Abstract
Sterile Inflammation (SI), a condition where damage associated molecular patterns (DAMPs) released from dying cells, leads to TLR (Toll-like receptor) activation and triggers hypoxemia in circulation leading to venous thrombosis (VT) through tissue factor (TF) activation, but its importance under acute hypoxia (AH) remains unexplored. Thus, we hypothesized that eRNA released from dying cells under AH activates TF via the TLR3-ERK1/2-AP1 pathway, leading to VT. Animals were exposed to stimulate hypoxia for 0–24 h at standard temperature and humidity. RNaseA and DNase1 were injected immediately before exposure. TLR3 gene silencing was performed through in vivo injection of TLR3 siRNA. 80 μg/kg BW of isolated eRNA and eDNA were injected 6 h prior to sacrifice. Antigens of TF pathway were determined by ELISA and TF activity by a chromogenic assay. AH exposure significantly induced release of SI markers i.e. eRNA, eDNA, HMGB1 and upregulated TLR3, ERK1/2 (Extracellular signal-regulated kinases), AP1 (Activator Protein-1) and TF, whereas RNaseA pre-treatment diminished the effect of AH, thus inhibiting TF expression as well as activity during AH. Hence, we propose a possible mechanism of AH-induced TF activation and thrombosis where RNaseA can become the novel focal point in ameliorating therapy for AH induced thrombosis.
Abbreviations: AH, acute hypoxia,; eRNA, extracellular RNA; SI, Sterile Inflammation; TF, tissue factor; VT, venous thrombosis
Keywords: Sterile Inflammation, Hypoxia, Tissue factor, TLR3, Thrombosis
Highlights
-
•
Acute hypoxia exposure leads to systemic Sterile Inflammation.
-
•
eRNA regulates upregulation of TF by activation of TLR3 pathway.
-
•
RNase A pre-treatment ameliorates effect of acute hypoxia on coagulation.
1. Introduction
Presence of less oxygen promotes the development of thrombosis when exposed to hypoxic environment such as ascent to high-altitude [1]. Increased susceptibility to thrombosis has been observed under decreased oxygen concentration in the atmosphere [2]. Occurrence of Venous thromboembolism (VTE), a widespread, possibly fatal incident which can be averted, is concomitant with the body's exposure to hypobaric hypoxia, either with ascent to high altitude or a long howl flight [3]. In the list of most common cardiovascular disorders, VTE comes right after Acute Coronary Syndrome (ACS) and stroke [4]. Increased risk of thrombosis has also been demonstrated in cases of Chronic Obstructive Pulmonary Disease (COPD) where there is a very high probability of the patients to develop (VTE) [5] and Pulmonary Embolism (PE) [6]. Hypoxemia in the deep veins stasis also can lead to initiation of thrombus formation. Previous studies from our lab demonstrate that hypoxia induced endothelial activation and inflammation lead to hyper coagulation through upregulation of tissue factor.
Toll-like receptors (TLRs) are a family of evolutionarily conserved Pattern Recognition Receptors (PRRs) which identify Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) [7]. Cellular damage and/or tissue-associated hypoxia lead to elevated RNA fragments, extracellular RNA (eRNA), in the circulation released from the disrupted, damaged cells [8]. As per background literature survey, we found that eRNA initiates cascades related to vascular diseases [9,10] i.e. that of blood coagulation along with inflammatory processes [11]. As observed earlier, TLR3 served as a receptor binding to dsRNA (double stranded RNA) of viral origin [12]. However, contemporary research has shown that TLR3 activation can also occur through binding of endogenous RNA (i.e., mRNA, miRNA, eRNA) [13,14]. Release of eRNA from wounded tissue or necrotic cells is proven to be pivotal in diseases such as atherosclerosis, cerebral stroke, pulmonary edema, and pancreatic β-cell apoptosis [10,15,16]. eRNA also initiates the activation of TLRs on the surface of Peripheral Blood Mononuclear Cells (PBMCs), leading to initiation of diverse signalling pathways [10,17]. eRNA has been demonstrated to activate intrinsic coagulation pathway which leads to thrombus formation [8]. However, eRNA mediated extrinsic coagulation activation in hypoxia remains obscure.
It has long been known that inflammation can activate coagulation. Cardiovascular diseases such as atherosclerosis and thrombosis have predominantly shown a progressive inflammation alongside [18,19]. Vascular inflammation is a fundamental cause of morbidity and mortality in hypoxia induced myocardial infarction (MI) and acute lung injury [20,21]. Biswas et al. showed that stimulation of TF activation and deposition of fibrin in lungs by hypobaric hypoxia is modulated via TLR3 signalling [22]. However, the molecular mechanism of TF upregulation due to oxygen deprivation remains obscure.
Thus, we designed our study with the aim to demonstrate the vital function of eRNA as the molecule affecting the initiation and advancement of thrombosis in a murine model of hypoxia. This study evaluated (i) the effect of hypoxia-induced release of eRNA on activation of TLR3 and (ii) the significance of TLR3 activation in up-regulation of TF expression and activity and stimulation of fibrin deposition in lungs through the transcriptional regulation of AP1.
2. Methods
2.1. Ethical clearance
Approval for all experimental protocols and procedures were obtained from the Internal Review Board of Defence Institute of Physiology and Allied Sciences (DIPAS) (IAEC/DIPAS/2015-03; authorization no: 27/GO/RBi/SL/99/CECSEA). For the following study, we used adult Swiss Albino mice weighing 25–30 g. The experimental animal facility of the institute was where the mice were housed during the course of work. They were provided the standard diet and water ad libitum. The institutional ethical committee provided approval for animal care and study protocols as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India. All procedures were performed in DIPAS under urethane (1.2 g/kg) induced general anaesthesia to minimize suffering.
2.2. Chemicals
The commercial source and catalogue numbers of the materials used for all experiments are as follows: ChIP-grade rabbit polyclonal anti-c-Jun antibody (ab13370), Rabbit polyclonal HSP90 antibody (Ab13495), Rabbit polyclonal HSP70 antibody (ab79852), rabbit polyclonal Fibrinogen antibody (ab34269), rabbit monoclonal p-c-Jun antibody (ab32385), Mouse monoclonal TLR3 antibody (ab13915), mouse monoclonal TLR3 antibody [TLR3.7] (ab12085), mouse monoclonal anti-PECAM-1 antibody (ab24590) from Abcam (MA, USA); rabbit polyclonal p-ERK1/2 antibody (sc-23759-R), mouse monoclonal ERK1/2 antibody (sc-514302), mouse monoclonal p-c-fos antibody (sc-81485), rabbit polyclonal c-fos antibody (sc-52), Santa Cruz Biotechnology, Inc. (CA, USA); Rabbit polyclonal anti-HMGB1 antibody (H9539), anti-β-actin antibody (A2228), Ficoll-Hypaque (Histopaque R1077), DNase (D4527), LTA (L2515), bovine serum albumin (BSA), RNaseA, TLR3 siRNA sense (5′-CGUUAUCACACACCAUUUA-3′) and antisense (5′-UAAAUGGUGUGUGAUAACG-3′), negative-control siRNA (scrambled sequence), and o-phenylenediamine dihydrogenchloride (OPD), Hydrogen peroxide, Sigma-Aldrich (MO, USA); MaxSuppressor In Vivo RNA-LANCEr II, Bio Scientific Corporation (TX, USA); HRP conjugated secondary antibodies [anti-mouse (62-6520), anti-rabbit (65-6120), anti-goat (81-1620)], Alexa Fluor 488-conjugated anti-mouse (A-10680) and Alexa Fluor 594-conjugated anti-rabbit (A-21211) secondary antibodies, Invitrogen (CA, USA); PVDF membrane (IPVH09120), Luminata chemiluminescent substrate (WBLUF0500), Merck Millipore (MA, USA); Trizol (15596026), DAPI (D1306), poly I:C (20148E), GeneRuler 100 bp DNA Ladder (SM0241), RiboRuler Low Range RNA Ladder (SM1833), Thermo Fischer (MA, USA); Goat polyclonal TF antibody (AF3178), R&D systems (MN, USA); Mouse monoclonal anti-TF antibody (Ab00516-1.1), Absolute Antibody (CLEV, UK); Antichrome TF activity assay kit (846), Sekisui Diagnostics (CT, USA); Quick-cfRNA Serum and Plasma kit (R1059) and Quick-cfDNA Serum and Plasma kit (D4076), Zymo Research (CA, USA); FDP (Fibrinogen degradation product) measurement kit (MBS2508316), Elabscience (TX, USA).
