Abstract
Coral reefs host hundreds of thousands of animal species that are increasingly threatened by anthropogenic disturbances. These animals host microbial communities at their surface, playing crucial roles for their fitness. However, the diversity of such microbiomes is mostly described in a few coral species and still poorly defined in other invertebrates and vertebrates. Given the diversity of animal microbiomes, and the diversity of host species inhabiting coral reefs, the contribution of such microbiomes to the total microbial diversity of coral reefs could be important, yet potentially vulnerable to the loss of animal species. Analysis of the surface microbiome from 74 taxa, including teleost fishes, hard and soft corals, crustaceans, echinoderms, bivalves and sponges, revealed that more than 90% of their prokaryotic phylogenetic richness was specific and not recovered in surrounding plankton. Estimate of the total richness associated with coral reef animal surface microbiomes reached up to 2.5% of current estimates of Earth prokaryotic diversity. Therefore, coral reef animal surfaces should be recognized as a hotspot of marine microbial diversity. Loss of the most vulnerable reef animals expected under present-day scenarios of reef degradation would induce an erosion of 28% of the prokaryotic richness, with unknown consequences on coral reef ecosystem functioning.
Keywords: skin microbiota, phylogenetic diversity, conservation, marine biodiversity, Octocorallia, Scleratinia
1. Background
Coral reefs provide habitats for at least 500 000 multicellular species [1], including more than 6000 described fish and 1000 coral species [2,3]. These animals are covered by diverse and abundant microbes (Bacteria, Archaea, viruses and micro-eukaryotes) which all together constitute the surface microbiome [4]. Animal surface microbiomes play crucial roles for their hosts, contributing to host resistance to pathogens and environmental perturbations [5,6], and to assimilation of nutrients [4]. However, animal surface microbiomes have been assessed in fewer than 50 and 100 coral and fish species, respectively [4,6–10].
Large-scale surveys of the marine water column demonstrated the important diversity of planktonic marine microbes [11,12]. However, these planktonic microbes are compositionally distinct from marine animal surface microbiomes, and many animal-associated microbes remain undetected in planktonic microbial communities [8,10,13]. In addition, marine animals have diverse biological traits that provide specific habitats at their surface, which ultimately favours the proliferation of microbial lineages that are different among different species [7]. Surface microbiomes and planktonic microbes form a microbial ‘metacommunity' [14], where local communities within it (individual animal microbiomes, as well as planktonic communities) are connected by dispersal of microbes through direct contacts and through the water column. Given the distinct composition of animal surface microbiomes between species, and the diversity of animal surfaces inhabiting coral reefs, surface microbiomes may constitute an important part of the coral reef microbial metacommunity. However, the contribution of animal surface microbiomes to the coral reef microbial diversity is currently unknown.
Coral reefs are facing increasing human pressures (including ocean warming, acidification and overfishing [15,16]) inducing an increased risk of extinction of a significant proportion of animal diversity (up to 8% and 10% of coral and fish species [16,17]). The documented erosion of animal biodiversity as well as the decrease of animal abundance may result in the loss of their associated microbial species. However, the effect of such erosion on microbial diversity has to date not been assessed.
In this study, we sampled surface microbiomes of abundant coral reef animals (e.g. fishes, corals, crustaceans, echinoderms, mollusks and sponges) from the same restricted area over a short period. In so doing, we avoided spatial and/or temporal variation of microbial diversity driven by environmental variability. We sampled the surface prokaryotic microbiome of 265 coral reef animals belonging to 74 different taxa, including 32 and 18 genera of teleost fishes and Anthozoa (hard and soft corals, and anemones), respectively, and 12 taxa of crustaceans, echinoderms, mollusks and sponges, from a single coral reef ecosystem (Mayotte lagoon, Indian Ocean). In order to test the hypothesis that surface microbiomes represent a major component of the coral reef microbial metacommunity, we compared the prokaryotic diversity hosted by animals to that of the surrounding planktonic communities. Ultimately, we compared the amount of microbial diversity to global microbial diversity estimates and estimated the erosion of microbes due to human-induced animal extinction.
2. Material and methods
(a). Sampling procedure
Sampling was conducted in November 2015 in Mayotte lagoon shallow (depth less than 10 metres) barrier and fringing coral reefs (Western Indian Ocean, electronic supplementary Material SM1-1 and 2). We sampled the most abundant taxa from each of the main animal groups (teleost fishes, Anthozoa, crustaceans, echinoderms, mollusks and sponges) within a radius of 50 m around each site (electronic supplementary material, SM1–3 and 4). Sampling procedure is provided in electronic supplementary material, SM1–5. Briefly, we sampled the skin microbiome by collecting surface mucus or swabbing.
Fishes were identified at species level. Corals were identified at genus level. Classification of other invertebrates was made at species level when possible (for crustaceans, starfish, urchins and anemones), or at higher taxonomic levels for a few clades (one sea cucumber, comatules, hermit crabs, brittle stars and giant clams; electronic supplementary material, SM1-4). A total of 138 fishes were sampled across two sites, as well as 82 colonies of hard and soft corals, gorgonians and anemones (referred collectively as Anthozoa) belonging to 13 genera of Scleractinia, three Octocorallia (soft corals and gorgonians) and one anemone species. We also sampled 43 individuals of crustaceans, echinoderms, mollusks and sponges. For each of 6 days of sampling, water samples were collected and filtrated to collect prokaryotic plankton as explained in electronic supplementary material, SM1-5. Prokaryotic diversity was assessed using high-throughput sequencing of the V4 hypervariable region of the 16S rDNA gene, as detailed in electronic supplementary material, SM1-5.
(b). Sequence processing and phylogenetic analyses
Sequence reads were processed using the ‘DADA2' R package v. 1.2 and R software v. 3.4.3 using script provided in electronic supplementary material, SM1-6. Around 7% of the 50 237 constructed ASVs were unclassified at this step. In order to define whether they could potentially correspond to remaining mitochondrial sequences, we mapped them onto the GenBank database (NCBI) using the BLASTn alignment tool. Around 7% of them showed more than 97% identity with a mitochondrial sequence and were removed before computing further analyses.
Sequence number ranged from 7074 to 56 927 across samples (electronic supplementary material, SM1-4). 7000 sequences were randomly sub-sampled within each sample in order to correct the uneven sequencing efficiency among samples using ‘rrarefy' function from ‘vegan' R package v. 2.5-5 [18]. To assess the effect of rarefaction on our results, all subsequent analyses were computed on both rarefied and unrarefied data. Analyses based on rarefied data are included in the main document, while the ones based on unrarefied data are included in electronic supplementary material, SM2. These two approaches provided similar results. Zhang & Huang's coverage estimator [19] was calculated using the ‘entropart’ R package v. 1.6-1 [20], using ‘coverage' function, and averaged 0.982 ± 0.015 across all samples (0.998 ± 0.001 before rarefaction). A phylogenetic tree was obtained by adding the ASV sequences into the GreenGenes phylogenetic tree v. 13.8 [21] using SEPP insertion tool [22] with default parameters.
