Abstract
During embryonic life, individuals should adjust their phenotype to the conditions that they will encounter after birth, including the social environment, if they have access to (social) cues that allow them to forecast future conditions. In birds, evidence indicates that embryos are sensitive to cues from clutch mates, but whether embryos adjust their development to cope with the expected level of sibling competition has not hitherto been investigated. To tackle this question, we performed a ‘match versus mismatch' experimental design where we manipulated the presence of clutch mates (i.e. clutch size manipulation) and the real (postnatal) level of sibling competition (i.e. brood size manipulation) in the yellow-legged gull (Larus michahellis). We provide evidence that the prenatal cues of sibling presence induced developmental changes (such as epigenetic profiles) that had programming effects on chick begging behaviour and growth trajectories after hatching. While receiving mismatching information favoured chick begging and growth, this came at the cost of reduced antioxidant defences and a premature loss of telomeres. Our findings highlight the role of the prenatal social environment in developmental plasticity and suggest that telomere attrition may be an important physiological cost of phenotype–environment mismatch.
Keywords: embryo, developmental plasticity, phenotypic programming, sibling cues
1. Introduction
Theory predicts that developing individuals should plastically adjust their phenotype if they have access to reliable cues that help them to forecast the conditions that they will encounter later in their life, commonly referred to as ‘predictive adaptive responses' [1,2]. Plasticity may occur during the whole development but it is especially expected during embryonic life [2], when most of the developmental control mechanisms, such as genome-wide DNA methylation in vertebrates, are established (e.g. [3,4]). These epigenetic changes (i.e. DNA methylation) during early life may translate environmental cues to physiological and behavioural adjustments to the environment [5,6]. However, in many species, embryos develop enclosed within the environment provided by mothers, which constrains the direct access to reliable environmental cues. In these circumstances, it is assumed that relevant information about the future environment is mainly transmitted by mothers to embryos [7]. This view, however, has been recently challenged by evidence indicating that embryos can adaptively adjust their development as a response to a variety of (social) environmental inputs [8,9].
In many animals, family represents the basic social environment during early life with long-lasting consequences for developing organisms. Growing in large families often reduces the number of parental resources and the level of parental care obtained by each individual [10], which may have negative consequences on developmental trajectories (e.g. [11–13]). While parents may adjust their level of care depending on their individual quality and/or resource availability to mitigate these effects [14], also developing individuals may adopt different developmental strategies according to the expected level of sibling competition [15–17], often in response to maternally mediated cues (i.e. adaptive maternal effects [7]). However, maternal-derived cues may not be informative of the level of sibling competition because cues that maximize the fitness of mothers may not necessarily maximize the fitness of their offspring (parent–offspring conflict [18]). In this context, it is possible that developing embryos rely on their perception of the future level of sibling competition. After all, embryos are very sensitive to a variety of direct and reliable cues of sibling presence, such as vibrations or calls performed by sibling embryos [9,19,20]. However, whether embryos perceive the presence of sibling competitors and adaptively adjust their phenotype to the postnatal (real) competitive conditions has not hitherto been investigated.
In natural conditions where resources are often limited, evidence suggests that living with a large number of siblings often reduces growth rates (see [21] for a review of studies) and increases oxidative stress levels [22] and telomere attrition [23], probably because siblings need to share and compete for parental resources [24]. Offspring may prepare for these harsh conditions by adopting strategic behavioural and developmental changes. An increased begging strategy may help developing individuals to get better access to parental resources and outcompete their siblings, as parents can increase food provisioning in response to begging [25,26]. Additionally, when high levels of sibling competition are perceived, individuals may change their developmental trajectories to increase competitive performance (e.g. a faster growth to obtain a higher competitive rank [27]) and/or to minimize the physiological costs of living in a competitive environment (e.g. by changes in responsiveness of the HPA axis [28]). However, these behavioural and developmental strategies to cope with sibling competition may produce fitness penalties. For instance, increased begging behaviour and growth rate enhance competitive ability but may also negatively affect offspring oxidative status [29,30] and telomere dynamics [31,32], both important factors that may influence long-term survival [33,34]. Hence, it is likely that embryos tune up their growth trajectories and begging behaviour to the expected level of competition to avoid such physiological costs [1,35].
Here, we investigate whether bird embryos perceive the presence of sibling competitors and strategically adjust their phenotype to the predicted postnatal competitive conditions. We investigated these questions using embryos of a long-lived semi-precocial seabird, the yellow-legged gull (Larus michahellis), and following a ‘match versus mismatch' approach. In this species, and our study colony, clutch size is a good predictor of postnatal sibling competition level; on average, 86% of three-egg clutches hatch successfully, and in 96% of them, the two oldest chicks survive until 8 days of age (J.C.N. & A.V. 2018, unpublished data). In gull broods, hierarchy is established in the first days after hatching, and adequate competitive strategies may be crucial to surviving under strong sibling competition at this time [36]. We carried out a field experiment where the first laid egg (senior) of 64 three-egg clutches was incubated either in contact with two sibling embryos or with two dummy eggs. This procedure allowed the experimental embryos to be ‘exposed' or ‘unexposed' to social cues informing about the presence of potential competitor (i.e. vibrations and calls emitted by their sibling embryos [9]). Then, after hatching, we further manipulated brood size to create four experimental groups where the postnatal levels of sibling competitions matched or mismatched sibling cues received prenatally (i.e. exposed/with two siblings; exposed/singleton; unexposed/singleton; unexposed/with two siblings; see electronic supplementary material, figure S1).
