Abstract
Matching habitat choice is a unique, flexible form of habitat choice based on self-assessment of local performance. This mechanism is thought to play an important role in adaptation and population persistence in variable environments. Nevertheless, the operation of matching habitat choice in natural populations remains to be unequivocally demonstrated. We investigated the association between body colour and substrate use by ground-perching grasshoppers (Sphingonotus azurescens) in an urban mosaic of dark and pale pavements, and then performed a colour manipulation experiment to test for matching habitat choice based on camouflage through background matching. Naturally, dark and pale grasshoppers occurred mostly on pavements that provided matching backgrounds. Colour-manipulated individuals recapitulated this pattern, such that black-painted and white-painted grasshoppers recaptured after the treatment aggregated together on the dark asphalt and pale pavement, respectively. Our study demonstrates that grasshoppers adjust their movement patterns to choose the substrate that confers an apparent improvement in camouflage given their individual-specific colour. More generally, our study provides unique experimental evidence of matching habitat choice as a driver of phenotype–environment correlations in natural populations and, furthermore, suggests that performance-based habitat choice might act as a mechanism of adaptation to changing environments, including human-modified (urban) landscapes.
Keywords: background colour matching, local adaptation, matching habitat choice, phenotype–environment correlation, urban adaptation
1. Background
Natural environments are not uniform and, as a result, individuals experience performance trade-offs across habitats that typically result in phenotype–environment correlations [1,2]. Whenever tests for local adaptation [1] are positive, patterns of phenotype–environment covariance are generally assumed to result from divergent natural selection through the selective removal of maladapted individuals [3]. However, the same pattern can be the result of alternative processes [4], including phenotypic plasticity [5,6] and matching habitat choice [7,8]. Natural selection and phenotypic plasticity have received considerable attention from evolutionary ecologists and many important aspects of these mechanisms, from their molecular bases to their adaptive significance and eco-evolutionary consequences, are well understood [9–12]. Nevertheless, although a theoretical framework has been established [4,8,13,14], research on matching habitat choice is still in its infancy and many questions remain open.
Matching habitat choice is defined as a specific habitat choice mechanism in which individuals assess local performance in different environments and then preferentially settle in the environment that is best suited to their phenotype, aiming to increase fitness [7,8]. This mechanism thereby differs from habitat choice due to imprinting [15] or genetic preference alleles [16], in which such self-assessment of local performance does not play a role [17]. Such self-assessment makes matching habitat choice inherently phenotype-dependent, and this should increase the phenotype–environment match compared to the other forms of habitat choice. Matching habitat choice, by creating patterns of phenotype–environment covariance and non-random gene flow between different habitats [18], is predicted to influence the degree and rate of local adaptation, population persistence, genetic structure, the maintenance of genetic variation, the evolution of niche width, and even reproductive isolation [8,13,14,19,20 and references therein, 21, and see electronic supplementary material, S1 for evidence on female-detection distances by males that suggests that matching habitat choice indirectly causes assortative mating between grasshoppers with similar colours]. Understanding the prevalence and relevance of this mechanism in nature is therefore important, but supporting evidence from natural settings is still rare and often indirect [22–28].
Much of this lack of empirical support for matching habitat choice is due to considerable logistical and inferential challenges. For instance, to distinguish matching habitat choice from other processes that may act simultaneously and also generate phenotype–habitat correlations, such as phenotypic plasticity and selective mortality, the phenotype of free-ranging individuals needs to be measured and then linked to their departure and settlement decisions [27,29]. Crucially, under matching habitat choice, departure and settlement decisions are based on performance variation across habitats and, therefore, a good biological understanding of the operation and strength of performance trade-offs between ecologically distinct environments is essential [8,24,25,28]. Next, to decouple the effect of self-assessment of phenotype–environment match from that of direct genetic preference or imprinting, an experimental manipulation of phenotypes should be performed [8,17], but this are generally difficult to implement in natural populations, and so most studies so far instead used controlled laboratory or microcosm settings [30,31].
Matching habitat choice has received considerable research attention in the last decade and, although several tests have been conducted under controlled indoor conditions [32–34], examples of phenotype manipulation experiments are restricted to a couple studies on grasshoppers. For example, in a series of laboratory experiments using a mosaic of solar radiation, Karpestam et al. [30] and Wennersten et al. [31] showed that pale-painted and dark-painted grasshoppers tended to settle in the thermal zone offering the better fitness prospects given their susceptibility to radiation. No such tendency was however seen in unmanipulated naturally dark and pale morphs, thus obscuring the applicability of these findings to more realistic natural scenarios. More recently, Edelaar et al. [35] demonstrated that laboratory-reared grasshoppers released in the field after a unidirectional hormone-induced cuticle darkening made greater use of dark substrates than unmanipulated (uninjected) individuals. Clearly, this finding provides support for matching habitat choice, but its relevance to nature is somewhat uncertain due to the use of laboratory-reared nymphs lacking prior knowledge of the study area. In addition, the hormone (corazonin) used for darkening also functions as a stress hormone, so injected grasshoppers could have preferred dark substrates for other reasons unrelated to their own colour and crypsis. Combining evidence from observational data and a phenotype manipulation experiment in both directions using free-ranging animals in their local natural environment is probably the best inferential approach, but no such study has been explicitly conducted. Consequently, the operation of matching habitat choice in natural populations remains to be conclusively demonstrated.
