowell, Charlotte Gaydos, Sosthenes Ketende, Afoke Kokogho, Jennifer Malia, Olumide Makanjuola, Nelson Michael, Nicaise Ndemb, Rebecca Nowak, Oluwasolape Olawore, Zahra Parker, Sheila Peel, Habib Ramadhani, Merlin Robb, Cristina Rodriguez-Hart, Eric Sanders-Buell, Elizabeth Shoyemi, Sodsai Tovanabutra, and Sandhya Vasan
Appendix A. Supplementary data
The following are the supplementary data to this article:
HPV Genotype Prevalence by Composite Screening Results, Stratified by HIV Infection.
Calibration Plot of Predicted and Expected from the Final Adjusted Logistic Model.
Note: E:O, ratio of expected and observed events; CITL, calibration-in-the-large (calculated on the logit scale); Slope, calibration slope; AUC, area under the curve represents a concordance index where 0.5 indicates discrimination no better than chance and 1.0 indicates perfect discrimination.
References
- 1.Machalek D.A., Poynten M., Jin F., Fairley C.K., Farnsworth A., Garland S.M., Hillman R.J., Petoumenos K., Roberts J., Tabrizi S.N., Templeton D.J., Grulich A.E. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 2.Schim van der Loeff M.F., Mooij S.H., Richel O., de Vries H.J.C., Prins J.M. HPV and anal cancer in HIV-infected individuals: a review. Curr. HIV AIDS Rep. 2014;11:250–262. doi: 10.1007/s11904-014-0224-x. [DOI] [PubMed] [Google Scholar]
- 3.Cachay E., Agmas W., Mathews C. Five-year cumulative incidence of invasive anal cancer among HIV-infected patients according to baseline anal cytology results: an inception cohort analysis. HIV Med. 2015;16:191–195. doi: 10.1111/hiv.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phanuphak N., Teeratakulpisarn N., Triratanachat S., Keelawat S., Pankam T., Kerr S.J., Deesua A., Tantbirojn P., Numto S., Phanuphak P., Palefsky J.M., Ananworanich J. High prevalence and incidence of high-grade anal intraepithelial neoplasia among young Thai men who have sex with men with and without HIV. AIDS. 2013;27:1753–1762. doi: 10.1097/QAD.0b013e328360a509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegenbeek van Heukelom M.L., Marra E., de Vries H.J.C., Schim van der Loeff M.F., Prins J.M. Risk factors for anal high-grade squamous intraepithelial lesions in HIV-positive MSM: is targeted screening possible? AIDS. 2017;31:2295–2301. doi: 10.1097/QAD.0000000000001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goddard S.L., Templeton D.J., Petoumenos K., Jin F., Hillman R.J., Law C., Roberts J.M., Fairley C.K., Garland S.M., Grulich A.E., Poynten I.M. Association of anal symptoms with anal high grade squamous intraepithelial lesions (HSIL) among men who have sex with men: baseline data from the study of the prevention of anal cancer (SPANC) Canc. Epidemiol. 2019;58:12–16. doi: 10.1016/j.canep.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Machalek D.A., Jin F., Poynten I.M., Hillman R.J., Templeton D.J., Law C., Roberts J.M., Tabrizi S.N., Garland S.M., Farnsworth A., Fairley C.K., Grulich A.E., Acraman B., Adams M., Carr A., Carroll S., Cooper D., Cornall A., Crampton L., Ekman D., Feeney L., Fraissard E., Howard K., Law M., McCaffery K., McDonald R., McGrath P., Mellor R., Norris R., O'Dwyer M., Pendlebury S., Petoumenos K., Phillips S., Prestage G., Richards A., Schema L., Seeds D., Segelov E., Thurloe J., Tong W., Varma R. Prevalence and risk factors associated with high-grade anal squamous intraepithelial lesions (HSIL)-AIN2 and HSIL-AIN3 in homosexual men. Papillomavirus Res. 2016;2:97–105. doi: 10.1016/j.pvr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillman R.J., Garland S.M., Gunathilake M.P.W., Stevens M., Kumaradevan N., Lemech C., Ward R.L., Meagher A., McHugh L., Jin F., Carroll S., Goldstein D., Grulich A.E., Tabrizi S.N. Human papillomavirus (HPV) genotypes in an Australian sample of anal cancers. Int. J. Canc. 2014;135:996–1001. doi: 10.1002/ijc.28730. [DOI] [PubMed] [Google Scholar]