If the social environment (number of potential competitors) does not have developmental programming effects, then exposed and unexposed embryos should have similar epigenetic profiles, and singletons should grow faster than chicks reared with siblings. However, if the prenatal environment has an anticipatory programming effect, then we expect that chicks strategically adjust their begging strategies, resulting in clear differences between exposed and unexposed groups of chicks. Importantly, if as theory predicts developing a phenotype that is not matched to the environment entails fitness cost for the chicks [1,35], when postnatal levels of sibling competition mismatched sibling cues received prenatally chicks should show reduced growth and/or experiencing a higher oxidative stress level and a faster rate of telomere attrition. Notably, if such costs of mismatching are mainly driven by the anticipatory effect that prenatal cues may have on postnatal competitive abilities, then the costs should be especially evident when unexposed chicks are reared with siblings (i.e. an unnatural social mismatch where unprepared chicks should have to face a high competitive postnatal environment). However, if programming a competitive phenotype is costly per se (i.e. plasticity costs), the costs of mismatching should be especially evident in the absence of postnatal competition (i.e. a possible natural scenario after brood reduction and where chicks should pay the costs even when living in a favourable postnatal environment).
2. Methods
(a). Study area, study species and general procedures
We carried out the field study between April and July 2018 in a large breeding colony of yellow-legged gulls on Sálvora Island, Spain. In this species, females normally lay the eggs at 1- to 3-day intervals until the (modal) three-egg clutch is completed. The experiment was carried out in an area of approximately 2.5 km2 that was mostly covered with sea thrift (Armeria maritima) and viper's bugloss (Echium vulgare), and where predation events use to occur sparsely and randomly through the breeding season. We surveyed the study area once daily during egg-laying and marked nests with numbered sticks. We visited each nest every day until clutch completion to mark the eggs and register their laying order. After clutch completion, we selected and measured (±0.01 mm) the first laid egg (i.e. A-egg) of 64 three-egg nests to calculate their volume as previously described [37]. The eggs are incubated, on average, 28 days and the chicks hatch asynchronously [37]. We focused on A-eggs because when clutch reduction occurs (approx. 4.3% of three-egg clutches lose two of their three eggs), in 75% of the cases the A-egg is the only egg that hatches and survives. Moreover, chicks from A-eggs hatch earlier are larger than their brood mates and they behaviourally monopolize parental food (see [36] and references therein). Thus, chicks from A-eggs raised as singletons or with siblings are expected to differ mainly due to their behavioural strategies. Senior (A) chicks have also a higher postnatal survival probability which allowed us to maximize sample sizes [38].
Marked first eggs were randomly assigned to one of two embryo treatment groups: unexposed or exposed to sibling eggs (hereafter, ‘unexposed' and ‘exposed' group, respectively). In the ‘unexposed' group (n = 32), the second and third laid egg (i.e. B- and C-egg) of each clutch was replaced by natural-sized dummy eggs made with plaster, whereas in the ‘exposed' group (n = 32), B- and C-eggs were handled in the same way as in the ‘unexposed' group (i.e. the B- and C-egg of each clutch was handled for few seconds by the experimenter) but the eggs were not removed from the nest. Afterwards, we also cross-fostered all senior (A) eggs between pairs of nests that had similar laying dates (±1 day) within each experimental group to disrupt any potential covariation between parental and offspring phenotype. Eggs removed from the unexposed group were relocated in a set of non-experimental nests with similar laying date but that had incomplete clutches.
We checked each nest daily beginning 2 days before the expected hatching date and fenced the nests with semi-transparent plastic mesh (2 m × 2 m × 30 cm) to avoid gull chicks escaping from their territories. Two unexposed embryos did not hatch but hatching success did not differ between the two prenatal treatment groups (see electronic supplementary material, §3). On the day of hatching, (experimental) A-eggs were again randomly allocated to one of two chick treatment groups: reared ‘with siblings’ or as ‘singletons'. To create these two experimental groups, in half of the nests that contained an unexposed embryo, the two dummy eggs were replaced by two pipped eggs (i.e. a B- and C-egg) of a nest that had the same laying date, whereas in the other half of the nests, we only removed the dummy eggs and left the experimental (senior) chick to be reared alone. Similarly, in half of the nests that contained an exposed embryo, we removed the B- and C-egg from the nests whereas, in the other half, we allowed the senior chick to be reared with two (unfamiliar) pipped eggs (i.e. a B- and C-egg) coming from a nest that had similar laying dates (±1 day). In all but one case (see Ethical statement for further details), the non-experimental eggs removed from exposed clutches (and assigned to be reared as singletons) were used to complete unexposed clutches (and assigned to be reared with siblings). This experimental design resulted in four experimental groups where the social cues of potential competitors received by the senior (A-) chick during its incubation either matched (i.e. exposed embryos reared with siblings (n = 16) or unexposed embryos reared as singletons (n = 16)) or mismatched (i.e. exposed embryos reared as singletons (n = 15) or unexposed embryos reared with siblings (n = 15)) to the real level of sibling competition encountered after hatching (see electronic supplementary material, figure S1). On average, B- and C-chicks hatched 0.91 days (±0.29 s.d.) and 1.8 days (±0.54 s.d.) after the senior (A) chick and the time difference was similar between experimental groups (GLM: embryo treatment: F1,59 = 0.120, p = 0.731; egg order: F1,59 = 59.48, p < 0.001; embryo treatment × egg order: F1,58 = 0.032, p = 0.859), so that our experimental manipulation did not alter the normal hatching sequence.
(b). Sampling procedure
We blood-sampled and weighed the experimental (A) chicks in each brood at hatching (day 0 of age) and day 8 of age. We collected blood samples (approx. 100 µl) from the brachial vein with heparinized capillary tubes and weighed them (±1 g) using a Pesola spring balance. Blood samples were kept cold until plasma was separated from red blood cells (within a few hours after collection) and stored in liquid nitrogen. To avoid the stress of reduced territory size, we removed the enclosures around the nests after blood sampling the chicks at day 8 of age and marked all of the experimental chicks with a numbered plastic ring with an individual two-digit combination to facilitate their long-term identification. At 30 days of age, when experimental chicks were fully grown and near fledging, we came back to the colony and searched for them around their territories. For all experimental chicks we found, we took a third blood sample and weighed them (±5 g).
