Abstract
Aged cotton rats (Sigmodon hispidus) from an established breeding colony displayed signs of spontaneous exophthalmos. Of a total of 118 colony animals that were older than 6 mo of age, 37 (31%) displayed signs of exophthalmos. These rats were clinically healthy and had no other signs of disease. Ophthalmic exams, molecular and microbiologic testing, and histopathology were performed to determine the cause of the exophthalmos and to provide appropriate treatment. Environmental monitoring records were also reviewed for vivarium rooms in which the cotton rats were housed. Histopathology findings supported that the exophthalmos in these cotton rats was secondary to retro-orbital thrombosis associated with cardiomyopathy. The exophthalmic eyes were treated by either removal of the affected eye (enucleation) or surgical closure of the eyelids (temporary tarsorraphy). Enucleation of the exophthalmic eye was the best intervention for these aged cotton rats. These findings demonstrate the potential for a high incidence of ocular problems occurring secondary to cardiomyopathy in aged cotton rats. Enucleation as a therapeutic intervention for exophthalmic eyes in aged cotton rats prolongs the morbidity-free time span during which these aged animals can be used experimentally.
Animals older than 6 mo of age in an established cotton rat (Sigmodon hispidus) breeding colony displayed spontaneous signs of exophthalmos. Breeding pairs had been established at 4 to 5 wk of age using animals derived from the existing colony or purchased from a vendor. Apart from exophthalmos, the affected rats appeared healthy and had no previous or concurrent clinical signs of disease. Our study summarizes data from 37 cases of exophthalmos that occurred in these cotton rats over a 13-mo period and the treatments taken to address the condition. We used several approaches to identify the cause of the exophthalmos and evaluated 2 treatment options. Of the possibilities tested, only retro-orbital thrombosis correlated with disease. Of the treatment options used, enucleation of the affected eye had the best outcome and prolonged the morbidity-free time span during which cotton rats could be used for studies in aged animals.
Materials and Methods
Animals.
Inbred cotton rats (Sigmodon hispidus) were purchased from Envigo (Indianapolis, IN). Breeding pairs were established as previously described.8 Cotton rats were free from mouse and rat parvovirus, minute virus of mice and rats, Parvovirus NS-1, mouse hepatitis virus, murine norovirus, Theiler murine encephalomyelitis virus, murine and rat rotavirus, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, adenovirus 1 and 2, ectromelia virus, mouse pneumonitis virus, polyoma virus, mouse cytomegalovirus, Hantaan virus, Encephalitozoon cuniculi, Filabacterium rodentium, mouse thymic virus, Prospect Hill virus, lactase dehydrogenase-elevating virus, Toolan H-1 virus, Kilham rat virus, rat sialodacryoadenitis virus, rat theilovirus, and Pneumoncystis carinii according to quarterly health monitoring of immunocompetent CD1 sentinel mice and rats exposed to 100% pooled dirty bedding from cotton rats at each cage change. Fresh fecal samples (n = 3) were collected from affected cotton rats and submitted to Charles River Laboratories (Wilmington, MA) for PCR testing for human adenoviruses. All experimental procedures received IACUC approval through the Ohio State University, and the cotton rats were cared for in accordance with the Guide for the Care and Use of Laboratory Animals.9
Husbandry.
Cotton rats were housed in polysulfone microisolation cages (NextGen Rat 900, Allentown, Allentown, NJ) in a barrier facility with a 12:12 h light:dark cycle. The room was maintained at a temperature of 72 ± 4 °F and 30% to 70% relative humidity. Cages contained 1/4-inch corncob bedding (Bed-o’ Cobs, The Andersons, Maumee, OH), a nylabone toy (Bio-Serv, Flemington, NJ), and a piece of polyvinyl chloride (PVC) pipe for environmental enrichment. Animals in breeding cages were housed on shredded aspen bedding (Teklad 7093, Envigo) instead of corncob. Cotton rats received unrestricted access to chlorinated reverse-osmosis water and standard rodent diet (Teklad 2019s, Envigo). Cages were sanitized by rack washer, and a tunnel washer sanitized items that came into contact with animals, such as PVC pipes. Cleaning in the room was completed with Opti-cide (Micro-Scientific Industries, Gurnee, IL) or Spor-Klenz (Steris Corporation, St Louis, MO) disinfectants.
Ophthalmic Examination.
Four of the affected cotton rats received a complete ophthalmic examination by a board-certified veterinary ophthalmologist (EJM or AGM). All examinations were performed immediately after euthanasia by carbon dioxide inhalation. Examinations included fluorescein staining (FUL-GLO, Akron, IL), rebound tonometry (Tonovet, Icare, Vantaa, Finland), direct illumination (3.5V Finoff ocular transilluminator, Welch Allyn, NY), biomicroscopy (Kowa SL-15, Kowa, Tokyo, Japan), and indirect ophthalmoscopy (30D lens, Volk Optical, OH) when applicable.
Therapeutic Procedures.
Enucleation.