Here, we combine behavioural observations of free-ranging individuals, a phenotype manipulation experiment, and a capture-recapture approach to test for matching habitat choice based on camouflage through background matching in a wild population of azure sand grasshoppers (Sphingonotus azurescens). Specifically, we investigated the association between body colour and substrate use by ground-perching grasshoppers in a recently developed urban area covered by a mosaic of adjoining patches of dark and pale pavements, and then used the same sample of individuals to examine their substrate use after manipulating their colour. Sphingonotus azurescens (Acrididae: Orthoptera) is a medium-sized (25–40 mm) omnivorous grasshopper almost exclusively found on sparsely vegetated or bare soils which may differ in colour and structure from sandy and clay substrates to gravel plains and even artificial pavements [36, P Edelaar 2013-2015, personal observation]. Sphingonotus azurescens ranges in colour from nearly black to very pale grey and from bluish-grey to orange-brown, and wild populations typically match the colour of the substrate on which they are found [37,38, A Sanabria-Fernández, P Edelaar 2013-2017, personal observation].
Background colour matching is a textbook example of crypsis (i.e. an adaptation to avoid detection by visual predators; see [39]), and there is strong evidence that predation risk of grasshoppers can be considerably reduced in matching backgrounds [40–42], also in our study species [43–45] (see electronic supplementary material, S2 for species-specific information). Grasshoppers colonizing our urban study area offer an excellent opportunity to assess matching habitat choice because: (i) their movement ability (on average, 12.3 m d−1; [35]) and the distances between distinct habitats are on a comparable scale, so grasshoppers are not constrained to assess and respond to spatial variation in local performance; (ii) in view of low grasshopper densities, there appears to be no competition for space that prevents individuals from seeking and settling in the preferred substrate; (iii) nymphs and adults are vulnerable to attack by visual predators, including wasps, jumping spiders, lizards, mice and birds (P Edelaar 2013-2015, personal observation); (iv) previous evidence indicates that they are indeed more colour-matched on their local urban pavement than they are on alternative pavements, supporting the potential for a trade-off in survival between distinctly coloured substrates [35,43]; (v) importantly, a recent survival analysis based on mark–capture–recapture data showed that the observed colour segregation across different pavements does not result from colour-dependent mortality (natural selection) favouring more cryptic individuals [35]; (vi) nor does it result from colour plasticity since adults no longer moult and only change colour very slowly, as experimentally confirmed in the laboratory [35,44]. Matching habitat choice has therefore the means and the motive to operate.
In addition, we take advantage of another aspect, namely the relative ease of manipulation (painting) of the relevant phenotypic trait (grasshopper colour) with no apparent negative effects on normal behaviour [30,46, A Sanabria-Fernández 2017, personal observation]. In this context, as a test for matching habitat choice we predict (i) that unmanipulated naturally darker individuals are more often found on dark substrates compared to paler individuals, and (ii) that after manipulation, dark-painted individuals are more often found on dark substrates regardless of their original colour, and vice versa for pale-painted individuals.
2. Methods
(a). Study area
The study was conducted in 2017 in a recently created (6–8 years before the study) urban-like habitat located in an abandoned housing development area near Dos Hermanas (Seville, Spain; 37.306° N, 5.932° E). This area consists of a network of orthogonal streets closed to traffic around blocks of sparsely vegetated natural soils that grasshoppers use as breeding sites. Due to the negligible level of use and maintenance of the streets, some colonizing food plants have started growing on roads and sidewalks, enough to provide a suitable alternative environment for grasshopper colonization and reproduction (see details in [35,43]). Streets are paved with dark asphalt in the centre, with pale sidewalks (tiles) and parking lots (cement) on both sides (figure 1a,b). Overall, the sampling area included a grid of four parallel streets (range: 223–335 m long) and one crossing street (395 m long).
Figure 1.
(a) Detail of one of the streets sampled, showing the stretch of dark asphalt in the centre and pale sidewalks (tiles) and parking lots (cement) on both sides. (b) Comparison of the degree of crypsis of pale and dark grasshoppers on pale (tile) versus dark (asphalt) habitat. Grasshopper images are standardized digital photographs of a pale (18% blackness) and a dark (74% blackness) individual, each superimposed on both background types to facilitate comparison. Background images are digital photographs (corrected using a reflectance standard) of a representative sidewalk tile (left) and asphalt pavement (right) of the study area. (c) Digital photographs of grasshoppers under standardized conditions illustrating natural variation in body colour from the upper to the lower extremes of the colour distribution. From left to right, estimates of blackness for these individuals are 18, 27, 35, 45, 58, 69 and 74%. (Online version in colour.)
(b). Field procedures
To detect grasshoppers, we walked up and down the streets sweeping from side to side a 30-cm diameter mesh net attached to a 1.5-m pole, which causes adults to jump up and fly a few metres. These were then caught with the same net. We conducted capture sessions during the period when fully-developed adults are present (May–September). Most grasshoppers disappeared from the streets between early afternoon and early evening (further supporting movement across the different pavements), and were sometimes seen to seek shade presumably as a thermoregulatory response to the high (greater than 40° C) afternoon temperatures [47]. Hence, capture sessions were conducted between 09.00–14.00 and 19.00–21.30 h.