(c). Biochemical, molecular and methylation analyses
In plasma sampled at hatching (day 0) and days 8 and 30 of age, we measured different plasma markers of oxidative status (total antioxidant capacity (TAC) and malondialdehyde (MDA)) following already established protocols for this species [9,37]. We also extracted DNA from the red blood cells (RBCs) and estimated the telomere length by real-time quantitative PCR following a previously validated protocol [39]. We further assessed the level of epigenetic marks by measuring the percentage of global DNA methylation (total 5-methylcytosine; 5-mC) at hatching using a commercially available kit and molecularly sexed the birds. Previous studies in birds have shown that both telomere length and methylation in RBCs is related to that in other tissues and organs [40,41]. A detailed description of the laboratory analyses can be found in the electronic supplementary material, §2.
(d). Begging behaviour tests
To assess whether the embryo and chick treatment influenced chick begging strategies, we tested for the intensity of two begging components in all experimental chicks at two days of age (hatching day = 0 day of age) and following a previously established standard protocol [29]. During the standardized begging tests (see electronic supplementary material, §1 for a detailed description), we recorded the number of distinct pecks delivered by each chick to a dummy head mimicking an adult gull's head and the total number of chatter calls emitted by the chicks during a minute as proxies of begging behaviour (see electronic supplementary material, §1). Because some chicks could not be tested as they never calmed and one chick was found dead, we were able to assess the begging behaviour in a total of 44 chicks (see electronic supplementary material, for further details on sample sizes, electronic supplementary material, table S1).
(e). Statistical analyses
We used generalized linear models (GLMs with Poisson error distribution) and linear models (LMs) to test the effect of the embryo treatment (i.e. exposed or unexposed) on the incubation time, body mass, oxidative stress markers (i.e. plasma antioxidants and MDA; LMs), telomere length and epigenetic marks (global DNA methylation level) at hatching. The effects of the experimental treatments on body mass, oxidative stress markers and telomere length during postnatal development (i.e. days 8 and 30 of age) were analysed with linear mixed effects models (LMMs). These models included the embryo and chick treatment, chick age and their three- and two-way interactions as fixed factors and chick identity (ID) as a random term. In all the above models (GLMs, LMs and LMMs), sex and egg volume were also included to account for any, even if subtle, influence of sex and eggs volume on chicks' growth and physiology. Moreover, since the effect of our experimental treatments on plasma antioxidants and telomere dynamics (see results) during the postnatal growth period may be the result of an indirect effect on growth trajectories (see e.g. [31,32,42]), we further performed partial correlation analyses to examine whether body mass gain correlated to the change in TAC and telomere length between 8 and 30 days.
We also examined whether the begging intensity of the chicks changed according to experimental treatments. The intensity of the begging components (i.e. the number of pecks and chatter calls) was analysed using GLMs fitted with Poisson error distribution and log link. The models included the embryo and chick treatment and their two-way interaction as fixed factors. Chick sex and body mass (at hatching) were also included in all models.
Before the analyses, TAC and MDA levels were log-transformed and the level of epigenetic marks (5mC) was Box–Cox-transformed to improve data distribution and meet model assumptions of normality and homoscedasticity of residuals. We confirmed there was no initial bias in laying, egg volume or sex ratio among experimental groups (see electronic supplementary material, §3). All analyses used Satterthwaite's approximation for degrees of freedom and post hoc comparisons among experimental groups were carried out using Fisher's (LSD) test, after performing planned contrast to compare differences between matched and mismatched groups and thus testing our main predictions [43]. Differences in sample sizes in some analyses reflect missing values because of death or loss of chicks and/or insufficient volume sample (see electronic supplementary material, table S1 for a detailed description of sample sizes). To avoid inflating type I error, we did not apply model selection in any analyses, and so report results for full models after removing non-significant interactions sequentially [44]. All analyses were conducted using IBM SPSS Statistics v. 24. Data are presented as means ± standard error (s.e.), the significance level was set at p = 0.05 and all statistical tests were two-tailed.
3. Results
(a). Effects of embryo treatment on developmental rate, body mass, oxidative status, telomere length and epigenetic marks at hatching
Embryo developmental rate was not affected by the embryo treatment, as both exposed and unexposed chicks showed a similar incubation time (Wald χ2 = 0.041, d.f. = 1, p = 0.840). Similarly, both exposed and unexposed chicks showed similar body mass (F1,58 = 2.575, p = 0.114), plasma antioxidants (F1,56 = 0.006, p = 0.941), lipid peroxidation levels (F1,55 = 0.255, p = 0.615) and telomere length at hatching (F1,57 = 1.053, p = 0.309). However, the presence of sibling cues during incubation did affect the level of epigenetic marks at hatching (F1,55 = 4.364, p = 0.041); chicks that hatched from eggs exposed to sibling cues had a lower level of global DNA methylation in the red blood cells than unexposed chicks (electronic supplementary material, figure S2). Egg volume was positively correlated to body mass at hatching (F1,58 = 30.983, p < 0.001) but in the rest of the above models neither egg volume nor the sex were significant (F < 1.614, p > 0.208 in all cases).
(b). Effects of the embryo and chick treatment on chick begging behaviour
The embryo and chick treatment interacted and affected the pecking behaviour of the gull chicks (embryo × chick treatment: Wald χ2 = 15.320, d.f. = 1, p < 0.001; see electronic supplementary material, table S1 and S2). This effect was mostly due to the high pecking rate in the mismatched singleton group (i.e. chicks hatched from exposed eggs but reared as singletons) compared with chicks in the other groups (LSD tests, p < 0.001 in all cases; figure 1a). The pecking rate was higher in males than females and negatively correlated with chick body mass (p < 0.018 in both bases; see electronic supplementary material, table S2).
Figure 1.
