Abstract
In species that care for their young, provisioning has profound effects on offspring fitness. Provisioning is important in honeybees because nutritional cues determine whether a female becomes a reproductive queen or sterile worker. A qualitative difference between the larval diets of queens and workers is thought to drive this divergence; however, no single compound seems to be responsible. Diet quantity may have a role during honeybee caste determination yet has never been formally studied. Our goal was to determine the relative contributions of diet quantity and quality to queen development. Larvae were reared in vitro on nine diets varying in the amount of royal jelly and sugars, which were fed to larvae in eight different quantities. For the middle diet, an ad libitum quantity treatment was included. Once adults eclosed, the queenliness was determined using principal component analysis on seven morphological measurements. We found that larvae fed an ad libitum quantity of diet were indistinguishable from commercially reared queens, and that queenliness was independent of the proportion of protein and carbohydrate in the diet. Neither protein nor carbohydrate content had a significant influence on the first principle component 1 (PC1), which explained 64.4% of the difference between queens and workers. Instead, the total quantity of diet explained a significant amount of the variation in PC1. Large amounts of diet in the final instar were capable of inducing queen traits, contrary to the received wisdom that queen determination can only occur in the third instar. These results indicate that total diet quantity fed to larvae may regulate the difference between queen and worker castes in honeybees.
Keywords: Apis mellifera, caste determination, diet, royal jelly
1. Introduction
Eusocial organisms have a division of labour in females between non-reproductive and reproductive individuals [1]. In bees, queens and workers have analogous genotypes; yet, these similar genomes produce distinct queen and worker phenotypes. Caste determination cues vary by species, but nutrition drives queen development in many social Hymenoptera [1,2]. In honeybees, nurse bees provision larvae with glandular secretions called jelly, which contain all the macronutrients and micronutrients required for growth and development [3]. It is thought that quality differences between royal jelly, which is fed to queen-destined larvae, and worker jelly controls this queen–worker dimorphism [2,3]; however, qualitative differences between royal and worker jelly have failed to fully explain caste determination. Recent findings suggest that diet quantity may have a significant role in honeybee caste determination [4,5]. However, the contribution of diet quantity has never been formally tested.
Since the 1890s, diet quality has been thought to determine caste in honeybees [6–10] through a ‘biological active substance’ found only in royal jelly [6,11] that activates a binary developmental switch for queen development [12]. The quality hypothesis arose from early observations of the queen and worker larvae receiving different proportions of water-clear and milky-white secretions from nurse bee glands [3,9]. The secretion fed to queen-destined larvae was termed royal jelly [13], and was thought to contain the major dietary component driving queen development [6]. As a result of these early observations, nearly every major component in royal jelly has been tested for its effects on caste determination, with most studies finding positive results. Lipids [14], proteins [15,16], carbohydrates [17–19] and water [20] all contribute to queen development in honeybees under some experimental conditions. However, most of these studies did not control for the quantity of diet. Nurse bees provide queen-destined larvae with an excess of diet [3,6,8,21]. Diet quantity appears to have a significant, but unaccounted role in honeybee caste determination and may explain why so many qualitative aspects of diet have been linked with caste.
Diet quantity controls queen–worker phenotypes in many other social Hymenoptera [22–26]. Increased provisioning drives queen differentiation in cape honeybees [22], bumblebees [24] and the oriental hornet [27]. Queen-destined bumblebees receive large quantities of diet during the final instar, resulting in both increased size and an elongated growth period [28,29]. In these examples, diet quantity elevates juvenile hormone, and triggers the development of reproductive potential [30]. The fact that elevated juvenile hormone also induces queen development in honeybees [31,32] suggests a conserved mechanism in which reproductive status is regulated by diet and mediated by juvenile hormone. The importance of food quantity in other bee species suggests that diet quantity may contribute to caste determination in honeybees.
Here, we test the relative contributions of diet quantity and quality during caste determination in honeybees. Larvae were reared in vitro on diets varying in quality (protein and carbohydrate proportion) and quantity in a factorial design. Using this experimental design, we test the effect of quantity and quality simultaneously. Our results indicate that diet quantity influences queen differentiation in honeybees.
2. Material and methods
(a). Artificial rearing
Apis mellifera larvae were collected from nine hives near Fargo, Cass County, North Dakota during a three-week period in the summer of 2015. Hives were supplemented with pollen patties (Mann Lake, MN, USA) and a 1 : 1 sucrose–water solution (Brushy Mountain Bee Farm, NC, USA) during poor foraging conditions. First instar larvae (0–21 h old) were transferred into 24-well cell culture plates (Falcon, Corning, Durham, NC) and placed onto 10 µl of diet. The 24-well plates were stored inside a modulator incubator chamber (Billups-Rothenberg, del Mar, CA, USA). Larvae were kept at a constant 34°C, darkness and relative humidity (RH) of 96% using potassium sulfate (K2SO4) [33]. Larvae were fed according to treatment in a factorial design of nine diet qualities and eight quantities with an additional ad libitum treatment for the medium diet, as described in the following sections. At the prepupal stage they were moved into 24-well cell culture plates containing Kimwipes (Kimtech Science, USA) sterilized in EtOH [34]. Pupae were maintained at a constant 34°C, darkness and 75% RH using NaCl until adult eclosion. Adults were stored at −20°C.
(b). Diet treatments
The study consisted of 72 treatment groups: nine diet qualities (table 1) combined with eight diet quantities in a factorial design. Additionally, an ad libitum quantity treatment was added using the medium-protein medium-carbohydrate diet (table 1). Each 24-well culture plate was randomly assigned to a diet quantity treatment. Within the plates, each row was assigned a diet quality treatment. Fresh diets were produced daily by homogenizing the ingredients for 10 min and warming in a 34°C water bath for 10 min before feeding. The volume of diet produced each day depended on the number of larvae in the study that were still in the feeding stage. For all the treatments, larvae were fed the same amount until the sixth day of development, while diet quality remained the same throughout development.
Table 1.