(c). Assessing microbial biodiversity
The average surface microbiome of each fish species, Anthozoa genus, or other invertebrate taxa, was computed as the mean relative abundance of each ASV across individuals from the same taxon (electronic supplementary material, SM1-4). Phylogenetic richness of each community was measured using Faith's PD [23], using ‘pd' function from ‘picante' R package v. 1.8 [24]. Phylogenetic diversity, taking into account the relative abundance of ASVs, was measured using Allen's index [25] using our own R function (https://github.com/marlenec/chao, q = 1). The evenness of ASVs abundances was measured for each microbial sample using O [26], which is robust against richness variation [27].
Phylogenetic dissimilarities between animal taxa were assessed using the unweigthed and weighted versions of Unifrac index (U- and W-Unifrac), computed using ‘GUniFrac' R package v. 1.1 [28], and were visualized using principal coordinates analysis (PCoA) computed using ‘ape' R package, and boxplots computed using function ‘boxplot.stats' from ‘grDevices' R package using default parameters.
(d). Statistical tests
Difference in phylogenetic α-diversity between plankton and animal surface microbiomes was tested using Kruskal–Wallis (KW) tests (999 permutations) computed with ‘vegan' R package. Correlation between fish vulnerability (see ‘Vulnerability of microbial diversity' below) and its associated microbiome diversity was assessed using Spearman's correlation tests (‘cor.test’) performed on phylogenetic independent contrasts (PIC) calculated on diversity indices, using the fish phylogenetic tree used before [7]. Due to the lower resolution of taxonomic identification of corals in our dataset (at genus level) and the polyphyly of genera in published coral trees [29], it was not possible to compute PIC on scleratinians. Therefore, correlation between coral vulnerability and its associated microbiome diversity was performed using a simple Spearman's correlation test.
Difference in microbial structure between plankton and animal surface microbiomes was tested using permutational multivariate ANOVAs (PERMANOVAs) performed on dissimilarities, using ‘adonis' function in ‘vegan’ R package and 999 permutations. To test if planktonic communities were more variable than surface-associated communities, a permutation analysis of dispersion (PERMDISP) was performed on both indices among planktonic communities and among animal surface microbiomes using ‘betadisper' provided in ‘vegan’, with 999 permutations.
Differences between surface microbiomes associated with the three major animal groups (44 teleostean fish species, 17 anthozoan genera and 12 other invertebrates' taxa) were tested using PERMANOVAs. The effect of animal's taxon to its associated surface microbiome was assessed for each animal group (before averaging the microbiome per animal taxon; electronic supplementary material, SM1-3 and 4) using separated PERMANOVAs.
Lastly, in order to identify prokaryotic clades that are different between plankton and animal surface microbiomes, and consistent enriched in all water samples or animal taxa, we performed a LEfSe analysis [30] using water samples and animal taxa as subclasses, with all-against-all parameter and p-value threshold for significance of 0.05. Then, to identify biomarkers for each animal group (teleostean fishes, Anthozoa and other vertebrates), a LEfSe analysis was performed using these groups as main classes, and the different animal taxa belonging to these groups (fish species, anthozoan genera and other invertebrates' taxa) as subclasses, using the same parameters.
(e). Contribution of surface microbiomes to total microbial diversity
We used two approaches to assess the importance of planktonic versus animal-associated prokaryotic diversity to total coral reef microbial diversity. We first computed the phylogenetic richness of ASVs that were unique to plankton or to animal surfaces. We then compared the diversity of planktonic and animal surface microbiomes, by randomly picking from 1 to 74 animal taxa and 1 to 35 planktonic samples and then computing the total ASV and phylogenetic richness for each sampling depth. This procedure was performed 100 times.
To get a conservative estimation of the total sampled + un-sampled prokaryotic species richness in our dataset, we first curated the ASVs in our dataset, removing all unclassified ASVs that could not match any sequence in GenBank database. The remaining ASVs were then grouped into 97%-identity operational taxonomic units (OTUs) using ‘pick_otus.py' function provided in QIIME v. 1.9 [31], with default de novo usearch parameter, and OTUs found in less than two animal taxa were removed. We obtained 20 021 OTUs from 37 758 curated ASVs. Then, each animal taxon was considered as an independent sampling unit to count the occurrence of each OTU (i.e. the number of sampling units where the OTU was recovered). Estimates of OTU richness were calculated using the estimators provided in ‘SpadeR' R package [32] using ‘ChaoSpecies' function and type 2 incidence frequency data option.
(f). Vulnerability of microbial diversity
To assess the vulnerability of reef microbial diversity to the loss of macro-organisms, we simulated an extinction scenario combining the effects of global warming and overfishing. Macro-organisms taxa were removed proportionally to their respective vulnerability to heat stress for hard corals, and to habitat loss (due to coral bleaching) plus fishing for fishes.
Vulnerability of scleractinian coral genera to global warming was based on their bleaching response in the western Indian Ocean [33]. The genus Isopora, for which we had no data, was excluded from the extinction scenario. Similarly, no data were available for non-scleratinian Anthozoa and for all other invertebrates. There were therefore excluded from the scenario. Vulnerability to fishing [34] was obtained from FishBase (http://www.fishbase.org/, 2017). Vulnerability to habitat loss due to global change in coral reefs was computed as in Graham et al. [35] using data from FishBase and expert knowledge for input variables. All vulnerability indices are provided in electronic supplementary material, SM1-7, and the script used to simulate such animal diversity extinction and subsequent losses of microbial taxonomic and phylogenetic diversity is available online (https://github.com/marlenec/MicroErosion).
We assessed the vulnerability of prokaryotic diversity hosted by the 44 fish species and 12 genera of scleractinian corals included in the scenario by simulating loss of the 1 to 100% most vulnerable taxa according to their individual vulnerability. At each level of extinction, the percentage of remaining microbial diversity was compared to the one obtained from a random loss of the same number of scleractinian corals and fishes, computed 100 times. Deviation from this random loss was assessed by computing a p-value, calculated as the rank of the mean diversity value in the 100 replicates of the extinction scenario, among the increasingly sorted diversity values of all 100 replicates of the random scenario. We considered that the deviation from the random scenario was significant when p < 0.05, meaning that the diversity value in the observed community was lower than the 5% lowest diversity values following the random loss of animal taxa.