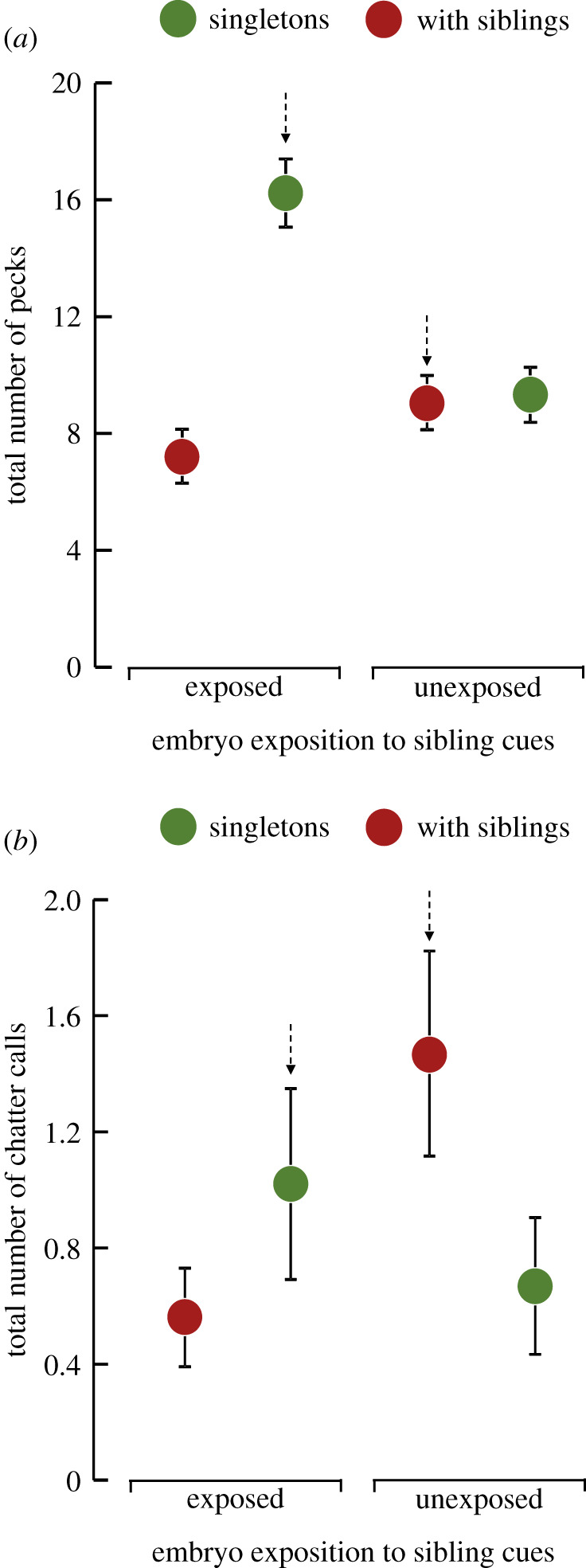
The total number of (a) pecks and (b) chatter calls in chicks that were ‘exposed' or ‘unexposed' to sibling cues during embryonic development. After hatching, half of each experimental group was either reared with two siblings (red dots) or as singletons (green dots), so that sibling cues received by the embryos matched or mismatched (dotted arrows) to the real level of sibling competition encountered after hatching. Data show estimated marginal mean ± s.e. (Online version in colour.)
The total number of chatter calls also differed according to the embryo and chick treatment (embryo × chick treatment: Wald χ2 = 4.514, d.f. = 1, p = 0.034; see electronic supplementary material, table S1 and S2). In this case, the effect was mostly due to chicks in the mismatched groups emitting more chatter calls than those in the matched groups (planned contrasts, p = 0.021; figure 1b) and especially if chicks were unexposed to sibling cues (LSD tests, mismatched group versus matched singleton, p = 0.05 and mismatched group versus matched with siblings group, p = 0.024). Females also emitted more chatter calls than males (Wald χ2 = 4.689, d.f. = 1, p = 0.030) but this begging component did not covary with chick body mass (p = 0.476; see electronic supplementary material, table S2).
(c). Effects of embryo and chick treatment on growth trajectories
We found a significant effect of the embryo and chick treatment on body mass growth but differences between groups were mostly evidenced at the end of the growth period (embryo × chick treatment × age: F1,41.75 = 17.103, p < 0.001; see electronic supplementary material, table S1 and S3 for further details). Near fledging (i.e. 30 days of age), mismatched chicks [i.e. when the real (postnatal) level of sibling competition did not match with the cues received prenatally] were heavier than the other chicks (Planned contrasts; p = 0.004; figure 2a), especially in the exposed group (LSD test; p < 0.001). Curiously, the smallest chicks were those reared with siblings and exposed to sibling cues, showing large differences form those reared with siblings but unexposed (LSD test, p = 0.009; figure 2a). Body mass growth was not related to egg volume (F1,49.70 = 0.309, p = 0.581) but differed between sexes, being the males heavier than the females (F1,45.40 = 5.436, p = 0.024).
Figure 2.
(a) Body mass, (b) plasma antioxidant capacity and (c) telomere length during postnatal development (i.e. 8 and 30 days of age) in chicks that were ‘exposed' or ‘unexposed' to sibling cues during embryonic development. After hatching, half of each experimental group was either reared with siblings (red dots) or as singletons (green dots), so that sibling cues received by the embryos matched (solid red or green lines) or mismatched (dashed red or green lines) to the real level of sibling competition encountered after hatching. Data show estimated marginal mean ± s.e. (Online version in colour.)