For each individual, we recorded sex [36], colour (% blackness, based on a 100-level grey scale (see electronic supplementary material, figure S2) ranging from white (0%) to black), type of substrate on which it was present upon disturbance (dark asphalt or pale sidewalks/parking lots), GPS coordinates, date, time of capture, and temperature conditions (coded as: 1 = cool, 2 = mild, 3 = warm, 4 = hot). Each grasshopper was marked with a unique combination of two letters on the posterior part of both forewings using a black permanent marker pen (Staedtler permanent Lumocolor), unless already marked. Marked grasshoppers were not recaptured, because in virtually all cases the letter code could be identified from a distance using binoculars. For visual recaptures, we also recorded the type of substrate upon encounter, coordinates, date, and time of resighting using the same field methods as for first captures.
(c). Experimental manipulation of colour
Upon first capture, individuals were alternatingly assigned to receive a dark (approx. 80% blackness) or pale (approx. 20% blackness) colour that resembled the extremes of the natural cline of colour variation, regardless of their original colour, sex or type of substrate on which they were found. The reason for manipulating the overall colour of individuals to either pale or dark is that, under matching habitat choice, the spatial responses of extreme phenotypes are expected to be stronger than that of intermediate phenotypes [28]. Furthermore, performance-based habitat selection decisions should be influenced primarily by the current colour of manipulated animals, independent of the magnitude of the difference between the original and newly acquired colour. To experimentally mimic the natural colour of the palest and darkest individuals in the population, one of us (A.S.-F.) applied white or black water-based (aquarelle) paint on those parts that are most likely to be relevant in self-colour assessment of grasshoppers [46]: circumocular mask, dorsal region between the eyes, cheeks, thorax, femur, tibia and forewings (figure 1c). The paint was applied thinly and diluted, so that the overall darkness of grasshoppers could be manipulated while keeping their original disruptive colour patterning visible. We took care not to paint the eyes, coxa, knee, tarsi, frons, ocelli or the antennae to avoid potential damage to soft tissues and receptors located there. Manipulation did not appear to cause undesirable effects as painted grasshoppers monitored under laboratory conditions showed no signs of negative locomotor or behavioural changes after the treatment, and bred for many months. Mark–recapture data indeed showed that some manipulated individuals were still alive 1.5 months later in the field and up to eight months later in the laboratory (unpublished data).
After painting, grasshoppers were released close (less than 5 m) to their capture site at the border between dark and pale substrates. Under the null hypothesis of random movement with respect to the degree of background matching, this should result in the lack of a correlation between the new phenotype and substrate use at resighting. The fact that the point of release is located at the border between pavements also enables an easy comparison between the two substrates by the grasshoppers.
(d). Data analyses
All statistical analyses were performed using R 3.6.3 [48]. To investigate the effect of grasshopper colour on substrate choice, we ran generalized linear mixed models (GLMM) with a binomial error structure and logit link function using the package ‘lmerTest’ [49]. First captures (unpainted individuals) and visual recaptures (resightings of painted individuals) were analysed in separate models to examine the effects of the original and new colour, respectively. For both models, the dependent variable was the type of substrate chosen by grasshoppers, coded as pale tiles/cement (0) or dark asphalt (1).
Measurements of the original colour of individuals (% blackness) were log-transformed and used as an explanatory variable not only in the model for first captures but also in the model for resightings. This is because colour has been shown to be genetically, developmentally and functionally associated with morphology, physiology, behaviour and life-history traits in other grasshopper species ([47,50], but see [51]). By including the original colour in the model, these potential associations, as well as any behavioural imprinting on their original colour or pavement type, can be accounted for. In addition, to test for differences between males and females in substrate use and/or in the effect of body colour on substrate use, we included sex and the original colour × sex interaction in the model. However, effects of sex were not considered for resightings because these were far from significant in the model for first captures (see Results), and we needed to avoid overfitting this smaller dataset.
New colour (0 = pale, 1 = dark) was included as a fixed effect only in the model for resightings. Many (58%) individuals were resighted within the first 48 h after colour manipulation, while some others were not seen again until 1.5 months later. Little information exists in the literature on the time it takes for a grasshopper to respond to their novel appearance; therefore, to test for any differences in the spatial response of recently painted versus long-painted individuals, the interaction between time since manipulation and new colour was included in the model as a fixed effect. Time since manipulation was modelled as binary variable (greater than 48 h versus less than or equal to 48 h) to improve model convergence and avoid overfitting. Because some (17%) of the colour-manipulated individuals were recorded more than once, we also included individual identity as a random factor in the model for resightings to account for repeated measurements of the same individuals in different trapping sessions. For model convergence reasons, sampling dates were grouped into 3-day periods and then included in all models as a random factor to account for any non-independence of observations made within the same period, effects of the weather, or other temporal effects. Prior to running the models, we z-transformed original colour values to a mean of zero and a standard deviation of one to achieve comparable data [52].
Free-ranging grasshoppers may not only consider predation risk for habitat selection, but the thermal attributes of the environment may also be important [30,47]. For instance, black-painted individuals might be more likely than pale-painted ones to use lighter, cooler microsites during the midday hours to reduce the risk of overheating due to solar radiation [47]. However, an exploratory analysis using the larger dataset of unmanipulated grasshoppers showed that the effects of time of capture or temperature conditions on pavement use did not interact with grasshopper colour (see electronic supplementary material, S4 and table S2). Hence, the effects of time of the day, temperature, and their interaction with grasshopper colour were not considered further to avoid model overfitting.