Macronutrient content of diet treatments. (Each diet combination (i.e. high protein, high carbohydrates) was fed at every diet quantity (160–370 µl). The ad libitum treatment was fed the medium protein, medium-carbohydrate diet.)
| diet treatments |
ingredients |
macronutrient (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| protein | carbohydrates | royal jelly (g) | glucose (g) | fructose (g) | yeast (g) | water (ml) | protein | carbohydrate | water | P : C ratio |
| high | high | 65 | 6 | 12 | 1 | 35 | 6.75 | 29.87 | 60.00 | 1 : 4.4 |
| medium | 65 | 4 | 8 | 1 | 35 | 7.10 | 26.15 | 63.19 | 1 : 3.7 | |
| low | 65 | 2 | 4 | 1 | 35 | 7.50 | 22.01 | 66.73 | 1 : 2.9 | |
| medium | high | 50 | 6 | 12 | 1 | 35 | 5.94 | 30.29 | 60.58 | 1 : 5.1 |
| medium | 50 | 4 | 8 | 1 | 35 | 6.30 | 26.02 | 64.29 | 1 : 4.1 | |
| low | 50 | 2 | 4 | 1 | 35 | 6.71 | 21.20 | 68.48 | 1 : 3.2 | |
| low | high | 35 | 6 | 12 | 1 | 35 | 4.86 | 30.84 | 61.35 | 1 : 6.3 |
| medium | 35 | 4 | 8 | 1 | 35 | 5.21 | 25.84 | 65.78 | 1 : 5.0 | |
| low | 35 | 2 | 4 | 1 | 35 | 5.61 | 20.06 | 70.91 | 1 : 6 | |
The reference diet (medium-protein medium-carbohydrate diet) was based on a previous study [35] which established the diet induced worker development. The other eight diets were produced by altering carbohydrates (glucose and fructose) and protein content (royal jelly: Pure Royal Jelly eBeeHoney.com, Ashland, OH, USA) in a full factorial design. The same batch of royal jelly was used for all diets for the duration of the experiment. Glucose and fructose have been previously used to alter carbohydrate content in in vitro diets [33,35]. However, royal jelly is the only protein source for in vitro diets because adding non-royal jelly proteins such as casein significantly decreases survival [36]. Altering amounts of royal jelly to manipulate protein content also changes carbohydrate content because commercial royal jelly contains sugars in addition to proteins. Therefore, the protein, carbohydrate and water content of the royal jelly was quantified using a Bradford assay and differential scanning calorimetry (see the electronic supplementary material, methods). The royal jelly contained 12.35% protein, 27% carbohydrates and 56% water. These values were used to calculate the percentage of macronutrients in each diet.
(c). Diet quantities
The lowest diet quantity (160 µl) was adopted from previous in vitro methods because this quantity produces workers [35]. Quantity was increased by 30 µl increments from 160 µl to 370 µl to produce the other treatments. There was an additional ad libitum treatment in which larvae were fed an excess of what they could consume. All larvae were fed the same amount during the first 5 days of development: day 1: 10 µl, day 2: 10 µl, day 3: 20 µl, day 4: 30 µl and day 5: 40 µl, totalling 110 µl of diet over the 5 days. During the sixth day of development, larvae were fed different amounts depending upon the diet quantity treatment so that total diet quantity ranged from 160 µl to 370 µl. In the ad libitum treatment, larvae were fed 200 µl per day until gut purge. Following gut purge, individuals were moved into pupation plates.
(d). Morphometrics
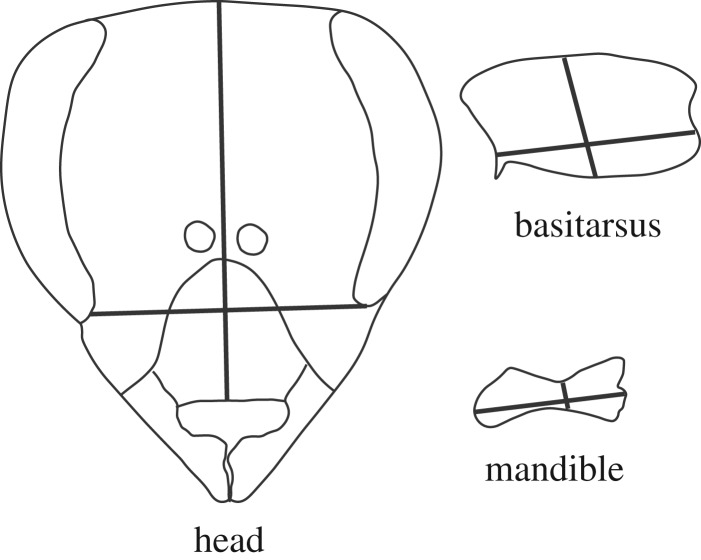
Adult morphometrics can separate and classify castes, even when ovariole number and spermathecal size are excluded [37]. The mandibles, basitarsus and head were dissected from adults (see the electronic supplementary material, table S1 for sample sizes by treatment) and photographed. Morphometric measurements included total body wet weight, width and length of the basitarsus, width and length of the mandible, and width and length of the head (figure 1). ImageJ software was used for measurements. In vitro reared adults were compared to two reference populations: commercially reared queens (Wildflower Meadows, Southern CA, USA) and hive-reared workers collected from the research hives in early spring.
Figure 1.
Measurements of honeybee head, basitarsus and mandible used in the morphometric analysis. Bold black lines represent the linear measurements of head length and width, basitarsus length and width, and mandible length and width.
(e). Data analysis and presentation of data
Statistical analyses were performed using R v.3.1.3 (R Core Team) [38]. Use of additional R packages are reported below where appropriate.
(i). Principal component analysis
A principal component analysis (PCA) was used to categorize an individual as a queen, worker or intercaste by comparing morphometric measurements between in vitro reared individuals and reference workers and queens. The principal components were calculated from hive-reared workers and commercially reared queens using the prcomp function in the stats package, and the predict function was used to produce principal components for the in vitro reared individuals. The assumptions for sphericity, sample adequacy and determinant of the matrix were tested and met. Principle component 1 (PC1) was used for downstream analysis.
(ii). Clustering analysis
Clustering is a statistical analysis for group classification and was used to determine if in vitro reared individuals were grouped with reference queens or workers. Linkage distances were calculated using the complete method for hierarchical clustering [39]. The cluster analysis was performed using the hclust function in the stats package. The results were graphed using the ColorDendrogram function within the sparcl package [40]. The optimal number of clusters was calculated using the K-means clustering method.
(iii). Measure of contribution of diet quantity and quality to principal component 1
The influence of diet quality and quantity on PC1 was compared for the 72 treatments, excluding the single ad libitum treatment. A generalized linear mixed model (GLMM) was performed using the lme4 package [41]. PC1 was the dependent variable and diet quantity and diet quality (protein, carbohydrate and water proportion) were the independent variables (table 1). Hive was treated as a random effect because bees from different hives are expected to differ in size and shape because of parental genetics. The assumptions of collinearity, independence of data and normality were tested and met for the model.