(d). Effects of embryo and chick treatment on oxidative status and telomere dynamics
Postnatal levels of antioxidant defences (TAC) changed with age during the postnatal growth period but differently among experimental groups (embryo × chick treatment × age: F1,25.35 = 0.181, p = 0.008; see electronic supplementary material, table S1 and S4). At fledging (i.e. 30 days of age), mismatched chicks had a lower level of plasma antioxidant defences than matched chicks (Planned contrasts; p = 0.015; figure 2b), particularly in the exposed group (singletons versus reared with siblings; LSD test, p = 0.026). Similarly, we also found that postnatal telomere dynamics varied differently among experimental groups (embryo × chick treatment × age: F1,29.61 = 4.606, p = 0.040; electronic supplementary material, table S5), which led to clear differences at 30 days of age; telomere length was significantly shorter if chicks were reared with a real level of sibling competition that did not match with the social cues received prenatally (planned contrasts, p = 0.007; figure 2c), an effect that was present in both the exposed and unexposed group of chicks (singletons versus reared with siblings; LSD tests, p = 0.043 and p = 0.032, respectively, in both pair-wise comparisons). Moreover, our complementary analyses also revealed that both telomere length and plasma antioxidant levels covaried with body mass growth between 8 and 30 days of age; chicks that grew up at a faster rate and gained more mass lost more antioxidant defences (r = −0.446, p = 0.023; see electronic supplementary material, figure S3a) and showed a faster rate of telomere attrition (r = −0.526, p = 0.006; see electronic supplementary material, figure S3b). The level of lipid peroxidation changed with age but did not differ between experimental groups (p > 0.83 in all cases; see electronic supplementary material, table S4 for further details). The rest of the variables included in the models were not significant (p > 0.149 in all cases; see electronic supplementary material, tables S4 and S5).
4. Discussion
In this study, we provide evidence supporting the hypothesis that social conditions experienced by embryos have a programming effect on the developmental trajectories of gull chicks. At first instance, these prenatally induced developmental changes were evidenced by differences in epigenetic profiles (i.e. unexposed hatchlings showed significantly more global DNA methylation compared to hatchlings prenatally exposed to sibling cues). Interestingly, after hatching, chicks that grew up with a number of siblings that did not match to that experienced prenatally showed an unusually high frequency of begging. Chatter calls are used to attract parental attention and care while pecking behaviour is often used to elicit parental provisioning [29], so when sibling competition levels were unexpectedly high (unexposed group), chicks increased chatter frequency, whereas when sibling competition levels were unexpectedly low (exposed group) chicks increased their pecking. These behavioural changes presumably contributed to a faster growth rate later during postnatal development. However, these developmental trajectories also involved some physiological costs, as only chicks that received sibling cues that mismatched the real number of competitors suffered from a significant loss of telomeres and antioxidant defences. Overall, our findings highlight the role of the prenatal social environment in developmental plasticity and suggest that accelerated cellular ageing via a significant loss of telomeres may be an important, but so far overlooked, physiological cost of phenotype-environment mismatch.
In our experiment, the presence (or absence) of sibling embryos during the entire incubation time did not affect embryo developmental rate, although this results could be influenced by a lack of precision due to the long time interval used during the nest monitoring (i.e. 24 h). However, we found evidence that the presence of sibling embryos probably affected developmental pathways, as revealed by differences in global DNA methylation. This type of epigenetic modifications was measured at hatching which probably reflects socially induced changes during incubation. Indeed, it is very well known that bird embryos are very sensitive to acoustic and tactile stimulation produced by sibling embryos ([9,20] and references therein), social cues that have been recently shown to promote prenatal epigenetic and neuroendocrine modifications that can alter the postnatal behaviour and growth trajectory in gull chicks [9], probably due to the causal role of DNA methylation in gene expression [45]. Therefore, is very likely that in our experiment perinatal vocalizations and vibrations of sibling embryos may have acted as social cues informing about the future level of sibling competition. In birds, it has recently been shown that peripheral blood epigenome relates to the level of epigenetic marks in other tissues such as the brain [41], and changes in RBCs methylation are associated with changes in the expression profiles of important functional genes contributing to behavioural changes during the postnatal period [41,46]. Similarly, in our experiment, social cue-induced epigenetic changes may have played a key role in determining the postnatal chick begging behaviour and growth. However, further studies in birds are still needed to evaluate the long-lasting effects of the embryonic environment on methylation patterns and the exact contribution of global DNA methylation on the expression of genes governing begging behaviour strategies.
In contrast with scramble sibling competition models (e.g. [47]), in our experiment, the number of chatter calls and pecks was not always higher in the chicks reared with siblings in comparison to those reared as singletons. Rather, we found that chick begging behaviour depended on current levels of sibling competition and the cues received during incubation. Chicks facing a sibling competition level that did not match to cues received prenatally did peck more intensively and emitted more chatter calls, suggesting that the mismatch between the pre- and the postnatal environment may alter behavioural strategies [1,48]. The group of chicks raised as singletons but previously exposed to sibling cues resemble a natural social mismatch occurring after brood reduction. Interestingly, these chicks showed high levels of pecking behaviour, suggesting that the programming effects of the social embryonic environment have some lasting effects after hatching, even when no postnatal competitors are present. On the other hand, chicks increased begging behaviour after two days of sibling competition, but only when not exposed to sibling embryos. This suggests that the postnatal level of competition triggers begging behaviour, which escalates in this (unnatural) mismatched situation.
By pecking more intensively and emitting more chatter calls, gull chicks may have had greater access to parental resources and care [25,49], what is in agreement with the faster growth rate showed by the chicks in the mismatched groups. Curiously, although the begging behaviour was tested soon after hatching (2 days of age), differences in growth rate were only evidenced between 8 and 30 days of age, probably because the benefits of having better access to parental provisioning become especially evident when the energetic demands for growth are maximal [50]. Attaining more body mass at fledging might bring some advantages for gull chicks receiving mismatched information if, for instance, it positively correlates with juvenile survival as it seems to be the case in other (passerine) birds species (see e.g. [51] and reference therein). While the benefits (if any) of these growth patterns in the mismatched groups of chicks still need further investigation, our results indicate that chicks receiving mismatching information endured increased oxidative stress levels as a result of their accelerated growth, as suggested by the decline in plasma antioxidant defences and its correlation with growth. This is in agreement with studies showing that elevated growth rates may increase oxidative stress levels during early postnatal life [42,52].