The surface area of pale pavement (5.5 m × 1399 m × 2 sides) is ca 35% greater than the asphalt surface (7 m × 1399 m). Nevertheless, unlike conventional habitat selection analyses, our analyses do not require the incorporation of the relative availability of each substrate type because we are not interested in evaluating habitat selection in the broad sense, but whether habitat use differs between dark and pale grasshoppers after experimental manipulation of their phenotypes and subsequent release at the border of contrasting substrates.
To assess the statistical support for each variable, we used the Akaike information criterion corrected for small sample sizes (AICc) and compared the AICc values for models with and without the focal variable (see electronic supplementary material, table S3 and S5 for a description of the set of models). Models whose AICc was ≥ 2 units larger than the most satisfactory model (i.e. with the lowest AICc value) were considered to have comparatively little support [53]. For comparison with more traditional ways of testing for statistical significance, the AICc difference is presented with an associated p-value obtained by a likelihood ratio test (R function anova with argument test set to ‘Chisq’) that compared the models as described above.
3. Results
A total of 218 individual grasshoppers (80 females, 138 males) were captured, marked and experimentally manipulated throughout the five months of the study, of which 33 (15.1%, 21 females and 12 males) were later resighted one (94% individuals) or more (2–3) times. Mean time between captures was 6.04 days (range: 0.34–47.36). Natural colour of grasshoppers ranged from 18% to 74% black, and the mode and mean of the colour-frequency distribution occurred at 42% and 41.9% ± 8.5 (s.d.), respectively, indicating a clear preponderance of intermediate phenotypes.
For the first captures, individuals made greater use of the pavement type that better matched their external appearance irrespective of their sex, as revealed by the strong positive relationship between the original colour of individuals and their respective substrates (table 1a). Darker individuals mostly occurred on dark asphalt, whereas paler individuals were more often found on pale pavement (figure 2).
Table 1.
Results of the GLMMs analysing the effects of original colour, new colour, sex, and time since manipulation on the type of substrate chosen (0 = pale pavement, 1 = dark asphalt) by (a) unmanipulated individuals captured for the first time and (b) individuals recaptured after colour manipulation. Changes in AICc values and p-values were obtained by comparing models with versus without the variable of interest (see text). AICc changes that (to us) indicate biologically and statistically meaningful support are highlighted in italics. Number of groups for random variables: grasshopper identity = 33; date = 11.
| variable | ΔAICc | p-value |
|---|---|---|
| (a) first captures (N = 218) | ||
| original colour | −62.18 | <0.001 |
| sex | 2.08 | 0.99 |
| original colour × sex | 1.37 | 0.40 |
| (b) resightings only (N = 36) | ||
| new colour | −17.08 | <0.001 |
| original colour | −3.70 | 0.011 |
| time since manipulation | 2.46 | 0.51 |
| new colour × time since manipulation | 1.83 | 0.26 |
Figure 2.
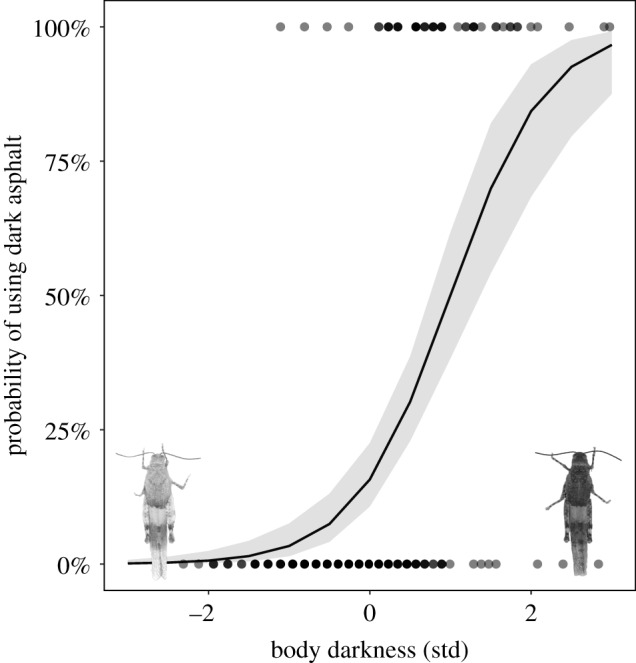
Probability of using dark asphalt versus pale pavement according to the original colour of unmanipulated grasshoppers. Body darkness corresponds to the z-transformed values of original colour of individuals after log-transformation (denoted as grey dots or black when overlapping). The shaded grey area represents the 95% confidence interval of the fitted regression line. (Online version in colour.)
Colour-manipulated resightings strikingly recapitulated the habitat selection behaviour of unmanipulated individuals, as shown by the significant effect of their new colour on substrate use (table 1b). Black-painted and white-painted individuals aggregated together on the dark asphalt and pale pavement, respectively (figure 3), although model comparisons indicated that there was a relatively minor remaining significant effect of their original colour on substrate use (table 1b). The interaction between time since manipulation and new colour did not predict substrate use by colour-manipulated individuals, indicating that the adjustment of substrate use after the experimental colour change occurred already within the first 2 days after manipulation (table 1).
Figure 3.