3. Results
(a). Principal component analysis
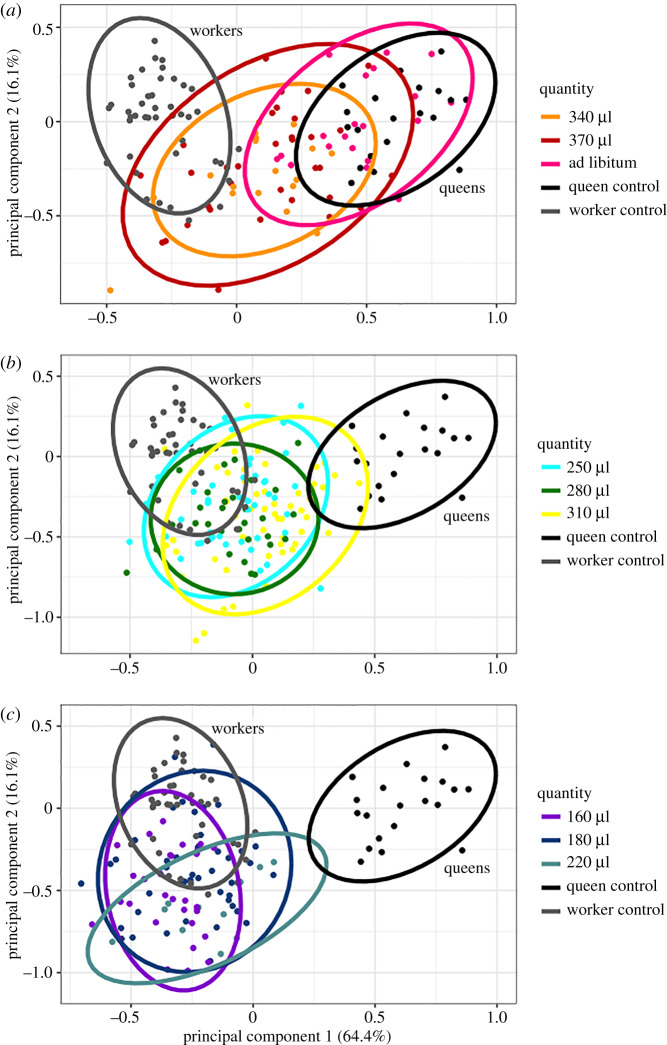
PCA was used to distinguish reference queens from reference workers based on six morphological measurements and body mass (figure 1). The PCA revealed a clear separation between reference workers and queens (figure 2), confirming morphometrics are a useful metric for caste classification. The results of the PCA also indicate that PC1 explains 64.42% of the variation between reference workers and queens, whereas principal component 2 (PC2) explained only 16.14% of the variation. Over 95% of the variation is explained by the first five components (PC1 = 64.42, PC2 = 16.14, PC3 = 8.78, PC4 = 4.34, PC5 = 2.82). The eigenvalues for PC1 were strongly influenced by adult weight and basitarsus length (table 2; electronic supplementary material, figure S1). PC2 was strongly influenced by head length (table 2; electronic supplementary material, figure S1). Because PC1 explained the majority of the variation between workers and queens, we used PC1 as a quantification of the queenliness of in vitro reared individuals. A total of 282 adults emerged from the in vitro treatments. Some treatments had high mortality (electronic supplementary material, table S1), particularly the low protein diets. The predict function in the stats package was used to calculate PC1 and PC2 for the in vitro reared individuals. PC1 increases as the quantity of diet increases (figure 2), but there was no discernable pattern with diet quality treatment (electronic supplementary material, figure S2). Statistical clustering and general linear modelling were used test the significance of the relationship between PC1 and diet quantity and quality.
Figure 2.
Principal component analysis (PCA) on seven traits visualized by diet quantity. Hive-reared queens (queen reference) and workers (worker reference) populations were analysed to establish principal component 1 (64.4%) and principal component 2 (16.14%). In vitro reared bees were compared to those reference populations. Colours represent amount of food during the final larval stage. (a) Bees fed larger quantities (340 µl, 370 µl and ad libitum) of food. (b) Bees fed medium quantities of food (250–310 µl). (c) Bees fed low quantities of food (160–220 µl). Ellipses represent the 95% confidence interval. Sample sizes were: 160 µl = 29, 190 µl = 45, 220 µl = 11, 250 µl = 44, 280 µl = 30, 310 µl = 54, 340 µl = 18, 370 µl = 33, ad libitum = 20, queen reference = 20, worker reference = 43. (Online version in colour.)
Table 2.
Eigenvalues of seven morphological traits of the first three principal components.
| trait | PC1 (64.42%) | PC2 (16.14%) | PC3 (8.78%) |
|---|---|---|---|
| adult weight | 55.2574 | 3.0590 | 7.4515 |
| basitarsus width | 0.1742 | 5.1875 | 0.0474 |
| basitarsus length | 32.5912 | 2.3740 | 0.3369 |
| head width | 4.0156 | 0.5515 | 90.7756 |
| head length | 5.2167 | 79.7015 | 1.3122 |
| mandible width | 2.5115 | 0.1337 | 0.0745 |
| mandible length | 0.2334 | 8.9928 | 0.0020 |
(b). Clustering analysis
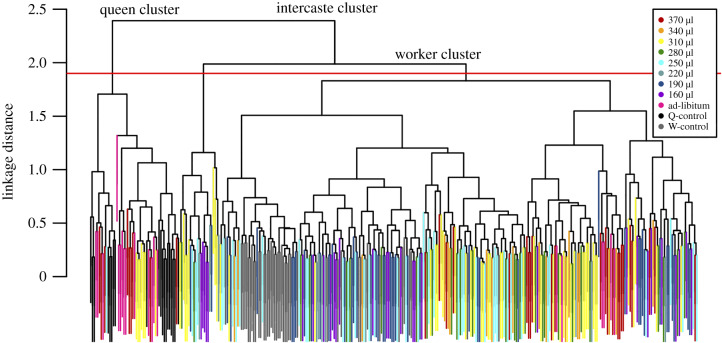
Once PC1 was calculated for all individuals, a cluster analysis was used to determine significant groupings between individuals. Specifically, the goal was to determine which in vitro reared individuals were grouped with queens or workers, versus which were determined to be intercaste. The K-means clustering revealed that three clusters were optimal because three minimized the within cluster variation (40.3%), compared to four clusters (47.4%). The high quantity treatments occupied the cluster with commercially reared queens while the low quantity treatments occupied the worker cluster (table 3). The cluster analysis distinguished the reference workers and queens into separate groups, while the in vitro individuals occupied all three clusters. Of the in vitro reared bees, 81 clustered with commercial queens, 75 clustered with hive-reared workers and 128 formed an intercaste cluster (table 3). The high quantity treatments produced a high proportion of queens. For example, the ad libitum treatment produced 100% (20 out of 20) queens, and the 370 µl treatment produced 58% queens (19 out of 33) (table 3). In the lower quantity treatments (220, 190, 160 µl), only three queens were produced. Clustering with the sparcl package produced a dendrogram with reference queens clustering with individuals from high quantity treatments (figure 3). By contrast, the diet quality treatments of in vitro reared bees were more evenly distributed across clusters, with 19–39% clustering with queens for the high and medium protein diets (electronic supplementary material, table S2).