2.3. Animal experiments
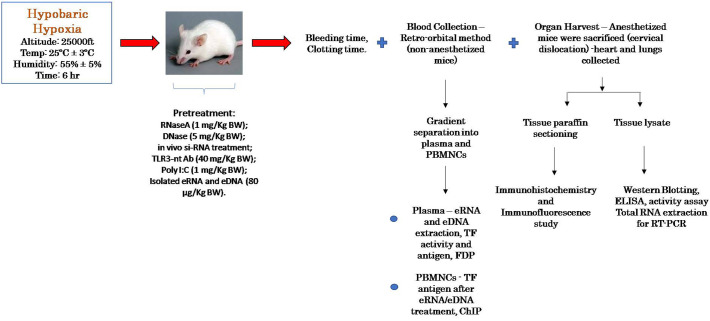
A specifically engineered decompression chamber was used to expose the mice to hypoxic conditions equivalent to high altitude climate at 7628 m i.e. pressure of 282 mm Hg, oxygen at ~8.5%, as previously described [[22], [23], [24]]. Humidity and temperature were efficiently controlled and maintained at 55% ± 5% and 25 ± 3 °C, respectively. Bovine pancreatic RNaseA and recombinant DNase were administered through the tail vein prior to exposure to AH at concentration of 1 mg/kg BW (body weight) and 5 mg/kg BW [8,25,26], respectively. After exposure for required duration, blood was drawn from the exposed and control animals and serum RNase activity measured [27]. siRNA was injected in vivo as described elsewhere [28]. mRNA and protein level of TLR3 was analysed to confirm gene silencing after complete siRNA injection procedure [29]. 1 mg/kg BW Poly I:C was also administered through tail-vein injection, as described [8,29]. 80 μg/kg BW of eRNA and eDNA (isolated from plasma of exposed animals, and purified by RNase/DNase treatment) were injected into the animal through the tail vein 6 h prior to sacrifice.
Chronological description of the animal experiments and the applied procedures.
2.4. Isolation of peripheral blood mononuclear cell (PBMC)
Retro-orbital blood withdrawal technique was used and blood collected in Sodium Citrate BD Vacutainer from non-anesthetized mice [30]. As described earlier, Ficoll reagent was used for density gradient isolation of PBMCs from whole blood [31]. These cells were pelleted, washed and immediately used for protein analysis, total RNA extraction and TF activity assay.
2.5. Total RNA isolation
Standard Trizol isolation was performed to obtain RNA which was then reverse transcribed according to standard protocol.
2.6. eRNA and eDNA isolation
Extracellular RNA and DNA were carefully extracted from fresh plasma of the animals using Trizol method and commercially available kit (Quick-cfDNA, Zymo Research) respectively.
2.7. Reverse transcription–polymerase chain reaction
Gene specific primers for mice were obtained for TF:
Forward 21-mer, 5′-GATTGTGTATTCTACACGCGGGAA-3′
Reverse 21-mer, 5′-CACAAGGATGTCCCAAGCAAAAAT-3′;
PCR was set up as per the following schedule, as previously described: 94 °C for 5 min followed by 33 cycles of 94 °C for 35 s, 54 °C for 35 s, and 72 °C for 50 s respectively [32]. A parallel PCR for 18S (GenBank accession X01117) was performed using the following primer set:
Forward 18-mer, 5′-GCCCGAGCCGCCTGGATA-3′
Reverse 17-mer, 5′-CCGCCGCATCGCCAGTC-3′;
PCR schedule for 18S was: 94 °C for 5 min followed by 33 cycles of 94 °C for 35 s, 55 °C for 35 s, and 72 °C for 50 s. PCR products were separated on 1% agarose gel with ethidium bromide and visualized under UV light.
2.8. Western blotting
As previously described, western blotting (or immunoblotting) was performed on fresh lysates of PBMCs or lungs (right lobe) [33]. 8–12% SDS-PAGE gels were prepared for molecular weight separation of proteins, which were then transferred onto PVDF membrane. The specific protein bands were visualized using a gel documentation system after incubation with specific primary and HRP-conjugated secondary antibodies.
2.9. Enzyme linked immunosorbent assay (ELISA)
As described earlier, ELISA was performed using plasma or PBMC and lung lysate [34]. Antigens from samples were coated onto an assay plate using 0.5 M carbonate buffer [pH 9.6]. After incubation with specific primary and HRP-conjugated secondary antibodies, 1 mg/ml p-nitrophenyl phosphate was used as a substrate for colour development and absorbance measured at 450 nm. Optical density (OD) values were used to analyse the relative expression of the specific proteins between various sample groups.
2.10. Flowcytometry
TLR3 expression was measured through flowcytometry in freshly isolated PBMCs after TLR3 siRNA treatment. Nonspecific binding of antibodies was blocked using Fc block (1 μg/mL, BD Fc Block). Freshly isolated cells were incubated with TLR3 antibody or isotype control in flow cytometry staining buffer (1:250) for 30 min at 4 °C. After 3 washes with PBS, cells were incubated with Alexa Fluor 488 conjugated secondary antibodies (Invitrogen) for 15 min. Cells were washed thrice with PBS and subjected to flow-cytometric analysis by using a BD-FACS Caliber system (BD Biosciences, Franklin Lakes, CA, USA).
2.11. ChIP assay
The methodology devised by Carey et al. Chromatin Immuno-Precipitation analysis along with the standard protocol IMGENEX Corporation (San Diego, CA, USA) was used for freshly isolated PBMC samples with some modifications [35]. Nuclear isolation from PBMCs was performed and the resulting solution was subjected to chromatin cross-linking using formaldehyde. Nuclear isolation was again done on the pellet obtained after cross-linking. The nuclear pellet now obtained was lysed and then sonicated using a probe bath sonicator (Sonics Vibra-Cell, Newtown, CT, USA) ten times at a power of 2 for 10 s each, and supernatant retained. Concentration of DNA was estimated using NanoDrop system of the Biotek Multimode Reader. 100 μg of isolated chromatin was treated with ChIP grade c-Jun antibody overnight under refrigerated conditions. Samples treated under similar conditions but without the antibody were used as a negative control. Pellet containing precipitated chromatin was sequentially washed with specific buffers and then eluted out. After reversing the cross-linking, the samples were treated with Proteinase K, precipitated with ethanol and then analysed by PCR.
2.12. In vivo experiments with TLR3 siRNA
Manufacturer protocol of MaxSuppressor kit was used was for in vivo delivery of TLR3 siRNA and non-specific siRNA to the mice by tail vein injection of 100 μl reaction mixture. Prior to injection using insulin syringe, mouse was restrained and tail was warmed to about 37 °C (approximately 10 min) using water as the medium. Once the vein was visualized, the site was injection was disinfected and siRNA solution was injected slowly (~20 μl/s) to avoid bulge formation. After incubation of 24 h, another dose was similarly injected till 4 doses is complete. Blood drawn retro-orbitally was immediately used for estimating activity and expression of TF and coagulation parameters at specific time points. Also, lung tissue was harvested for further experiments.
2.13. Tissue factor activity assay
Activity assessment of TF expressed on surface of PBMCs was done by measurement of the generated amounts of activated factor X (FXa) using commercially available Antichrome TF activity kit. Equal quantities of freshly isolated PBMCs were incubated with factor VIIa, factor X and substrate. Kinetic measurement of absorbance at 405 nm was done using a temperature-regulated multiwell reader (BioTek Instruments, Winooski, VT, USA). Maximal reaction velocity of treated samples w.r.t control was calculated.
2.14. Immunohistochemistry & immunofluorescence microscopy
4% PFA and neutral buffered saline were used as a fixative for preservation of the right lobe of lung harvested from the mice. Tissues were processed for paraffin embedding and sectioning done using a standard microtome. Paraffin sections of lung were collected on lysine-coated microscopic slides and stored for further usage. Sections were deparaffinised and rehydrated using xylene and alcohol gradient. Antigen retrieval was done using heat and sodium citrate buffer (pH 6.0) at 85 °C for 45 min. Endogenous peroxidase activity was blocked prior to specific primary and HRP-conjugated secondary antibody treatment after which DAB reagent was used for colour development on the sections. The sections were counterstained with haematoxylin, mounted using DPX solution and the visualized. The obtained images were analysed using ImageJ, an open-source imaging software.