3. Results and discussion
(a). High diversity of animal microbiomes
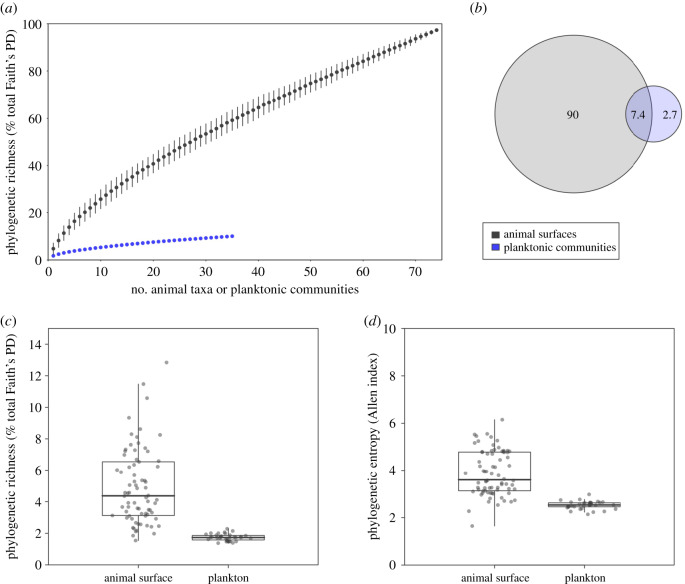
In our entire dataset made of 74 animal taxa and 35 samples of plankton, prokaryotic diversity hosted by animal surfaces contributed to 95% of total phylogenetic richness (Faith's PD) on rarefied data (figure 1a,b; unrarefied data in electronic supplementary material, SM2-1). Individual fish and Anthozoa samples contained slightly more ASVs than 200 ml planktonic samples (electronic supplementary material, SM2-2). A recent study found on average five times more 97%-identity bacterial OTUs in 1 l of seawater than in coral mucus [36]. Such different results may be due to the contrasting volume of coral microbiome versus seawater compared, a coral surface of 15–20 cm2 (see electronic supplementary material, SM1-5) to 0.2 l water samples in our study versus approximately 2 cm2 to 1 l of seawater in this former study. Similarly, differences in the protocols used (i.e. biomolecular methods and data analysis pipelines) may explain such discrepancies.
Figure 1.
Phylogenetic diversity of reef microbial communities. (a) Accumulation curves showing the phylogenetic richness (Faith's PD, expressed in percentage of maximum PD of the entire dataset) obtained from animal surface microbiomes and planktonic communities, depending on the number of randomly selected planktonic communities or animal taxa, represented as the mean ± the s.d. across 100 random subsamples. (b) Venn diagram representing the percentage of phylogenetic richness unique to animal surface microbiomes after pooling all animal taxa and to planktonic communities, and shared between both compartments. (c,d) Boxplots of (c) percentage of PD and (d) phylogenetic diversity (Allen's index) recovered from animal surface microbiomes and plankton. The same figure based on unrarefied data is provided in electronic supplementary material, SM2-1. Accumulation curves and Venn diagram based on ASV richness are provided in electronic supplementary material, SM2-3. (Online version in colour.)
Average surface microbiome recovered from each animal taxon (fish species, coral genus or other invertebrate taxon) was phylogenetically richer and more diverse than a 200 ml water sample (KW on Faith's PD and Allen's index on rarefied and unrarefied data, p < 0.001), with one animal taxon hosting on average 4.9 ± 2.4% of the total branch length of the phylogenetic tree grouping all ASVs recovered in the rarefied dataset, while one 200 ml water sample contained on average only 1.7 ± 0.2% of the total branch length (figure 1c,d; unrarefied data in electronic supplementary material, SM2-1). Additionally, 35 randomly chosen average animal microbiomes reached roughly eightfold higher phylogenetic richness than our 35 water samples (figure 1a), indicating that at a similar sampling effort, animal taxa host higher prokaryotic diversity compared to seawater. When considering unrarefied data, the gap between both types of communities was narrower (electronic supplementary material, SM2-1). Similar results were obtained based on ASV richness (electronic supplementary material, SM2-3).
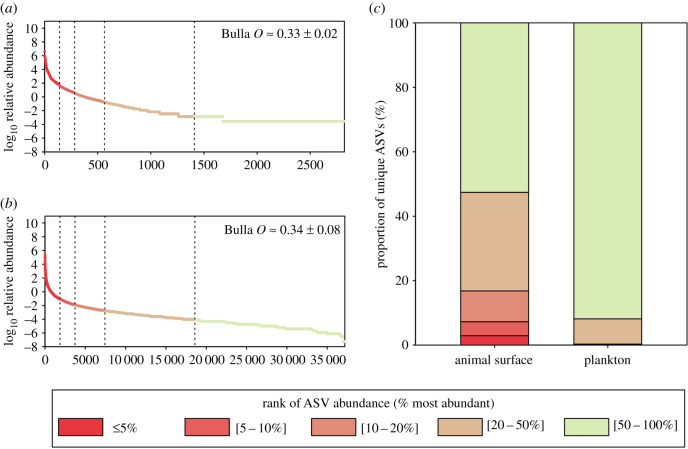
ASVs unique to animal surfaces (i.e. not detected in plankton) made more than 85% of total phylogenetic richness (figure 1b; unrarefied data in electronic supplementary material, SM2-1), demonstrating that most of the prokaryotic phylogenetic richness was associated with animal microbiomes. Around 16.8% of such ASVs were abundant to moderately abundant on animal surfaces, i.e. belonging to the top 20% most abundant ASVs (figure 2; unrarefied data in electronic supplementary material, SM2-4). By contrast, only 0.3% of the ASVs unique to plankton belonged to the 20% most abundant ASVs in planktonic samples. These results suggest that a significant portion of animal microbiome is specialized to a host-associated lifestyle and may not thrive in the water column, while the most abundant planktonic prokaryotes were also capable of colonizing animal skin. Such transient colonization of animal surface microbiomes may be a result of water contamination [37] or may permit host adaptation to environmental fluctuations [38].
Figure 2.
Abundance of ASVs in animal surface microbiomes and plankton. (a,b) Average rank-abundance curves obtained from surface microbiomes of (a) animal surface microbiomes and (b) planktonic communities. Vertical dotted lines represent 5, 10, 20 and 50% threshold for most abundant ASVs. The average evenness of ASVs abundances (Bulla's O) in each compartment is indicated on the corresponding plot. (c) Rank of abundances of ASVs unique to animal surface microbiomes or to plankton within respective microbial communities. The same figure based on unrarefied data is provided in electronic supplementary material, SM2-4.