Table 3.
Number of individuals found in each hierarchical cluster determined by ward linkage.
| cluster | queen control | ad libitum | 370 µl | 340 µl | 310 µ | 280 µl | 250 µl | 220 µl | 190 µl | 160 µl | worker control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 20 | 19 | 8 | 20 | 3 | 8 | 1 | 2 | 0 | 0 |
| 2 | 0 | 0 | 12 | 7 | 26 | 18 | 17 | 9 | 20 | 19 | 0 |
| 3 | 0 | 0 | 2 | 3 | 8 | 9 | 19 | 1 | 23 | 10 | 43 |
| in queen cluster | 100% | 58% | 44% | 37% | 10% | 18% | 9% | 4% | 0% | ||
Figure 3.
Dendrogram using ward linkage method for hierarchal clustering. Each colour represents either a quantitative treatment or a queen/worker control. The red line represents the significance threshold, which established three significant clusters. The three clusters are labelled worker, queen and intercaste based on the majority of the individuals occupying each cluster. (Online version in colour.)
(i). Effect of diet quantity and quality on final adult caste
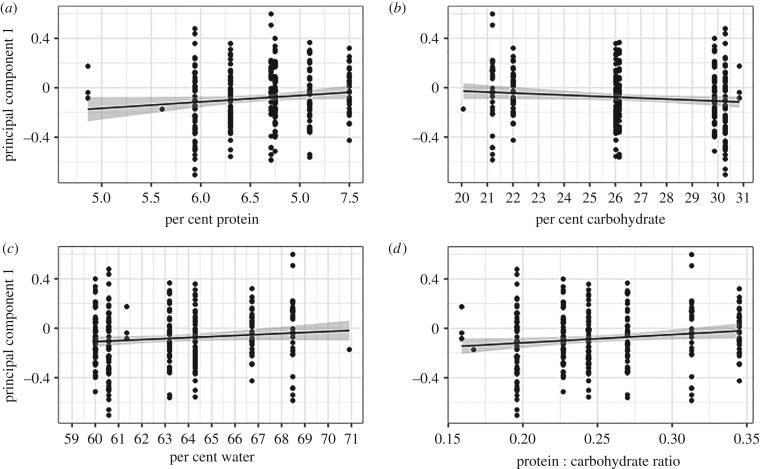
A GLMM was used to determine whether PC1 was significantly influenced by diet quantity for in vitro reared individuals. The individuals from the ad libitum treatment were not included in the model because the treatment was not part of the factorial design. The GLMM results indicate quantity has a significant influence on PC1 (p < 0.001; table 4), whereas quality (protein, carbohydrate and water proportion) does not. Interaction terms were tested and excluded because they were not significant. The overall model explained 38.8% of the variation in PC1. Because the quality variables were not significant, the R2 of 38.8% indicates that diet quantity, together with the random effect of hive origin, is explaining that amount of variation in PC1. In order to further examine the contributions of quantity and quality, a separate GLMM was run to test the influence of protein to carbohydrate ratio and quantity. Quantity was significant (p < 0.0001), but protein to carbohydrate ratio was not (p = 0.244). To visualize these relationships, diet quantity was plotted (figure 4), and increasing diet quantity had a significant effect on queenliness (p < 0.0001, R2 = 0.3814). The effects of different quality metrics (protein content, carbohydrate content, water content) were not significant (figure 5). However, protein to carbohydrate ratio did have a significant effect (p = 0.0278) when quantity was not taken into account. When each diet treatment was tested separately, quantity consistently had a positive influence on queenliness (electronic supplementary material, figure S3).
Table 4.
Results of generalized linear mixed model. (The effects of diet quantity and quality (protein and carbohydrate proportion) were tested on principal component 1, which explains 64.42% of the variation between the reference workers and queens.)
| principle component 1 (64.42%) |
|||
|---|---|---|---|
| β | t-value | p-value | |
| fixed parts | |||
| (intercept) | −710.2 | 1.161 | 0.247 |
| total quantity | 0.002 | 12.047 | p < 0.0001 |
| protein proportion | −8.884 | 1.161 | 0.247 |
| carbohydrate proportion | −7.109 | 1.163 | 0.246 |
| water proportion | −7.309 | 1.162 | 0.246 |
| random effects | |||
| hive | 0.009 | ||
| residual | 0.185 | ||
| NHive | 8 | ||
| observations | 264 | ||
| R2 | 0.388 | ||
Figure 4.
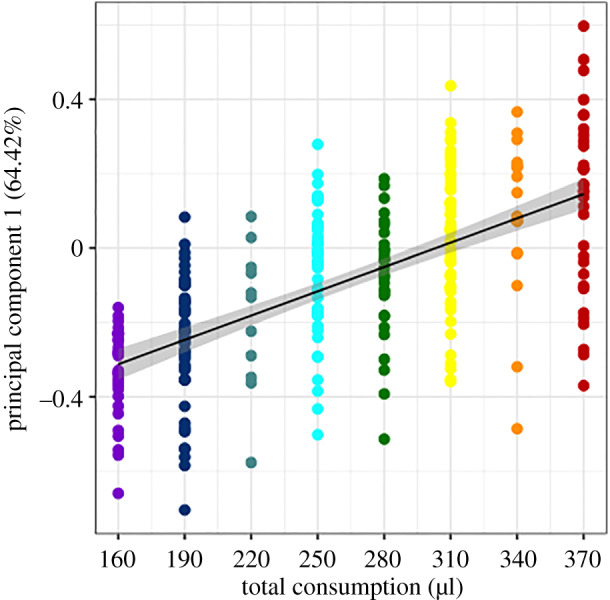
Influence of diet quantity on principal component 1 (p < 0.0001, R2 = 0.3814). (Online version in colour.)