Similarly, for immunofluorescence analysis, Alexa-Fluor 488 tagged secondary antibody was used to stain the sections along with DAPI as the nuclear counterstain. The sections were visualized under a fluorescence microscope (Ti2-E Motorized Inverted Microscope; Nikon, Tokyo, Japan) wherein green colour indicated fibrin deposition and blue indicated nucleus.
2.15. Clotting time
The protocol describer by Lemini et al. was used for evaluation of clotting time [36]. Mouse tail was briefly warmed in water at 40 °C, dried and cut at the tip. Small amount of blood was collected in a micro haematocrit glass capillary and blood was allowed to flow by the force of gravity and time was noted until blood ceased to flow in the capillary tube.
2.16. Bleeding time
Dejana et al. provided a method for assessing the bleeding time in animal model [37]. Restrained mice were placed on the working bench and a distal 2 mm segment of the tail was severed. Immediately, the cut end was immersed in an isotonic saline solution at 37 °C and time noted till the stream of blood ceased to flow.
2.17. Statistical analysis
Triplicate analysis of all experimental procedures was performed and data was tabulated and expressed as Mean ± SEM. One-way ANOVA analysis was done to calculate the statistical significance between the different groups, followed by Bonferroni's multiple compression test. Statistical significance was by a p-value of <0.05.
3. Results
3.1. Hypoxia induces circulating nucleic acids (CNAs) in time dependent manner
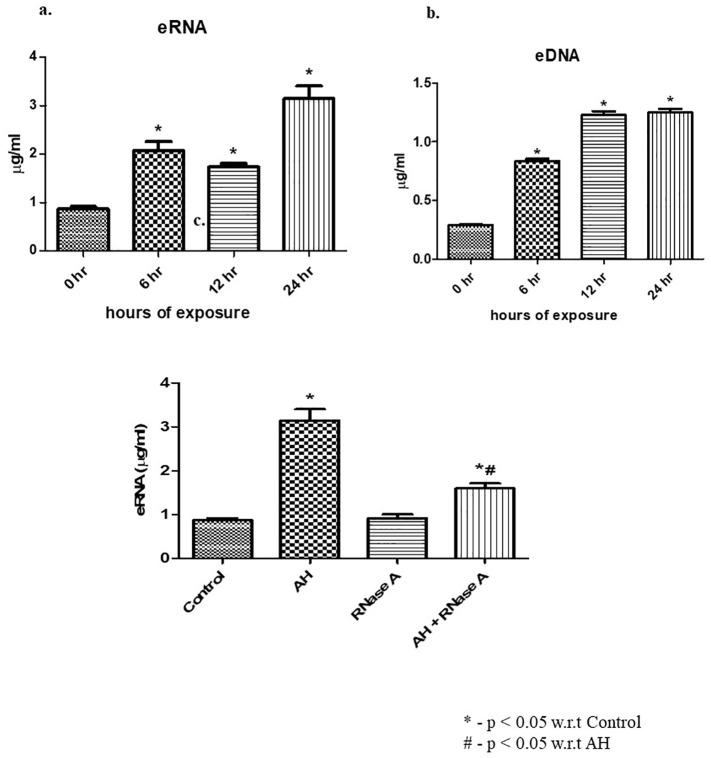
Experiments were conducted to establish the time-dependent impact of AH exposure on plasma level of CNA i.e. eRNA and eDNA. Mice subjected to AH indicate highest liberation of eRNA (Fig. 1a) and eDNA (Fig. 1b) at 24 h as compared to control. We also found that RNase pre-treatment significantly decreased the eRNA level in circulation (Fig. 1c). For our further experiments, we chose to use the 6-h timepoint of hypoxia exposure for mice model.
Fig. 1.
Effect of AH on release of CNAs in plasma. (a) eRNA expression levels estimated in plasma (in μg/ml); (b) eDNA expression levels estimated in plasma (in μg/ml); (c) concentration of eRNA in circulation upon pre-treatment of RNaseA prior to AH exposure. Data are shown as mean ± SEM (n = 5/group/each time point) from one experiment representative of three independent experiments, all performed in triplicate. One-way ANOVA revealed statistical significance in the results (*p < 0.05) at distinct time points. eRNA, extracellular RNA; eDNA, extracellular DNA; AH, acute hypoxia.
3.2. eRNA but not eDNA is involved in hypoxia induced TF activation
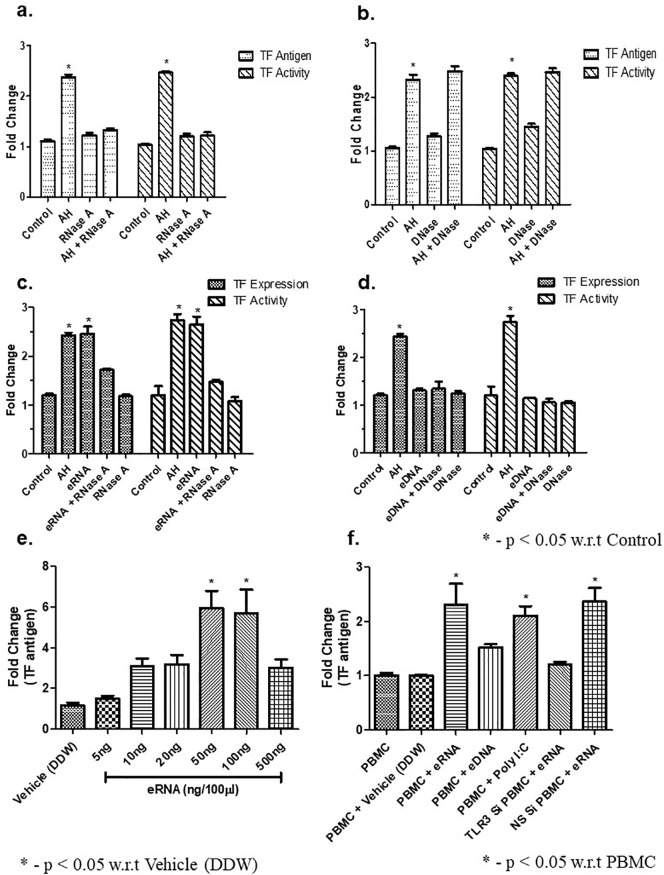
The above results showed that plasma level of CNAs was significantly increased due to AH exposure. However, the involvement of CNAs in inducing the up-regulation of expression and activity of TF stands to be elucidated still. Consequently, we carried out multiple tests by administering mice with RNaseA (1 mg/kg BW) or DNase (5 mg/kg BW) as well as isolated eRNA or eDNA (80 μg/kg BW). Subsequently, the expression of TF and its activity in PBMCs was quantified. Our results showed that pre-treatment of mice with RNaseA 2 h before AH exposure, the TF expression and activity significantly inhibited (Fig. 2a) whereas pre-treatment of DNase (2 h before AH exposure) failed to do so in compared to control (Fig. 2b). To reconfirm it, we treated the animal either with purified eRNA or eDNA. Our results showed that purified eRNA significantly upregulated TF expression as well as activity which was abrogated upon RNaseA pre-treatment (Fig. 2c). However, treatment of purified eDNA failed to upregulate TF antigen and/or activity when comparison to control, and DNase treatment had no effect either (Fig. 2d). To further prove the above results, isolated PBMNCs (1 × 105) were treated with different concentration of eRNA in assay buffer (final volume 100 μl) for 1 h. Our results showed that 100 ng eRNA (100 μl) exerts maximum TF activity (Fig. 2e). In another set of experiments, we showed that PMBCs isolated from TLR3-siRNA treated animal failed to induced TF expression in comparison to PBMCs isolated from Non-specific siRNA treatment (Fig. 2f). Poly I:C treatment used as positive control. These observations implied that upregulation of TF in PBMCs under hypoxic stress is controlled by means of eRNA and not eDNA.
Fig. 2.
eRNA but not eDNA is involved in AH-induced TF expression and activation. (a) Effect of RNase treatment. Mice were exposed to AH in presence or absence of RNaseA. TF expression and activity were measured through ELISA and TF activity assay; (b) Effect of DNase treatment. Same as (a), except the mice were treated with DNase instead of RNaseA; (c) Effect of eRNA injection on TF expression. Same as (a), except the mice were injected with eRNA in the presence or absence of RNaseA; (d) Effect of eDNA injection on TF. Same as (c), except the mice were injected with eDNA in the presence or absence of DNase; (e) Effect of different concentrations of eRNA on isolated control PBMCs. Control PBMCs were treated with different amounts of eRNA and the expression of TF was measured through ELISA; (f) Role of TLR3 in eRNA-mediated TF expression analysed through the treatment of eRNA in TLR3 siRNA and non-specific siRNA treated mice. PBMCs were isolated from control, TLR3 siRNA treated and non-specific siRNA treated mice and eRNA-mediated TF expression was analysed through ELISA. Data are shown as mean ± SEM (n = 5/group/treatment) from one experiment representative of three independent experiments, all performed in triplicate. One-way ANOVA revealed statistical significance in the results (*p < 0.05) at distinct time points. AH, acute hypoxia; TF, tissue factor; eRNA, extracellular RNA; eDNA, extracellular DNA; PBMCs, peripheral blood mononuclear cells; TLR3, Toll-like receptor 3; siRNA, small interfering RNA.