Intriguingly, we did not detect any change in oxidative damage level in lipids (MDA). However, the lower availability of antioxidant defences does not always reduce the level of all biomarkers of oxidative damage [53,54]. Hence, it is plausible that chicks in the mismatched groups accumulated more damage as a consequence of fast growth (including mitochondrial damage; see e.g. [55]) than chicks in the matched groups but such an effect was not mirrored in MDA levels. Importantly, previous studies have shown that increased postnatal growth rate and oxidative stress levels are important factors accelerating telomere attrition (reviewed in [56,57]). We also found that chicks in the mismatched groups lost telomere length during development and had the shortest telomeres near independence. Since both reduced telomere length and a faster rate of telomere loss during postnatal development have been related to a lower phenotypic quality (e.g. reduced adult foraging behaviour and breeding performance [58,59]) and reduced lifespan across taxa [60], these interesting results suggest that chicks developing a phenotype that did not match to the real level of sibling competition may undergo long-term fitness costs. Furthermore, the fact that both exposed and unexposed chicks lost telomere length when receiving mismatching postnatal information indicates that the costs of mismatching are not only the result of chicks failing to develop the appropriate competitive abilities but also the result of plasticity costs per se.
Our results also suggest that gull embryos may perceive and use prenatal cues informing about the presence of potential competitors to finely adjust their postnatal behaviour and growth rates to the expected level of sibling competition. In this study, we focused on senior chicks which have a higher probability to survive when brood reduction occurs (see Methods). We found that senior chicks reared with siblings emitted fewer chatter calls and grew up at a slower rate if they were prenatally exposed to sibling embryos than if they did not. There is good evidence that animals generally do not grow at their maximal rate because of the costs associated with a faster growth (reviewed in [61]), including the loss of telomeres [31,32]. In our experiment, chicks reared with siblings lost telomere length if they did not receive sibling cues during incubation. It is plausible that chicks exposed to sibling cues may have adopted a restraint strategy when the real level of sibling competition matched to cues received prenatally––for example, limiting their growth to avoid (negative) carryover effects on fitness-related traits as a result of premature attrition in their telomeres [33]. It might be possible that similar restraint strategies explain why the experimental enlargement of brood size in birds does not always lead to reduced growth and body mass at fledging (see [21] for a review of studies), although it may also be explained by the ability of parents to increase their level of care [14]. While postnatal brood size manipulation is a common experimental approach in ecological and evolutionary studies, at least in species where clutch size strongly predicts final brood size, the results should be interpreted with caution because our results would suggest that these type of experimental studies may be disrupting the prenatal information and the postnatal environment, with possible fitness costs. Further studies should evaluate if developmental plasticity, including the prenatal traits (e.g. embryo size), varies among species with different clutch size, degree of hatching asynchrony or according to differences in prenatal predictability of the postnatal level of competition (i.e. final brood size).
In conclusion, our experimental manipulation demonstrates that social conditions experienced during embryonic development may have informative value and exert a programming influence over how organisms respond to their postnatal social environment. Although our sample sizes were slightly reduced at 30 days of age, we provide clear evidence that sibling cues induced developmental changes (such as epigenetic profiles) that had a programming effect on chick begging behaviour and growth trajectories. While receiving mismatching information favoured chick begging and growth during postnatal development, this came at the cost of reduced antioxidant defences and a premature loss of telomeres. Because in this and other bird species other social factors such as intra-brood hierarchy, the number of individuals of each sex or agonistic interactions with other conspecifics may have negative effects of chick growth and survival [27,38], future studies with larger sample sizes should investigate the programming effect of other types of social cues occurring prenatally (e.g. from population density to intra-clutch sex ratio and egg-laying order).
Supplementary Material
Acknowledgements
We are grateful to the staff at the Atlantic Islands of Galicia National Park, especially to Pablo Mallo, Roberto Castiñeira and José Arca. We also thank Alberto da Silva for helping with the telomere analyses, Naya Alvarez-Quintero for her assistance during the fieldwork and two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Ethics
To reduce to the minimum the impact of our experimental manipulation, the removed eggs from the unexposed group were relocated in non-experimental nests that had similar laying date but had lost some of their eggs. Similarly, the eggs removed from exposed clutches (and assigned to be reared as singletons) were used to complete the unexposed clutches (and assigned to be reared with siblings). However, in one nest, this procedure was not possible and so the two pipped eggs that had to be removed were relocated in two different non-experimental nests that had an incomplete clutch. The egg exchange needed during our experimental manipulations was possible to be done because every year we also monitor egg order, and laying and hatching date of a large number of non-experimental nests (greater than 300) in the population for other purposes. The study complied with the standards of animal experimentation and animal welfare established under current Spanish law (RD53/2013), and all experimental procedures and permissions were granted by the authorities of Parque Nacional de las Islas Atlánticas and approved by the Xunta de Galicia review board (263/RX583146).
Data accessibility
All data needed to evaluate the conclusions of the study are presented in the paper and/or the electronic supplementary material. Raw data can also be found in the Figshare digital repository [62].
Authors' contributions
J.C.N. designed the study, conducted the experimental work and analysed the data. A.V. had inputs on study design and data analysis and both J.C.N. and A.V. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
J.C.N. was supported by Programa de Retención de Talento (Universidad de Vigo) and the project was funded by MICINN (grant no. PGC2018-095412-B-I00).
References
- 1.Nettle D, Bateson M. 2015. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B 282, 20151005 ( 10.1098/rspb.2015.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Le Luyer J, Laporte M, Beacham TD, Kaukinen KH, Withler RE, Leong JS, Rondeau EB, Koop BF, Bernatchez L. 2017. Parallel epigenetic modifications induced by hatchery rearing in a Pacific salmon. Proc. Natl. Acad. Sci. USA 114, 12 964–12 969. ( 10.1073/pnas.1711229114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, et al. 2018. Dynamic epigenomic landscapes during early lineage specification in mouse embryos. Nat. Gen. 50, 96–105. ( 10.1038/s41588-017-0003-x) [DOI] [PubMed] [Google Scholar]
- 5.Beldade P, Mateus ARA, Keller RA. 2011. Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 20, 1347–1363. ( 10.1111/j.1365-294X.2011.05016.x) [DOI] [PubMed] [Google Scholar]
- 6.Laubach ZM, Perng W, Dolinoy DC, Faulk CD, Holekamp KE, Getty T. 2018. Epigenetics and the maintenance of developmental plasticity: extending the signalling theory framework. Biol. Rev. 93, 1323–1338. ( 10.1111/brv.12396) [DOI] [PubMed] [Google Scholar]
- 7.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 8.Mariette MM, Buchanan KL. 2016. Prenatal acoustic communication programs offspring for high posthatching temperatures in a songbird. Science 353, 812–814. ( 10.1126/science.aaf7049) [DOI] [PubMed] [Google Scholar]
- 9.Noguera JC, Velando A. 2019. Bird embryos perceive vibratory cues of predation risk from clutch mates. Nat. Ecol. Evol. 3, 1225–1232. ( 10.1038/s41559-019-0929-8) [DOI] [PubMed] [Google Scholar]
- 10.Parker GA, Royle NJ, Hartley IR. 2002. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. Lond. B 357, 295–307. ( 10.1098/rstb.2001.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atchley WR, Logsdon T, Cowley DE, Eisen E. 1991. Uterine effects, epigenetics, and postnatal skeletal development in the mouse. Evolution 45, 891–909. ( 10.1111/j.1558-5646.1991.tb04358.x) [DOI] [PubMed] [Google Scholar]
- 12.De Kogel C. 1997. Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J. Anim. Ecol. 66, 167–178. ( 10.2307/6019) [DOI] [Google Scholar]
- 13.Saino N, Ambrosini R, Rubolini D, Romano M, Caprioli M, Romano A, Parolini M. 2018. Carry-over effects of brood size on morphology, reproduction, and lifespan in barn swallows. Behav. Ecol. Sociobiol. 72, 30 ( 10.1007/s00265-018-2446-1) [DOI] [Google Scholar]
- 14.Royle NJ, Smiseth PT, Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Lloyd JD, Martin TE. 2003. Sibling competition and the evolution of prenatal development rates. Proc. R. Soc. Lond. B 270, 735–740. ( 10.1098/rspb.2002.2289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace JK, Froud L, Meillère A, Angelier F. 2017. House sparrows mitigate growth effects of post-natal glucocorticoid exposure at the expense of longevity. Gen. Comp. Endocrinol. 253, 1–12. ( 10.1016/j.ygcen.2017.08.011) [DOI] [PubMed] [Google Scholar]
- 17.Senner NR, Conklin JR, Piersma T. 2015. An ontogenetic perspective on individual differences. Proc. R. Soc. B 282, 20151050 ( 10.1098/rspb.2015.1050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuijper B, Johnstone RA. 2018. Maternal effects and parent–offspring conflict. Evolution 72, 220–233. ( 10.1111/evo.13403) [DOI] [PubMed] [Google Scholar]
- 19.Spencer KA, Minderman J. 2018. Developmental programming via activation of the hypothalamic–pituitary–adrenal axis: a new role for acoustic stimuli in shaping behavior? Adv. Study Behav. 50, 87–126. ( 10.1016/bs.asb.2017.11.003) [DOI] [Google Scholar]
- 20.Rumpf M, Tzschentke B. 2010. Perinatal acoustic communication in birds: why do birds vocalize in the egg? Open Ornithol. J. 3, 141–149. ( 10.2174/1874453201003010141) [DOI] [Google Scholar]
- 21.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Costantini D, Casagrande S, Filippis S, Brambilla G, Fanfani A, Tagliavini J, Dell'Omo G. 2006. Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J. Comp. Physiol. B 176, 329–337. ( 10.1007/s00360-005-0055-6) [DOI] [PubMed] [Google Scholar]
- 23.Costanzo A, et al. 2016. Brood size, telomere length, and parent-offspring color signaling in barn swallows. Behav. Ecol. 28, 204–211. ( 10.1093/beheco/arw147) [DOI] [Google Scholar]
- 24.Nettle D, Monaghan P, Boner W, Gillespie R, Bateson M. 2013. Bottom of the heap: having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS ONE 8, e83617 ( 10.1371/journal.pone.0083617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguera JC, Kim SY, Velando A. 2013. Maternal testosterone influences a begging component that makes fathers work harder in chick provisioning. Horm. Behav. 64, 19–25. ( 10.1016/j.yhbeh.2013.04.008) [DOI] [PubMed] [Google Scholar]
- 26.Whittingham LA, Dunn PO, Clotfelter ED. 2003. Parental allocation of food to nestling tree swallows: the influence of nestling behaviour, sex and paternity. Anim. Behav. 65, 1203–1210. ( 10.1006/anbe.2003.2178) [DOI] [Google Scholar]
- 27.Gil D, Bulmer E, Celis P, Lopez-Rull I. 2007. Adaptive developmental plasticity in growing nestlings: sibling competition induces differential gape growth. Proc. R. Soc. B 275, 549–554. ( 10.1098/rspb.2007.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitousek MN, Jenkins BR, Hubbard JK, Kaiser SA, Safran RJ. 2017. An experimental test of the effect of brood size on glucocorticoid responses, parental investment, and offspring phenotype. Gen. Comp. Endocrinol. 247, 97–106. ( 10.1016/j.ygcen.2017.01.021) [DOI] [PubMed] [Google Scholar]
- 29.Noguera JC, Morales J, Pérez C, Velando A. 2010. On the oxidative cost of begging: antioxidants enhance vocalizations in gull chicks. Behav. Ecol. 21, 479–484. ( 10.1093/beheco/arq005) [DOI] [Google Scholar]
- 30.Moreno-Rueda G, Redondo T, Trenzado CE, Sanz A, Zuniga JM. 2012. Oxidative stress mediates physiological costs of begging in magpie (Pica pica) nestlings. PLoS ONE 7, e40367 ( 10.1371/journal.pone.0040367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F.. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 32.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol. Lett. 11, 20140938 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguera JC, Kim SY, Velando A. 2012. Pre-fledgling oxidative damage predicts recruitment in a long-lived bird. Biol. Lett. 8, 61–63. ( 10.1098/rsbl.2011.0756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz-Real J, Kim SY, Velando A. 2016. Hatching hierarchy but not egg-related effects governs behavioral phenotypes in gull chicks. Behav. Ecol. 27, 1782–1789. [Google Scholar]
- 37.Noguera JC, Kim SY, Velando A. 2017. Family-transmitted stress in a wild bird. Proc. Natl Acad. Sci. USA 114, 6794–6799. ( 10.1073/pnas.1706242114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SY, Noguera JC, Tato A, Velando A. 2013. Vitamins, stress and growth: the availability of antioxidants in early life influences the expression of cryptic genetic variation. J. Evol. Biol. 26, 1341–1352. ( 10.1111/jeb.12136) [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Velando A. 2015. Antioxidants safeguard telomeres in bold chicks. Biol. Lett. 11, 20150211 ( 10.1098/rsbl.2015.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8, e81496 ( 10.1371/journal.pone.0081496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhulst EC, Mateman AC, Zwier MV, Caro SP, Verhoeven KJ, Van Oers K.. 2016. Evidence from pyrosequencing indicates that natural variation in animal personality is associated with DRD 4 DNA methylation. Mol. Ecol. 25, 1801–1811. ( 10.1111/mec.13519) [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Noguera JC, Morales J, Velando A. 2011. Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol. Ecol. 25, 461–472. ( 10.1007/s10682-010-9426-x) [DOI] [Google Scholar]
- 43.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Engqvist L. 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971. ( 10.1016/j.anbehav.2005.01.016) [DOI] [Google Scholar]
- 45.Jones PA, Takai D. 2001. The role of DNA methylation in mammalian epigenetics. Science 293, 1068–1070. ( 10.1126/science.1063852) [DOI] [PubMed] [Google Scholar]
- 46.Baker-Andresen D, Ratnu VS, Bredy TW. 2013. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 36, 3–13. ( 10.1016/j.tins.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 47.Harper AB. 1986. The evolution of begging: sibling competition and parent–offspring conflict. Am. Nat. 128, 99–114. ( 10.1086/284542) [DOI] [Google Scholar]
- 48.Stamps JA, Frankenhuis WE. 2016. Bayesian models of development. Trends Ecol. Evol. 31, 260–268. ( 10.1016/j.tree.2016.01.012) [DOI] [PubMed] [Google Scholar]
- 49.Morales J, Alonso-Álvarez C, Pérez C, Torres R, Serafino E, Velando A. 2009. Families on the spot: sexual signals influence parent–offspring interactions. Proc. R. Soc. B 276, 2477–2483. ( 10.1098/rspb.2008.1942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starck JM, Ricklefs RE. 1998. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford, UK: Oxford University Press. [Google Scholar]
- 51.Schwagmeyer P, Mock DW. 2008. Parental provisioning and offspring fitness: size matters. Anim. Behav. 75, 291–298. ( 10.1016/j.anbehav.2007.05.023) [DOI] [Google Scholar]
- 52.Noguera JC, Lores M, Alonso-Álvarez C, Velando A. 2011. Thrifty development: early-life diet restriction reduces oxidative damage during later growth. Funct. Ecol. 25, 1144–1153. ( 10.1111/j.1365-2435.2011.01856.x) [DOI] [Google Scholar]
- 53.Noguera JC, Monaghan P, Metcalfe NB. 2015. Interactive effects of early and later nutritional conditions on the adult antioxidant defence system in zebra finches. J. Exp. Biol. 218, 2211–2217. ( 10.1242/jeb.120956) [DOI] [PubMed] [Google Scholar]
- 54.Sumien N, Forster MJ, Sohal RS. 2003. Supplementation with vitamin E fails to attenuate oxidative damage in aged mice. Exp. Gerontol. 38, 699–704. ( 10.1016/S0531-5565(03)00068-8) [DOI] [PubMed] [Google Scholar]
- 55.Velando A, Noguera JC, da Silva A, Kim SY. 2019. Redox-regulation and life-history trade-offs: scavenging mitochondrial ROS improves growth in a wild bird. Sci. Rep. 9, 2203 ( 10.1038/s41598-019-38535-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young AJ. 2018. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil. Trans. R. Soc. B 373, 20160452 ( 10.1098/rstb.2016.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichert S, Stier A. 2017. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463 ( 10.1098/rsbl.2017.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelier F, Weimerskirch H, Barbraud C, Chastel O. 2019. Is telomere length a molecular marker of individual quality? Insights from a long-lived bird. Funct. Ecol. 33, 1076–1087. ( 10.1111/1365-2435.13307) [DOI] [Google Scholar]
- 59.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 ( 10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whittemore K, Vera E, Martínez-Nevado E, Sanpera C, Blasco MA. 2019. Telomere shortening rate predicts species life span. Proc. Natl Acad. Sci. USA 116, 15 122–15 127. ( 10.1073/pnas.1902452116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 62.Noguera JC, Velando A. 2020. Data from: Gull chicks grow faster but lose telomeres when prenatal cues mismatch the real presence of sibling competitors Figshare digital repository. ( 10.6084/m9.figshare.9255662) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Noguera JC, Velando A. 2020. Data from: Gull chicks grow faster but lose telomeres when prenatal cues mismatch the real presence of sibling competitors Figshare digital repository. ( 10.6084/m9.figshare.9255662) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions of the study are presented in the paper and/or the electronic supplementary material. Raw data can also be found in the Figshare digital repository [62].