Figure 5.
Influence of diet quality on principal component 1. (a) Protein proportion (p = 0.0596, R2 = 0.0097), (b) carbohydrate proportion (p = 0.0621, R2 = 0.0095), (c) water proportion (p = 0.0997, R2 = 0.0065), and (d) protein to carbohydrate ratio (p = 0.0278, R2 = 0.0146).
4. Discussion
Eusocial insects are an excellent example of phenotypic plasticity because they have reproductive and non-reproductive castes [1]. In many bee species, environmental cues drive female larvae into irreversible queen or worker developmental pathways [10]. These cues vary by species among eusocial Hymenoptera; however, only a few studies have fully characterized the cues that drive this divergence [25,26,28,29]. In honeybees, the cues driving caste bifurcation remain enigmatic [42]. Our goal was to determine the nutritional factors regulating caste, specifically testing whether diet quantity has a significant role in queen differentiation.
(a). Increasing diet quantity induces queen traits
Diet quantity had a significant influence on adult caste. Larger quantities of diet increased the queenliness of adults regardless of diet quality. All of the ad libitum individuals (20 out of 20) clustered with the commercially reared queens, even though they were reared on a medium quality diet. Most of the 370 µl treatment (19 out of 33) clustered with commercially reared queens, while no queens were produced in the lowest quantity treatment (160 µl), independent of diet quality. In the GLMM, diet quantity significantly influenced PC1, our measurement of queenliness. Our study is, to our knowledge, the first to directly test the role of diet quantity on honeybee queen development, and observations of nurse behaviour support our findings. Nurse bees provide queen larvae with more food than workers throughout development [3,6]. When emergency queen rearing is necessary, nurses increase worker-destined larvae cell size in order to rear queens [2]. Increased cell size probably allows nurse bees to increase provisioning for queen rearing.
Traditionally, caste is empirically determined based upon reproductive metrics, such as ovariole number and spermatheca size [43]. While these metrics assess reproductively viability of queens, the measurements are time-consuming and must be done immediately after queens are collected. Our study resulted in 282 in vitro reared adults, and dissecting individuals immediately upon eclosion was not feasible. Other studies have used PCA on morphology to overcome the challenge of depending on internal traits. De Souza et al. [37] determined that morphometrics could successfully distinguish intercastes from queens and workers. Like De Souza et al. [37] our study did not include binary traits like the presence or absence of corbiculae and mandible notches because binary traits cannot be used in a PCA. In our study, PC1 explained 64.4% of the variation between reference queens and workers, which is more of the variation between workers and queens seen by De Souza et al. [37] (PC1 = 32.71%). Our PC1 was probably higher because we performed the PCA on just the reference queens and workers, and then used a prediction model to determine the principal components for the in vitro reared individuals. Morphometrics, including live weight, highly correlate with reproductive metrics, and are excellent predictors of reproductive viability of queens [44].
Many social hymenopterans use diet quantity to regulate caste determination [22,24–27,29]. Quantity controls caste in bees including bumblebees [28,29] and stingless bees [45]. Honeybees were one of the few exceptions with quality thought to play the primary role. Similar to honeybees, workers of other species increase cell size, and provision more diet in order to raise queens [46]. In Bombus, diet quantity elevates juvenile hormone in queen-destined larvae [29], indicating a conserved endocrine mechanism across bees [32]. In honeybees, the insulin pathways link nutritional status to juvenile hormone production [31,47–49], with the involvement of target of repamycin [31,49,50]. Ostensibly, these pathways can either trigger juvenile hormone release from the corpora allata [32] or be triggered by juvenile hormone, as observed during external application of the hormone [31]. Our findings that quantity regulates caste in honeybee suggests a conserved regulator of caste determination in bees.
(b). Diet quality did not influence caste determination
We found that quantity, not protein proportion, carbohydrate proportion, nor water content, had a significant influence on final adult caste. This result is counter to previous studies, which identified these macronutrients as influencing caste [15,18,20]. Our results probably differ from previous studies because ours was, to our knowledge, the first study to systematically regulate consumption. Our results indicate these qualitative components do not seem to determine caste when food quantity is controlled. High carbohydrate diets produced more queens when diet quantity is not controlled [33]. When quantity was accounted for, Aupinel et al. [35] could not distinguish queen development between high and low carbohydrate diets, results that are corroborated by our study. Royalactin (MRJP-1) [15] and Major Royal Jelly Protein-3 (MRJP-3) [16] are considered the main royal jelly components influencing queen development, but neither study controlled for diet quantity. Our results indicate these qualitative components do not seem to determine caste when food quantity is controlled, and these qualitative factors should be reassessed to determine whether or not they play an important role in caste development.
Proteins have been widely studied for their role in caste determination, specifically royalactin (MRJP-1) [15] and MRJP-3 [16]. While studies initially showed these proteins were key regulators of caste [15,16], follow up studies have failed to reinforce these initial findings [51,52]. For example, Buttstedt et al. [51] added different amounts of monomeric royalactin in vitro and found royalactin did not induce queen development. Moreover, the major difference between the studies were Buttstedt et al. [51] controlled for diet quantity while Kamakura [15] did not. Since then, studies have revealed royalactin has multiple functions: the monomeric MRJP-1 is necessary for basic growth and development and the oligomeric MRJP-1 binds with 10-hydroxy-2-decenoic acid to increase royal jelly viscosity [53]. A current limitation of in vitro rearing is the inability to culture honeybee larvae on artificial diets components, such as casein [36], that would allow us to isolate the function of specific compounds. MRJPs have basic functions for larval growth and development, but it is unlikely one MRJP or a specific macronutrient determines caste alone.
Diet quality is important for juvenile growth, development and survival [34,35,54]. Growth is the result of not one but many interacting nutrients, including but not limited to proteins and carbohydrates. When protein and carbohydrate content are altered under controlled diet quantities, as done under a geometric framework for nutrition, growth rate, development and survival are affected, particularly on low protein diets [54]. We observed a similar result of high mortality on low protein diets. However, the proportion of proteins and carbohydrates do not appear to determine caste in honeybees. Carbohydrates can act as phagostimulants across insects [19,55–57], encouraging greater food consumption when present in high ratios. Sugars alone excite chemoreceptors as much as royal jelly [58]. Royal jelly contains significantly more carbohydrates than worker jelly [6,59]; thus, carbohydrates may be the phagostimulant that increases food consumption in queen-destined larvae. Royal jelly proteins are thought to regulate caste, but royal jelly and worker jelly do not always differ in the proportion of MRJPs. One study found royal jelly had higher protein content than worker jelly [59], whereas others reported lower protein content [8,60]. Protein and carbohydrates have important roles for larval growth and development, but the proportion of their components may not be the important difference between the larval diets of queens and workers.