3.3. Induction of TF upregulation and activation is facilitated via TLR3 in AH
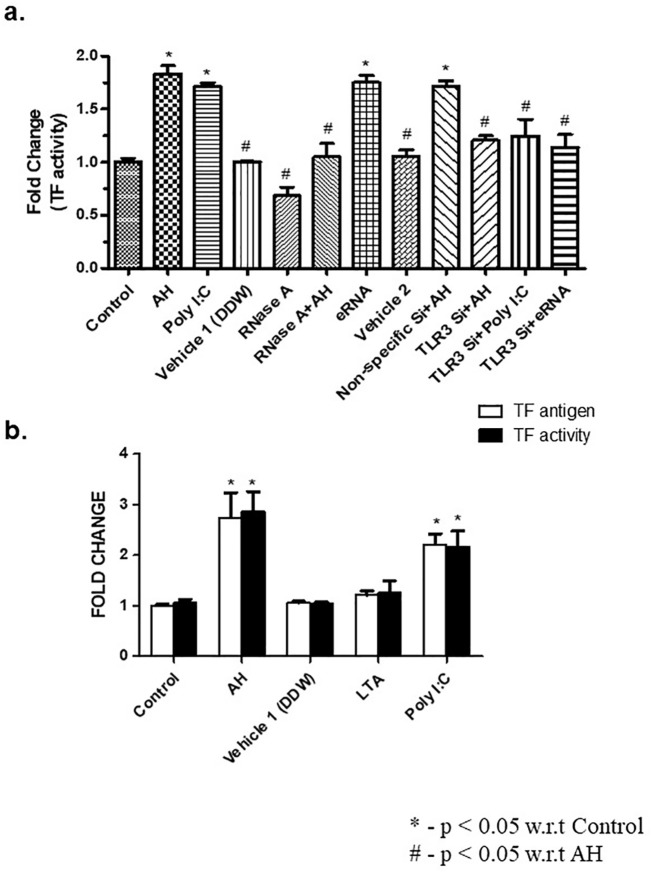
AH exposure lead to an increase in eRNA (Fig. 1a), eDNA (Fig. 1b), high mobility group box 1 (HMGB1) (Supp Fig. 1a), and TLR3 (Supp Fig. 1b). Our result showed that eRNA but not eDNA or HMGB1 induce TLR3 (Supp Fig. 1c). Next, we explored the possible role of eRNA-TLR3 pathway in the upregulation of TF. To confirm it, mice were treated with TLR3 siRNA for four consecutive days and TLR3 level was measured at mRNA and protein level (Supp Fig. 2a, b). DDW (vehicle 1) was used for dissolution of RNaseA and siRNA was dissolved in RNALancer II solution (vehicle 2). TF activity was assayed to analyse the effect of TLR3 gene silencing. It was observed that RNase treatment or TLR3 gene silencing markedly inhibited AH, poly I:C or eRNA-induced TF activity in PBMCs (Fig. 3a) in comparison to non-specific siRNA treated control. Our group earlier showed that hypoxic stress induces the expression of TLR2 and TLR3 both. Nevertheless, the association between TLR expression and TF regulation is still obscure. Hence, we carried out various experiments wherein mice were being treated with either Lipoteichoic acid (LTA) (a TLR2 agonist, 100 μg/kg BW) or with poly I:C (a TLR3 agonist, 1 mg/kg BW) and the level of TF antigen and activity in PBMCs were quantified. Our results showed that treatment of poly I:C or AH exposure significantly increased the plasma level of TF (Fig. 3b). However, treatment with LTA did not have any effect (Fig. 3b). These results suggested that AH induced upregulation of TF is regulated through TLR3 but not TLR2.
Fig. 3.
TLR3 regulates AH-induced TF expression up-regulation and activity. (a) TF activity assayed using a chromogenic-substrate based activity assay. TLR3 siRNA or non-specific siRNA treated mice were exposed to AH and TF activity was measured in PBMCs; (b) Expression of TF antigen and TF activity in PBMCs were measured in AH, Poly I:C or LTA treated mice. Data are shown as mean ± SEM (n = 5/per group/treatment) from one experiment representative of three independently performed experiments, all performed in triplicate. The results showed significant (*p < 0.05) difference in between treatment using one-way ANOVA. AH, acute hypoxia; TF, tissue factor; PBMCs, peripheral blood mononuclear cells; TLR3, Toll-like receptor 3; siRNA, small interfering RNA; poly I:C, Polyinosinic: polycytidylic acid; LTA, lipoteichoic acid.
3.4. eRNA released due to AH exposure induced ERK1/2, c-Jun phosphorylation & TF expression and activation
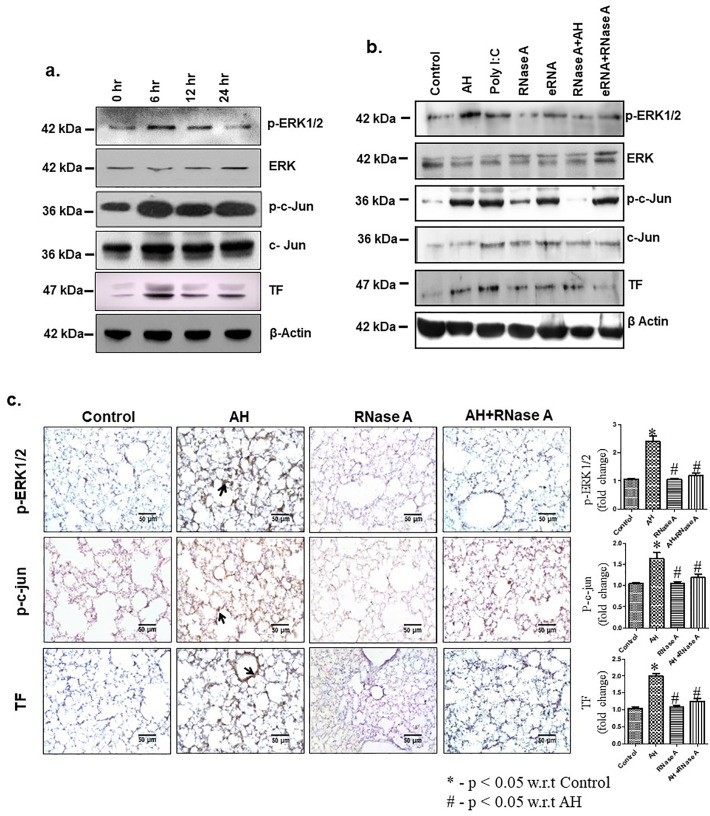
To explore the involvement of signalling molecules in AH-induced TF expression, immunoblotting analysis revealed that AH induced phosphorylation of ERK1/2, c-Jun and TF expression was maximum at 6 h in the PBMCs (Fig. 4a, Supp Fig. 3a). In vivo injection of RNaseA through tail vein (2 h prior to AH exposure) showed inhibition of ERK1/2, c-Jun phosphorylation and TF expression in compared to AH exposure or eRNA treatment (Fig. 4b, Supp Fig. 3b). Immunohistochemistry analysis of lung tissue revealed that AH exposure also induced ERK1/2 and c-Jun phosphorylation as well as TF expression (Fig. 4c). Treatment with RNaseA significantly inhibits the AH-mediated increase in ERK1/2 and c-Jun phosphorylation as well as TF expression. These results suggest that eRNA plays a crucial role in AH-mediated phosphorylation of ERK1/2 & c-Jun as well as TF expression in mice.
Fig. 4.