- 9.Ouhoummane N., Steben M., Coutlee F., Vuong T., Forest P., Rodier C., Louchini R., Duarte E., Brassard P. Squamous anal cancer: patient characteristics and HPV type distribution. Canc. Epidemiol. 2013;37:807–812. doi: 10.1016/j.canep.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz L., Jacquard A.-C., Jaroud F., Haesebaert J., Siproudhis L., Pradat P., Aynaud O., Leocmach Y., Soubeyrand B., Dachez R., Riethmuller D., Mougin C., Pretet J.-L., Denis F. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int. J. Canc. 2011;129:433–439. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 11.Phanuphak N., Teeratakulpisarn N., Pankam T., Kerr S.J., Barisri J., Deesua A., Rodbamrung P., Hongchookiat P., Chomchey N., Phanuphak P., Sohn A.H., Ananworanich J., Palefsky J.M. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J. Acquir. Immune Defic. Syndr. 2013;63:472–479. doi: 10.1097/QAI.0b013e3182918a5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geskus R.B., González C., Torres M., Del Romero J., Viciana P., Masiá M., Blanco J.R., Iribarren G.M., De Sanjosé S., Hernández-Novoa B., Ortiz M., Del Amo J., Pena A., Garcia F., Ocampo A., Da Silva A.R., Miralles C., Gonzalez-Carrero J., Madrid N., Benito A., Sanz I., Vera M., Rodriguez C., Puerta T., Carrio J.C., Raposo M., Trastoy M., Fontillon M., Robledano C., Gutierrez F., Padilla S., Andrada E., Cervero M., Perez L., Portilla J., Portilla I., Vonwichmann M.A., Iribarren J.A., Camino X., Sendagorta E., Herranz P., Rodriguez P., Gomez J.L., Rosado D., Rodriguez-Arenas M.A., Alejos B., Sobrino-Vegas P. Incidence and clearance of anal high-risk human papillomavirus in HIV-positive men who have sex with men: estimates and risk factors. AIDS. 2016;30 doi: 10.1097/QAD.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Pokomandy A., Rouleau D., Ghattas G., Vezina S., Cote P., Macleod J., Allaire G., Franco E.L., Coutlee F. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J. Infect. Dis. 2009;199:965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 14.Sahasrabuddhe V.V., Castle P.E., Follansbee S., Borgonovo S., Tokugawa D., Schwartz L.M., Lorey T.S., LaMere B.J., Gage J.C., Fetterman B., Boyle S., Sadorra M., Tang S.D., Darragh T.M., Wentzensen N. Human papillomavirus genotype attribution and estimation of preventable fraction of anal intraepithelial neoplasia cases among HIV-infected men who have sex with men. J. Infect. Dis. 2013;207:392–401. doi: 10.1093/infdis/jis694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018;18 doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornall A.M., Roberts J.M., Garland S.M., Hillman R.J., Grulich A.E., Tabrizi S.N. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int. J. Canc. 2013;133:2253–2258. doi: 10.1002/ijc.28228. [DOI] [PubMed] [Google Scholar]
- 17.Hillman R.J., Cuming T., Darragh T., Nathan M., Berry-Lawthorn M., Goldstone S., Law C., Palefsky J., Barroso L.F., Stier E.A., Bouchard C., Almada J., Jay N. IANS international guidelines for practice standards in the detection of anal cancer precursors. J. Low. Genit. Tract Dis. 2016;20(2016):283–291. doi: 10.1097/LGT.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 18.Dias Goncalves Lima F., Viset J.D., Leeflang M.M.G., Limpens J., Prins J.M., de Vries H.J.C. The accuracy of anal swab-based tests to detect high-grade Anal intraepithelial neoplasia in HIV-infected patients: a systematic review and meta-analysis. Open Forum Infect. Dis. 2019;6:ofz191. doi: 10.1093/ofid/ofz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak R.G., Ndembi N., Dauda W., Jibrin P., Bentzen S.M., Nnaji C.H., Olaomi O., Darragh T.M., Madukwe J., Crowell T.A., Baral S.D., Blattner W.A., Charurat M.E., Palefsky J.M., Cullen K.J. Implementation of and early outcomes from anal cancer screening at a community-engaged Health care facility providing care to Nigerian men who have sex with men. J. Glob. Oncol. 2019:1–11. doi: 10.1200/JGO.19.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charurat M.E., Emmanuel B., Akolo C., Keshinro B., Nowak R.G., Kennedy S., Orazulike I., Ake J., Njoku O., Baral S., Blattner W. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. J. Acquir. Immune Defic. Syndr. 2015;68(Suppl 2):S114–S123. doi: 10.1097/QAI.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baral S.D., Ketende S., Schwartz S., Orazulike I., Ugoh K., Peel S.A., Ake J., Blattner W., Charurat M. Evaluating respondent-driven sampling as an implementation tool for universal coverage of antiretroviral studies among men who have sex with men living with HIV. J. Acquir. Immune Defic. Syndr. 2015;68(Suppl 2):S107–S113. doi: 10.1097/QAI.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambulos N.P.J., Schumaker L.M., Mathias T.J., White R., Troyer J., Wells D., Cullen K.J. Next-generation sequencing-based HPV genotyping assay validated in formalin-fixed, paraffin-embedded oropharyngeal and cervical cancer specimens. J. Biomol. Tech. 2016;27:46–52. doi: 10.7171/jbt.16-2702-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak R.G., Ambulos N.P., Schumaker L.M., Mathias T.J., White R.A., Troyer J., Wells D., Charurat M.E., Bentzen S.M., Cullen K.J. Genotyping of high-risk anal human papillomavirus (HPV): ion torrent-next generation sequencing vs. linear array. Virol. J. 2017;14:112. doi: 10.1186/s12985-017-0771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machalek D.A., Poynten I.M., Jin F., Hillman R.J., Templeton D.J., Law C., Roberts J.M., Tabrizi S.N., Garland S.M., Farnsworth A., Fairley C.K., Grulich A.E. A composite cytology-histology endpoint Allows a more accurate estimate of anal high-grade squamous intraepithelial lesion prevalence. Cancer Epidemiol. Biomark. Prev. 2016;25:1134–1143. doi: 10.1158/1055-9965.EPI-15-1106. [DOI] [PubMed] [Google Scholar]
- 25.Hoots B.E., Palefsky J.M., Pimenta J.M., Smith J.S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Canc. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 26.de Pokomandy A., Rouleau D., Ghattas G., Trottier H., Vezina S., Cote P., Macleod J., Allaire G., Hadjeres R., Franco E.L., Coutlee F. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin. Infect. Dis. 2011;52:1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 27.Burgos J., Curran A., Tallada N., Guelar A., Navarro J., Landolfi S., Villar J., Crespo M., Ribera E., Falcó V. Risk of progression to high-grade anal intraepithelial neoplasia in HIV-infected MSM. AIDS. 2015;29 doi: 10.1097/QAD.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Sigel K., Gaisa M.M. Human papillomavirus genotypes predict progression of anal low-grade squamous intraepithelial lesions. J. Infect. Dis. 2018;218:1746–1752. doi: 10.1093/infdis/jiy463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palefsky J.M., Holly E.A., Efirdc J.T., Da Costa M., Jay N., Berry J.M., Darragh T.M. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 30.Palefsky J.M. Practising high-resolution anoscopy. Sex. Health. 2012;9:580–586. doi: 10.1071/SH12045. [DOI] [PubMed] [Google Scholar]
- 31.Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnurch H.G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc. Natl. Acad. Sci. U.S.A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland S.M., Steben M., Sings H.L., James M., Lu S., Railkar R., Barr E., Haupt R.M., Joura E.A. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 33.Lacey C.J.N., Lowndes C.M., Shah K.V. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Suppl 3):35–41. doi: 10.1016/j.vaccine.2006.06.015. S3. [DOI] [PubMed] [Google Scholar]
- 34.Winer R.L., Kiviat N.B., Hughes J.P., Adam D.E., Lee S.-K., Kuypers J.M., Koutsky L.A. Development and duration of human papillomavirus lesions, after initial infection. J. Infect. Dis. 2005;191:731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 35.Lacey C.J.N. Therapy for genital human papillomavirus-related disease. J. Clin. Virol. 2005;32(Suppl 1):S82–S90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Patel H., Wagner M., Singhal P., Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oriel J.D. Natural history of genital warts. Br. J. Vener. Dis. 1971;47:1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jay N., Berry J.M., Miaskowski C., Cohen M., Holly E., Darragh T.M., Palefsky J.M. Colposcopic characteristics and Lugol's staining differentiate anal high-grade and low-grade squamous intraepithelial lesions during high resolution anoscopy. Papillomavirus Res. (Amsterdam, Netherlands) 2015;1:101–108. doi: 10.1016/j.pvr.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreuter A., Siorokos C., Oellig F., Silling S., Pfister H., Wieland U. High-grade dysplasia in anogenital warts of HIV-positive men. JAMA Dermatol. 2016;152:1225–1230. doi: 10.1001/jamadermatol.2016.2503. [DOI] [PubMed] [Google Scholar]
- 40.Daling J.R., Madeleine M.M., Johnson L.G., Schwartz S.M., Shera K.A., Wurscher M.A., Carter J.J., Porter P.L., Galloway D.A., McDougall J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 41.Blomberg M., Friis S., Munk C., Bautz A., Kjaer S.K. Genital warts and risk of cancer: a Danish study of nearly 50 000 patients with genital warts. J. Infect. Dis. 2012;205:1544–1553. doi: 10.1093/infdis/jis228. [DOI] [PubMed] [Google Scholar]
- 42.Holly E.A., Whittemore A.S., Aston D.A., Ahn D.K., Nickoloff B.J., Kristiansen J.J. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J. Natl. Cancer Inst. 1989;81:1726–1731. doi: 10.1093/jnci/81.22.1726. [DOI] [PubMed] [Google Scholar]
- 43.Brown D.R., Schroeder J.M., Bryan J.T., Stoler M.H., Fife K.H. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J. Clin. Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richel O., Hallensleben N.D.L., Kreuter A., van Noesel C.J.M., Prins J.M., de Vries H.J.C. High-resolution anoscopy: clinical features of anal intraepithelial neoplasia in HIV-positive men. Dis. Colon Rectum. 2013;56:1237–1242. doi: 10.1097/DCR.0b013e3182a53568. [DOI] [PubMed] [Google Scholar]
- 45.Pimenoff V.N., Felez-Sanchez M., Tous S., Clavero O., Godinez J.M., Klaustermeier J., Saunier M., Molijn A., Alemany L., Quint W., Bosch F.X., de Sanjose S., McCloskey J., Bravo I.G. Disagreement in high-grade/low-grade intraepithelial neoplasia and high-risk/low-risk HPV infection: clinical implications for anal cancer precursor lesions in HIV-positive and HIV-negative MSM. Clin. Microbiol. Infect. 2015;21:605. doi: 10.1016/j.cmi.2015.02.009. e11–9. [DOI] [PubMed] [Google Scholar]
- 46.Siegenbeek van Heukelom M.L., Richel O., de Vries H.J.C., van de Sandt M.M., Beck S., van den Munckhof H.A.M., Pirog E.C., de Koning M.N.C., Prins J.M., Quint K.D. Low- and high-risk human papillomavirus genotype infections in intra-anal warts in HIV-positive men who have sex with men. Br. J. Dermatol. 2016;175:735–743. doi: 10.1111/bjd.14567. [DOI] [PubMed] [Google Scholar]
- 47.Dunne E.F., Friedman A., Datta S.D., Markowitz L.E., Workowski K.A. Updates on human papillomavirus and genital warts and counseling messages from the 2010 Sexually Transmitted Diseases Treatment Guidelines. Clin. Infect. Dis. 2011;53(Suppl 3):S143–S152. doi: 10.1093/cid/cir703. [DOI] [PubMed] [Google Scholar]
- 48.Lacey C.J.N., Woodhall S.C., Wikstrom A., Ross J. European guideline for the management of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2012;27(2013):e263–e270. doi: 10.1111/j.1468-3083.2012.04493.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPV Genotype Prevalence by Composite Screening Results, Stratified by HIV Infection.
Calibration Plot of Predicted and Expected from the Final Adjusted Logistic Model.
Note: E:O, ratio of expected and observed events; CITL, calibration-in-the-large (calculated on the logit scale); Slope, calibration slope; AUC, area under the curve represents a concordance index where 0.5 indicates discrimination no better than chance and 1.0 indicates perfect discrimination.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. As a precautionary measure due to the criminalization of same-sex behavior in Nigeria, the research data is kept confidential.