Enucleation was performed in 26 animals to remove exophthalmic globes. Rats were anesthetized with 5% isoflurane gas in an induction chamber and then transferred to a nose cone and maintained in a surgical plane of anesthesia, as confirmed by toe pinch. The periorbital hair was clipped, and the skin was aseptically prepared with alternating chlorhexidine and alcohol wipes without affecting the contralateral eye. The affected eye was proptosed from the orbit using gentle pressure with curved forceps caudal to the globe. The optic nerve sheath was then clamped with forceps. Curved iris scissors were used to cut the optic nerve and extraocular muscle cone proximal to the forceps to remove the globe. Pressure was applied to the orbit using a sterile cotton tip applicator or gauze square until hemostasis was achieved. Some animals required cautery to achieve hemostasis. Next, the orbit was inspected to ensure that all visible globe was removed. One to 2 drops of tissue adhesive (Vetbound, 3M, St Paul, MN) were used to appose lid margins. In some animals, lid margins were freshened by trimming with scissors prior to closure, but skipping this step had no impact on the successful closure of the orbit. Both buprenorphine and either ibuprofen administered in water (Children's Motrin, Johnson and Johnson, New Brunswick, NJ) or carprofen gel cups (MediGel CPF, Clear H2O, Westbrook, ME) were provided as pre and postoperative analgesia.
Temporary Tarsorrhaphy.
Temporary tarsorrhaphy was performed on 2 rats with early signs of exophthalmos and minimal exposure keratitis. Rats were anesthetized with 5% isoflurane gas in induction chambers, and then transferred to a nose cone and maintained in a surgical plane of anesthesia. The periorbital hair was clipped, and the skin was aseptically prepared using a diluted betadine solution. Sterile ophthalmic triple antibiotic ointment was applied to the affected cornea. Toe pinch was performed to confirm a surgical plane of anesthesia. Using forceps, the lid margins were pulled over the globe and held together as they were lifted away from the cornea. Tissue adhesive or 4-0 poliglecaprone 25 suture (Monocryl, Ethicon, Somerville, NJ) in a partial thickness mattress pattern was used to close the lid margins. Pre and postoperative analgesia were provided as described above. The lids remained closed for 2 to 3 d before opening naturally. Upon opening, the globe was no longer proptosed. The animals were then maintained until their study endpoint.
Microbiology.
Culture swabs (BBL CultureSwab Plus, BD, Baltimore, MD) were used to identify the microbiota of the affected cotton rat eyes. Swabs from the conjunctiva of 6 affected (2 males, 4 females) and 3 unaffected cotton rats were obtained either while under isoflurane anesthesia, or immediately after euthanasia. Swabs were placed into 600 µL of brain heart infusion broth to create a sample suspension (BBL, BD). Two to 3 drops of this suspension were placed on various plates to identify the microbiota from the eyes. Plates and broths used include: Columbia Blood Agar (BBL, BD), Chocolate II Agar (BBL, BD), Columbia CNA Agar (BBL, BD), MacConkey II Agar (BBL, BD), Mycoplasma broth (Myco JD Broth, University of California-Davis, Davis, CA), and Thioglycollate (fluid) prepared media (BBL, BD). Blood agar, chocolate agar, CNA, and mycoplasma broth were incubated for one week at 35 °C with 5% CO2. MacConkey plates and thioglycollate media were incubated for one week at 33 °C ambient. All recovered microorganisms were identified by MALDI/TOF, with the exception of Pasteurella pneumotropica, which was biochemically identified by using bioMérieux's API 20NE, a test kit designed to identify Gram negative non-Enterobacteriaceae within 24 to 48 h.
Culture of conjunctival swabs identified Staphylococcus aureus. Two methods were used to test the antimicrobial susceptibility of this strain. First, 2 different microdilution plates, Sensititre JOEYE2 (1 animal) and Sensititre Companion Animal Gram Positive MIC Plate (1 animal), were used. Sensititre demineralized water was inoculated to a 0.5 McFarland concentration with the isolate. Then, 10ul of the suspension was combined with Sensititre cation adjusted Mueller Hinton broth containing TES. The inoculum was dispensed to the corresponding plates by a Sensititre Autoinoculator in 50 uL increments. The plates were incubated at 33 °C ambient for 24 h and then read with a Sensititre Vizion reader. Second, a Kirby-Bauer test was performed for 2 animals in tandem using the same water suspension and plated on Mueller Hinton II plates (BBL, BD). Antibiotic disks used included: Oxacillin (1 µg, BD), Polymyxin B (300 IU/IE/UI, BD), Cefoxitin (30 µg, BD), Levofloxacin (5 µg, BD), and Nitrocefin (BBL, BD). Plates were incubated at 33 °C ambient for 24 h. After incubation, plates were read and interpreted according to the Clinical and Laboratory Standards Institute guidelines.
Pathology.
Cotton rats with exophthalmos were euthanized by carbon dioxide inhalation at the acute, mild or severe stage of disease. Complete postmortem evaluations were performed on 10 cotton rats (6 males, 4 females), and enucleated eyes from 3 additional female cotton rats were evaluated. All tissues were fixed in 10% neutral buffered formalin for 48 h. Whole heads were then decalcified for 48 h (Decalcifier I, Surgipath Medical Industries, Richmond, IL) prior to making coronal sections. All tissues were processed by routine methods and embedded in paraffin wax. Sections of eyes or coronal sections of heads ± sections of hearts, lungs and livers (4 μm) were stained with hematoxylin and eosin (HE), coronal sections demonstrating bacteria were gram-stained with modified Lillie-Twort, hearts and livers were stained with Masson trichrome to detect collagen, and lungs and liver were stained with Prussian blue to detect hemosiderin/iron. All slides were evaluated with an Olympus BX45 light microscope with attached DP25 digital camera (B and B Microscopes Limited, Pittsburgh, PA) by a veterinary pathologist certified by the American College of Veterinary Pathologists (ACVP).