AH induced eRNA expression facilitates ERK1/2 and c-Jun phosphorylation and TF expression. (a) ERK1/2, c-Jun phosphorylation and TF expression were analysed by IB at indicated time-points. Densitometry analysis of the western blots shown in Supp Fig. 3a; (b) Expression of TF and ERK1/2, c-Jun phosphorylation was analysed by IB after different treatment. Densitometry analysis of the western blots shown in Supp Fig. 3b; (c) Expression of TF and phosphorylation of ERK1/2, c-Jun were observed by IHC (40×) in lung vasculature. β-Actin used as loading control. Data are representative of three independent experiments. Data are presented as mean ± SEM (n = 5/group) and are from a single experiment. The results showed significant (*p < 0.05) difference in between treatment using one-way ANOVA. ERK, extracellular-signal-regulated kinase; TF, tissue factor; IB, immunoblotting; IHC, immunohistochemistry; TLR3, Toll-like receptor 3; poly I:C, polyinosinic: polycytidylic acid; eRNA, extracellular RNA.
3.5. Hypoxia induced TF expression and activation is mediated through AP1
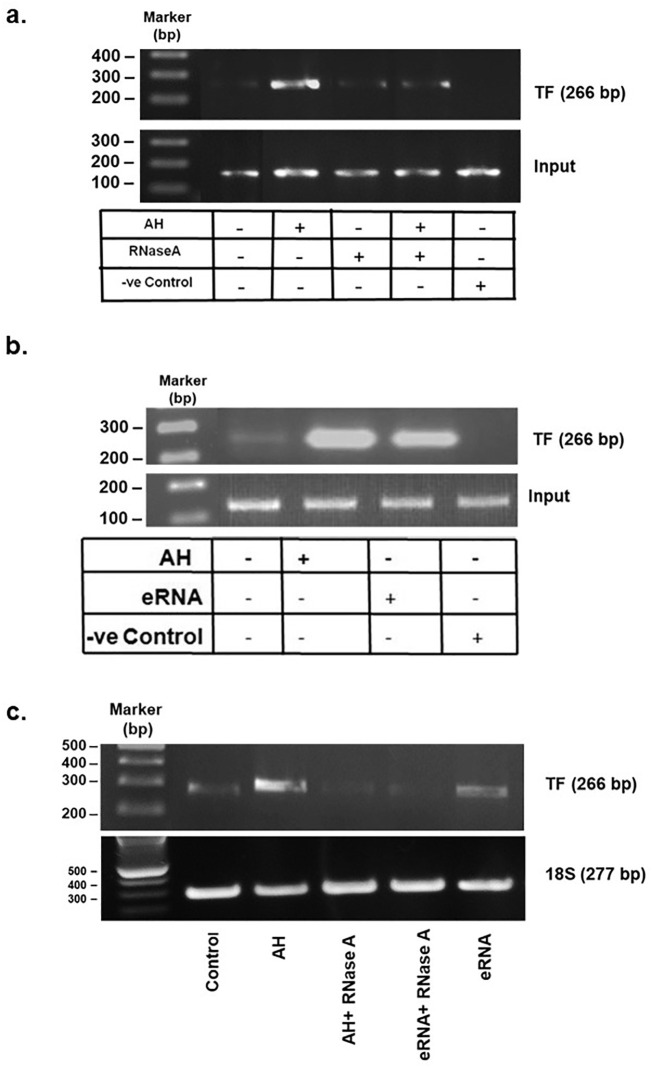
Transcription factors regulate the expression of proteins by binding to the promoter region of their corresponding genes. Therefore, chromatin immunoprecipitation (ChIP) assay was performed to evaluate whether c-Jun (a component of AP1 transcription factor) could bind to the promoter of TF and control its expression. eRNA was inhibited by treating the mice with RNaseA (2 h prior to AH) and in another set of experiment eRNA was injected into the mice and ChIP assay was performed. Our results show that AH or eRNA induced TF transcription through binding of c-Jun to the promoter of TF (Fig. 5a, b). However, pre-treatment of RNaseA significantly inhibits AH induced TF transcription. Treatment of eRNA was used as positive control for the assay. Furthermore, pre-treatment of RNaseA significantly abrogated either AH or eRNA induced TF mRNA expression (Fig. 5c). Taken together, these results suggest that AH-induced upregulation of TF is mediated by AP1.
Fig. 5.
Involvement of AP1 in TLR3-mediated TF upregulation in AH exposure. (a, b) ChIP assay for TF after different treatments. Negative control used in ChIP assay is the reaction mixture without TF antibody treatment; (c) RT-PCR of TF mRNA was performed in PBMCs after respective treatment. 18S RNA was used as loading control. Data are shown as representative of three independent experiment. ChIP, chromatin immunoprecipitation; TF, tissue factor; RT-PCR, reverse-transcriptase polymerase chain reaction; mRNA, messenger RNA; PBMCs, peripheral blood mononuclear cells.
3.6. Hypoxia induced TF activation is mediated through TLR3-ERK1/2-AP1 pathways
As established earlier, AH induced coagulation by activation of TF [22]. Our observation also implies that AH induces quantitative and qualitative changes in TF through eRNA-mediated activation of TLR3 and binding of AP1 to its cognate binding site on the TF promoter. However, the exact pathway for TF activation by eRNA during AH exposure is not known. Therefore, we employed both pharmacological and genetic approaches to determine whether inhibition of TF expression and fibrin deposition in lungs during AH is mediated by the TLR3-ERK1/2-AP1 pathway.
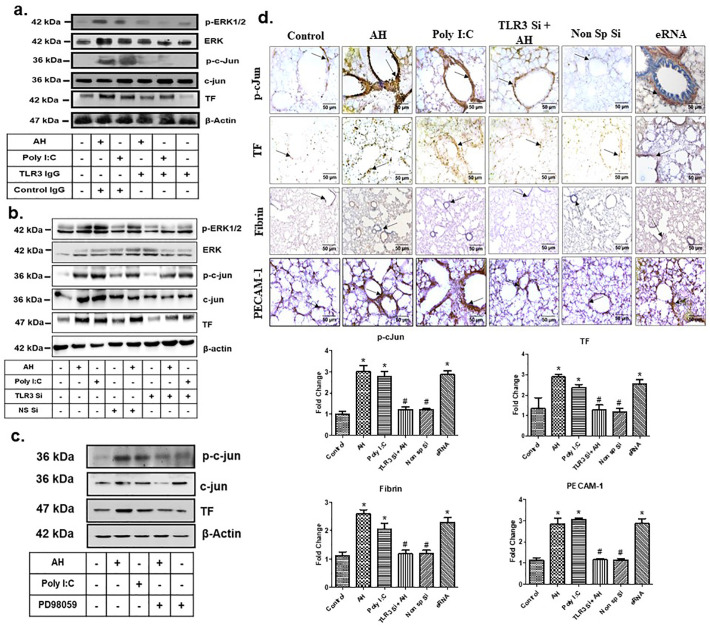
Specifically, we pre-treated mice with TLR3-neutralizing antibody (Anti-TLR3 IgG) prior to AH exposure. To confirm the role of TLR3, we also used TLR3 siRNA. Results were analysed by immunoblotting and immunohistochemistry. Pre-treatment with TLR3-neutralizing antibody, or TLR3 gene silencing significantly abrogated the AH-induced ERK1/2, c-Jun phosphorylation and TF expression (Fig. 6a, b and Supp Fig. 4a, b). ERK1/2 phosphorylation was also specifically inhibited by treatment of PD98059. Subsequent analysis also revealed that ERK inhibition significantly reduced c-Jun phosphorylation as well as TF expression in PBMCs (Fig. 6c, Supp Fig. 4c).
Fig. 6.
TLR3-ERK1/2-AP1 pathway promotes TF up-regulation during AH exposure. (a) Anti-TLR3 IgG treatment. Densitometry analysis of the western blots shown in Supp Fig. 4a; (b) TLR3 siRNA treatment. Densitometry analysis of the western blots shown in Supp Fig. 4b; (c) Treatment of ERK1/2 inhibitor (PD98059). Densitometry analysis of the western blots shown in Supp Fig. 4c; (d) Expression of phospho c-Jun, TF, PECAM1, fibrin deposition and c-Jun phosphorylation were analysed in lung by IHC in TLR3 gene-silenced mice. Poly I:C and eRNA were used as positive control. Data are shown as representative of three independent experiments. TLR3, Toll like receptor 3; siRNA, small interfering RNA; ERK, extracellular-signal-regulated kinase; TF, tissue factor; IHC, immunohistochemistry; poly I:C, polyinosinic: polycytidylic acid; eRNA, extracellular RNA.