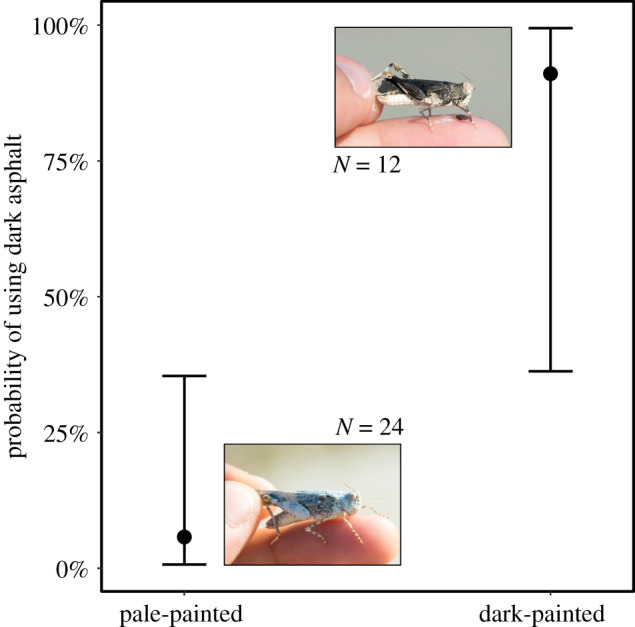
Effect of colour treatment on the probability of using dark asphalt versus pale pavement (corrected for the effect of original colour). Black dots are model-estimated medians. Error bars denote 95% CI. Grasshopper photographs positioned next to each bar illustrate the external appearance of individuals after a white-paint (left bottom) and a black-paint (right upper) treatment (both become a bit paler after drying up). Photos: Alberto Sanabria-Fernández. (Online version in colour.)
4. Discussion
Our observational data indicated that the habitat use of grasshoppers is biased towards pavements that appear to provide more matching backgrounds, so individuals of the same colour tend to cluster together on the same pavement type. This is in agreement with the almost universally accepted—though often not strictly confirmed—assumption that improved background colour matching conceals prey from visual predators [43,44,54–57] (see also electronic supplementary material, S2 for supporting data for the study species). Similar presumably adaptive phenotype–environment associations for colour have been reported in a range of taxa, from arachnids [58] and crustaceans [34,59,60] to amphibians [27] and birds [56]. However, in many cases, the mechanism causing this association is unknown due to the difficulty in ascribing spatial clustering of similar phenotypes to the effects of self-assessment of local performance. Here, the experimental manipulation of the colour of wild, free-ranging grasshoppers allowed us to demonstrate that differential habitat use in response to individual colour was responsible for the observed phenotype–environment match in unmanipulated individuals, after accounting for the effects of genetic background and imprinting. This study, therefore, provides unique experimental evidence of matching habitat choice as a driver of phenotype–environment correlations (i.e. a pattern of local adaptation) in natural populations.
Differential mortality of mismatched phenotypes (natural selection) or adaptive colour change through pigment deposition in the integument during moulting (phenotypic plasticity) cannot explain the phenotype–environment match in the unmanipulated grasshoppers. The daily mortality rate of adult grasshoppers in the study population of only 3.8% (as determined by multistate capture-recapture modelling, N = 172) is far from the estimated 58–72% rate needed to create and maintain the observed colour segregation across different pavements if movement is random with respect to colour match [35]. Dark-painted grasshoppers were resighted less often than pale-painted ones, and this difference might be related to mortality due to overheating, since dark-painted individuals tended to be associated with hot, dark asphalt. However, mortality of dark individuals on dark asphalt would create the opposite pattern to what we actually observed. Phenotypically plastic colour change of S. azurescens imagoes, although possible, would take weeks to months to achieve the required change and is therefore too slow given their rate of movement between pavements [35,44]. Even though colour scoring was not done blind with respect to the initial capture substrate, an unconscious observer bias cannot explain the observed substrate choice of manipulated individuals (nor the effect of original colour on substrate choice).
Our finding that grasshoppers adjust their movement patterns to choose the substrate that confers an apparent improvement in camouflage given their individual-specific colour strengthens the interpretations of previous studies on e.g. unicellular ciliates [32], insects [29,46], crustaceans [34] amphibians [27], fish [22,61] and birds [23,25,26], suggesting a role of matching habitat choice as a mechanism of local adaptation. More specifically, our study supports the findings of earlier phenotype manipulation experiments using laboratory-reared individuals [30,31,35]. But, importantly, the approach used in this study is more relevant to natural populations because, unlike in previous studies, it combines evidence from observational and experimental data on the same sample of wild-born individuals in their local, natural habitat. By manipulating the phenotype of wild-caught grasshoppers, our study accounts for the effects of past experience and genetic background and, therefore, provides unequivocal evidence for the operation of matching habitat choice in wild settings. The fact that manipulated grasshoppers preferentially use matching substrates given their current colour indicates that habitat choice does not reflect major fixed genetic or acquired preferences for a given habitat, but is primarily based on flexible assessment of local performance. Still, our results revealed a significant but smaller effect of original colour on substrate choice. This effect might be due to misuse of information from unpainted parts for self-assessment of colour match or because the original integument colour was still partly visible through the thin layer of paint we applied. One could also argue that imprinting during early life stages might have a concomitant effect on habitat selection by adults [15,62], emphasizing the importance of using wild-raised individuals to avoid misidentification of the mechanism(s) that enable populations to optimize the match of phenotype to the environment.