In this study, we chose to focus on proteins, carbohydrates and water because previous studies showed these diet components had the strongest influence on queen development [15,18,20], even though royal jelly contains lipids and micronutrients [6] which also differed between our diets. Despite these limitations, our study corroborates other studies that have indicated relative protein content does not control queen differentiation [51,52]. Our results indicate diet quantity has a larger role on queen development than quality, and future studies should control for diet quantity when systematically testing macronutrients on caste development.
5. Conclusion
Nutritional status regulates many insect polyphenisms, such as the horns of dung beetles, and increased production of non-worker castes in termites [61–63]. A biological active substance in royal jelly has been thought to determine caste in honeybees since the late 1890s [3,6,21]. Many studies have evaluated caste determination both in vivo and in vitro [31,47,49,50], and while these studies offer important insights, the influence of diet quantity has not been explicitly tested. Our results indicate diet quantity is a significant factor in caste determination. Nutritional stress is a simple and elegant mechanism for controlling reproductive potential. In social vertebrates, nutritional stress regulates reproductive status [64]. During the evolution of eusociality in wasps, lower nutritional status in workers probably regulated their non-reproductive status [65]. In solitary bee species, which represent the ancestral state with respect to sociality [66], all female larvae become reproductive adults. Therefore, caste determination is a loss of reproductive potential in worker larvae, not necessarily a gain of reproductive potential caused by ingestion of a special type of food. During the evolution of eusociality, the loss of reproductive potential in workers coincides with the development of multiple morphological and behavioural traits that support hive fitness. miRNAs and p-coumaric acid found in pollen inhibit ovary development and are only present in the diet of worker-destined larva [67,68]. In our study, low food quantities inhibited queen development. The third day has long been thought the critical window for queen determination [12], but our results suggest reproductive status can be retained up to the sixth day if sufficient food is present. Thus, our study and [68] suggests honeybee larvae become workers through dietary suppression, such as food restriction and p-coumaric acid. In this framework, the mechanism that underlies eusociality in bees is food restriction, which suppresses reproduction in the worker caste.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank M. Palmersheim and E. Jensen for contributing to data collection. A. Rajamohan helped develop experimental procedures and contributed to data collection. J. Rinehart and M. Larson provided technical support. B. Heidinger provided comments on the manuscript. We thank four anonymous reviewers for their comments.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h44j0zpgc [69].
Author contributions
G.P.S. and J.H.B. designed the experiment. G.P.S. performed the experiment and statistical analyses. J.H.B. and G.D.Y. contributed statistical expertise. G.P.S., J.H.B. and G.D.Y. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a grant from the National Science Foundation (grant nos NSF IOS-155794 and NSF-RII-1826834), resources from the USDA ARS Insect Genetics and Biochemistry (grant no. 3060-21000-041-00D) and funding from the Department of Biological Sciences at North Dakota State University.
References
- 1.Wheeler DE. 1986. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am. Nat. 128, 13–34. ( 10.1086/284536) [DOI] [Google Scholar]
- 2.Winston ML. 1991. The biology of the honey bee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Haydak MH. 1970. Honey bee nutrition. Annu. Rev. Entomol. 15, 143–156. ( 10.1146/annurev.en.15.010170.001043) [DOI] [Google Scholar]
- 4.Leimar O, Hartfelder K, Laubichler MD, Page RE Jr. 2012. Development and evolution of caste dimorphism in honeybees: a modeling approach. Ecol. Evol. 2, 3098–3109. ( 10.1002/ece3.414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linksvayer TA, Kaftanoglu O, Akyol E, Blatch S, Amdam GV, Page RE Jr. 2011. Larval and nurse worker control of developmental plasticity and the evolution of honey bee queen-worker dimorphism. J. Evol. Biol. 24, 1939–1948. ( 10.1111/j.1420-9101.2011.02331.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rembold H, Dietz A. 1966. Biologically active substances in royal jelly. Vitam. Horm. 23, 359–382. ( 10.1016/S0083-6729(08)60385-4) [DOI] [PubMed] [Google Scholar]
- 7.Haydak MH. 1968. Nutrition des larves d'abeilles. Traité de Biologie de l'Abeille 1, 302–333. [Google Scholar]
- 8.Haydak MH. 1943. Larval food and development of castes in the honeybee. J. Econ. Entomol. 36, 778–792. ( 10.1093/jee/36.5.778) [DOI] [Google Scholar]
- 9.Johansson TS. 1955. Royal jelly. Bee World 36, 21–32. ( 10.1080/0005772X.1955.11094863) [DOI] [Google Scholar]
- 10.Weaver N. 1966. Physiology of caste determination. Annu. Rev. Entomol. 11, 79–102. ( 10.1146/annurev.en.11.010166.000455) [DOI] [PubMed] [Google Scholar]
- 11.Rembold H, Lackner B, Geistbeck I. 1974. The chemical basis of honeybee, Apis mellifera, caste formation. Partial purification of queen bee determinator from royal jelly. J. Insect. Physiol. 20, 307–314. ( 10.1016/0022-1910(74)90063-8) [DOI] [Google Scholar]
- 12.Evans JD, Wheeler DE. 2000. Expression profiles during honeybee caste determination. Genome Biol. 2, research0001.1 ( 10.1186/gb-2000-2-1-research0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber F, Bonnet C. 1792. Nouvelles observations sur les abeilles, adressées à M. Charles Bonnet: Chez Barde, Manget & Compagnie.
- 14.Wang WX, Tian LQ, Huang Q, Wu XB, Zeng ZJ. 2014. Effects of 10-hydroxy-2-decenoic acid on the development of honey bee (Apis mellifera) larvae. J. Apic. Res. 53, 171–176. ( 10.3896/IBRA.1.53.1.19) [DOI] [Google Scholar]
- 15.Kamakura M. 2011. Royalactin induces queen differentiation in honeybees. Nature 473, 478–483. ( 10.1038/nature10093) [DOI] [PubMed] [Google Scholar]
- 16.Huang C-Y, Chi L-L, Huang W-J, Chen Y-W, Chen W-J, Kuo Y-C et al. 2012. Growth stimulating effect on queen bee larvae of histone deacetylase inhibitors. J. Agric. Food Chem. 60, 6139–6149. ( 10.1021/jf300815b) [DOI] [PubMed] [Google Scholar]
- 17.Asencot M, Lensky Y. 1984. Juvenile hormone induction of ‘queenliness’ on female honey bee (Apis mellifera L. larvae reared on worker jelly and on stored royal jelly. Comp. Biochem. Physiol. B 78, 109–117. ( 10.1016/0305-0491(84)90153-6) [DOI] [Google Scholar]
- 18.Asencot M, Lensky Y. 1988. The effect of soluble sugars in stored royal jelly on the differentiation of female honeybee (Apis mellifera L.) larvae to queens. Insect Biochem. 18, 127–133. ( 10.1016/0020-1790(88)90016-9) [DOI] [PubMed] [Google Scholar]
- 19.Asencot M, Lensky Y. 1985. The phagostimulatory effect of sugars on the induction of ‘queenliness’ in female honeybee (Apis mellifera L.) larvae. Comp. Biochem. Physiol. A 81, 203–208. ( 10.1016/0300-9629(85)90289-0) [DOI] [Google Scholar]
- 20.Dietz A, Haydak MH. 1971. Caste determination in honey bees. I. The significance of moisture in larval food. J. Exp. Zool. 177, 353–357. ( 10.1002/jez.1401770309) [DOI] [PubMed] [Google Scholar]
- 21.von Planta A. 1888. Ueber den Futtersaft der Bienen. Z. Physiol. Chem. 12, 327–354. [Google Scholar]
- 22.Allsopp MH, Calis JNM, Boot WJ. 2003. Differential feeding of worker larvae affects caste characters in the Cape honeybee, Apis mellifera capensis. Behav. Ecol. Sociobiol. 54, 555–561. ( 10.1007/s00265-003-0666-4) [DOI] [Google Scholar]
- 23.de Camargo CA, de Almeida MG, Parra MGN, Kerr WE. 1976. Genetics of sex determination in bees. IX. Frequencies of queens and workers from larvae under controlled conditions (Hymenoptera: Apoidea). J. Kansas Entomol. Soc. 49, 120–125. [Google Scholar]
- 24.Pereboom J. 2000. The composition of larval food and the significance of exocrine secretions in the bumblebee Bombus terrestris. Insectes Soc. 47, 11–20. ( 10.1007/s000400050003) [DOI] [Google Scholar]
- 25.Judd TM, Teal PE, Hernandez EJ, Choudhury T, Hunt JH. 2015. Quantitative differences in nourishment affect caste-related physiology and development in the paper wasp Polistes metricus. PLoS ONE 10, e0116199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karsai I, Hunt JH. 2002. Food quantity affects traits of offspring in the paper wasp Polistes metricus (Hymenoptera: Vespidae). Environ. Entomol. 31, 99–106. ( 10.1603/0046-225X-31.1.99) [DOI] [Google Scholar]
- 27.Ishay J. 1975. Caste determination by social wasps: cell size and building behaviour. Anim. Behav. 23, 425–431. ( 10.1016/0003-3472(75)90090-1) [DOI] [Google Scholar]
- 28.Plowright R, Jay S. 1977. On the size determination of bumble bee castes (Hymenoptera: Apidae). Can. J. Zool. 55, 1133–1138. ( 10.1139/z77-146) [DOI] [Google Scholar]
- 29.Cnaani J, Borst DW, Huang Z-Y, Robinson GE, Hefetz A. 1997. Caste determination in Bombus terrestris: differences in development and rates of JH biosynthesis between queen and worker larvae. J. Insect. Physiol. 43, 373–381. ( 10.1016/S0022-1910(96)00106-0) [DOI] [PubMed] [Google Scholar]
- 30.Hartfelder K, Cnaani J, Hefetz A. 2000. Caste-specific differences in ecdysteroid titers in early larval stages of the bumblebee Bombus terrestris. J. Insect. Physiol. 46, 1433–1439. ( 10.1016/S0022-1910(00)00067-6) [DOI] [PubMed] [Google Scholar]
- 31.Mutti NS, Dolezal AG, Wolschin F, Mutti JS, Gill KS, Amdam GV. 2011. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J. Exp. Biol. 214(Pt 23), 3977–3984. ( 10.1242/jeb.061499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachinsky A, Hartfelder K. 1990. Corpora allata activity, a prime regulating element for caste-specific juvenile hormone titre in honey bee larvae (Apis mellifera carnica). J. Insect. Physiol. 36, 189–194. ( 10.1016/0022-1910(90)90121-U) [DOI] [Google Scholar]
- 33.Kaftanoglu O, Linksvayer TA, Page RE. 2011. Rearing honey bees, Apis mellifera, in vitro 1: effects of sugar concentrations on survival and development. J. Insect Sci. 11, 96 ( 10.1673/031.011.9601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Z. 2009. A standardized procedure for the in vitro rearing of honey bee larvae. Sacramento, CA: California Agricultural Research Inc.
- 35.Aupinel P, Fortini D, Dufour H, Tasei J, Michaud B, Odoux J et al. 2005. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 58, 107. [Google Scholar]
- 36.Singh P. 1977. Artificial diets for insects, mites, and spiders. Berlin, Germany: Springer. [Google Scholar]
- 37.De Souza DA, Wang Y, Kaftanoglu O, De Jong D, Amdam GV, Goncalves LS et al. 2015. Morphometric identification of queens, workers and intermediates in in vitro reared honey bees (Apis mellifera). PLoS ONE 10, e0123663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Everitt BS. 1974. Cluster analysis. London, UK: Heinemann Educational Publishers. [Google Scholar]
- 40.Witten DM, Tibshirani R. 2013. sparcl: Perform sparse hierarchical clustering and sparse k-means clustering. R package version, 1(3). See https://cran.r-project.org/web/packages/sparcl/index.html .
- 41.Bates D, Mächler M, Bolker B, Walker S.2014. Fitting linear mixed-effects models using lme4. See http://arxiv.org/14065823 .