To confirm these results, we performed TLR3 gene-silencing in vivo using TLR3 siRNA and exposed the mice to AH. Our results showed that TLR3 gene silencing markedly abrogated AH induced p-c-Jun expression, TF expression as well as fibrin deposition in lung as compared to control (Fig. 6d). Taken together, these results suggest that AH-induced TF activation and fibrin deposition in the lungs is mediated through the TLR3-ERK1/2-AP1 pathway. To confirm the TF expression in the endothelial cells of lungs, we stained the lung tissues for TF and PECAM1(CD31) as an endothelial cell's marker (Fig. 6d). Our result showed that AH exposure increases the TF expression on endothelial cells as analysed by TF and PECAM1 colocalization in immunofluorescence staining of lung tissue (Supp Fig. 5). AH exposure also leads to proinflammatory monocyte infiltration in the lung. To analyse the TF expression in inflammatory monocytes, lung tissues were stained for TF and CD11b (inflammatory monocyte marker). Our results showed AH exposure induce TF expression in infiltrated monocytes (Supp Fig. 6).
3.7. AH induced coagulation activation is attenuated by RNase treatment
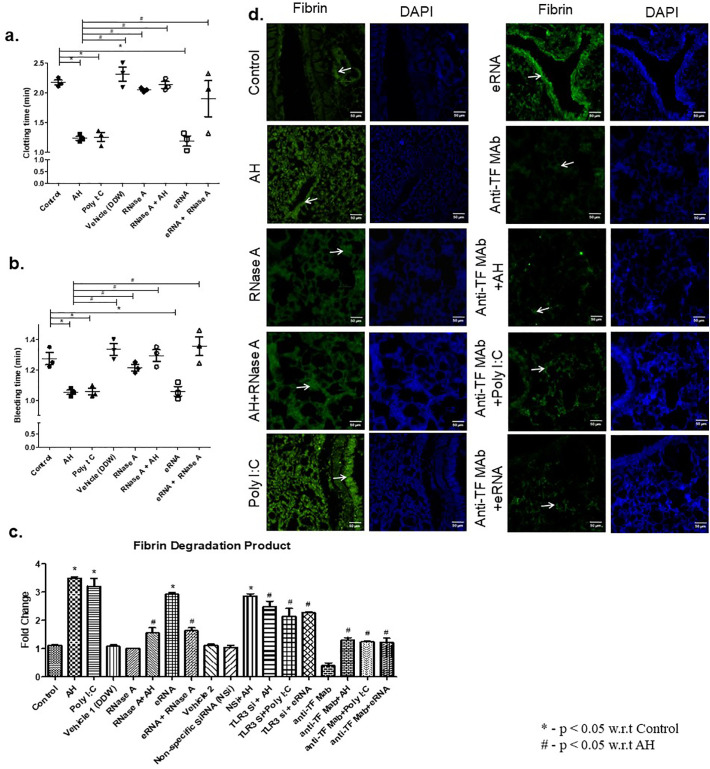
The above results suggest that AH-induced TF activation is due to eRNA release during hypoxia. TF activation leads to activation of coagulation through extrinsic pathways of coagulation. Therefore, to delineate the role of eRNA in AH induced coagulation, we performed a set of experiments and coagulation parameters were assayed. Our results showed that pre-treatment of RNaseA (2 h prior to AH exposure) significantly increased BT & CT (Fig. 7a, b) and diminished the level of FDP (Fig. 7c). eRNA and poly I:C were used as positive control and anti-TF antibody was used as an experimental control. We further showed that TLR3 silencing and TF pathway inhibition by anti-TF antibody significantly abrogated plasma level of FDP in the situation of exposure to AH and/or treatment of poly I:C (Fig. 7c). DDW (vehicle 1) was used for dissolution of RNaseA and siRNA was dissolved in RNALancer II solution (vehicle 2).
Fig. 7.
RNaseA attenuates AH-induced coagulation activation. (a) Clotting time, (b) Bleeding time and (c) Fibrin degradation product were analysed after indicated treatments. TLR3 agonist (poly I:C) and eRNA were used as positive controls and anti-TF monoclonal antibody treatment was used as an experimental control. Non-specific (NS) siRNA control was used similarly in another group of mice. Data are shown as mean ± SEM (n = 5/group/each treatment) and are pooled from three independently performed experiments with three mice per experiment. The results showed significant (*p < 0.05) difference in between treatment using one-way ANOVA. (d) Fibrin deposition (green) in lung vasculature was identified by immunofluorescence microscopy (40×) after indicated treatment. DAPI (blue) was used as a nuclear stain. Immunofluorescence experiments are representative of three independent times with three mice per experiment. (Scale bar: 50 μm). TLR3, Toll like receptor 3; poly I:C, polyinosinic: polycytidylic acid; eRNA, extracellular RNA; TF, tissue factor; siRNA, small interfering RNA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To assess the impact on eRNA on hypoxia-induced maladies, coagulation and fibrin deposition was analysed in the presence of RNaseA. Our result showed that AH exposure increased fibrin deposition in the lung vasculature, whereas pre-treatment of RNaseA significantly decreased AH-induced fibrin deposition (Fig. 7d). eRNA and poly I:C treatment was used as positive control and anti-TF antibody was used as an experimental control. We found that anti-TF antibody treatment, inhibit AH, eRNA and poly I:C induced fibrin deposition. We further try to explore whether eRNA has any role in platelet aggregation. Our results showed that eRNA failed to initiate platelet aggregation in compare to control (Supp Fig. 7). These findings might provide a crucial role of eRNA in simulation of coagulation and fibrin deposition but not platelet aggregation under AH exposure.
4. Discussion
Of late it was recognised that medical situations of local or systemic hypoxemia can cause a prothrombotic state [38]. The incidence of VT, which is primarily due to increased build-up of activated PBMCs in the lung, has been well established in murine models of hypoxic stress. These activated PBMCs mediate the initial response of TF activation, ultimately leading to fibrin deposition in the lungs [39]. Still, the process of activation of TF due to hypoxemia remains unclear. Considering the aforementioned information and the persistent clinical significance of VT in a number of ailments, we pursued to investigate the underlying means by which coagulation is altered through TF stimulation under hypoxia exposure. Here, we have established that AH-induced release of eRNA brought about an increase in TF activation. We have also validated the role of TLR3-ERK1/2-AP1 axis in the intervention of TF activation in AH.
Tissue factor, a glycoprotein expressed on the cellular surface, facilitates the cells to induct clotting via the initiation of the extrinsic pathway of coagulation [40]. After activation of TF it binds to factor VIIa and X and initiates a cascade of reactions which commence thrombin generation, triggers the platelets and leads to fibrin production from the precursor molecule fibrinogen [41]. Epithelium and perivascular barriers of the lung, brain, skin, placenta and intestine fundamentally express TF [42]. There are varied sources of TF at the cellular level in the vasculature and it is the cellular stimulation that mostly controls TF mediated prothrombotic activity [43]. Even though platelet expression of TF has been debated, platelet-like particles originating from in vitro differentiated human megakaryocytes reveals that platelets can not only contain and express protein and mRNA subpopulations of TF but activation of platelets can initiate intron-retained splicing for TF protein translation [44,45].
Identification of endogenous ligands for innate immune receptors by contemporary studies has created a substantial interest due to their high significance in SI. Exposure to hypoxia can stimulate apoptosis and necrosis of affected tissue resulting in liberation of DAMPs (eDNA, eRNA, and histone and non-histone DNA binding proteins) into circulation, which facilitates induction of the innate immune system [46]. eRNA is a well-documented endogenous ligand for TLR3 [14,16]. The grounds for analysing the functional role of eRNA in case of hypoxia exposure was centered upon the existing literature which states that arterial thrombosis is elevated due to these poly-anionic compounds [8]. In the past studies of acute models of vessel stenosis or chronic models of atherosclerosis it has been shown that there is an elevated plasma and tissue concentrations of eRNA [15]. Our study showed that tail vein injection of eRNA upregulates expression and activation of TF and alteration in the coagulatory parameters comparable to that of mice exposed to AH. eRNA and eDNA was also observed in circulation in plasma of hypoxia exposed animals. Yet, in vivo injection of RNaseA, and not DNase, significantly decreased TF expression as well as activity. It suggests that the eRNA released into circulation during the exposure to AH is the pivotal molecule responsible for coagulation. Also, the role of eRNA on fibrin deposition in lung vasculature after exposure to hypoxia was also assessed. Our results showed that AH exposure or eRNA treatment induced fibrin deposition in lung vasculature. However, pre-treatment of RNaseA significantly inhibited AH-induced fibrin deposition. RNaseA significantly abrogated the functional effects of eRNA, thereby emerging as a vital player for vessel-and tissue-protection [47]. However, our study further suggests that eRNA has no role in platelet aggregation. This suggests that AH-induced eRNA release leads to accumulation of fibrin in the vasculature of lungs.