The observed microhabitat shifts of grasshoppers after colour manipulation allow for several additional inferences. First, grasshoppers appear to be able to self-evaluate their level of camouflage relative to the pavement, consistent with previous laboratory studies on different grasshopper species showing that manipulation of some parts of the body changed their choice of substrate towards greater background colour matching [21,35,46] and confirming that mismatched individuals behave as if they are aware of this [43]. Grasshoppers have trichromatic colour vision [63], and at any rate should not have any trouble comparing the darkness of their own body against that of the substrate, given their large eyes, which are placed somewhat on top of their mobile headsand should enable them to see many parts of their body and the substrate. Second, it seems likely that grasshoppers continuously track and update information on the degree of match between their external appearance and the colour of the substrate to adjust their habitat preferences, as suggested by Gillis [46] and Wennersten et al. [31].
5. Conclusion
To sum up, our study provides the best experimental evidence to date that matching habitat choice operates in natural populations. More generally, our results suggest that this flexible assessment of local performance across habitats might enable individuals to improve ecological performance in changing environments and thus facilitate population persistence in a variable world, something increasingly relevant as environments change more and more under global change. More combined evidence from observational and experimental field studies is needed to determine the actual prevalence and importance of matching habitat choice in nature.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Graciela Escudero for assistance at various stages of this study and Simone Santoro for statistical advice. A.S.-F. thanks José Carrasco and Antonio Sanabria for help with fieldwork. Two anonymous reviewers and the associate editor made insightful comments on an earlier version that substantially improved the quality of this manuscript.
Data accessibility
The data supporting the results of this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qbzkh18dv [64].
Authors' contributions
C.C. carried out the statistical analyses and wrote the manuscript. A.S.-F. collected field data, participated in data analysis and critically revised the manuscript. A.B.-V. participated in data collection and analysis, and critically revised the manuscript. P.E. conceived and designed the study and helped analysing the data and writing the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (grants ref. no. CGL2013-49460-EXP and CGL2016-79483-P to P.E.; grant ref. BES-2013-062905 to A.B.-V.) with support from the European Regional Development Fund.
References
- 1.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 2.Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588. ( 10.1086/597611) [DOI] [PubMed] [Google Scholar]
- 3.Schluter D. 2001. Ecology and the origin of species. Trends Ecol. Evol. 16, 372–380. ( 10.1016/S0169-5347(01)02198-X) [DOI] [PubMed] [Google Scholar]
- 4.Edelaar P, Bolnick DI. 2019. Appreciating the multiple processes increasing individual or population fitness. Trends Ecol. Evol. 34, 435–446. ( 10.1016/j.tree.2019.02.001) [DOI] [PubMed] [Google Scholar]
- 5.West–Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278. ( 10.1146/annurev.es.20.110189.001341) [DOI] [Google Scholar]
- 6.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 7.Holt RD, Barfield M. 2008. Habitat selection and niche conservatism. Isr. J. Ecol. Evol. 54, 279–285. ( 10.1560/IJEE.54.3-4.279) [DOI] [Google Scholar]
- 8.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 462–472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 9.Endler JA. 1986. Natural selection in the wild. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Schlichting CD, Smith H. 2002. Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evol. Ecol. 16, 189–211. ( 10.1023/A:1019624425971) [DOI] [Google Scholar]
- 11.Nosil P, Vines TH, Funk DJ. 2005. Perspective: reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59, 705–719. ( 10.1111/j.0014-3820.2005.tb01747.x) [DOI] [PubMed] [Google Scholar]
- 12.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 13.Ravigné V, Dieckmann U, Olivieri I. 2009. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. Am. Nat. 174, 141–169. ( 10.1086/605369) [DOI] [PubMed] [Google Scholar]
- 14.Bolnick DI, Otto SP. 2013. The magnitude of local adaptation under genotype-dependent dispersal. Ecol. Evol. 3, 4722–4735. ( 10.1002/ece3.850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis JM, Stamps JA. 2004. The effect of natal experience on habitat preferences. Trends. Ecol. Evol. 19, 411–416. ( 10.1016/j.tree.2004.04.006) [DOI] [PubMed] [Google Scholar]
- 16.Jaenike J, Holt RD. 1991. Genetic variation for habitat preference: evidence and explanations. Am. Nat. 137, 67–90. ( 10.1086/285140) [DOI] [Google Scholar]
- 17.Akcali CK, Porter CK. 2017. Comment on Van Belleghem et al. 2016, Habitat choice mechanisms in speciation and other forms of diversification. Evolution 71, 2754–2761. ( 10.1111/evo.13375) [DOI] [PubMed] [Google Scholar]
- 18.Edelaar P, Bolnick DI. 2012. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. ( 10.1016/j.tree.2012.07.009) [DOI] [PubMed] [Google Scholar]
- 19.Berner D, Thibert-Plante X. 2015. How mechanisms of habitat preference evolve and promote divergence with gene flow. J. Evol. Biol. 28, 1641–1655. ( 10.1111/jeb.12683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelaar P. 2018. Ecological speciation: when and how variation among environments can drive population divergence. In Bird species—how they arise modify and vanish (ed. Tietze DT.), pp. 195–215. Berlin, Germany: Springer. [Google Scholar]
- 21.Pellerin F, Cote J, Bestion E, Aguilée R. 2019. Matching habitat choice promotes species persistence under climate change. Oikos 128, 221–234. ( 10.1111/oik.05309) [DOI] [Google Scholar]
- 22.Bolnick DI, Snowberg LK, Patenia C, Stutz WE, Ingram T, Lau OL. 2009. Phenotype-dependent native habitat preference facilitates divergence between parapatric lake and stream stickleback. Evolution 63, 2004–2016. ( 10.1111/j.1558-5646.2009.00699.x) [DOI] [PubMed] [Google Scholar]
- 23.Dreiss AN, Antoniazza S, Burri R, Fumagalli L, Sonnay C, Frey C, Roulin A. 2012. Local adaptation and matching habitat choice in female barn owls with respect to melanic coloration. J. Evol. Biol. 25, 103–114. ( 10.1111/j.1420-9101.2011.02407.x) [DOI] [PubMed] [Google Scholar]
- 24.Camacho C, Canal D, Potti J. 2015. Testing the matching habitat choice hypothesis in nature: phenotype–environment correlation and fitness in a songbird population. Evol. Ecol. 29, 873–886. ( 10.1007/s10682-015-9793-4) [DOI] [Google Scholar]
- 25.Benkman CW. 2017. Matching habitat choice in nomadic crossbills appears most pronounced when food is most limiting. Evolution 71, 778–785. ( 10.1111/evo.13146) [DOI] [PubMed] [Google Scholar]
- 26.Holtmann B, Santos ES, Lara CE, Nakagawa S. 2017. Personality-matching habitat choice rather than behavioural plasticity is a likely driver of a phenotype–environment covariance. Proc. R. Soc. B 284, 20170943 ( 10.1098/rspb.2017.0943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe WH, Addis BR. 2019. Matching habitat choice and plasticity contribute to phenotype–environment covariation in a stream salamander. Ecology 100, e02661 ( 10.1002/ecy.2661) [DOI] [PubMed] [Google Scholar]
- 28.Camacho C, Hendry AP. 2020. Matching habitat choice: it's not for everyone. Oikos 129, 689–699. (doi:101111/oik06932) [Google Scholar]
- 29.Boyle J, Start D. 2020. Plasticity and habitat choice match colour to function in an ambush bug. Funct. Ecol. 34, 822–829. (doi:101111/1365-243513528) [Google Scholar]
- 30.Karpestam E, Wennersten L, Forsman A. 2012. Matching habitat choice by experimentally mismatched phenotypes. Evol. Ecol. 26, 893–907. ( 10.1007/s10682-011-9530-6) [DOI] [Google Scholar]
- 31.Wennersten L, Karpestam E, Forsman A. 2012. Phenotype manipulation influences microhabitat choice in pygmy grasshoppers. Curr. Zool. 58, 392–400. ( 10.1093/czoolo/58.3.392) [DOI] [Google Scholar]
- 32.Jacob S, Legrand D, Chaine AS, Bonte D, Schtickzelle N, Huet M, Clobert J. 2017. Gene flow favours local adaptation under habitat choice in ciliate microcosms. Nat. Ecol. Evol. 1, 1407 ( 10.1038/s41559-017-0269-5) [DOI] [PubMed] [Google Scholar]
- 33.Eacock A, Rowland HM, van't Hof AE, Yung CJ, Edmonds N, Saccheri IJ. 2019. Adaptive colour change and background choice behaviour in peppered moth caterpillars is mediated by extraocular photoreception. Commun. Biol. 2, 286 ( 10.1038/s42003-019-0502-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green SD, Duarte RC, Kellett E, Alagaratnam N, Stevens M. 2019. Colour change and behavioural choice facilitate chameleon prawn camouflage against different seaweed backgrounds. Commun. Biol. 2, 230 ( 10.1038/s42003-019-0465-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelaar P, Baños-Villalba A, Quevedo DP, Escudero G, Bolnick DI, Jordán-Andrade A. 2019. Biased movement drives local cryptic coloration on distinct urban pavements. Proc. R. Soc. B 286, 20191343 ( 10.1098/rspb.2019.1343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Husemann M, Llucià-Pomares D, Hochkirch A. 2013. A review of the Iberian Sphingonotini with description of two novel species (Orthoptera: Acrididae: Oedipodinae). Zool. J. Linn. Soc. 168, 29–60. ( 10.1111/zoj.12023) [DOI] [Google Scholar]
- 37.Vosseler J. 1902. Beitrage zur faunistik und biologie der Orthopteren Algeriens und Tunesiens Zoologische Jahrbücher: Abteilung für Systematik. Geographie und Biologie der Tiere 16, 337–404. ( 10.5962/bhl.part.17152) [DOI] [Google Scholar]
- 38.Eisentraut M. 1927. Beitrag zur frage der farbanpassung der orthopteren an die färbung der umgebung. Zoomorphology 7, 609–642. ( 10.1007/bf00540534) [DOI] [Google Scholar]
- 39.Merilaita S, Stevens M. 2011. Crypsis through background matching. In Animal camouflage: mechanisms and function (eds Stevens M, Merilaita S), pp. 17–33. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 40.Merilaita S, Tuomi J, Jormalainen V. 1999. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 67, 151–161. ( 10.1111/j.1095-8312.1999.tb01858.x) [DOI] [Google Scholar]
- 41.Karpestam E, Merilaita S, Forsman A. 2012. Reduced predation risk for melanistic pygmy grasshoppers in post-fire environments. Ecol. Evol. 2, 2204–2212. ( 10.1002/ece3.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karpestam E, Merilaita S, Forsman A. 2013. Detection experiments with humans implicate visual predation as a driver of colour polymorphism dynamics in pygmy grasshoppers. BMC Ecol. 13, 17 ( 10.1186/1472-6785-13-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baños-Villalba A, Quevedo DP, Edelaar P. 2018. Positioning behavior according to individual color variation improves camouflage in novel habitats. Behav. Ecol. 29, 404–410. ( 10.1093/beheco/arx181) [DOI] [Google Scholar]
- 44.Peralta-Rincón JR, Escudero G, Edelaar P. 2017. Phenotypic plasticity in color without molt in adult grasshoppers of the genus Sphingonotus (Acrididae: Oedipodinae). J. Orthoptera Res. 26, 21–27. ( 10.3897/jor.26.14550) [DOI] [Google Scholar]
- 45.Edelaar P, Baños-Villalba A, Escudero G, Rodríguez-Bernal C. 2017. Background colour matching increases with risk of predation in a colour-changing grasshopper. Behav. Ecol. 28, 698–705. ( 10.1093/beheco/arx016) [DOI] [Google Scholar]
- 46.Gillis JE. 1982. Substrate colour-matching cues in the cryptic grasshopper Circotettix rabula rabula (Rehn, Hebard). Anim. Behav. 30, 113–116. ( 10.1016/S0003-3472(82)80244-3) [DOI] [Google Scholar]
- 47.Ahnesjö J, Forsman A. 2006. Differential habitat selection by pygmy grasshopper color morphs; interactive effects of temperature and predator avoidance. Evol. Ecol. 20, 235–257. ( 10.1007/s10682-006-6178-8) [DOI] [Google Scholar]
- 48.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistial Computing: http://wwwR-projectorg [Google Scholar]
- 49.Kuznetsova A, Brockhoff PB, Christensen RHB. 2016. lmerTest: tests in linear mixed effects models R package. See https://cranr-projectorg/web/packages/lmerTest/indexhtml.
- 50.Forsman A, Ringblom K, Civantos E, Ahnesjö J. 2002. Coevolution of color pattern and thermoregulatory behavior in polymorphic pygmy grasshoppers Tetrix undulate. Evolution 56, 349–360. ( 10.1111/j.0014-3820.2002.tb01345.x) [DOI] [PubMed] [Google Scholar]
- 51.Hochkirch A, Deppermann J, Gröning J. 2008. Phenotypic plasticity in insects: the effects of substrate color on the coloration of two ground-hopper species. Evol. Dev. 10, 350–359. ( 10.1111/j.1525-142X.2008.00243.x) [DOI] [PubMed] [Google Scholar]
- 52.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 53.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 54.Stevens M, Ruxton GD. 2019. The key role of behaviour in animal camouflage. Biol. Rev. 94, 116–134. ( 10.1111/brv.12438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN. 2016. Camouflage predicts survival in ground-nesting birds. Sci. Rep. 6, 19966 ( 10.1038/srep19966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens M, Troscianko J, Wilson-Aggarwal JK, Spottiswoode CN. 2017. Improvement of individual camouflage through background choice in ground-nesting birds. Nat. Ecol. Evol. 1, 1325 ( 10.1038/s41559-017-0256-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte RC, Stevens M, Flores AAV. 2018. The adaptive value of camouflage and colour change in a polymorphic prawn. Sci. Rep. 8, 16028 ( 10.1038/s41598-018-34470-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonte D, Maelfait J. 2004. Colour variation and crypsis in relation to habitat selection in the males of the crab spider Xysticus sabulosus (Hahn, 1832) (Araneae: Thomisidae). Belg. J. Zool. 134, 3–7. [Google Scholar]
- 59.Todd PA, Briers RA, Ladle RJ, Middleton F. 2006. Phenotype–environment matching in the shore crab (Carcinus maenas). Mar. Biol. 148, 357–1367. ( 10.1007/s00227-005-0159-2) [DOI] [Google Scholar]
- 60.Stevens M, Broderick AC, Godley BJ, Lown AE, Troscianko J, Weber N, Weber SB. 2015. Phenotype–environment matching in sand fleas. Biol. Lett. 11, 20150494 ( 10.1098/rsbl.2015.0494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobson B, Dubois F, Peres-Neto PR. 2017. Phenotype-dependent selection underlies patterns of sorting across habitats: the case of stream-fishes. Oikos 126, 1660–1671. ( 10.1111/oik.04126) [DOI] [Google Scholar]
- 62.Camacho C, Canal D, Potti J. 2016. Natal habitat imprinting counteracts the diversifying effects of phenotype-dependent dispersal in a spatially structured population. BMC Evol. Biol. 16, 158 ( 10.1186/s12862-016-0724-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. ( 10.1146/annurev.ento.46.1.471) [DOI] [PubMed] [Google Scholar]
- 64.Camacho C, Sanabria-Fernández A, Baños-Villalba A, Edelaar P. 2020. Data from: Matching habitat choice in azure sand grasshoppers Dryad Digital Repository. ( 10.5061/dryad.qbzkh18dv) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Camacho C, Sanabria-Fernández A, Baños-Villalba A, Edelaar P. 2020. Data from: Matching habitat choice in azure sand grasshoppers Dryad Digital Repository. ( 10.5061/dryad.qbzkh18dv) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the results of this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qbzkh18dv [64].