- 42.Bloch G, Shpingler H, Wheeler D, Robinson G. 2009. Endocrine influences on the organization of insect societies. In Hormones, brain and behavior, vol. 3 (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 195–235. New York, NY: Academic Press. [Google Scholar]
- 43.Eckert J. 1934. Studies in the number of ovarioles in queen honeybees in relation to body size. J. Econ. Entomol. 27, 629–635. ( 10.1093/jee/27.3.629) [DOI] [Google Scholar]
- 44.Akyol E, Yeninar H, Kaftanoglu O. 2008. Live weight of queen honey bees (Apis mellifera L.) predicts reproductive characteristics. J. Kansas Entomol. Soc. 81, 92–101. ( 10.2317/JKES-705.13.1) [DOI] [Google Scholar]
- 45.Hartfelder K, Makert GR, Judice CC, Pereira GAG, Santana WC, Dallacqua R et al. 2006. Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie 37, 144–163. ( 10.1051/apido:2006013) [DOI] [Google Scholar]
- 46.Lisboa L, Serrão J, Cruz-Landim C, Campos L. 2005. Effect of larval food amount on ovariole development in queens of Trigona spinipes (Hymenoptera, Apinae). Anat. Histol. Embryol. 34, 179–184. ( 10.1111/j.1439-0264.2005.00591.x) [DOI] [PubMed] [Google Scholar]
- 47.de Azevedo SV, Hartfelder K. 2008. The insulin signaling pathway in honey bee (Apis mellifera) caste development: differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect. Physiol. 54, 1064–1071. ( 10.1016/j.jinsphys.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Azevedo SV, Hartfelder K, Amdam GV. 2013. Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L). J. Exp. Biol. 216, 4347–4357. ( 10.1242/jeb.085779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wheeler DE, Buck NA, Evans JD. 2014. Expression of insulin/insulin-like signalling and TOR pathway genes in honey bee caste determination. Insect. Mol. Biol. 23, 113–121. ( 10.1111/imb.12065) [DOI] [PubMed] [Google Scholar]
- 50.Patel A, Fondrk MK, Kaftanoglu O, Emore C, Hunt G, Frederick K et al. 2007. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2, e509 ( 10.1371/journal.pone.0000509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buttstedt A, Ihling CH, Pietzsch M, Moritz RF. 2016. Royalactin is not a royal making of a queen. Nature 537, E10–E12. [DOI] [PubMed] [Google Scholar]
- 52.Kucharski R, Foret S, Maleszka R. 2015. EGFR gene methylation is not involved in royalactin controlled phenotypic polymorphism in honey bees. Sci. Rep. 5, 14070 ( 10.1038/srep14070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buttstedt A, Muresan CI, Lilie H, Hause G, Ihling CH, Schulze SH et al. 2018. How honeybees defy gravity with royal jelly to raise queens. Curr. Biol. 28, 1095–1100.e3. ( 10.1016/j.cub.2018.02.022) [DOI] [PubMed] [Google Scholar]
- 54.Helm BR, Slater GP, Rajamohan A, Yocum GD, Greenlee KJ, Bowsher JH. 2017. The geometric framework for nutrition reveals interactions between protein and carbohydrate during larval growth in honey bees. Biol. Open. 6, 872–880. ( 10.1242/bio.022582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen AC. 2015. Insect diets: science and technology. Boca Raton, FL: CRC Press. [Google Scholar]
- 56.Singer M, Bernays E, Carrière Y. 2002. The interplay between nutrient balancing and toxin dilution in foraging by a generalist insect herbivore. Anim. Behav. 64, 629–643. ( 10.1006/anbe.2002.3082) [DOI] [Google Scholar]
- 57.Mitchell B, Schoonhoven L. 1974. Taste receptors in Colorado beetle larvae. J. Insect. Physiol. 20, 1787–1793. ( 10.1016/0022-1910(74)90208-X) [DOI] [PubMed] [Google Scholar]
- 58.Goewie EA. 1978. Regulation of caste differentiation in the honey bee (Apis mellifera L). Meded. Landbouwhogesch. Wageningen 78, 1–76. [Google Scholar]
- 59.Wang Y, Ma L, Zhang W, Cui X, Wang H, Xu B. 2015. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 47, 48–56. ( 10.1007/s13592-015-0374-x) [DOI] [Google Scholar]
- 60.Shuel R, Dixon S. 1959. Studies in the mode of action of royal jelly in honeybee development: II. Respiration of newly emerged larvae on various substrates. Can. J. Zool. 37, 803–813. ( 10.1139/z59-081) [DOI] [Google Scholar]
- 61.Scharf ME, Buckspan CE, Grzymala TL, Zhou X. 2007. Regulation of polyphenic caste differentiation in the termite Reticulitermes flavipes by interaction of intrinsic and extrinsic factors. J. Exp. Biol. 210(Pt 24), 4390–4398. ( 10.1242/jeb.010876) [DOI] [PubMed] [Google Scholar]
- 62.Emlen DJ. 1997. Diet alters male horn allometry in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 264, 567–574. ( 10.1098/rspb.1997.0081) [DOI] [Google Scholar]
- 63.Moczek AP. 1998. Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behav. Ecol. 9, 636–641. ( 10.1093/beheco/9.6.636) [DOI] [Google Scholar]
- 64.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 010. ( 10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunt J. 2012. A conceptual model for the origin of worker behaviour and adaptation of eusociality. J. Evol. Biol. 25, 1–19. ( 10.1111/j.1420-9101.2011.02421.x) [DOI] [PubMed] [Google Scholar]
- 66.Brothers DJ. 1999. Phylogeny and evolution of wasps, ants and bees (Hymenoptera, Chrysidoidea, Vespoidea and Apoidea). Zool. Scr. 28, 233–250. ( 10.1046/j.1463-6409.1999.00003.x) [DOI] [Google Scholar]
- 67.Zhu K, Liu M, Fu Z, Zhou Z, Kong Y, Liang H et al. 2017. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genet. 13, e1006946 ( 10.1371/journal.pgen.1006946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao W, Schuler MA, Berenbaum MR. 2015. A dietary phytochemical alters caste-associated gene expression in honey bees. Sci. Adv. 1, e1500795 ( 10.1126/sciadv.1500795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slater GP, Yocum GD, Bowsher JH. 2020. Data from: Diet quantity influences caste determination in honeybees (Apis mellifera) Dryad Digital Repository. ( 10.5061/dryad.h44j0zpgc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Slater GP, Yocum GD, Bowsher JH. 2020. Data from: Diet quantity influences caste determination in honeybees (Apis mellifera) Dryad Digital Repository. ( 10.5061/dryad.h44j0zpgc) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h44j0zpgc [69].