Thus far, the definitive signalling mechanism involved in AH-induced TF activation remains unexplored. Stress induction on the cells on exposure to hypoxia leads to advancement of ERK1/2 phosphorylation and its nuclear translocation to impart its biological activity [48]. ERK is a stress kinase belonging to the MAPK subfamily and stimulates the action of various transcription factors for regulation of gene expression along with cellular proliferation, differentiation and cell survival mechanisms [49]. Upon detailed investigation of human TF promoter region, it was confirmed that the induction of TF response is mediated by a 56-bp enhancer (−227 to −172) which comprises of two AP1 and one NFκβ-like site [50]. Evaluation of the AP1 sites by EMSA method led to the discovery that these sites integrally bind to the heterodimer of c-Fos & c-Jun to expedite the transcription of TF gene. For the above reason, we studied the likely role of ERK1/2-AP1 pathway in AH induced TF activation.
As we know, hypoxia plays a vital role in both ischemic and neoplastic diseases. Analysis of earlier studies directly connect HIF to be the foremost regulator of entire RNA transcriptome in case of hypoxia exposure [51]. Hence, it can be postulated that eRNA expression and release in case of hypoxia can occur not only through dying cells but also be regulated via HIF expression.
TLR3, involved in the IFN response, in case of hypoxia upregulates the expression of IFNα and IFNγ [16]. The release of eRNA upon hypoxia exposure upregulates ICAM and PECAM causing infiltration of proinflammatory monocytes and neutrophils. As already established, inflammatory monocytes are responsible for TF expression and hence it is possible that IFNs play an important role in hypoxia induced TF expression [29]. We have also shown that AH induced the phosphorylation of ERK1/2 and TF expression as well as activation are mediated through TLR3 [22]. Here, we showed AH–induced eRNA release as well as coagulation activation. However, injection of eRNA in animal also showed ERK1/2 phosphorylation as shown in AH exposure. The study implies that eRNA-TLR3 axis is similarly responsible for mediating ERK1/2 phosphorylation. Prevention of TF antigen and activity stimulation and ERK1/2 phosphorylation was observed after pre-treatment of the mice with RNaseA and/or TLR3 siRNA prior to exposure to AH or poly I:C treatment. Correspondingly, similar abrogation of the above parameters was observed upon PD98059 (a specific ERK inhibitor) pre-treatment before exposure to AH. Additionally, these results validate the dominant role of ERK phosphorylation on TF stimulation mediated by TLR3 under hypoxic conditions.
Dysfunction in coagulation and fibrinolysis has been observed in various virus infections such as Ebola virus [52], dengue virus [53], etc. that impair coagulation by infecting the endothelial cells [54] and monocytes [55] with their RNA genome [56]. Direct up-regulation of TF in EC has been demonstrated in the case of influenza, adenovirus, etc. [57].
Endothelial cells, which vividly respond to the viral infection, can get infected by COVID-19 and lead to TLR3 activation by viral RNA [58]. It has also been reported that TLR3 activation by viral RNA analogue poly I:C can activate tissue factor in endothelial cells [12]. Interestingly, hypoxic condition, as found in COVID-19, is also known to activate TLR3 activation through eRNA lead to monocyte infiltration, TF expression and hypercoagulation [22,29]. This suggests that viral genetic material could activate TF and coagulation. Therefore, TLR3 may also be involved in COVID-19-mediated coagulation disorder leading to overt clot formation, pulmonary embolism with detrimental effects in patient recovery and survival.
5. Conclusions
Our study presents evidence that eRNA released due to AH exposure activates TLR3, which further mediates upregulation of expression of TF and fibrin deposition in lungs through the TLR3˗ERK1/2˗AP1 axis. Thus, we attempt to define the underlying mechanism of TF mediated coagulation upregulation and lung fibrin deposition associated with hypoxia-induced thrombosis. Also, RNaseA treatment comes up as a conceivable therapeutic remedy for coagulation disorders.
Acknowledgments
Acknowledgement
We would like to acknowledge and thank various division of DIPAS-DRDO who helped us in the work by providing permission to use certain instruments in their labs. Madiha Khan, The Heritage School, Kolkata for English languages correction.
Funding
This work was supported by grants from the Ministry of Defence, Govt. of India (DIP-253) & Grant Challanges Canada (R-ST-POC-1807-13914), Canada to GAK.
Editor: Mohandas Narla
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bcmd.2020.102459.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Anand A.C., Saha A., Seth A.K., Chopra G.S., Nair V., Sharma V. Symptomatic portal system thrombosis in soldiers due to extended stay at extreme altitude. Eur. J. Gastroen. Hepat. 2005;20:777–783. doi: 10.1111/j.1440-1746.2005.03723.x. [DOI] [PubMed] [Google Scholar]
- 2.Malone P.C. A hypothesis concerning the aetiology of venous thrombosis. Med. Hypotheses. 1977;3:189–201. doi: 10.1016/0306-9877(77)90005-6. [DOI] [PubMed] [Google Scholar]
- 3.Ezgü F.S., Atalay Y., Hasanoglu A., Gücüyener K., Koç E., Ergenekon E. Intracranial venous thrombosis after hypoxia-ischemia brain insult in two newborns: could low serum carnitine levels have contributed? Nutr. Neurosci. 2004;7:63–65. doi: 10.1080/1028415042000194612. [DOI] [PubMed] [Google Scholar]
- 4.Goldhaber S.Z. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J. Am. Coll. Cardiol. 1992;19:246–247. doi: 10.1016/0735-1097(92)90473-Z. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosetti M., Ageno W., Spanevello A., Salerno M., Pedretti R.F. Prevalence and prevention of venous thromboembolism in patients with acute exacerbations of COPD. Thromb. Res. 2003;112:203–207. doi: 10.1016/j.thromres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Rizkallah J., Man S.F.P., Sin D.D. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135:786–793. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- 7.Brunelle J.K., Chandel N.S. Oxygen deprivation induced cell death: an update. Apoptosis. 2002;7:475–482. doi: 10.1023/A:1020668923852. [DOI] [PubMed] [Google Scholar]
- 8.Kannemeier C., Nakazawa F., Trusheim H., Ruppert C., Markart P., Song Y. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Prot. Natl. Acad. Sci. USA. 2007;104:6388–9363. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deindl E., Fischer S., Preissner K.T. New directions in inflammation and immunity: the multi-functional role of the extracellular RNA/RNase system, Indian. J. Biochem. Biophys. 2009;46:461–466. http://nopr.niscair.res.in/handle/123456789/7249 [PubMed] [Google Scholar]
- 10.Fischer S., Gerriets T., Wessels C., Walberer M., Kostin S., Stolz E. Extracellular RNA mediates endothelial-cell permeability via vascular endothelial growth factor. Blood. 2007;110:2457–2465. doi: 10.1182/blood-2006-08-040691. [DOI] [PubMed] [Google Scholar]
- 11.Fischer S., Grantzow T., Pagel J.I., Tschernatsch M., Sperandio M., Preissner K.T. Extracellular RNA promotes leukocyte recruitment in the vascular system by mobilising proinflammatory cytokines. Thromb. Haemost. 2012;108:730–741. doi: 10.1160/TH12-03-0186. [DOI] [PubMed] [Google Scholar]
- 12.Shibamiya A., Hersemeyer K., Wöll T.S., Sedding D., Daniel J.M., Bauer S. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood. 2009;113:714–722. doi: 10.1182/blood-2008-02-137901. [DOI] [PubMed] [Google Scholar]
- 13.Karikó K., Ni H., Capodici J., Lamphier M., Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.m310175200. [DOI] [PubMed] [Google Scholar]
- 14.Cavassani K.A., Ishii M., Wen H., Schaller M.A., Lincoln P.M., Lukacs N.W. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simsekyilmaz S., Cabrera-Fuentes H.A., Meiler S., Kostin S., Baumer Y., Liehn E.A. Response to letter regarding article “Role of extracellular RNA in atherosclerotic plaque formation in mice”. Circulation. 2014;130:e144–e145. doi: 10.1161/circulationaha.114.012346. [DOI] [PubMed] [Google Scholar]