(b). Bacterial composition
Bacteria dominated communities, accounting for 99.1 ± 1.8% of sequences. The 18 dominant bacterial classes in animal microbiome are depicted in figure 3a. Around 72% of them were also detected among the dominant classes in the whole microbiome of corals [39–41], and 61% and 50% were also detected on temperate fish skin and on tropical fish gills, respectively [42,43]. Two classes, Gracilibacteria and Campylobacteria, were not reported to be associated with animals in the aforementioned studies. Gracilibacteria belongs to the candidate phyla radiation (CPR), containing ultra-small bacteria that are recovered from diverse environments and have a probable symbiotic lifestyle [44]. Campylobacteria contained mostly the genus Arcobacter in our study (up to 8% of abundance). This genus usually inhabits the human or animal gastrointestinal system, though free-living strains have been isolated in various environments [45]. This genus has been isolated from coral disease lesions [46,47]. It has also been recovered from corals exposed to fish farm effluents and in thermally stressed corals [48,49]. Here, while the corals were apparently healthy and sampled far from coastal cities (electronic supplementary material, SM1-1), the high temperatures at the time of sampling (electronic supplementary material, SM1-2), together with a potential contamination of the lagoon by human faeces due to the absence of effluent treatment in Mayotte [50] may explain the presence of this genus.
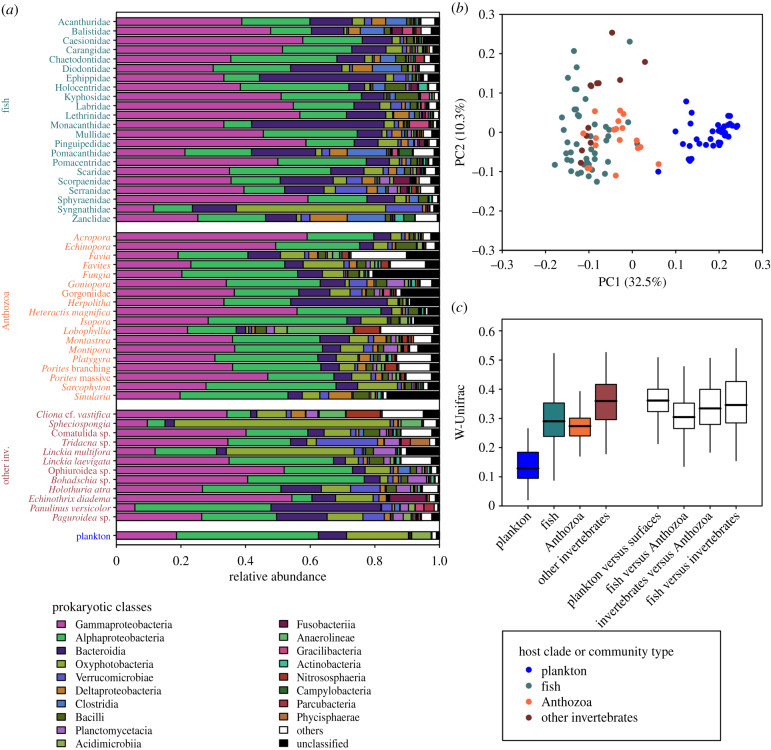
Figure 3.
Dissimilarity in taxonomic and phylogenetic structure of microbial communities from plankton or animal surfaces. (a) Dominance of 18 prokaryotic classes for animal surface microbiomes (averaged by fish families, Anthozoa genera and other invertebrates' taxa) and for plankton (averaged across all 35 planktonic communities). (b) Microbial communities are plotted on the two first axes of a PCoA computed on W-Unifrac. (c) Intra- and inter-group W-Unifrac. Results based on unrarefied data and on U-Unifrac are provided in electronic supplementary material, SM2-5 and 6.
Structure of prokaryotic communities recovered on animal surfaces was significantly distinct from plankton (PERMANOVA on W-Unifrac on rarefied data, p = 0.001, R2 = 0.29; figure 3; unrarefied data and U-Unifrac in electronic supplementary material, SM2-5 and 6). Plankton was mostly enriched in Alphaproteobacteria (especially SAR11 and SAR86 clades), Cyanobacteria (especially Prochlorococcus and Synechococcus) and the actinobacterial family Actinomarinaceae (figures 3a; electronic supplementary material, SM2-6 and 7). All these clades are abundant and widely distributed marine photoautotrophs and photoheterotrophs displaying small cell sizes and genomic reduction [51–55], that may be selected by the very oligotrophic conditions of surface ocean [56]. The presence of such taxa on our sites suggests that the reefs were well preserved from coastal eutrophication at the time of sampling.
By contrast, animal surfaces were mostly enriched in Gammaproteobacteria (especially Endozoicomonas), Firmicutes (especially Clostridia), Betaproteobacteriales (especially Burkholderiaceae), the α-proteobacterial Rhizobiales and Sphingomonadaceae, and Verrucomicrobia. Such clades have been previously identified in temperate and tropical marine fish skin, gut and gill microbiomes [42,43,57–61], and at the surface or inside marine invertebrates [9,58,62,63]. Endozoicomonas, especially, is associated with a wide range of hosts, from fish to hard and soft corals, sponges and tunicates, and is hypothesized to play a significant role in host's nutrition and health [58]. Particularly, Endozoicomonas genomes show enrichment in several functions that may help to cooperate with various types of hosts (e.g. protein secretion and carbohydrate transport), but also potential functional specialization depending on their hosts [64], that might explain the ubiquity of such genus on the wide diversity of animals studied here. To confirm this hypothesis, such taxonomic assessment of animal-associated microbiomes should be completed by studies assessing the functions that are necessary for bacteria to thrive on marine hosts.
(c). High dissimilarity between animal microbiomes
The high phylogenetic diversity of animal surface microbiomes was paired with a 2.3 times higher variability among those microbiomes than among planktonic samples (PERMDISP on W-Unifrac on rarefied data, p < 0.001; figure 3c; electronic supplementary material, SM2-5). The three major animal groups sampled (i.e. teleostean fishes, Anthozoa and other invertebrates) hosted significantly different prokaryotic communities at their surface, with the distinction between such groups explaining between 5% and 11% of variability (PERMANOVAs, p < 0.001, SM2-8). However, no significant biomarker was identified using LEfSe analysis for each of these groups, indicating that there is no consistently enriched bacterial clade in all animal taxa of these groups, as highlighted by the high dissimilarity between taxa within each group (figure 3b,c; electronic supplementary material, SM2-5).
Among each main animal group, there was a significant effect of animal taxon on surface-associated microbiome structure and composition (PERMANOVAs, electronic supplementary material, SM2-8). Interestingly, while the larger animal groups explained up to 11% of microbiome dissimilarity, the effect of such smaller taxa explained from 34% (for coral genera) to 49% (for fish species) of surface microbiome variability (PERMANOVAs on rarefied data, p < 0.001; electronic supplementary material, SM2-8).
While the species specificity of marine animal skin microbiome has already been reported [7], here we report that this interspecific variability within a clade is as high as between clades that diverged approximately 800 Mya (figures 3c; electronic supplementary material, SM2-5, divergence time estimate between Teleostei and Anthozoa according to http://www.timetree.org/). This finding suggests that the correlation between host phylogenetic relationships and microbiome composition, a pattern called ‘phylosymbiosis', which has been evidenced in numerous animal models including microbiomes of tropical fish skin, sponges and coral skeleton [7,36,65], may be unobservable at deeper phylogenetic level due to the very large phenotypic differences between the hosts that would saturate this signal.
(d). Exceptional reef microbial diversity
As a consequence of the coupled high diversity and high variability of animal surface microbiomes, the prokaryotic diversity sampled on animal surface microbiomes in this study is far from reaching an asymptote (figure 1a; electronic supplementary material, SM2-1, SM2-3). We estimated that the total sampled and un-sampled bacterial diversity associated with the subset of reef animals we sampled would range from 8700 to more than 20 000 97%-identity bacterial OTUs, respectively, on rarefied and unrarefied data (electronic supplementary material, SM2-9).
A recent estimate of world's bacterial diversity predicted that there exist 0.8–1.6 million prokaryotic OTUs worldwide [66]. Our richness estimates correspond to 0.5% to 2.5% of such global diversity. This is striking, given that coral reefs occupy less than 0.1% of surface worldwide, with southwestern Indian Ocean coral reefs making 1.8% of that surface (0.001% of global surface, www.reefbase.org/). Here, we based our estimates on carefully curated ASVs grouped as 97%-identity OTUs, which prevented any overestimation of prokaryotic richness at a local scale [67]. In addition, we only sampled a less than 7% subset of animal diversity in Mayotte's lagoon, hosting more than 700 fish species [68], 57 soft corals [69], 22 sea cucumbers [70], more than 200 scleractinian species and subspecies, 13 Asteroideae, 36 ascidians, 56 bivalves and 23 Ophiuroideae [71]. We also did not sample any species from other phylogenetically contrasted clades such as sharks and rays, sea turtle and mammal and vegetal species inhabiting Mayotte's lagoon. Likewise, we did not sample the microbes living within the tissue and skeleton of corals nor those living in fish guts. Our claim is that coral reef communities, just as they host a large portion of marine macrobial diversity compared with their surface [1], may also support a significant proportion of global marine prokaryotic diversity. More extensive sampling on the thousands of coral reef animals on different coral reefs will allow refining the estimates provided here.
(e). Vulnerability of reef microbial diversity
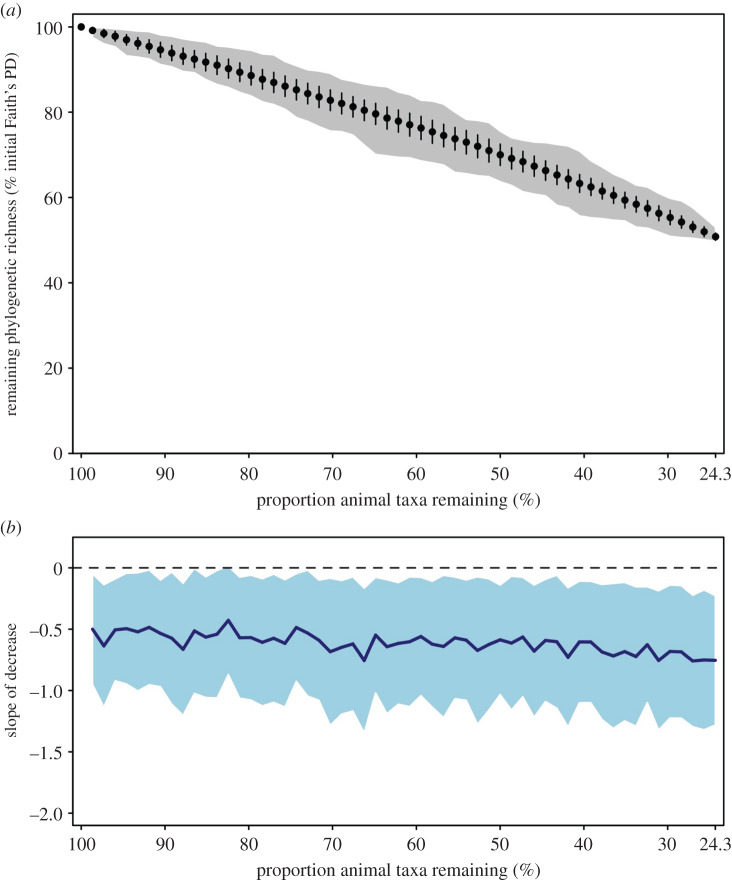
The loss of an animal species due to environmental disturbances at a given location induces the loss of its associated unique microbial diversity. We classified fish species and scleratinian genera depending on their respective vulnerability, to the combined overfishing and habitat loss in the case of fish, and ocean warming in the case of corals (see Material and methods; electronic supplementary material, SM1-7). There was no correlation between animal vulnerability and microbial diversity at its surface (Spearman correlation tests, p > 0.05). Microbial diversity erosion following macroscopic extinction scenarios revealed that the loss of the 50% most vulnerable coral and fish species for which we had vulnerability data would induce a loss of 28–29% of ASVs (electronic supplementary material, SM2-10) and 23% of phylogenetic richness (figure 4a; electronic supplementary material, SM2-11). Such lower phylogenetic erosion indicates a moderate phylogenetic redundancy between animal surface-associated ASVs. The slope of microbial diversity extinction was only slightly steeper with increasing loss of macroscopic species (figure 4b; electronic supplementary material, SM2-11). Accordingly, the levels of prokaryotic diversity loss were not significantly different from that expected under a random extinction scenario, because the most vulnerable animal species and less vulnerable ones host partially redundant prokaryotic diversity.
Figure 4.
Vulnerability of coral reef microbial phylogenetic richness to loss of fishes and scleratinian corals. (a) Mean (±s.d.) of remaining prokaryotic phylogenetic richness on the studied coral reef for a given proportion of animal taxa lost according to the simulated erosion scenario (100 replicates). When 100% of coral and fish species included in the scenario are lost, the remaining microbial phylogenetic richness corresponds to the one of prokaryotic communities associated with plankton and animal taxa that were not included in the extinction scenario (i.e. 24% of taxa, corresponding to Isopora, soft corals, gorgonians, anemone and all non-anthozoan invertebrates). Scenario simulating a random loss of fishes and corals (i.e. species removed independently from their vulnerability) is illustrated with the grey area representing the range of remaining phylogenetic richness among 100 replicates. (b) Mean (solid line) ±s.d. (shaded area) of slopes of phylogenetic richness loss calculated at each level of extinction on all 100 replicates. The same analyses based on unrarefied data and on ASV richness are provided in electronic supplementary material, SM2-10 and 11. (Online version in colour.)
To our knowledge, no quantified vulnerability measures exist for invertebrates other than sessile Anthozoa, so we did not include them in our extinction scenario. In our dataset, microbiomes of invertebrates other than Anthozoa showed the highest diversity and variability (figure 3c; electronic supplementary material, SM2-2, SM2-5), suggesting that the loss of such warming-sensitive taxa [72], as well as those targeted by humans [73], would induce a more severe erosion of coral reef microbial diversity than the one simulated here. Our results should be further completed by an assessment of the functional erosion that may result from anthropic activities, as such unique phylogenetic diversity in animal microbiomes may also host unique microbial functions in coral reef ecosystems [74]. Finally, our scenario did not account for potential direct effects of anthropic stressors (warming, acidification) that could alter the diversity and functions of microbiomes [75].
4. Conclusion
Reef animals macro-organisms host a high and unique diversity of microbes at their surface. Hence, the thousands of species living in coral reef ecosystems may support a substantial part of marine microbial diversity. For now, roles of animal surface microbiomes for ecosystem functioning are only partially described, but could be essential (e.g. production and degradation of dimethylsulfoniopropionate, a crucial molecule in sulfur cycling [4]). Future studies should investigate to what extent these unique and vulnerable microbial lineages present on animals contribute to coral reef functioning and possible consequence of their loss in the Anthropocene.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Emily Darling and Jérémie Vidal-Dupiol for helping us identify hard corals, and Frederic Ducarme for his help identifying echinoderms.
Data accessibility
Sequence data is available in the NCBI Sequence Read Archive database under BioProject accession numbers PRJNA506563 and PRJNA419057. Bacterial ASV table and associated metadata and sequences are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.wh70rxwjw [76].
Authors' contribution
M.C., T.B. and S.V. contributed to the conceptualization of the study. M.C., C.B., N.A.J.G., T.B. and S.V. contributed to the methodology. M.C., J.-C.A., C.B., T.C., C.X.R.-O., F.R., E.S. and S.V. helped in the investigation. M.C. conducted the analyses and writing of the original draft. M.C., J.-C.A., Y.B., C.B., T.C., N.A.J.G., F.R., E.S., T.B. and S.V. contributed to writing, reviewing and editing of the manuscript. T.B., M.C. and S.V. helped in the acquisition of funding. T.B. and S.V. supervised the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This project was funded by the TOTAL Foundation (MICMAC projet BIO-2015-135-49, grant no. 138295). The funders had no role in study design, sampling and analysis decision to publish, or preparation of the manuscript.
References
- 1.Fisher R, O'Leary R, Low-Choy S, Mengersen K, Knowlton N, Brainard RE, Caley JM. 2015. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 25, 500–505. ( 10.1016/j.cub.2014.12.022) [DOI] [PubMed] [Google Scholar]
- 2.Knowlton N. 2008. Coral reefs. Curr. Biol. 18, R18–R21. ( 10.1016/j.cub.2007.11.018) [DOI] [PubMed] [Google Scholar]
- 3.Kulbicki M, et al. 2013. Global biogeography of reef fishes: a hierarchical quantitative delineation of regions. PLoS ONE 8, e81847 ( 10.1371/journal.pone.0081847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne DG, Morrow KM, Webster NS. 2016. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 70, 317–340. ( 10.1146/annurev-micro-102215-095440) [DOI] [PubMed] [Google Scholar]
- 5.Krediet CJ, Ritchie KB, Alagely A, Teplitski M. 2013. Members of native coral microbiota inhibit glycosidases and thwart colonization of coral mucus by an opportunistic pathogen. ISME J. 7, 980–990. ( 10.1038/ismej.2012.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasl B, Herndl GJ, Frade PR. 2016. The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. ISME J. 10, 2280–2292. ( 10.1038/ismej.2016.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiarello M, et al. 2018. Skin microbiome of coral reef fish is highly variable and driven by host phylogeny and diet. Microbiome 6, 147 ( 10.1186/s40168-018-0530-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen A, Tao Z, Bullard SA, Arias CR. 2013. Diversity of the skin microbiota of fishes: evidence for host species specificity. FEMS Microbiol. Ecol. 85, 483–494. ( 10.1111/1574-6941.12136) [DOI] [PubMed] [Google Scholar]
- 9.Carlos C, Torres TT, Ottoboni LMM. 2013. Bacterial communities and species-specific associations with the mucus of Brazilian coral species. Sci. Rep. 3, 1624 ( 10.1038/srep01624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiarello M, Paz-Vinas I, Veyssière C, Santoul F, Loot G, Ferriol J, Boulêtreau S. 2019. Environmental conditions and neutral processes shape the skin microbiome of European catfish (Silurus glanis) populations of Southwestern France. Environ. Microbiol. Reports 11, 605–614. ( 10.1111/1758-2229.12774) [DOI] [PubMed] [Google Scholar]
- 11.Sunagawa S, et al. 2015. Structure and function of the global ocean microbiome. Science 348, 1261359 ( 10.1126/science.1261359) [DOI] [PubMed] [Google Scholar]
- 12.de Vargas C, et al. 2015. Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1261605 ( 10.1126/science.1261605) [DOI] [PubMed] [Google Scholar]
- 13.Troussellier M, Escalas A, Bouvier T, Mouillot D. 2017. Sustaining rare marine microorganisms: macroorganisms as repositories and dispersal agents of microbial diversity. Front. Microbiol. 8, 947 ( 10.3389/fmicb.2017.00947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleary DFR, et al. 2019. The sponge microbiome within the greater coral reef microbial metacommunity. Nat. Commun. 10, 1644 ( 10.1038/s41467-019-09537-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 16.Hughes TP, Bellwood DR, Connolly SR, Cornell HV, Karlson RH. 2014. Double jeopardy and global extinction risk in corals and reef fishes. Curr. Biol. 24, 2946–2951. ( 10.1016/j.cub.2014.10.037) [DOI] [PubMed] [Google Scholar]
- 17.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 18.Dixon P. 2003. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. ( 10.1111/j.1654-1103.2003.tb02228.x) [DOI] [Google Scholar]
- 19.Zhang Z, Huang H. 2007. Turing's formula revisited. J. Quant. Linguist. 14, 222–241. ( 10.1080/09296170701514189) [DOI] [Google Scholar]
- 20.Marcon E, Hérault B.2014. entropart: an R package to measure and partition diversity. See www.jstatsoft.org/article/view/v067i08 .
- 21.DeSantis TZ, et al. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. ( 10.1128/AEM.03006-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen S, et al. 2018. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems 3, e00021-18 ( 10.1128/mSystems.00021-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. ( 10.1016/0006-3207(92)91201-3) [DOI] [Google Scholar]
- 24.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. ( 10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 25.Allen B, Kon M, Bar-Yam Y. 2009. A new phylogenetic diversity measure generalizing the shannon index and its application to phyllostomid bats. Am. Nat. 174, 236–243. ( 10.1086/600101) [DOI] [PubMed] [Google Scholar]
- 26.Bulla L. 1994. An index of evenness and its associated diversity measure. Oikos 70, 167–171. ( 10.2307/3545713) [DOI] [Google Scholar]
- 27.Mouillot D, Wilson JB. 2002. Can we tell how a community was constructed? A comparison of five evenness indices for their ability to identify theoretical models of community construction. Theor. Popul. Biol. 61, 141–151. ( 10.1006/tpbi.2001.1565) [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Orphaned M.2012. Package ‘GUniFrac’. See https://cran.r-project.org/web/packages/GUniFrac/index.html .
- 29.Huang D, Roy K. 2015. The future of evolutionary diversity in reef corals. Phil. Trans. R. Soc. B 370, 20140010 ( 10.1098/rstb.2014.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 ( 10.1186/gb-2011-12-6-r60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. ( 10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao A, Ma KH, Hsieh TC, Chiu CH.2016. SpadeR: species-richness prediction and diversity estimation in R. See http://chao.stat.nthu.edu.tw/wordpress/software_download .
- 33.McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Sebastin CR, Guillaume MMM, Bruggemann JH. 2007. Western Indian Ocean coral communities: bleaching responses and susceptibility to extinction. Mar. Ecol. Prog. Ser. 337, 1–13. ( 10.3354/meps337001) [DOI] [Google Scholar]
- 34.Cheung WWL, Pitcher TJ, Pauly D. 2005. A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol. Conserv. 124, 97–111. ( 10.1016/j.biocon.2005.01.017) [DOI] [Google Scholar]
- 35.Graham NAJ, et al. 2011. Extinction vulnerability of coral reef fishes. Ecol. Lett. 14, 341–348. ( 10.1111/j.1461-0248.2011.01592.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock FJ, McMinds R, Smith S, Bourne DG, Willis BL, Medina M, Thurber RV, Zaneveld JR. 2018. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat. Commun. 9, 4921 ( 10.1038/s41467-018-07275-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweet MJ, Bulling MT. 2017. On the importance of the microbiome and pathobiome in coral health and disease. Front. Mar. Sci. 4, 10 ( 10.3389/fmars.2017.00009) [DOI] [Google Scholar]
- 38.Hester ER, Barott KL, Nulton J, Vermeij MJ, Rohwer FL. 2016. Stable and sporadic symbiotic communities of coral and algal holobionts. ISME J. 10, 1157–1169. ( 10.1038/ismej.2015.190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharp KH, Pratte ZA, Kerwin AH, Rotjan RD, Stewart FJ. 2017. Season, but not symbiont state, drives microbiome structure in the temperate coral Astrangia poculata. Microbiome 5, 120 ( 10.1186/s40168-017-0329-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apprill A, Weber LG, Santoro AE. 2016. Distinguishing between microbial habitats unravels ecological complexity in coral microbiomes. mSystems 1, e00143-16 ( 10.1128/mSystems.00143-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quigley KM, Roa CA, Torda G, Bourne DG, Willis BL. 2020. Co-dynamics of Symbiodiniaceae and bacterial populations during the first year of symbiosis with Acropora tenuis juveniles. MicrobiologyOpen 9, e959 ( 10.1002/mbo3.959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minniti G, Hagen LH, Porcellato D, Jørgensen SM, Pope PB, Vaaje-Kolstad G. 2017. The skin-mucus microbial community of farmed Atlantic Salmon (Salmo salar). Front. Microbiol. 8, 2043/ ( 10.3389/fmicb.2017.02043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratte ZA, Besson M, Hollman RD, Stewart FJ. 2018. The gills of reef fish support a distinct microbiome influenced by host-specific factors. Appl. Environ. Microbiol. 84, e00063-18 ( 10.1128/AEM.00063-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Méheust R, Burstein D, Castelle CJ, Banfield JF. 2019. The distinction of CPR bacteria from other bacteria based on protein family content. Nat. Commun. 10, 1–12. ( 10.1038/s41467-019-12171-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pérez-Cataluña A, Salas-Massó N, Diéguez AL, Balboa S, Lema A, Romalde JL, Figueras MJ. 2018. Revisiting the taxonomy of the genus Arcobacter: getting order from the chaos. Front. Microbiol. 9, 2077 ( 10.3389/fmicb.2018.02077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, DeSalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M. 2009. Bacterial diversity and white plague disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 3, 512–521. ( 10.1038/ismej.2008.131) [DOI] [PubMed] [Google Scholar]
- 47.Meyer JL, Castellanos-Gell J, Aeby GS, Häse CC, Ushijima B, Paul VJ. 2019. Microbial community shifts associated with the ongoing stony coral tissue loss disease outbreak on the florida reef tract. Front. Microbiol. 10, 2244 ( 10.3389/fmicb.2019.02244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garren M, Raymundo L, Guest J, Harvell CD, Azam F. 2009. Resilience of coral-associated bacterial communities exposed to fish farm effluent. PLoS ONE 4, e7319 ( 10.1371/journal.pone.0007319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiu J-H, Keshavmurthy S, Chiang P-W, Chen H-J, Lou S-P, Tseng C-H, Hsieh HJ, Chen CA, Tang S-L. 2017. Dynamics of coral-associated bacterial communities acclimated to temperature stress based on recent thermal history. Sci. Rep. 7, 1–13. ( 10.1038/s41598-017-14927-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gourbesville P, Thomassin BA. 2000. Coastal environment assessment procedure for sustainable wastewater management in tropical islands: the Mayotte example. Ocean Coastal Manage. 43, 997–1014. ( 10.1016/S0964-5691(00)00072-7) [DOI] [Google Scholar]
- 51.Burkill PH, Leakey RJG, Owens NJP, Mantoura RFC. 1993. Synechococcus and its importance to the microbial foodweb of the northwestern Indian Ocean. Deep Sea Res. Part II 40, 773–782. ( 10.1016/0967-0645(93)90057-T) [DOI] [Google Scholar]
- 52.Dupont CL, et al. 2012. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J. 6, 1186–1199. ( 10.1038/ismej.2011.189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F. 2013. Metagenomics uncovers a new group of low GC and ultra-small marine Actinobacteria. Sci. Rep. 3, 1–8. ( 10.1038/srep02471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grébert T, et al. 2018. Light color acclimation is a key process in the global ocean distribution of Synechococcus cyanobacteria. PNAS 115, E2010–E2019. ( 10.1073/pnas.1717069115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kent AG, Dupont CL, Yooseph S, Martiny AC. 2016. Global biogeography of Prochlorococcus genome diversity in the surface ocean. ISME J. 10, 1856–1865. ( 10.1038/ismej.2015.265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giovannoni SJ, Cameron Thrash J, Temperton B. 2014. Implications of streamlining theory for microbial ecology. ISME J. 8, 1553–1565. ( 10.1038/ismej.2014.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiarello M, Villéger S, Bouvier C, Bettarel Y, Bouvier T. 2015. High diversity of skin-associated bacterial communities of marine fishes is promoted by their high variability among body parts, individuals and species. FEMS Microbiol. Ecol. 91, fiv061 ( 10.1093/femsec/fiv061) [DOI] [PubMed] [Google Scholar]
- 58.Neave MJ, Apprill A, Ferrier-Pagès C, Voolstra CR. 2016. Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl. Microbiol. Biotechnol. 100, 8315–8324. ( 10.1007/s00253-016-7777-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egerton S, Culloty S, Whooley J, Stanton C, Ross RP. 2018. The gut microbiota of marine fish. Front. Microbiol. 9, 873 ( 10.3389/fmicb.2018.00873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullam KE, Essinger SD, Lozupone CA, O'connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. 2012. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol. Ecol. 21, 3363–3378. ( 10.1111/j.1365-294X.2012.05552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Sun G, Li S, Li X, Liu Y. 2018. Intestinal microbiota of healthy and unhealthy Atlantic salmon Salmo salar L. in a recirculating aquaculture system. J. Ocean. Limnol. 36, 414–426. ( 10.1007/s00343-017-6203-5) [DOI] [Google Scholar]
- 62.Cooney RP, Pantos O, Tissier MDAL, Barer MR, O'Donnell AG, Bythell JC. 2002. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ. Microbiol. 4, 401–413. ( 10.1046/j.1462-2920.2002.00308.x) [DOI] [PubMed] [Google Scholar]
- 63.Maravić A, Skočibušić M, Šprung M, Šamanić I, Puizina J, Pavela-Vrančić M. 2012. Occurrence and antibiotic susceptibility profiles of Burkholderia cepaciacomplex in coastal marine environment. Int. J. Environ. Health Res. 22, 531–542. ( 10.1080/09603123.2012.667797) [DOI] [PubMed] [Google Scholar]
- 64.Neave MJ, Michell CT, Apprill A, Voolstra CR. 2017. Endozoicomonas genomes reveal functional adaptation and plasticity in bacterial strains symbiotically associated with diverse marine hosts. Sci. Rep. 7, 1–12. ( 10.1038/srep40579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Easson CG, Thacker RW. 2014. Phylogenetic signal in the community structure of host-specific microbiomes of tropical marine sponges. Front. Microbiol. 5, 532 ( 10.3389/fmicb.2014.00532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Louca S, Mazel F, Doebeli M, Parfrey LW. 2019. A census-based estimate of Earth's bacterial and archaeal diversity. PLoS Biol. 17, e3000106 ( 10.1371/journal.pbio.3000106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue Z, Kable ME, Marco ML. 2018. Impact of DNA sequencing and analysis methods on 16S rRNA gene bacterial community analysis of dairy products. mSphere 3, e00410-18 ( 10.1128/mSphere.00410-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickel J, Jamon A, Pinault M, Durville P, Pascale C.. 2014. Composition et structure des peuplements ichtyologiques marins de l’île de Mayotte (sud-ouest de l'océan Indien). Paris, France: Société Française d'Ichtyologie.
- 69.Schleyer MH, Benayahu Y. 2016. The soft coral fauna (Octocorallia: Alcyonacea) of Mayotte. Mar. Biodiv. 48, 1643–1650. ( 10.1007/s12526-016-0621-z) [DOI] [Google Scholar]
- 70.Eriksson H, Byrne M, de la Torre-Castro M. 2012. Sea cucumber (Aspidochirotida) community, distribution and habitat utilization on the reefs of Mayotte, Western Indian Ocean. Mar. Ecol. Prog. Ser. 452, 159–170. ( 10.3354/meps09665) [DOI] [Google Scholar]
- 71.INPN. In press. Inventaire National du Patrimoine Naturel. See https://inpn.mnhn.fr/accueil/index (accessed on 13 November 2018).
- 72.Przeslawski R, Ahyong S, Byrne M, Wörheide G, Hutchings P. 2008. Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Change Biol. 14, 2773–2795. ( 10.1111/j.1365-2486.2008.01693.x) [DOI] [Google Scholar]
- 73.Polovina JJ, Haight WR, Moffitt RB, Parrish FA. 1995. The role of benthic habitat, oceanography, and fishing on the population dynamics of the spiny lobster, Panulirus marginatus (Decapoda, Palinuridae), in the Hawaiian Archipelago. Crustaceana 68, 203–212. ( 10.1163/156854095X00106) [DOI] [Google Scholar]
- 74.Martiny JBH, Jones SE, Lennon JT, Martiny AC. 2015. Microbiomes in light of traits: a phylogenetic perspective. Science 350, aac9323 ( 10.1126/science.aac9323) [DOI] [PubMed] [Google Scholar]
- 75.Zaneveld JR, et al. 2016. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 7, 11833 ( 10.1038/ncomms11833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiarello M, et al. 2020. Data from: Exceptional but vulnerable microbial diversity in coral reef animal surface microbiomes. Dryad Digital Repository. ( 10.5061/dryad.wh70rxwjw) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chiarello M, et al. 2020. Data from: Exceptional but vulnerable microbial diversity in coral reef animal surface microbiomes. Dryad Digital Repository. ( 10.5061/dryad.wh70rxwjw) [DOI]
Supplementary Materials
Data Availability Statement
Sequence data is available in the NCBI Sequence Read Archive database under BioProject accession numbers PRJNA506563 and PRJNA419057. Bacterial ASV table and associated metadata and sequences are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.wh70rxwjw [76].