Results
Incidence, Clinical Examination, and Treatment.
Cotton rats of an established breeding colony displayed signs of exophthalmos. Cotton rats had been paired for breeding at 4 to 5 wk of age. During breeding and subsequent retirement, animals began showing signs of exophthalmos from the age of 6 mo. The 37 affected cotton rats ranged in age from 6 to 13 mo (average 10 mo). Of the 37 animals, 14 were male and 23 were females. All animals had unilateral exophthalmos, with the exception of 2 animals in which both eyes were affected consecutively (Table 1). Exophthalmos progressed rapidly, with visible clinical signs developing within one day and advancing to severe exophthalmos a day or 2 later (Figure 1 A through C). To further characterize the exophthalmos and help determine the underlying cause, clinical examinations were performed.
Table 1.
Incidence, treatment, and procedures performed for all 37 cotton rats with exophthalmos. H, histopathology; OE, ophthalmic exam; M, microbiology; *, bacteria noted on histopathology by HE or gram stains
| Animal ID | Age (mo) | Sex (M/F) | Origin | Treatment | Procedure |
| AD | 10 | F | Inhouse | Enucleation | |
| AS | 6 | F | Inhouse | Enucleation | |
| BL | 11 | M | Inhouse | Enucleation | |
| BH | 11 | M | Inhouse | Enucleation | |
| AZ | 13 | M | Inhouse | Enucleation | |
| BX | 12 | F | Inhouse | Enucleation | |
| CM | 10 | M | Inhouse | Enucleation | |
| CG | 12 | F | Inhouse | Enucleation | |
| DH | 8 | M | Vendor | Enucleation | |
| CZ | 10 | M | Inhouse | Enucleation | |
| CZ | 10 | F | Inhouse | Enucleation | |
| DC | 10 | F | Inhouse | Enucleation | |
| CQ | 12 | F | Inhouse | Enucleation | |
| CV | 12 | F | Inhouse | Enucleation | |
| DO | 10 | F | Inhouse | Enucleation | |
| DH | 10 | F | Vendor | Enucleation | |
| CW | 12 | F | Inhouse | Enucleation | |
| DC (both eyes) | 12 | M | Inhouse | N/A | H, * |
| DP | 10 | M | Vendor | N/A | H |
| DS | 10 | M | Vendor | N/A | H, * |
| DA | 12 | F | Inhouse | Enucleation | H, * |
| DR | 10 | F | Vendor | Enucleation | H |
| DE | 12 | F | Vendor | Enucleation | H |
| DF (both eyes) | 12 | F | Vendor | N/A | H, OE, M, * |
| DR | 11 | M | Vendor | N/A | H, OE, M |
| DD | 12 | M | Vendor | N/A | H, M, * |
| DT | 11 | F | Vendor | N/A | H, OE, M |
| DQ | 11 | F | Vendor | Enucleation | M |
| DI | 12 | F | Inhouse | N/A | H, OE, M, * |
| DD | 13 | F | Vendor | Tarsorrhaphy-Glued | H |
| DV | 9 | M | Inhouse | Tarsorrhaphy-Suture | H |
| DZ | 9 | M | Inhouse | Failed Tarsorrhaphy | |
| EB | 10 | M | Inhouse | Enucleation | |
| EG (both eyes) | 10 | F | Inhouse | Enucleation | |
| EI | 10 | F | Inhouse | Enucleation | |
| DV | 11 | F | Inhouse | Enucleation | |
| EE | 10 | F | Inhouse | Enucleation |
Figure 1.
(A) Representation of a clinically normal cotton rat eye. (B) Representation of an exophthalmic cotton rat eye in the acute, mild stage. (C) Representation of an exophthalmic cotton rat eye in the severe stage. (D) Representation of an enucleated cotton rat eye.
A complete ophthalmic examination was performed on 4 cotton rats immediately after euthanasia by carbon dioxide inhalation and prior to postmortem evaluation (Table 2). All cotton rats exhibited mild or severe unilateral exophthalmos at the time of examination, with the condition occurring in 2 left eyes and 2 right eyes. Two animals had acute, mild exophthalmos; the other 2 had severe exophthalmos. Rat DF had been previously enucleated due to having the same condition in the contralateral eye. All animals showed varying degrees of subconjunctival hemorrhage and corneal lesions, ranging from diffuse corneal edema with geographic corneal ulceration (2) to complete corneal desiccation (2). In 2 rats, the anterior chamber and lens could be visualized and immature anterior cortical cataracts were noted. Intraocular pressure (IOP) was low (0 to 4 mmHg) in all affected and unaffected eyes except DR, with an IOP of 12 mmHg in the affected eye, which is still considered normal in cotton rats. The posterior segment could only be visualized in normal eyes, with no significant abnormalities detected. All findings were consistent with spontaneous exophthalmos and secondary exposure keratitis.
Table 2.
Clinical results from complete ophthalmic examinations of exophthalmic cotton rat eyes
| Fluorescein stain | IOP (Tonovet)/ mmHG | Anterior segment exam | ||||
| Animal ID | OD | OS | OD | OS | OD | OS |
| DF (Female) | N/A | + | N/A | 4 | Enucleated | Mild exophthalmos, subconjunctival hemorrhage, 3+ diffuse corneal edema, large axial geographic corneal ulcer, immature anterior cortical cataract |
| DR (Male) | + | — | 12 | 4 | Mild exophthalmos, slight resistance to retropulsion, subconjunctival hemorrhage, 1+ diffuse corneal edema, paraxial geographic corneal ulcer, miotic pupil, no aqueous flare, immature anterior cortical cataract | Normal |
| DT (Female) | N/A | — | 0 | 3 | Severe exophthalmos, hemorrhagic periocular discharge, subconjunctival hemorrhage, complete corneal desiccation, anterior chamber and lens not visualized | Normal |
| DI (Female) | — | N/A | 4 | 0 | No aqueous flare, punctate anterior cortical cataract | Severe exophthalmos, purulent hemorrhagic ocular discharge, complete desiccation of anterior globe, anterior chamber and lens not visualized |
After identification of consistent clinical signs in multiple animals, 2 treatment options were evaluated: the removal of the affected eye (enucleation) and closure of the lid (temporary tarsorrhaphy). Enucleations were performed on 26 cotton rats to remove the exophthalmic eye (Figure 1 D). Once the procedure was performed, rats recovered quickly and did not appear to be adversely affected by the enucleation. Temporary tarsorrhaphies were investigated as a possible intervention in 2 cotton rats. This procedure was only useful during the acute stages of exophthalmos. However, the exophthalmos progressed so rapidly such that identifying acutely affected animals was difficult. Advanced protrusion and exposure keratitis during later stages precluded tarsorrhaphy; enucleations were performed in these cases. Both cotton rats that underwent temporary tarsorrhaphy were maintained until study endpoints. Histopathologic evaluations of these rats were performed on specified structures at the conclusion of the study.
Molecular and Microbiologic Testing.
With successful treatment options implemented, the underlying cause of the exophthalmos was investigated. Putatively, exophthalmos could be caused by experimental manipulation, environmental factors, or spontaneous disease. While some of the cotton rats were being used for experimentation, no orbital procedures or manipulations had been performed on the animals that developed exophthalmos. Because experimental manipulation could be excluded as a cause of the exophthalmos, environmental conditions were considered. Both prior to and throughout the entire course of the disease, husbandry remained constant. No changes were made to the housing for the cotton rats or the changing and cleaning of the cages. Environmental factors also remained constant with the exception of humidity, which ranged from 12% to 85% relative humidity. While the husbandry did not change, exposure to bacteria in the animal facilities could lead to eye infection and exophthalmos.
To examine a potential role of bacteria in the cases of exophthalmos, swabs from exophthalmic and normal eyes were tested. Overall, no bacterial species could be found specifically in cotton rats with exophthalmos. The most predominate isolates recovered from the conjunctiva of affected animals were Coryneforms, α hemolytic Streptococcus species, Pasteurella pneumotropica, and Staphylococcus aureus (Table 3). In the 9 cotton rat samples, 7 (78%) had growth of Coryneforms and α hemolytic Streptococcus species from their conjunctival swabs, and P. pneumotropica and S. aureus were recovered from 4 (44%) of the 9 swabs. Bacteria were noted laterally at the limbus or along the anterior aspect of the cornea in HE- and/or gram-stained sections from 6 of 13 cotton rats. The histopathologic evaluation was compared with culture results from eye swabs for these 6 animals and 3 healthy controls. In 3 of the 6 affected cotton rats, gram-positive cocci were noted by histopathology, but were not recovered by microbiologic culture.
Table 3.
Microbiology characterization of isolates recovered from culture swabs of the cotton rat conjunctiva. P. pneumotropica: Pasteurella pneumotropica, S. aureus: Staphylococcus aureus
| Isolate | |||
| Animal ID | Health status | OD | OS |
| DI (Male) | Healthy-12 mo old | N/A | Coryneform, Streptococcus – α hemolytic, P. pneumotropica, Staphylococcus capitus |
| DY (Male) | Healthy-9 mo old | N/A | Coryneform, Streptococcus – α hemolytic |
| EI (Male) | Healthy-7 wk old | N/A | Coryneform, Streptococcus – α hemolytic, P. pneumotropica, Staphylococcus sciuri |
| DI (Female) | OS affected | Coryneform, Streptococcus – α hemolytic | Streptococcus agalactiae, Staphylococcus xylosus, Klebsiella pneumoniae |
| DT (Female) | OD affected | Streptococcus – α hemolytic, S. aureus, Streptococcus merionis | Coryneform, Streptococcus – α hemolytic, Staphylococcus xylosus |
| DD (Male) | OS affected | N/A | Coryneform, Corynebacterium aurimucosum, Staphylococcus saprophyticus |
| DF (Female) | OS affected | N/A | Streptococcus- α hemolytic, P. pneumotropica, S. aureus, Streptococcus – nonhemolytic, Staphylococcus capitus |
| DE (Female) | OS affected | N/A | P. pneumotropica, S. aureus |
| DR (Male) | OD affected | Streptococcus – α hemolytic, S. aureus, Staphylococcus succinus | Coryneform, Streptococcus merionis, Micrococcus luteus |
Histopathology.
Histopathologic evaluations of 13 cotton rats were performed to evaluate the possible underlying causes of the spontaneous exophthalmos. The unilateral enucleated eyes were examined from 3 cotton rats, while coronal sections from whole heads (including either one or both eyes) were evaluated from 10 cotton rats. Mild histopathologic changes were noted in 5 eyes (38%) from cotton rats that presented peracutely with exophthalmos. These 5 eyes included one enucleated eye and unilateral in situ eyes from 4 cotton rats; 2 were from animals in which the contralateral eye was unaffected or had already been enucleated, and 2 had been treated with a temporary tarsorrhaphy via sutures (DV) or tissue glue (DD-F) that was opened 3 d later. The cornea from these rats was characterized by a near total absence of corneal epithelium, a small amount of fibrin and/or proteinaceous fluid in the anterior chamber, and mild serosuppurative inflammation at the limbus, with migration of neutrophils from the vascularized limbus into the adjacent anterior stroma and along the posterior aspect of Descemet's membrane (Figure 2 A). The corneal epithelium was intact in the unilateral affected eyes that received temporary tarsorrhaphy, but was multifocally detached from the underlying stroma in DV; the cleft frequently contained small numbers of macrophages and neutrophils. The underlying corneal stroma was diffusely edematous, frequently neovascularized and contained scattered neutrophils (Figure 2 B). In the glued eye, anisocytosis and anisokaryosis were prominent in the basal corneal epithelium, the basement membrane was segmentally mineralized and focally extensive neovascularization was present in the superficial stroma (Figure 2 C); rare lymphocytes and plasma cells were limited to the limbus bilaterally.
Figure 2.
(A) Corneal limbus from acutely affected cotton rat (DF-female) with segmental loss of corneal epithelium (↔), neutrophilic stromal infiltrates starting at the limbus and extending anteriorly, neutrophils adhered to Descemet's membrane (↑), and fibrinosuppurative exudate (*) within the anterior chamber adhered to the iris (I). HE, bar = 200 μm. (B) Cornea from a cotton rat (DV-male) treated with tarsorrhaphy via suture and opened 3 d later, in which there is multifocal clefting between the corneal epithelium and the underlying stroma (arrow) which is further characterized by edema, infiltrating neutrophils, and neovascularization. HE, bar = 50 μm. (C) Cornea from a cotton rat (DD-female) treated with tarsorrhaphy via glue and opened 3 d later, in which corneal epithelium is intact and the stroma is characterized by fewer neutrophilic infiltrates, milder neovascularization and a focal, linear, basophilic band of mineralization. HE, bar = 50 μm. (D) Subgross of in situ eye from cotton rat (DD-male) with chronic keratitis involving the entire cornea, exudate in the anterior (A) and posterior (P) chambers, as well as exudate and free hairs within the conjunctiva (*). HE, bar = 1 mm. Rectangle represents inset, HE, bar = 50 μm.
The severe histopathologic changes of the remaining enucleated eye resembled those of the eyes that had remained in situ (Figure 2 D), with near total absence of epithelium lining all affected corneas. Serofibrinosuppurative inflammation, hemorrhage and edema were aggregated at the limbus, forming a mass-like effect, while the anterior and posterior chambers were full of exudate and proteinaceous fluid that began to migrate caudally into the vitreous. In addition, the fibrinosuppurative inflammation, hemorrhage, and edema extended into the conjunctiva, as well as the retrobulbar tissues. Cortical liquefactive necrosis of lens fibers with formation of Morgagnian globules (cataracts) were noted in 4 of 7 rats (57%), and retinal detachment with prominent hypertrophy (“tomb stoning”) of retinal pigmented epithelium was observed in 2 of 7 (29%).
Conjunctival goblet cells were assessed when inclusion of the eyelid and/or conjunctiva permitted. In general, the overall number of goblet cells associated with each eyelid was markedly reduced in affected eyes as compared with conjunctiva on the contralateral side, which was unaffected or had been enucleated. However, goblet cell numbers varied widely in the latter, ranging from approximately 10 to greater than 100. Goblet cells in affected eyes were also decreased in size.
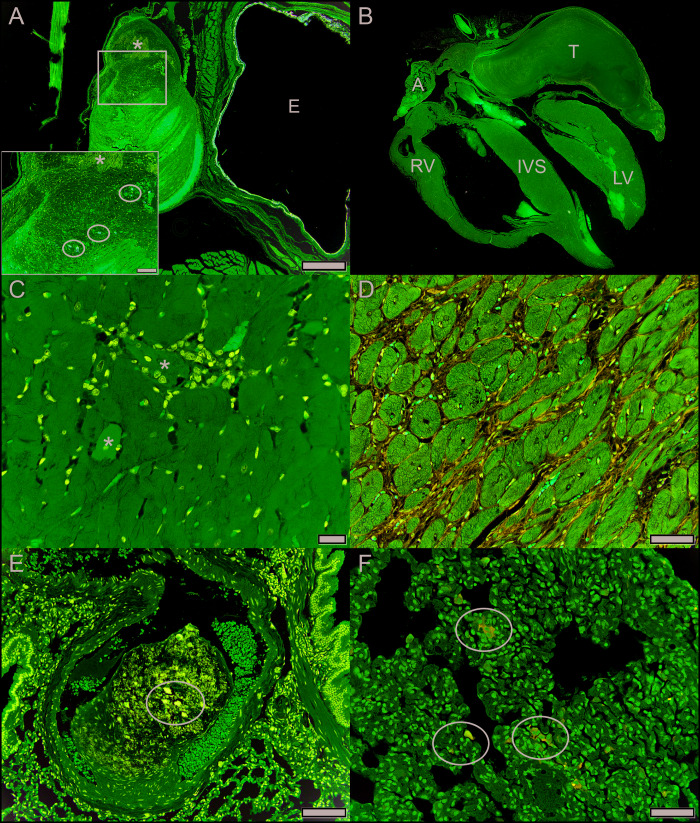
Of the 10 cotton rats in which coronal sections of whole heads were evaluated, orbital venous sinus thrombosis was evident in 5 (50%) (Figure 3 A). The orbital venous sinus thrombosis was unilateral in 4 of 5 (80%), specifically in the affected eye, but not in the contralateral normal eye or the contralateral sinus corresponding to an eye that had previously been enucleated. Bilateral thrombi in cotton rat DC involved both the affected eye and the side that had been enucleated. Bilateral, retro-orbital aggregates of hemosiderophages without distinct thrombi were present in cotton rat DR-M.
Figure 3.
(A) Retro-orbital, organizing fibrin thrombus containing mineralization (*) and hemosiderophages (circles) (cotton rat DI-female). E = eye with lens out of the plane of section. HE, bar = 500 μm. Square represents inset, HE, bar = 100 μm. (B) Subgross of entire heart with large left auricular fibrin thrombus (T) (cotton rat DD-female). A: right auricle; RV: right ventricular free wall; IVS: interventricular septum; LV: left ventricular free wall. HE. (C) Cardiomyopathy characterized by hypereosinophilic necrotic cardiomyofibers (*) associated with infiltrating macrophages (cotton rat DR-male). HE, bar = 20 μm. (D) Cardiomyopathy characterized by moderate amounts of interstitial fibrous connective tissue separating cardiomyofibers (cotton rat DT-female). Masson's trichrome, bar = 50 μm. (E) Organizing fibrin thrombus containing mineralization (circle) within a pulmonary artery (cotton rat DC-male). HE, bar = 100 μm. (F) Macrophages containing iron (heart failure cells, circles) within pulmonary alveolar spaces (cotton rat DD-female). Prussian blue, bar = 50 μm.
Because heart failure has been reported to lead to exophthalmos,13 hearts and lungs were evaluated. Livers were also evaluated to see whether changes in circulation or metabolism were present in these cotton rats. Hearts and lungs (9 of 13; 70%), and livers (8 of 13; 62%) were evaluated for a subset of cotton rats with affected eyes. A large atrial thrombus obstructing the left auricle of the heart was present in 1 cotton rat (11%) (Figure 3 B). All cotton rats had histologic evidence of mild to moderate chronic cardiomyopathy throughout the heart, characterized by myofiber necrosis, dystrophic mineralization and atrophy with replacement by macrophages and interstitial fibrosis. (Figure 3 C and D). One or both ventricular lumens were dilated in 8 of 9 (89%) cotton rats. Four of these rats (44%) had focal chronic pulmonary thromboemboli (Figure 3 E), and one rat (11%) also had multifocal syncytial trophoblast emboli as previously reported.10 Macrophages laden with cytoplasmic hemosiderin (heart failure cells) were scattered throughout the lung in 3 of 9 rats (33%), present in a focally extensive area of the lung parenchyma (1 of 9, 11%) (Figure 3 F) or the tunica media of an otherwise normal pulmonary artery (1 of 9, 11%). All lungs had equivocal evidence of perivascular interstitial edema. Hemosiderin-laden kupffer cells were also scattered throughout the livers of 7 of 8 (88%) cotton rats. All together, these results suggest the exophthalmos in this colony resulted from retro-orbital thrombosis associated with cardiomyopathy-induced subclinical heart failure.
To evaluate the occurrence of heart failure in this colony, a histologic examination was performed on 17 cotton rats of the same age as the affected rats in this case study. At the time of euthanasia, the rats appeared healthy and had no previous or concurrent clinical signs of disease. In these 17 rats, 6 had no significant findings in their hearts, lungs, or livers. The other 11 had histologic evidence of mild cardiomyopathy throughout the heart, but no significant changes in their lungs or livers. A histologic examination of hearts, lungs and livers was also performed on 20 younger animals (8 to 10 wk old). Of these 20 rats, only 6 had histologic evidence of mild cardiomyopathy, with the rest having no significant findings in the heart, lung, or liver. Although both age groups showed histologic evidence of mild cardiomyopathy, none presented with the lung or liver lesions suggestive of heart failure that were seen in animals with exophthalmos.
Discussion
The significant number of exophthalmos cases in this aged cotton rat colony led to the testing of various parameters to identify the underlying cause and subsequent treatment for affected animals. Ophthalmic exams, molecular and microbiologic testing, and histopathology were performed. Environmental monitoring records were also reviewed for vivarium rooms in which cotton rats were housed. Age correlated directly with incidence, with exophthalmos occurring once cotton rats were 6 mo or older. Molecular and microbiologic testing failed to identify a consistent causative infectious etiologic agent. Histopathology suggests that the exophthalmos is due to retro-orbital thrombosis associated with cardiomyopathy-induced subclinical heart failure. Ophthalmic examinations facilitated successful therapeutic interventions for affected eyes, preferably enucleation, to prolong the morbidity-free time span during which affected animals could be used for studies using aged animals.
Exophthalmos can occur in several animal species and can have a variety of causes. These include trauma (self-inflicted or cage-mate),2 husbandry issues,1 experimental manipulation,12,14-16 orbital neoplasia,3 orbital cellulitis,7,17 or cardiomyopathy with secondary retro-orbital thrombosis.13 Treatment depends heavily on the underlying cause of the exophthalmos. In an attempt to exclude causes, we examined the manipulation of the cotton rats described in this study and the condition of their facilities. The affected animals from this colony were breeding animals that experienced minimal handling, other than during weekly cage changes. By approximately 8 mo of age, female cotton rats have typically given birth to multiple litters and are separated from their respective mates for retirement. Many of the affected cotton rats were already retired and singly housed, greatly reducing the potential for cage mate-induced trauma. Clinically, all of the findings were attributed to spontaneous exophthalmos and did not indicate self-trauma. Husbandry factors, including the choice of bedding, cleaning supplies and environmental conditions can also affect the eyes of the cotton rats. Husbandry practices, including alterations in the room, cages, bedding or cleaning supplies, were not changed before or during the period of greatest exophthalmos incidence. Low humidity and high airflow in an environment can induce a significant decrease in lacrimal gland secretion and an increase in corneal fluorescein staining.1 Humidity levels in the room varied greatly, but did not correlate with the occurrence of exophthalmos in this colony. The lack of variability in the husbandry practices excludes it as a cause of exophthalmos in this colony.
Cotton rats are a valuable small animal model for studying viral respiratory diseases. The colony described here is used to study these pathogens, including adenovirus. Adenovirus types 5 and 8, inoculated topically in the eyes of cotton rats, replicate in ocular tissue and induce ocular pathology.15 Although the affected cohort of cotton rats were not being used for experimental adenoviral studies, the highly contagious nature of adenovirus was a concern. However, adenovirus was excluded as the cause of exophthalmos in this cotton rat colony by fecal testing. Isoflurane is used as an inhaled anesthesia for handling cotton rats and performing procedures. Inhaled and injectable anesthesia can lead to corneal opacities and lesions in rats.16 In addition, retro-orbital bleeding under isoflurane anesthesia is a common technique for blood collection in rats.14 Any orbital phlebotomy technique generates a risk for eye injury, including exophthalmia.12 For the breeding colony, isoflurane was not used for cage changes, and these animals were not exposed to isoflurane before exophthalmos occurred. In addition, no orbital manipulation or procedure was performed on the rats before exophthalmos occurred. Because the affected animals had no experimental manipulation, use-related damage can be excluded as a cause of exophthalmos.
Orbital neoplasia and cellulitis are common causes of orbital disease that can lead to exophthalmos in dogs, cats and to a lesser degree,3 hedgehogs17. However, such lesions were not apparent by clinical ophthalmic or histopathologic exam of these cotton rats. Various natural bacterial and viral pathogens, excluded by sentinel testing, can cause ocular lesions in rodents. In addition, enhanced susceptibility to S. aureus-induced keratitis has been documented in various strains of aging mice.7 Therefore, swabs from the conjunctiva of the exophthalmic and normal eyes were obtained for microbiology. The only published microbiota study using cotton rats evaluated the nasal microbiome.5 Due to their close proximity, the nose and eye conjunctiva could have comparable bacterial floras. Dominant species in the cotton rat nose were in the Campylobacter, Acholeplasma, Streptobacillus and Catonella genera.5 None of the dominant species in the nose were among the bacteria cultured from conjunctiva of these aged cotton rat eyes. However, our microbiology results determined that commensal bacterial strains did not differ between healthy or affected rats. Consequently, a primary infectious bacterial etiology was excluded as an underlying cause of exophthalmos.
With all other causes excluded, histopathology findings support that the exophthalmos in these cotton rats is secondary to retro-orbital thrombosis associated with cardiomyopathy. A previous publication found that exophthalmos resulted from orbital venous sinus thrombosis caused by stasis of venous blood, secondary to right heart failure associated with a heritable cardiomyopathy.13 Thus, the exophthalmos seems to have been a consequence of orbital sinus thrombosis.13 While the histologic findings are similar in the hearts of cotton rats in both this study and the previous one, these cardiac findings did not result in death in our animals as they did for many of the rats in the previous study. Once the exophthalmic eye was enucleated, the cotton rats showed no further morbidity. In addition, while the cotton rat is an inbred strain, the affected cotton rats were derived from both an inhouse colony and from a commercial vendor. The cardiomyopathy and resultant exophthalmos may be heritable; however, if this were the case, incidence and severity would be expected to vary between cotton rat populations.
Our results demonstrate the potential for a high incidence of ocular problems in aged cotton rats. In cases of spontaneous or experimentally induced corneal pathology, temporary tarsorrhaphy may be used to protect the cornea.4 Although temporary tarsorrhaphy seemed successful in 2 rats, in most rats the exophthalmos progressed so rapidly that the identification of acutely affected animals in order to perform the tarsorrhaphy in a timely fashion before the development of advanced protrusion and exposure keratitis was generally not feasible. Enucleation is a refinement that can be used to reduce the loss of valuable animals with severe ocular disease.18 In all cases, cotton rats that underwent enucleation returned to a clinically normal state after the eye was removed. Prompt diagnosis and treatment of the exophthalmos in this colony led to clinical management and prolonged life expectancy for the aged cotton rats.
Cotton rats are often used as a small animal model for the study of infectious diseases,11 including the study of respiratory syncytial virus (RSV) in various age groups.6 With increasing studies on the elderly population, aged colonies of cotton rats are being maintained to model disease in this demographic. The therapeutic interventions for retro-orbital thrombosis-induced exophthalmos presented in this paper will facilitate studies in aged cotton rats.
Acknowledgments
We thank Ms Julie Rectenwald in the Comparative Pathology and Mouse Phenotyping Shared Resource (CPMPSR) of The Ohio State University Comprehensive Cancer Center for assistance with animal necropsies, and Ms Clara Zumpetta and Dr Joshua Daniels in the Clinical Diagnostic Laboratories of The Ohio State University Veterinary Medical Center for microbiologic analysis of culture swabs.
This work was supported in part by The Ohio State Comprehensive Cancer Center and the National Institutes of Health under grant number P30 CA016058.
References
- 1.Barabino S, Rolando M, Chen L, Dana MR. 2007. Exposure to a dry environment induces strain-specific responses in mice. Exp Eye Res 84:973–977. 10.1016/j.exer.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Bedford PG. 1987. Ocular emergencies in the dog and cat. Br Vet J 143:489–497. 10.1016/0007-1935(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 3.Betbeze C. 2015. Management of orbital diseases. Top Companion Anim Med 30:107–117. 10.1053/j.tcam.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Brown C. 2007. Temporary tarsorrhaphy in the rat. Lab Anim (NY) 36:25–26. 10.1038/laban0107-25. [DOI] [PubMed] [Google Scholar]
- 5.Chaves-Moreno D, Plumeier I, Kahl S, Krismer B, Peschel A, Oxley APA, Jauregui R, Pieper DH. 2015. The microbial community structure of the cotton rat nose. Environ Microbiol Rep 7:929–935. 10.1111/1758-2229.12334. [DOI] [PubMed] [Google Scholar]
- 6.Curtis SJ, Ottolini MG, Porter DD, Prince GA. 2002. Age-dependent replication of respiratory syncytial virus in the cotton rat. Exp Biol Med (Maywood) 227:799–802. 10.1177/153537020222700912. [DOI] [PubMed] [Google Scholar]
- 7.Girgis DO, Sloop GD, Reed JM, O'Callaghan RJ. 2009. Susceptibility of aged mice to Staphylococcus aureus keratitis. Curr Eye Res 29:269–275. 10.1080/02713680490516783. [DOI] [PubMed] [Google Scholar]
- 8.Green MG, Huey D, Niewiesk S. 2013. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab Anim (NY) 42:170–176. 10.1038/laban.188. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 10.La Perle KM, Green MG, Niewiesk S. 2014. Trophoblast deportation to the lungs of cotton rats (Sigmodon hispidus). Comp Med 64:448–455. [PMC free article] [PubMed] [Google Scholar]
- 11.Niewiesk S, Prince G. 2002. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim 36:357–372. 10.1258/002367702320389026. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Fish BL, Moulder JE, Medhora M, Baker JE, Mader M, Cohen EP. 2014. Safety and blood sample volume and quality of a refined retro-orbital bleeding technique in rats using a lateral approach. Lab Anim (NY) 43:63–66. 10.1038/laban.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorden SD, Watts TC. 1996. Spontaneous cardiomyopathy and exophthalmos in cotton rats (Sigmodon hispidus). Vet Pathol 33:375–382. 10.1177/030098589603300402. [DOI] [PubMed] [Google Scholar]
- 14.Suber RL, Kodell RL. 1985. The effect of three phlebotomy techniques on hematological and clinical chemical evaluation in Sprague-Dawley rats. Vet Clin Pathol 14:23–30. 10.1111/j.1939-165X.1985.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JC, Garlinghouse G, McDonnell PJ, Trousdale MD. 1992. An experimental animal model of adenovirus-induced ocular disease. The cotton rat. Arch Ophthalmol 110:1167–1170. 10.1001/archopht.1992.01080200147043. [DOI] [PubMed] [Google Scholar]
- 16.Turner PV, Albassam MA. 2005. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med 55:175–182. [PubMed] [Google Scholar]
- 17.Wheler CL, Grahn BH, Pocknell AM. 2001. Unilateral proptosis and orbital cellulitis in eight African hedgehogs (atelerix albiventris). J Zoo Wildl Med 32:236–241. 10.1638/1042-7260(2001)032[0236:UPAOCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Wilding LA, Uchihashi M, Bergin IL, Nowland MH. 2015. Enucleation for treating rodent ocular disease. J Am Assoc Lab Anim Sci 54:328–332. [PMC free article] [PubMed] [Google Scholar]