- 16.Rasschaert J., Ladrière L., Urbain M., Dogusan Z., Katabua B., Sato S. Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA+ interferon-gamma-induced apoptosis in primary pancreatic beta-cells. J. Biol. Chem. 2005;280:33984–33991. doi: 10.1074/jbc.m502213200. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y., Chao W. Toll-like receptors and myocardial inflammation. Int. J. Inflam. 2011;2011 doi: 10.4061/2011/170352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksu K., Donmez A., Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr. Pharm. Des. 2012;18:1478–1493. doi: 10.2174/138161212799504731. [DOI] [PubMed] [Google Scholar]
- 19.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:135–143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 20.Frijns C.J., Kappelle L.J. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- 21.Fröhlich S., Boylan J., McLoughlin P. Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am. J. Respir. Cell Mol. Biol. 2012;48:271–279. doi: 10.1165/rcmb.2012-0137tr. [DOI] [PubMed] [Google Scholar]
- 22.Biswas I., Garg I., Singh B., Khan G.A. A key role of Toll-like receptor 3 in tissue factor activation through extracellular signal regulated kinase 1/2 pathway in a murine hypoxia model. Blood Cells Mol. Dis. 2012;49:92–101. doi: 10.1016/j.bcmd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Sethy N.K., Singh M., Kumar R., Ilavazhagan G., Bhargava K. Upregulation of transcription factor NRF2-mediated oxidative stress response pathway in rat brain under short-term chronic hypobaric hypoxia. Funct. Integr. Genomics. 2011;11:119–137. doi: 10.1007/s10142-010-0195-y. [DOI] [PubMed] [Google Scholar]
- 24.De Meyer S.F., Suidan G.L., Fuchs T.A., Monestier M., Wagner D.D. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:1884–1891. doi: 10.1161/atvbaha.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walberer M., Tschernatsch M., Fischer S., Ritschel N., Volk K., Friedrich C. RNase therapy assessed by magnetic resonance imaging reduces cerebral edema and infarction size in acute stroke. Curr. Neurovasc. Res. 2009;6:12–19. doi: 10.2174/156720209787466037. [DOI] [PubMed] [Google Scholar]
- 26.Nair A.B., Jacob A. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C., Feng Y., Zou L. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J. Am. Heart Assoc. 2014;3 doi: 10.1161/jaha.113.000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiggins J.F., Ruffino L., Kelnar K., Omotola M., Patrawala L., Brown D. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;25:5923–5930. doi: 10.1158/0008-5472.can-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas I., Singh B., Sharma M., Agrawala P.K., Khan G.A. Extracellular RNA facilitates hypoxia-induced leukocyte adhesion and infiltration in the lung through TLR3-IFN-?-STAT1 signaling pathway. Eur. J. Immunol. 2015;9:3158–3173. doi: 10.1002/eji.201545597. [DOI] [PubMed] [Google Scholar]
- 30.van Herck H., Baumans V., Boere H.A., Hesp A.P., van Lith H.A., Beynen A.C. Orbital sinus blood sampling in rats: effects upon selected behavioural variables. Lab. Anim. 2000;34:10–19. doi: 10.1258/002367700780577993. [DOI] [PubMed] [Google Scholar]
- 31.Hofman F.M., Kuenessberg B., Smith M.D., Garrison D., Sevier E.D. Stability of T- and B-cell numbers in human peripheral blood. Am. J. Clin. Pathol. 1982;1:710–713. doi: 10.1093/ajcp/77.6.710. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita T., Sekiguchi A., Iwasaki Y.K., Sagara K., Hatano S., Iinuma H.et.al. Thrombomodulin and tissue factor pathway inhibitor in endocardium of rapidly paced rat atria. Circulation. 2003;108:2450–2452. doi: 10.1161/01.cir.0000102969.09658.f2. [DOI] [PubMed] [Google Scholar]
- 33.Khan G.A., Girish G.V., Lala N., Di Guglielmo G.M., Lala P.K. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol. Endocrinol. 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 1972;1091:135. (PMID: 4113792) [PubMed] [Google Scholar]
- 35.Carey M.F., Peterson C.L., Smale S.T. Chromatin immunoprecipitation (ChIP) Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5279. [DOI] [PubMed] [Google Scholar]
- 36.Lemini C., Rubio-Póo C., Silva G., García-Mondragón J., Zavala E., Mendoza-Patiño N. Anticoagulant and estrogenic effects of two new 17 beta-aminoestrogens, butolame [17 beta-(4-hydroxy-1-butylamino)-1,3,5(10)-estratrien-3-ol] and pentolame [17 beta-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol] Steroids. 1993;58:457–461. doi: 10.1016/0039-128X(93)90002-5. [DOI] [PubMed] [Google Scholar]
- 37.Dejana E., Callioni A., Quintana A., de Gaetano G. Bleeding time in laboratory animals. II - a comparison of different assay conditions in rats. Thromb. Res. 1979;15:191–197. doi: 10.1016/0049-3848(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 38.Dickson B.C. Virchow’s triad. Brit. J. Haematol. 2009;145:433. doi: 10.1111/j.1365-2141.2009.07617.x. [DOI] [PubMed] [Google Scholar]
- 39.Lawson C.A., Yan S.D., Yan S.F., Liao H., Zhou Y.S., Sobel J. Monocytes and tissue factor promote thrombosis in a murine model of oxygen deprivation. J. Clin. Invest. 1997;99:1729–1738. doi: 10.1172/jci119337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach R.R. Initiation of coagulation by tissue factor. Crit. Rev. Biochem. 1988;23:336–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 41.Owens A.P., 3rd, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb. Hemost. 2010;104:432–439. doi: 10.1160/th09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Østerud B., Bjørklid E. Sources of tissue factor. Semin. Thromb. Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 43.Chu A.J. Tissue factor, blood coagulation, and beyond: an overview. Int. J. Inflam. 2011;2011 doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brambilla M., Facchinetti L., Canzano P., Rossetti L., Ferri N., Balduini A. Human megakaryocytes confer tissue factor to a subset of shed platelets to stimulate thrombin generation. Thromb. Hemost. 2015;114:579–592. doi: 10.1160/th14-10-0830. [DOI] [PubMed] [Google Scholar]
- 45.Schwertz H., Tolley N.D., Foulks J.M., Denis M.M., Risenmay B.W., Buerke M. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J. Exp. Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczmarek A., Vandenabeele P., Krysko D.V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:206–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Fischer S., Preissner K.T. Extracellular nucleic acids as novel alarm signals in the vascular system. Mediators of defence and disease. Hamostaseologie. 2013;33:37–42. doi: 10.5482/hamo-13-01-0001. [DOI] [PubMed] [Google Scholar]
- 48.Schweppe R.E., Cheung T.H., Ahn N.G. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J. Biol. Chem. 2006;281:20993–21003. doi: 10.1074/jbc.m604208200. [DOI] [PubMed] [Google Scholar]
- 49.Irving E.A., Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Parry G.C.N., Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1995;15:612–621. doi: 10.1161/01.ATV.15.5.612. [DOI] [PubMed] [Google Scholar]
- 51.Choudhry H., Schödel J., Oikonomopoulos S., Camps C., Grampp S., Harris A.L., Ratcliffe P.J., Ragoussis J., Mole D.R. Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 2014;15:70–76. doi: 10.1002/embr.201337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hensley L.E., Geisbert T.W. The contribution of the endothelium to the development of coagulation disorders that characterize Ebola hemorrhagic fever in primates. Thromb. Haemost. 2005;94:254–261. doi: 10.1160/th05-03-0153. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Z., Tang X., Xiao R., Jiang L., Chen X. Dengue virus regulates the expression of hemostasis-related molecules in human vein endothelial cells. J. Inf. Secur. 2007;55:e23–e28. doi: 10.1016/j.jinf.2007.04.351. [DOI] [PubMed] [Google Scholar]
- 54.Peters C.J., Zaki S.R. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 2002;30:S268–S273. doi: 10.1097/00003246-200205001-00016. [DOI] [PubMed] [Google Scholar]
- 55.Stroher U., West E., Bugany H., Klenk H.D., Schnittler H.J., Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 2001;75:11025–11033. doi: 10.1128/jvi.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J.P., Cosgriff T.M. Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul. Fibrinolysis. 2000;11:461–483. doi: 10.1097/00001721-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Visseren F.L., Bouwman J.J., Bouter K.P., Diepersloot R.J., de Groot P.H., Erkelens D.W. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb. Haemost. 2000;84:319–324. (PMID: 10959707) [PubMed] [Google Scholar]
- 58.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures